Diluent capable of improving stability of acridinium ester antigen-antibody conjugate and reducing background and preparation method thereof
A technology of antigen-antibody and conjugates, which is applied in the field of immunoassay, can solve the problems of reagent performance and stability discount, non-specific binding increase, diluent effect is not good, etc., to achieve good protection effect, less components, and good blocking Sexual and protective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
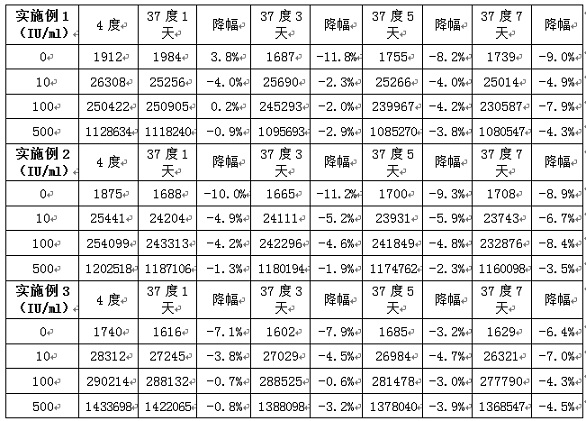
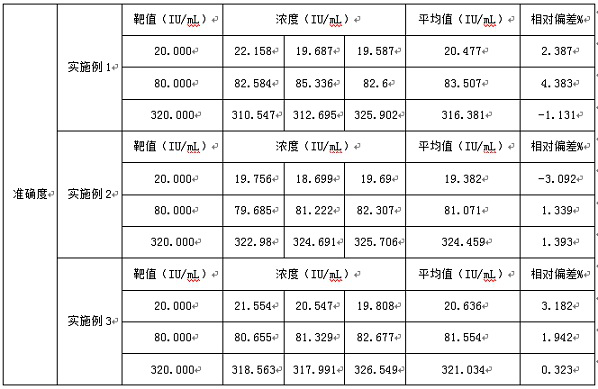
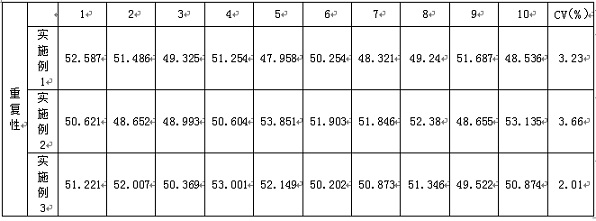
Examples
Embodiment 1
[0059] Embodiment 1: A diluent that can improve the stability of the acridinium ester antigen-antibody conjugate and reduce the background, including the following components:
[0060] Tris-base: 5.0g;
[0061] Concentrated hydrochloric acid: 3.5mL;
[0062] Polyethylene glycol-6000: 9.0g;
[0063] 10% sodium lauryl sulfate: 0.95mL;
[0064] Tween: 9.5mL;
[0065] Disodium edetate: 0.8g;
[0067] Proclin300: 0.95mL;
[0068] Triton X-100: 9.5mL;
[0069] Use purified water for in vitro diagnostic reagents, add to 1000mL.
[0070] A method for preparing a diluent capable of improving the stability of an acridinium ester antigen-antibody conjugate and reducing the background, comprising the following steps:
[0071] In the first step, according to the above formula, each component is weighed for subsequent use;
[0072] In the second step, take an appropriate amount of purified water, add Tris-base, polyethylene glycol 6000, 10% sodium laur...
Embodiment 2
[0077] A diluent capable of improving the stability of an acridinium ester antigen-antibody conjugate and reducing the background, comprising the following components:
[0078] Tris-base: 6.057g
[0079] Concentrated hydrochloric acid: 3.8mL
[0080] Polyethylene glycol-6000: 10g
[0081] 10% sodium lauryl sulfate: 1.0mL
[0082] Tween: 10mL
[0083] Disodium EDTA: 0.93g
[0085] Proclin300: 1.0mL
[0086] Triton X-100: 10mL
[0087] Purified water, add to 1000mL.
[0088] A method for preparing a diluent capable of improving the stability of an acridinium ester antigen-antibody conjugate and reducing the background, comprising the following steps:
[0089] In the first step, according to the above formula, each component is weighed for subsequent use;
[0090] In the second step, take an appropriate amount of purified water, add Tris-base, polyethylene glycol 6000, 10% sodium lauryl sulfate, Tween, disodium edetate, Proclin300, and Triton X-...
Embodiment 3
[0095] A diluent capable of improving the stability of an acridinium ester antigen-antibody conjugate and reducing the background, comprising the following components:
[0096] Tris-base: 7.0g
[0097] Concentrated hydrochloric acid: 4.0mL
[0098] Polyethylene glycol-6000: 11.0g
[0099] 10% sodium lauryl sulfate: 1.05mL
[0100] Tween: 10.5mL
[0101] Disodium EDTA: 1.0g
[0103] Proclin300: 1.05mL
[0104] Triton X-100: 10.5mL
[0105] Use purified water for in vitro diagnostic reagents, add to 1000mL.
[0106] A method for preparing a diluent capable of improving the stability of an acridinium ester antigen-antibody conjugate and reducing the background, comprising the following steps:
[0107] In the first step, according to the above formula, each component is weighed for subsequent use;
[0108] In the second step, take an appropriate amount of purified water, add Tris-base, polyethylene glycol 6000, 10% sodium lauryl sulfate, Twee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com