Protein for specifically detecting Mycobacterium tuberculosis infection
A Mycobacterium tuberculosis, specific technology, applied in the field of medical products, can solve the problems of unsatisfactory sensitivity and specificity of Mycobacterium tuberculosis antibody, insensitivity of latent infection diagnosis of Mycobacterium tuberculosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Rv3899c-pDEST17 expression vector construction basic process
[0041] by LR Enzyme mix catalysis Rv3899c The entry carrier (provided free of charge by PFGRC under the Craig VentorInstitute in the United States) and pDEST TM 17 vectors were recombined to generate the expression vector of Rv3899c.
[0042] specific method:
[0043] A. Cloning reaction system: 1.5 μL of entry vector of Rv3899c, pDEST TM 17 Vehicle 1 μL, BP II Enzyme mix 2.5μL; Reaction conditions: 25℃, react overnight.
[0044] B. Transformation method: Add 100 μL Escherichia coli DH5α competent (self-made) to the reaction system, ice bath for 30 minutes, heat shock at 42°C for 90 seconds, place the system on ice for 10 minutes, add 200 μL LB medium for renaturation, and spread it on ampicillin-containing On LB solid medium of penicillin (100mg / l), culture at 37°C for 20h.
[0045] C. Plasmid extraction: The plasmid was extracted with N96 high-purity plasmid mini-extraction kit...
Embodiment 2
[0048] Example 2. Expression and renaturation of target protein Rv3899c
[0049] 1. Protein expression
[0050] a) LB plates were activated by streaking (adding kan), and left at 37°C overnight (about 16 hours).
[0051] b) Inoculate the Escherichia coli host cells transformed with the Rv3899c-integrated expression vector prepared in Example 1 in 5ml liquid LB medium (5ul kan added) at 37°C and 200rpm, and cultivate until the OD is greater than 0.6-0.8 (about 3h).
[0052] c) Inoculate 5 ml of the inoculum into 300 ml of LB liquid medium, culture at 37° C. and 200 rpm until OD600 ≈ 0.6-0.8 (about 2 hours). Cool down to 16°C, add final concentration 0.04mM IPTG
[0053] d) Cultivate at 16° C. at 200 rpm for 16 hours, then centrifuge at 4000 rpm for 10 minutes at 4° C. to collect bacteria.
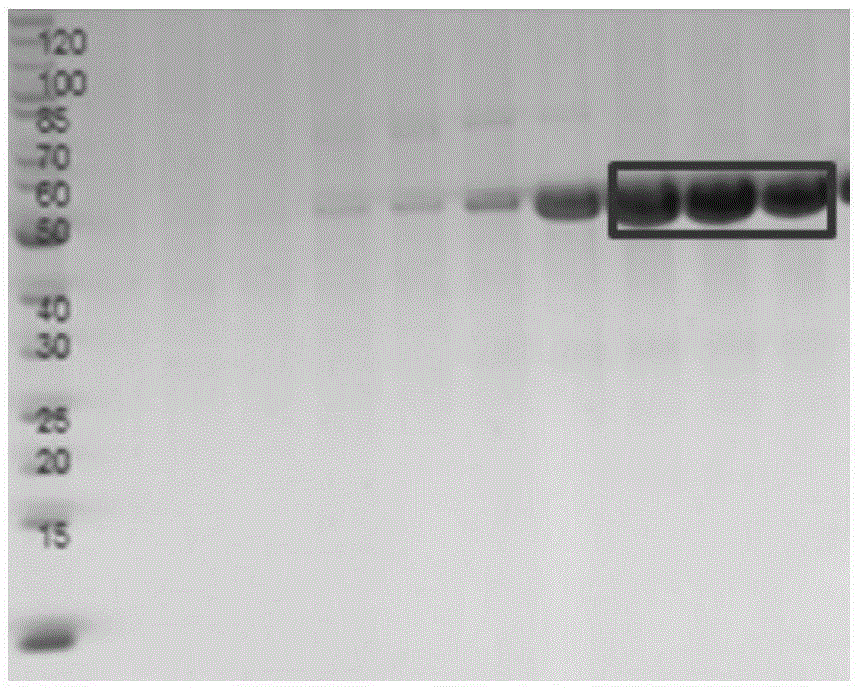
[0054] 2. Protein purification
[0055]a) Bacterial resuspension: Resuspend the collected bacteria with about 40ml of lysis buffer per 1L of culture medium (add 1% protease inhibitor PMS...
Embodiment 3
[0069] Example 3. Immunogenicity test of target protein Rv3899c
[0070] In order to detect the immunogenicity of Rv3899c and its enhancement effect on the existing antigen detection kits, further antigen detection screening was carried out on multiple cases of clinical diagnosis from Beijing Chest Hospital.
[0071] Test blood samples: from patients in Beijing Chest Hospital
[0072] Positive control reagents and consumables: T-SPOT kit (ESAT-6 and CFP 10 double antigen kit), Oxford immunotec (UK) product,
[0073] Negative control: Rv2327 protein antigen, another randomly selected piece of known protein fragment Rv2327 derived from Mycobacterium tuberculosis, its nucleotide sequence and amino acid sequence are shown in SEQ ID NO.3 and SEQ ID NO.4 respectively
[0074] SEQ ID No.3
[0075]
[0076]
[0077] SEQ ID No.4
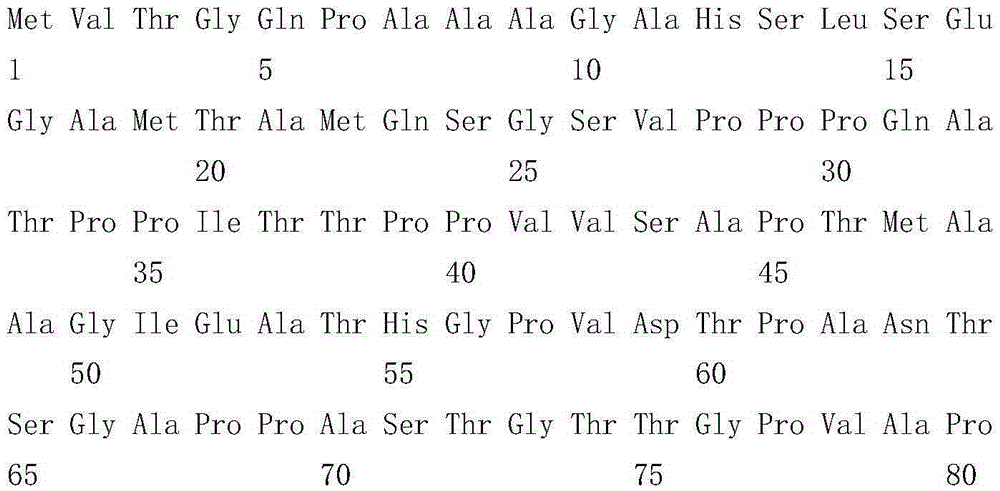
[0078]
[0079] The experimental methods and evaluation methods are as follows:
[0080] 1) Heparin anticoagulant blood, anticoagulant blood sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com