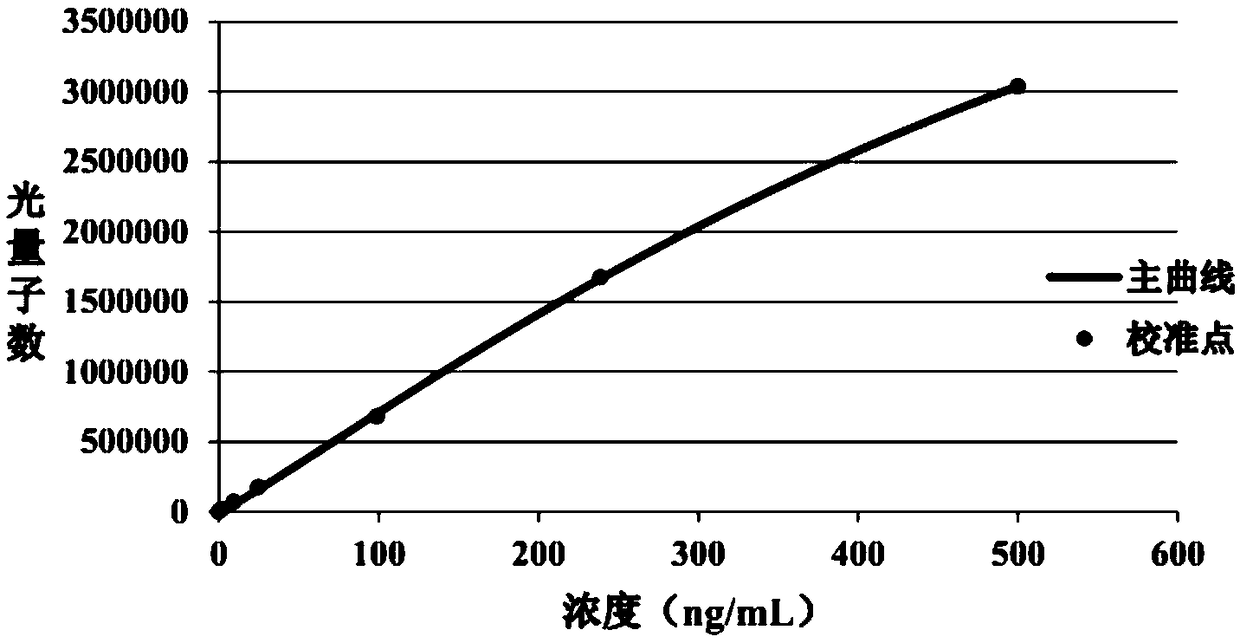
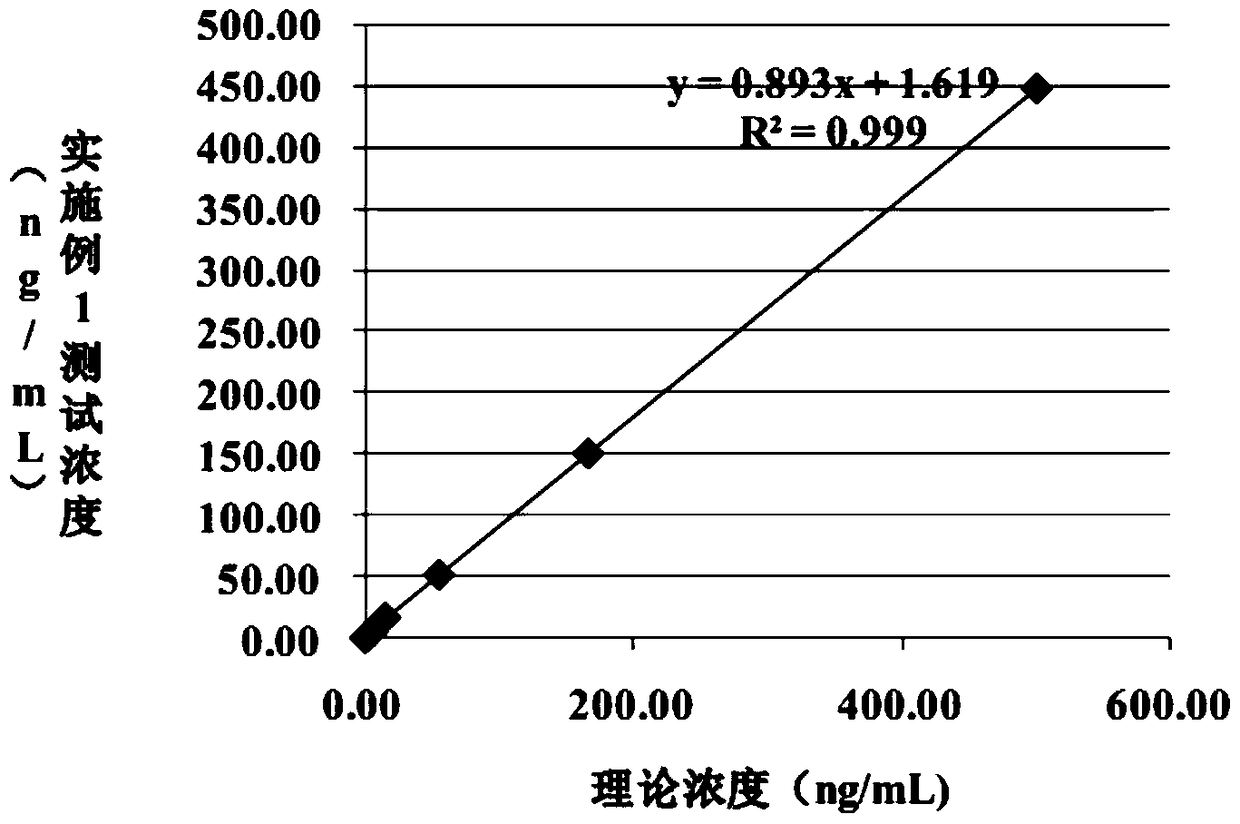
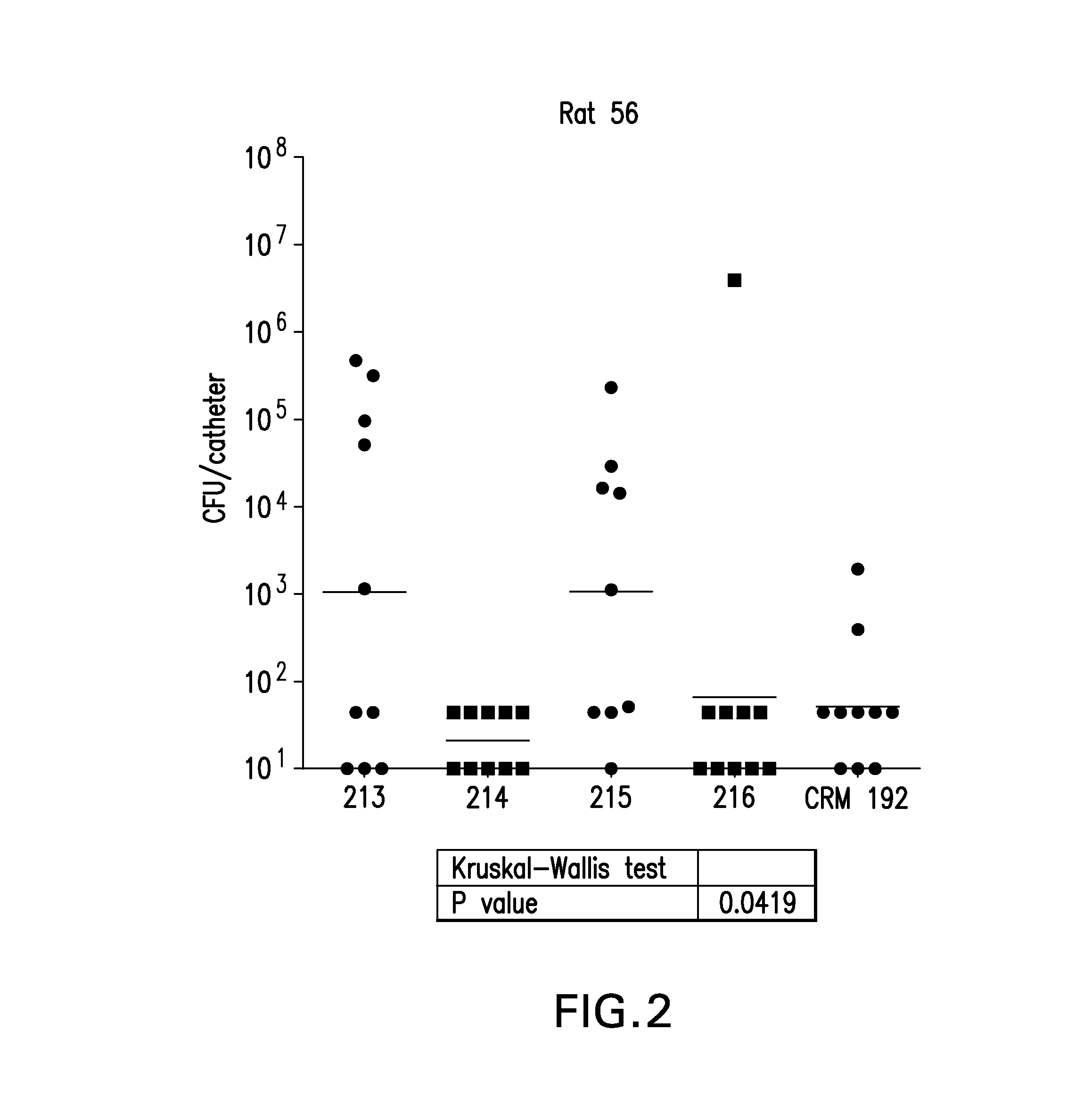
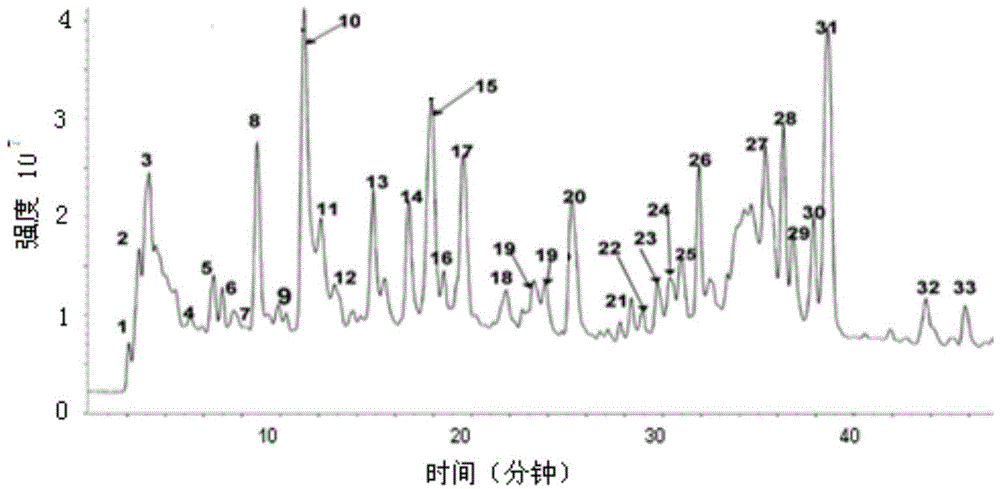
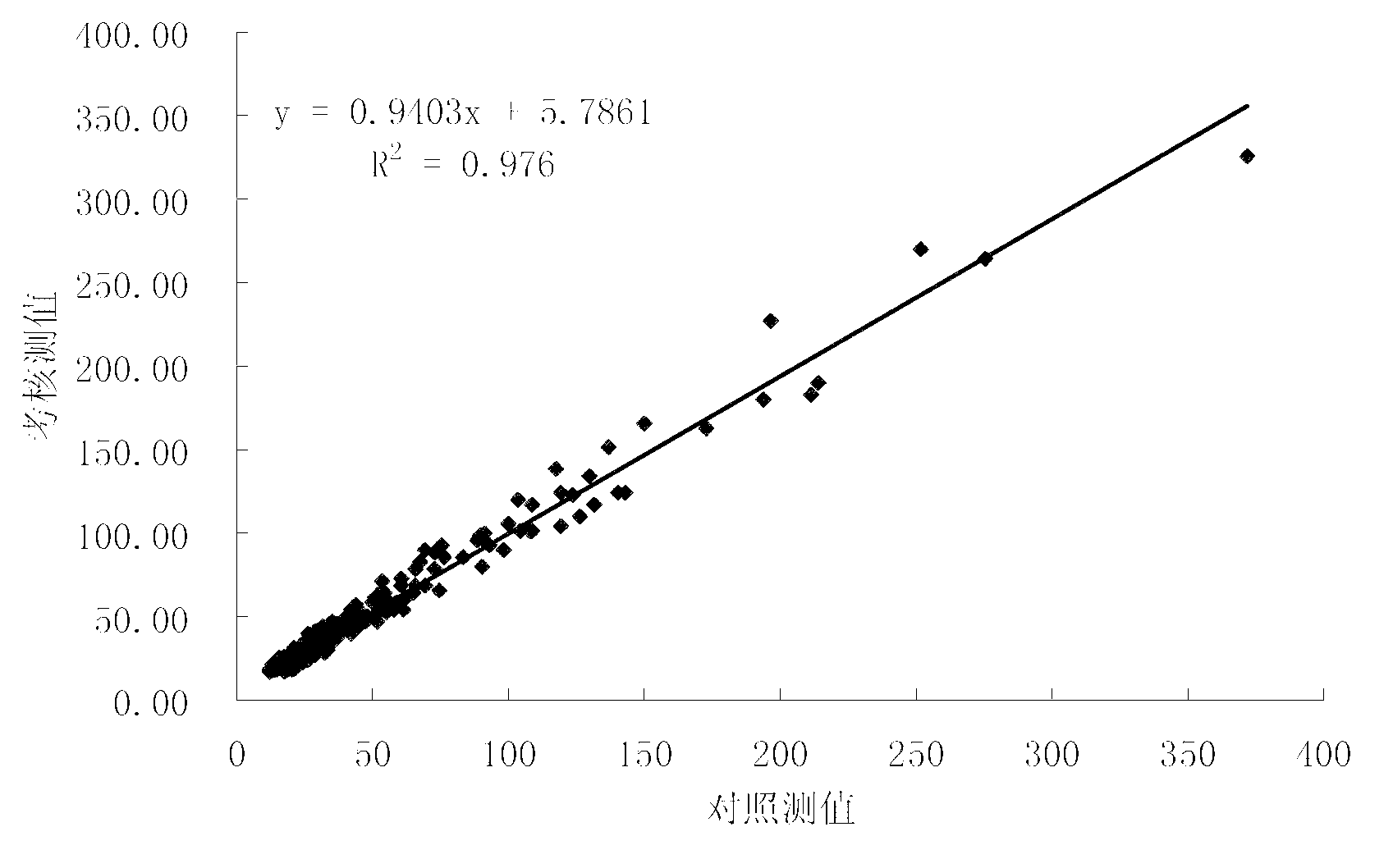Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
