Detection method and detection kit for SARS-CoV-2 neutralizing antibody
A technology of antibody detection and detection method, applied in the field of biomedical detection, can solve the problems of not being able to know the infection risk of the tested person, slow response of neutralizing antibody detection, narrow linear range, etc., to achieve high accuracy, widen linear range, and sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Reagent Preparation 1
[0040] Preparation of Reagent R1
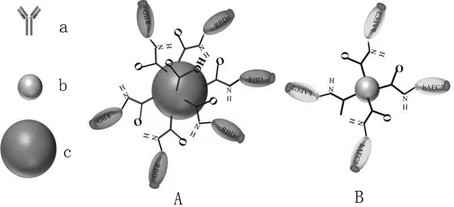
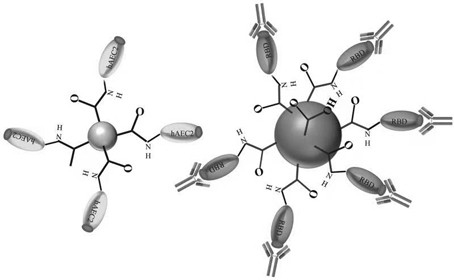
[0041] The latex microspheres coupled with the S protein receptor binding domain (RBD) of SARS-CoV-2 in R1 in the kit are polystyrene latex microspheres with a diameter of 278nm and surface carboxyl modification (category number P0113, Manufacturer JSR Life Sciences Corporation).
[0042] Marking: Polystyrene latex microspheres with a diameter of 278nm and surface carboxyl modification are placed in 0.1M Hepes solution (pH=7.5), and 0.1M EDC (pH 6.3) is added to the latex solution to activate at 37°C for 0.5h, stirring at a constant speed Stir and react on the instrument (300r / min) for 0.5h, then add 0.01M NHS and react for 20min, then add RBD protein for coupling reaction for 1.5h, add blocking agent after the reaction is completed for 2.5h, and finally age at 37°C for 24h.
[0043] In this embodiment, the reaction solution R1 includes RBD-labeled latex microspheres, buffer solution, sugar, salt, ...
Embodiment 2
[0052] Example 2 Reagent Preparation 2
[0053] Preparation of Reagent R1
[0054] The latex microspheres coupled with the S protein receptor binding domain (RBD) of SARS-CoV-2 in R1 in the kit are polystyrene latex microspheres with a diameter of 100 nm and surface carboxyl modification (category number P0113, Manufacturer JSR Life Sciences Corporation).
[0055] Marking: Polystyrene latex microspheres with a diameter of 100nm and surface carboxyl group modification were activated in 0.1M phosphate buffer (pH=6.8), and 0.05M EDC (pH 6.0) was added to the latex solution for 0.5h at 37°C. Stir and react on a stirrer (100r / min) for 0.5h, then add 0.01M NHS and react for 10min, then add RBD protein for coupling reaction for 1h, after the reaction is completed, add a blocking agent to seal for 2h, and finally age at 37°C for 12h.
[0056] In this embodiment, the reaction solution R1 includes RBD-labeled latex microspheres, buffer solution, sugar, salt, stabilizer (sealing agen...
Embodiment 3
[0065] Example 3 Reagent Preparation 3
[0066] Preparation of Reagent R1
[0067] The latex microspheres coupled with the S protein receptor binding domain (RBD) of SARS-CoV-2 in R1 in the kit are polystyrene latex microspheres with a diameter of 400 nm and surface carboxyl modification (category number P0113, Manufacturer JSR Life Sciences Corporation).
[0068] Marking: Polystyrene latex microspheres with a diameter of 400nm and surface carboxyl modification were activated in 0.1M MES buffer (pH=6.0), and 0.2M EDC (pH 6.5) was added to the latex solution for 0.5h at 37°C. Stir and react on a stirrer (500r / min) for 0.5h, then add 0.2M NHS and react for 30min, then add RBD protein for coupling reaction for 2h, after the reaction is completed, add a blocking agent for blocking for 3h, and finally age at 37°C for 48h.
[0069] In this embodiment, the reaction solution R1 includes RBD-labeled latex microspheres, buffer solution, sugar, salt, stabilizer (sealing agent), prese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com