105 results about "Enolase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
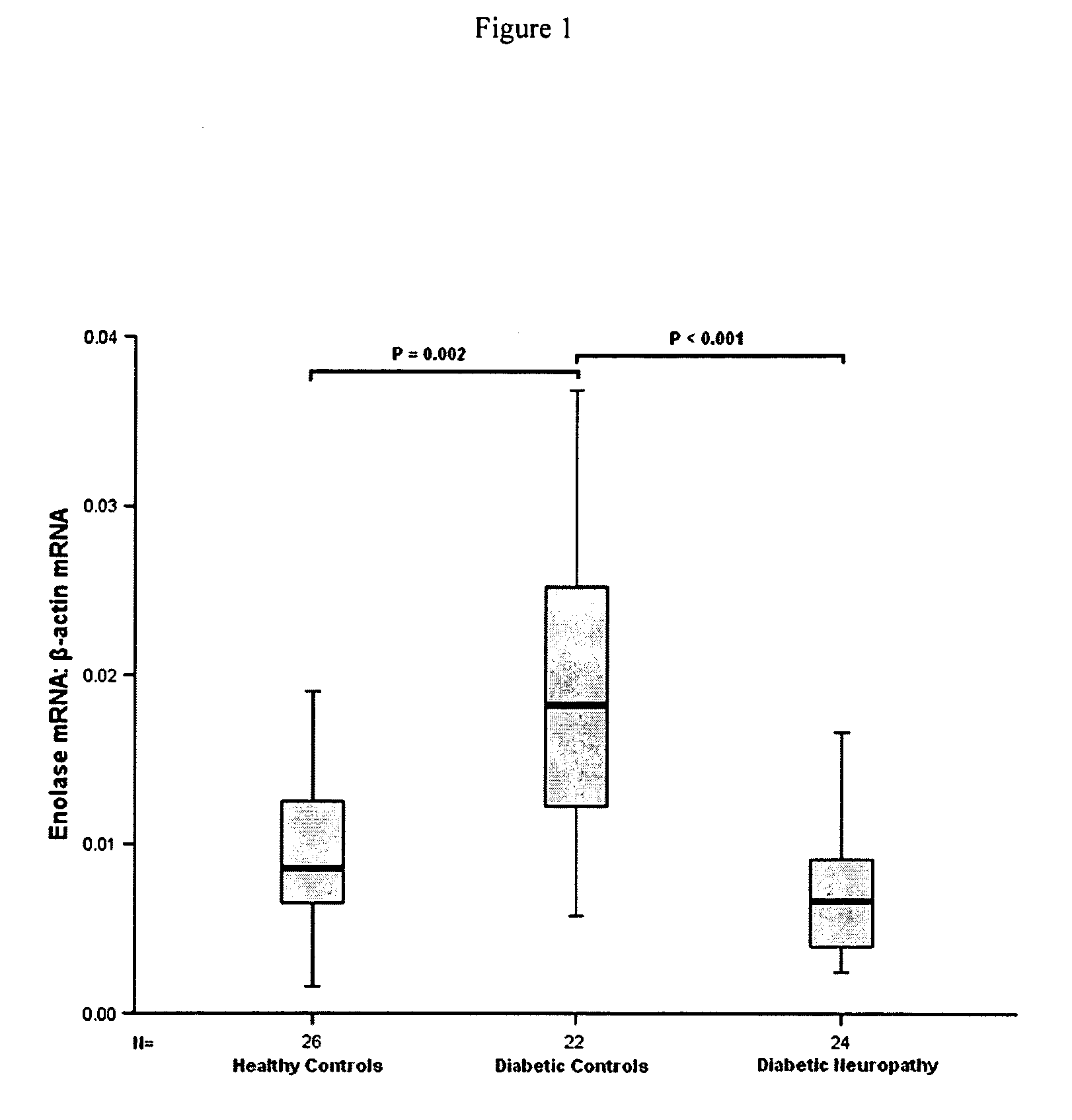
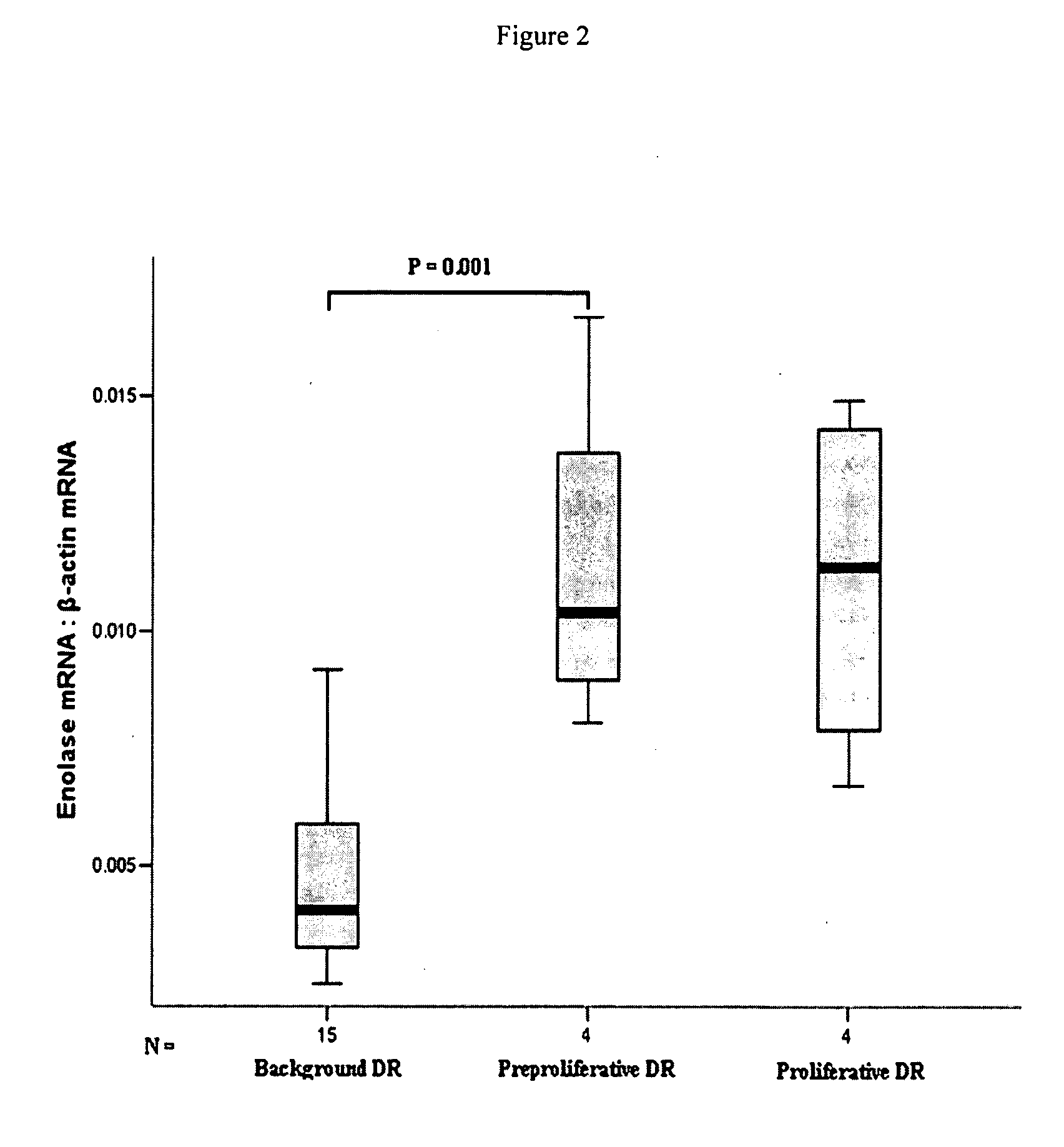
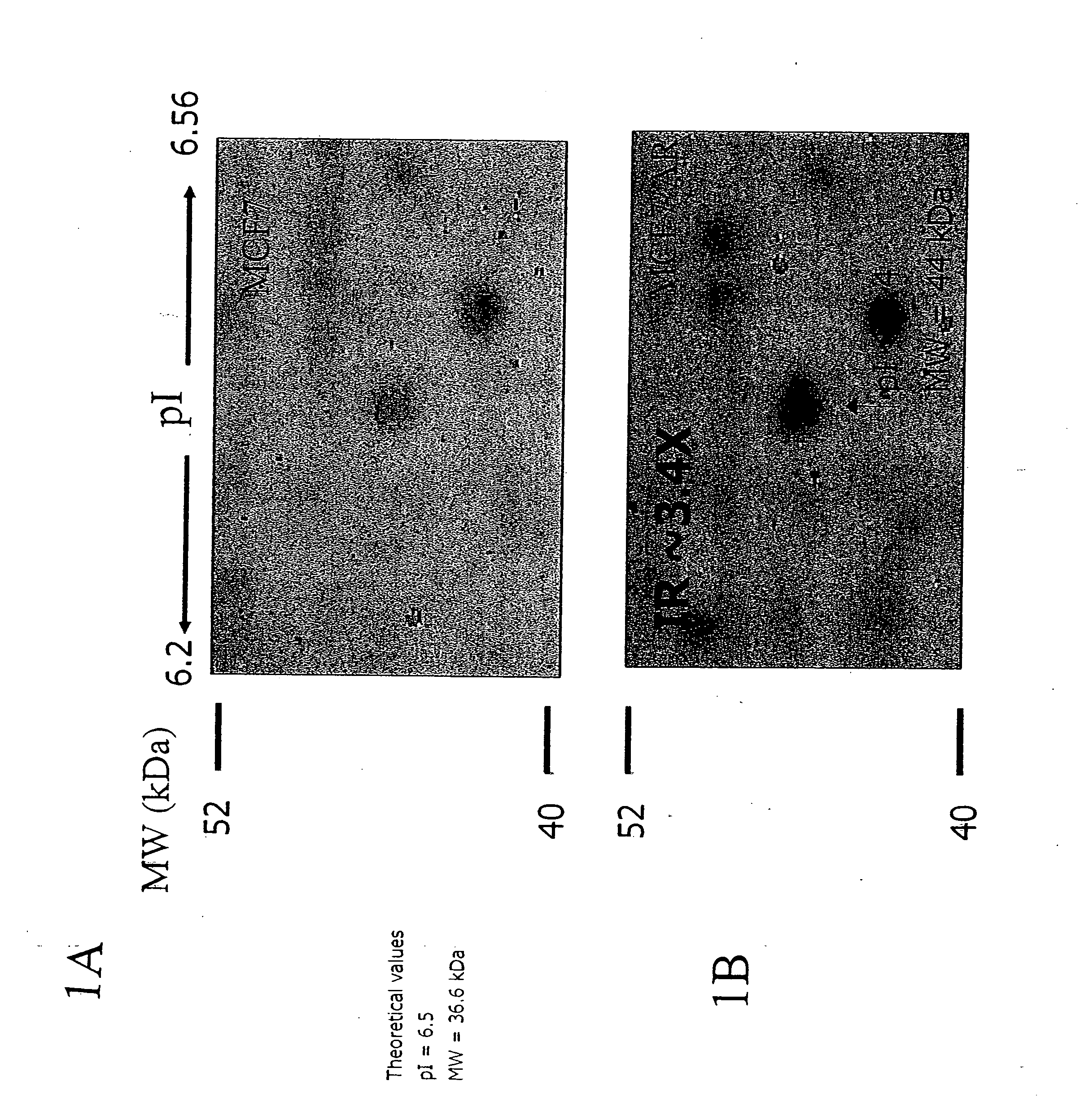
Enolase, also known as phosphopyruvate hydratase, is a metalloenzyme responsible for the catalysis of the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP), the ninth and penultimate step of glycolysis. The chemical reaction catalyzed by enolase is: 2-phospho-D-glycerate ⇌ phosphoenolpyruvate + H₂O Enolase belongs to the family of lyases, specifically the hydro-lyases, which cleave carbon-oxygen bonds.
Choline binding proteins for anti-pneumococcal vaccines
InactiveUS6245335B1Improve efficiencySufficient quantityAntibacterial agentsBacteriaBacteroidesCholine binding protein
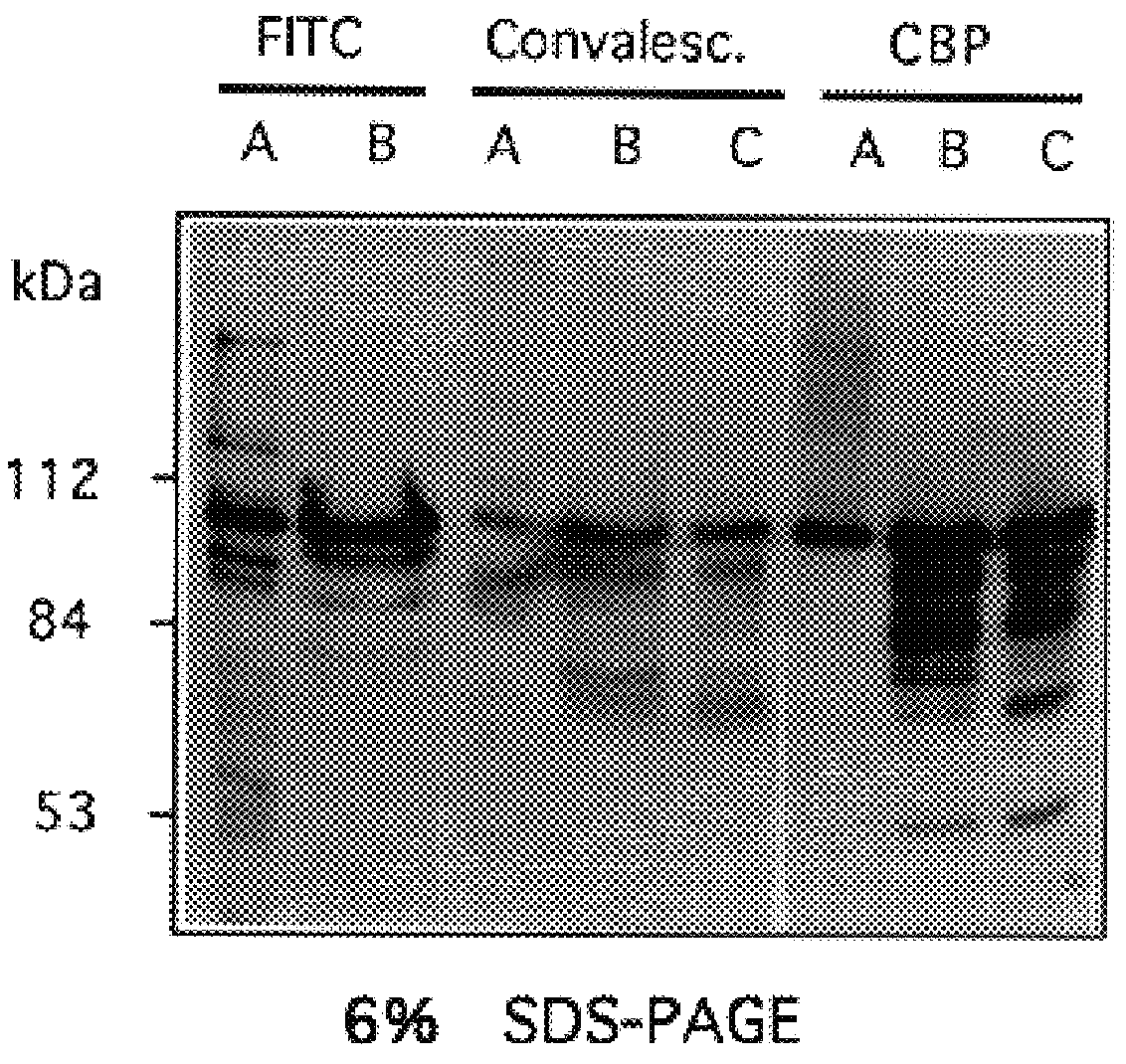
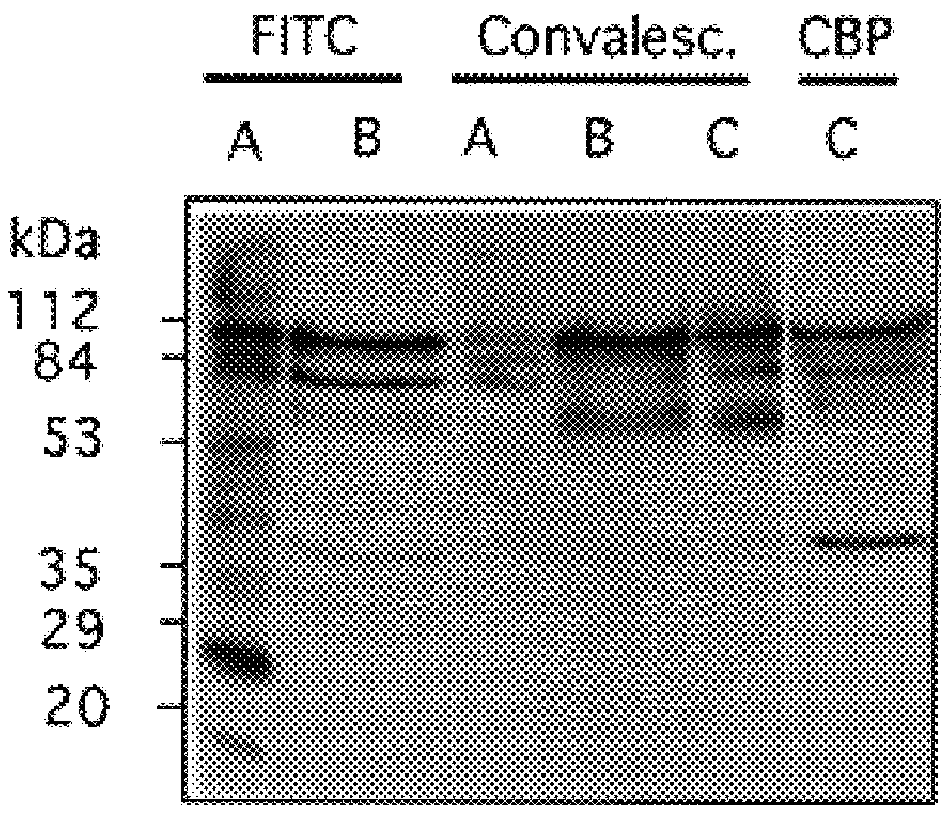
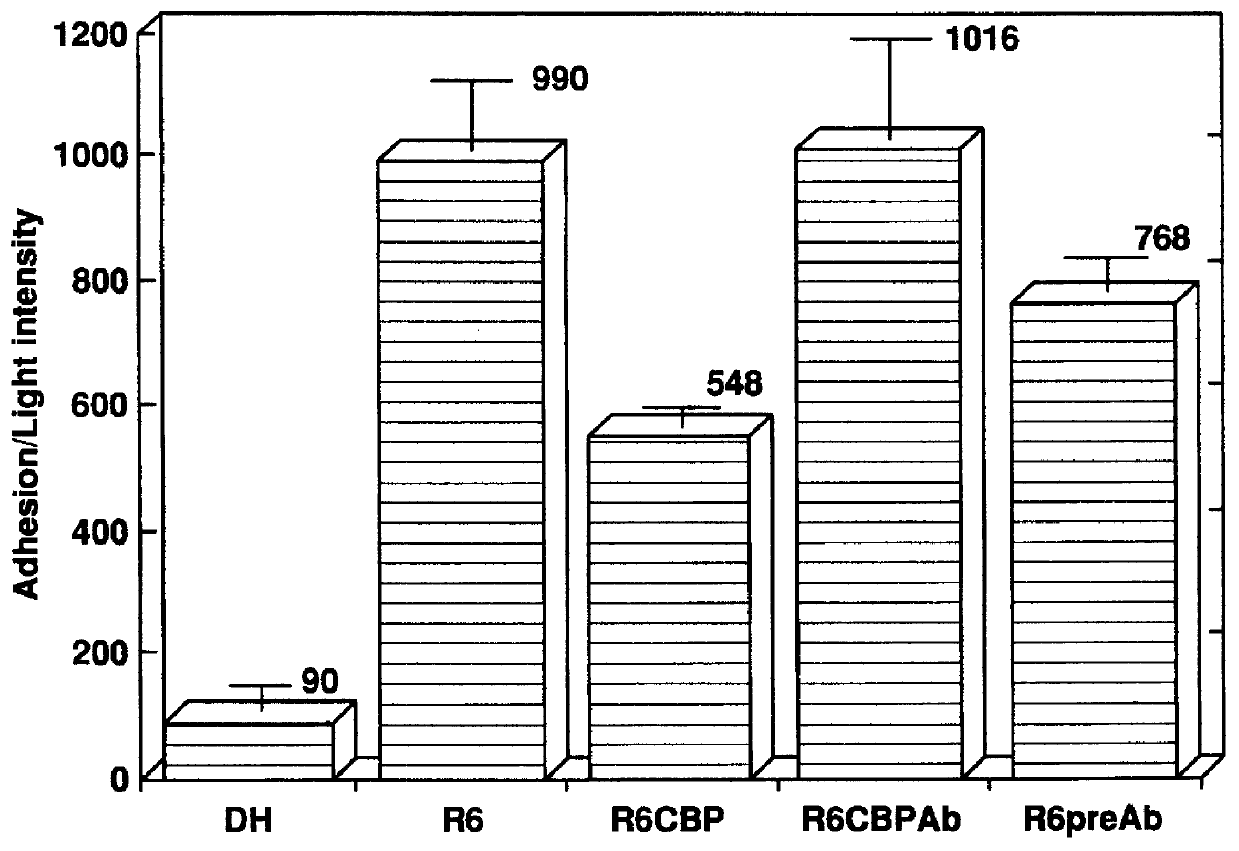
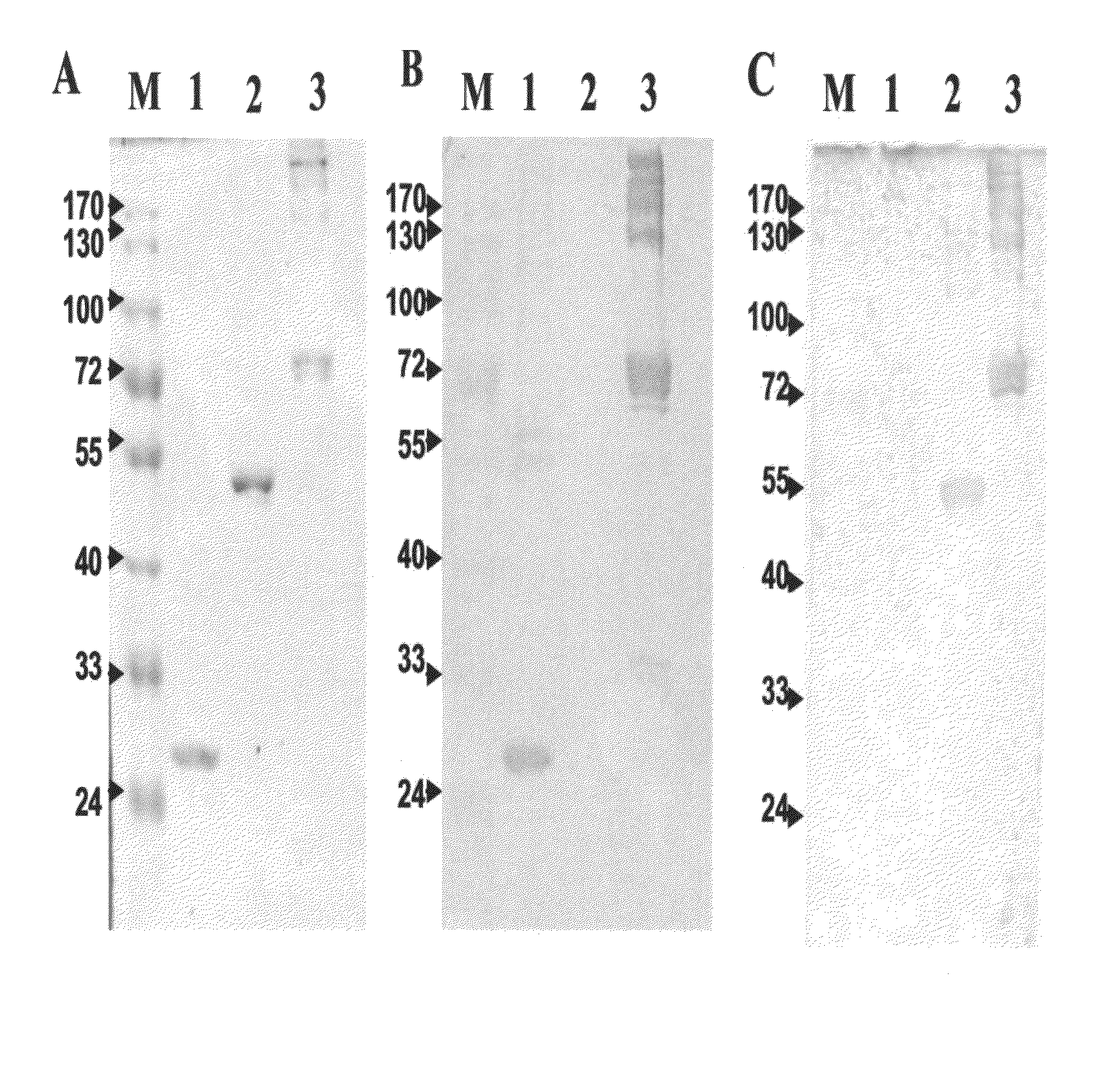
The invention relates to bacterial choline binding proteins (CBPs) which bind choline. Such proteins are particularly desirable for vaccines against appropriate strains of Gram positive bacteria, particularly streptococcus, and more particularly pneumococcus. Also provided are DNA sequences encoding the bacterial choline binding proteins or fragment thereof, antibodies to the bacterial choline binding proteins, pharmaceutical compositions comprising the bacterial choline binding proteins, antibodies to the bacterial choline binding proteins suitable for use in passive immunization, and small molecule inhibitors of choline binding protein mediated adhesion. Methods for diagnosing the presence of the bacterial choline binding protein, or of the bacteria, are also provided. In a specific embodiment, a streptococcal choline binding protein is an enolase, which demonstrates strong affinity for fibronectin.
Owner:THE ROCKEFELLER UNIV
Anti-alpha-Enolase I Antibodies for Diagnosis and Treatment of alpha-Enolase I-Associated Diseases
The invention relates to antibodies against α-enolase I, their pharmaceutical compositions and diagnosis and treatment uses. Particularly, the invention provides polyclonal anti-α-enolase I antibodies and monoclonal single-chain variable fragment (scFv) anti-α-enolase antibodies, pharmaceutical compositions containing the same and their uses in uses in diagnosis and treatment of cancers, autoimmune disorders, ischemia and bacterial infection.
Owner:TAIPEI MEDICAL UNIV
Brain specific exosome based diagnostics and extracorporeal therapies
InactiveUS20170014450A1Reliable and inexpensive and portable and rapid and simple approachMinimally invasive, inexpensive, portable, and reliableCell receptors/surface-antigens/surface-determinantsImmunoglobulins against animals/humansPsa ncamPhosphorylation
Disclosed are methods, compositions, devices, and kits for the isolation of brain-specific exosomes. Specifically, methods, compositions, devices, and Unbound kits comprising an isolated brain-specific extracellular vesicle or exosome joined to a first binding agent that is specific for tau, β-amyloid, SlOO β, neuron-specific enolase, glycoprotein A2B5, CD133, NQ01, synaptophysin, neuronal nuclei, MAB 1569, polysialic acid-neural cell adhesion molecule (PSA-NCAM), or neurogenic differentiation 1 (NeuroD or Beta2), or glycosylated or phosphorylated forms of these molecules, are provided.
Owner:EXOSOME SCI
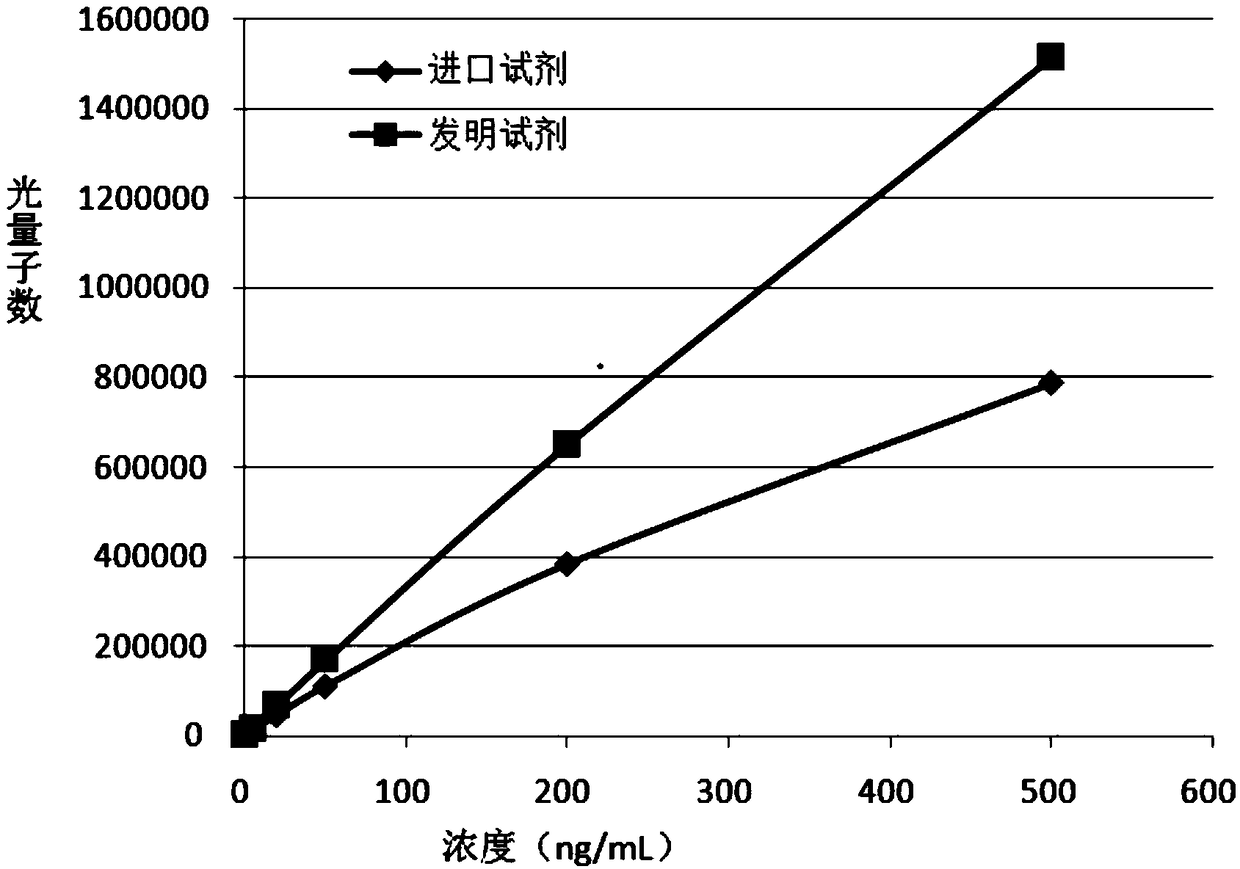
Quantitative detection kit for neuronspecific enolase (NSE) and preparation method and application thereof
InactiveCN102914650AImprove stabilityEasy to operate manuallyMaterial analysisBiotin-streptavidin complexAntigen
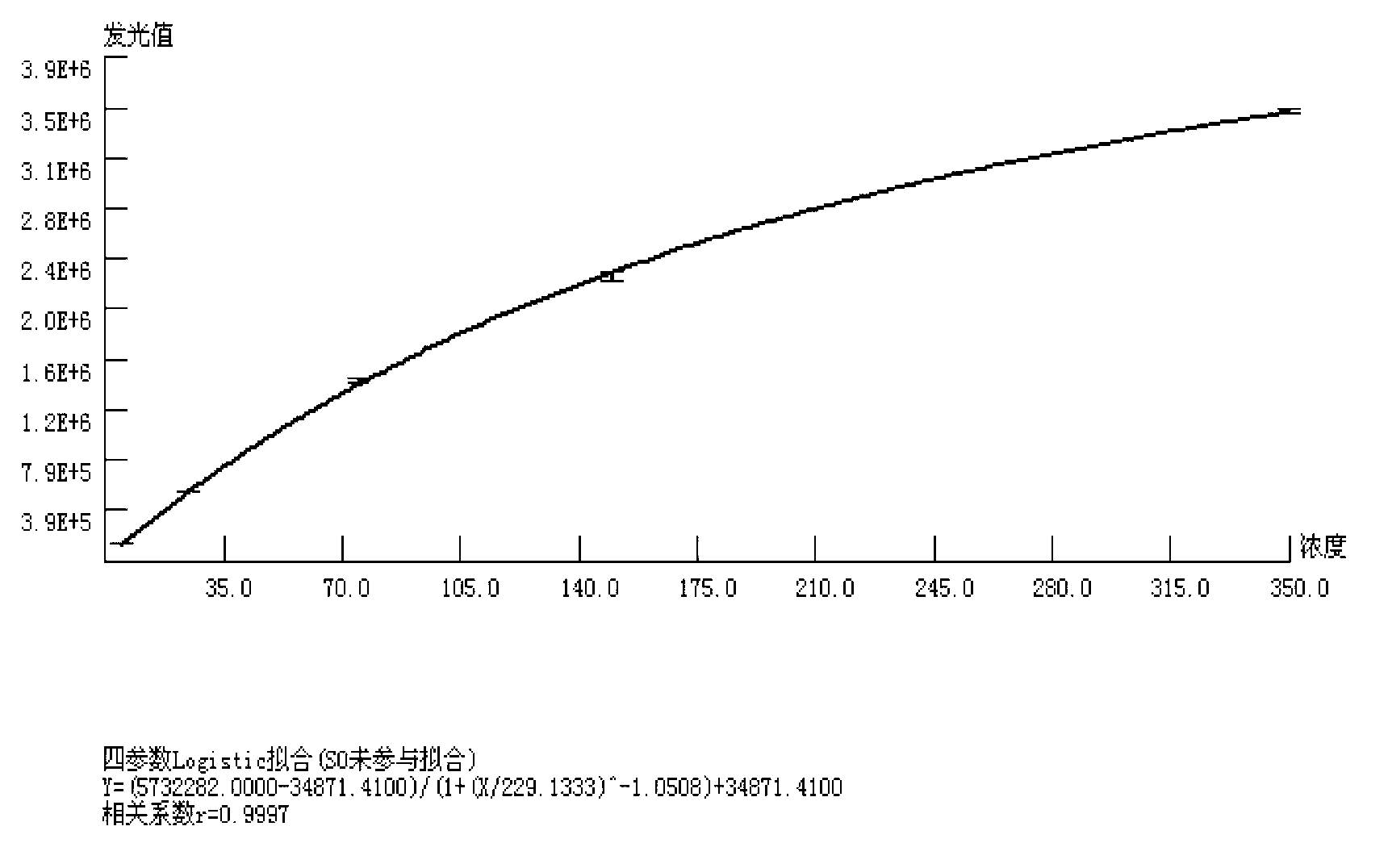
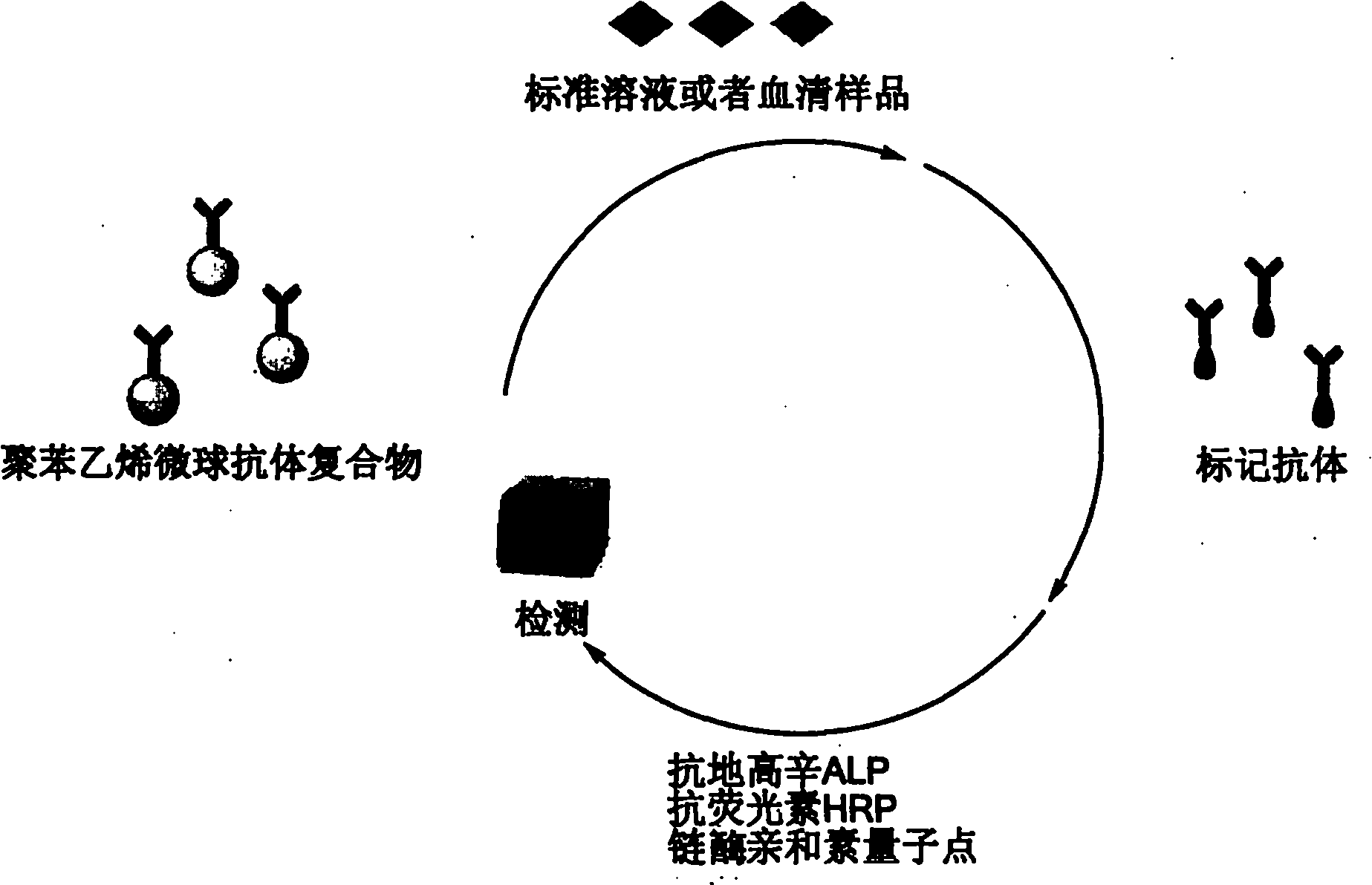
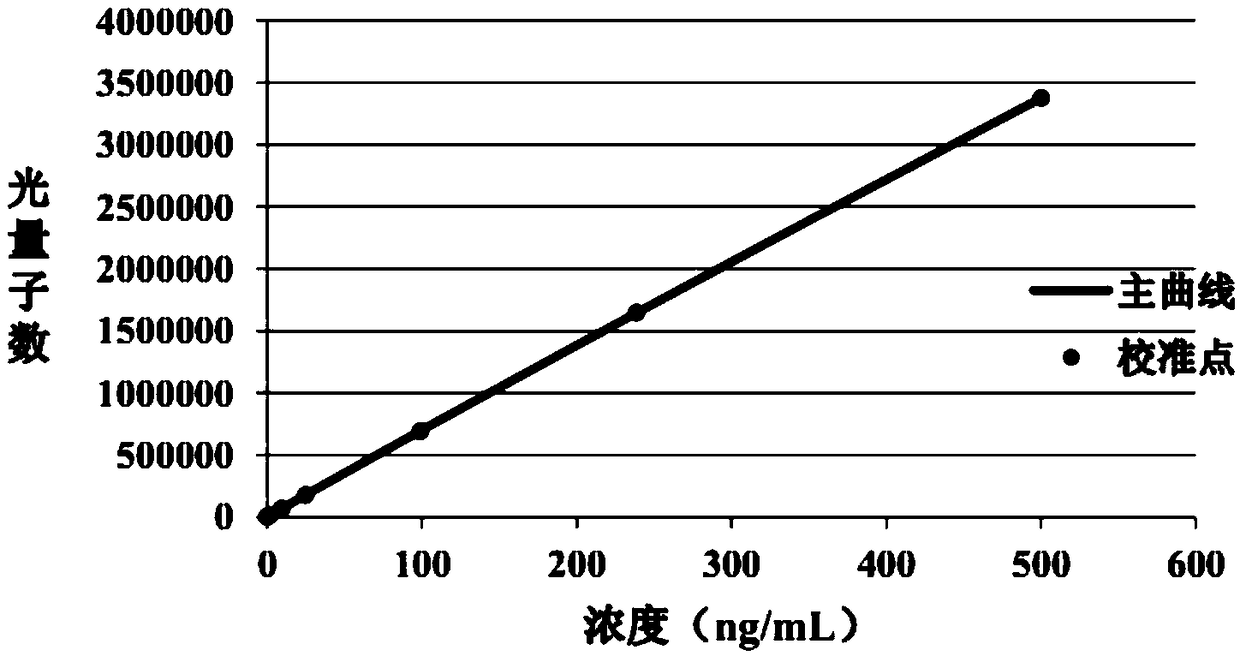
The invention relates to a quantitative detection kit for NSE and a preparation method and application of the quantitative detection kit. The kit comprises a calibrator, a magnetic separation reagent, an enzyme reactant, a stable reinforcing agent and a chemiluminiscent substrate, wherein the calibrator is obtained by treating NSE antigen through a reducing agent solution and diluting the NSE antigen to a buffer solution containing a nonionic surfactant; the magnetic separation reagent is obtained by immunofixation of a biotinylation antibody and streptavidin magnetic particles; the enzyme reactant comprises a NSE tracing antibody marked by alkaline phosphatase; and the stable reinforcing agent comprises a multicomponent immune compound interfered by an anti-heterophilic antibody. The invention further relates to a preparation method of the kit and a method of applying the kit to quantitatively detect a tumor marker NSE. The kit is reliable in performance, high in flexibility, and wide in linear range, and matched up with an automatic instrument for use. At present, the kit has already obtained a third registration certificate of a diagnostic reagent in SFDA (State Food and Drug Administration).
Owner:BEIJING DIACHA BIO ENG
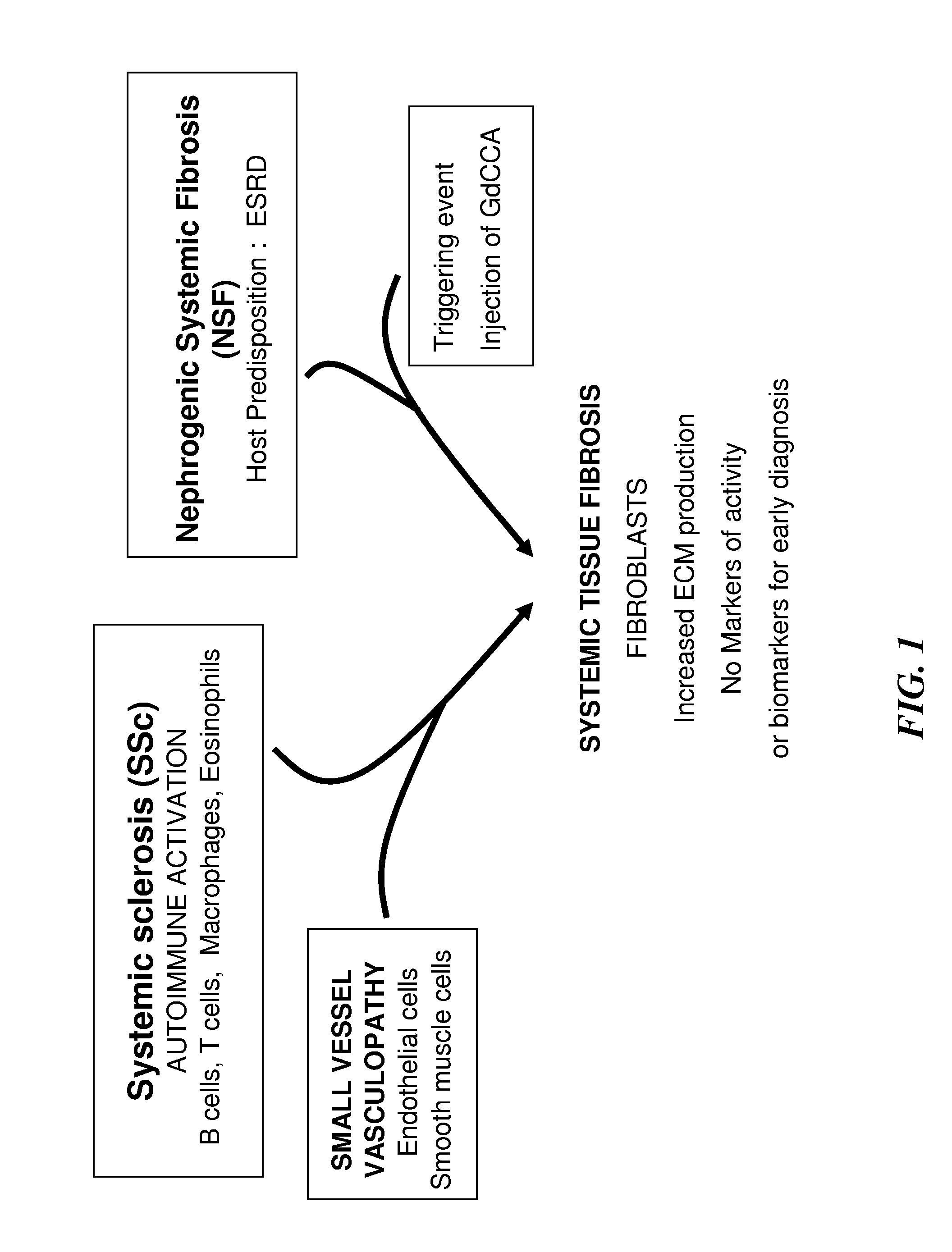
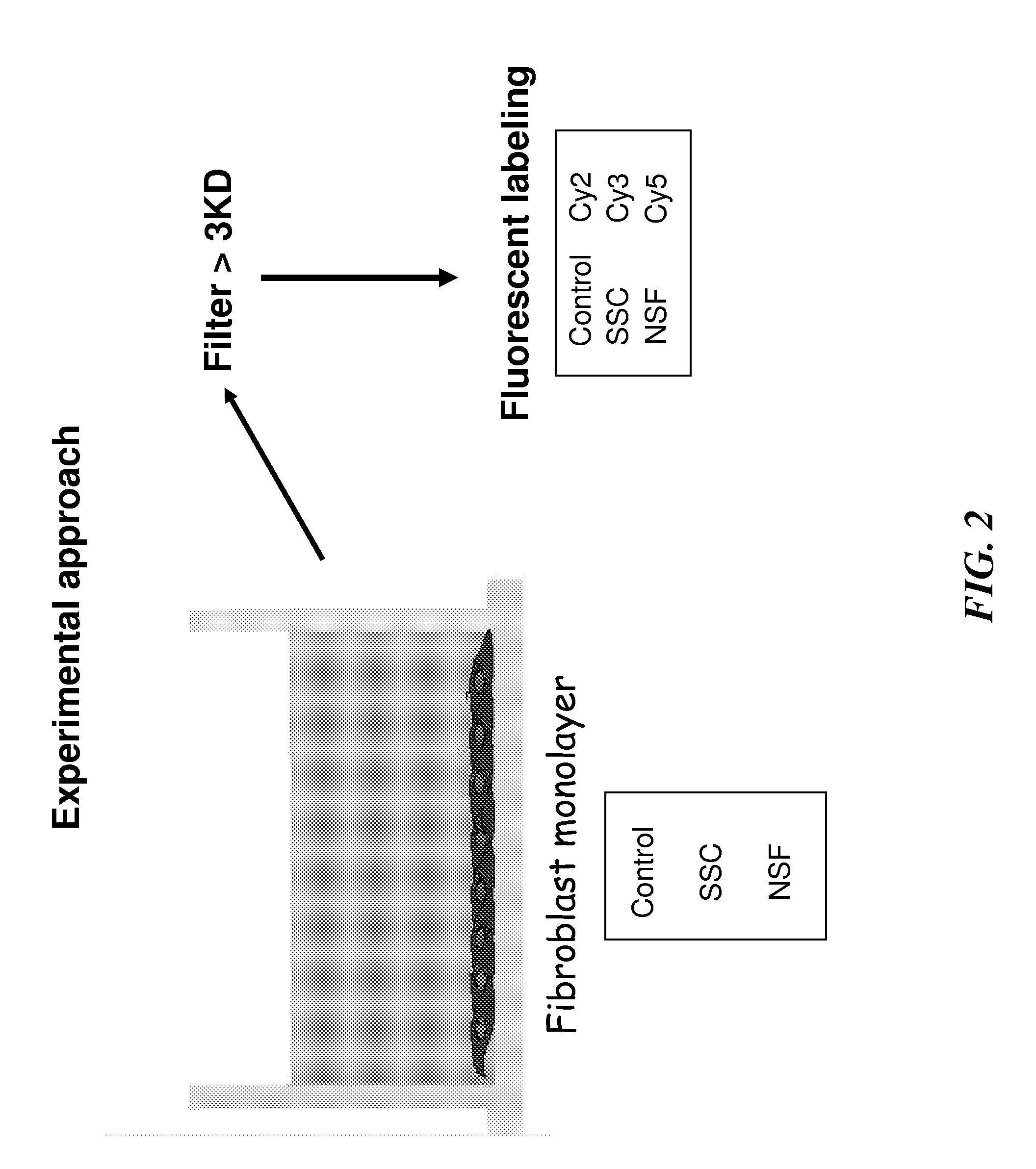
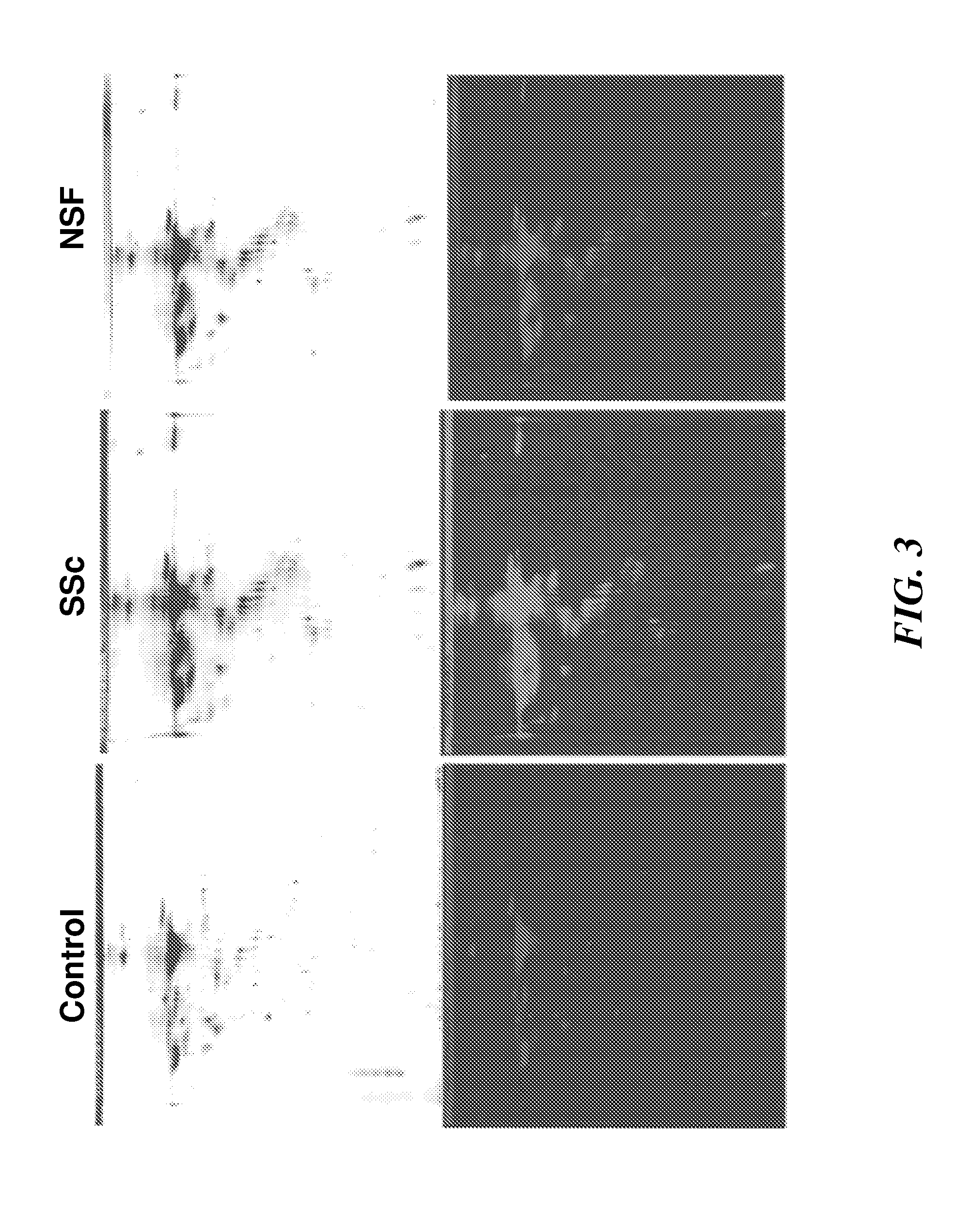
Biomarkers for early diagnosis of systemic tissue fibrosis
InactiveUS20120015368A1Increase volumeEarly diagnosisBioreactor/fermenter combinationsBiological substance pretreatmentsPIGMENT EPITHELIUM-DERIVED FACTORAlpha-enolase
Embodiments of the invention provides methods, devices and kits for determining the likelihood of an individual having an active fibrotic condition and / or early diagnosis, and subsequently prognosis evaluation of an individual having an active fibrotic condition such as systemic sclerosis (SSc) and / or nephrogenic systemic fibrosis (NSF) by measuring the levels of several biomarkers: α-enolase (ENO1), reticulocalbin 3 (RCN-3), alpha smooth muscle actin (α-SMA), reticulocalbin 1 (RCN-1) and pigment epithelium-derived factor (PEDF) in the individual.
Owner:THOMAS JEFFERSON UNIV
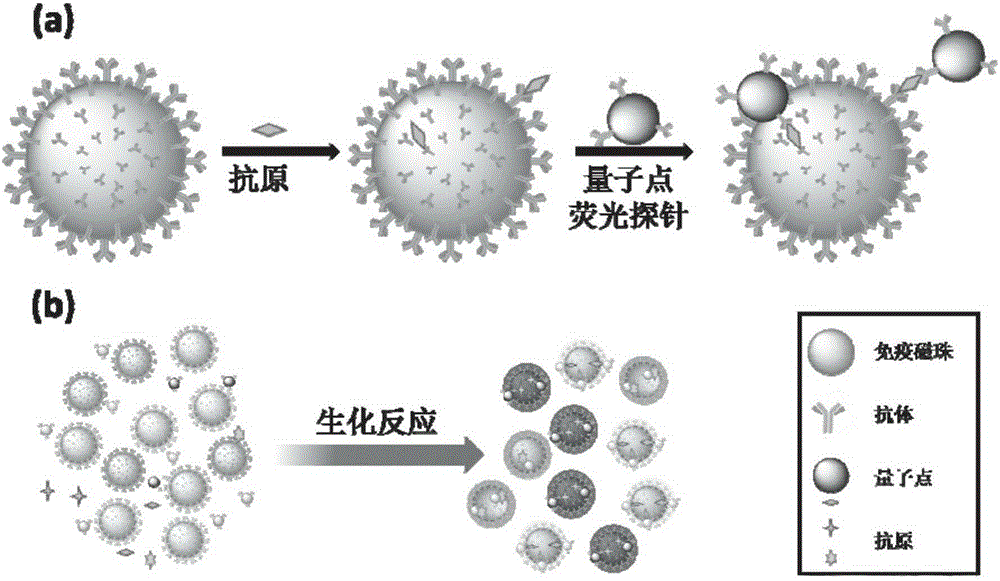
Quantum dot fluorescent probe and application thereof
The invention relates to a quantum dot fluorescent probe and application thereof. The invention is characterized in that the quantum dot fluorescent probe utilizes a difunctional cross-linking agent long-chain succinimidyl-4-[N-maieimidomethyl]cyclohexane-1-carboxy-[6-amidocaproate] (SMCC) to undergo a cross-linking reaction with an amino group on the surface of an amino quantum dot; a maleimide group is produced on the surface of the quantum dot; a to-be-labeled antibody is reduced by a reducing agent dithiothreitol (DTT) so as to reduce a disulfide bond into a mercapto group; and the mercapto group and the maleimide group form a covalent bond so as to realize labeling of the quantum dot with the antibody. The quantum dot fluorescent probe is applied to (1) detection of one protein oncofetal antigen or (2) detection of a plurality of protein CEA, euron-specific enolase (NSA) and a cytokerain fragment 19CYFRA21-1, wherein the detection limit of detection (1) is 38 pg / ml, and the detection limit of detection (2) is 0.9 ng / ml.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI +1
Biomarkers
InactiveUS20100047779A1Rapid and early detectionQuick checkMicrobiological testing/measurementBiomarker (petroleum)RETINOSCHISIN
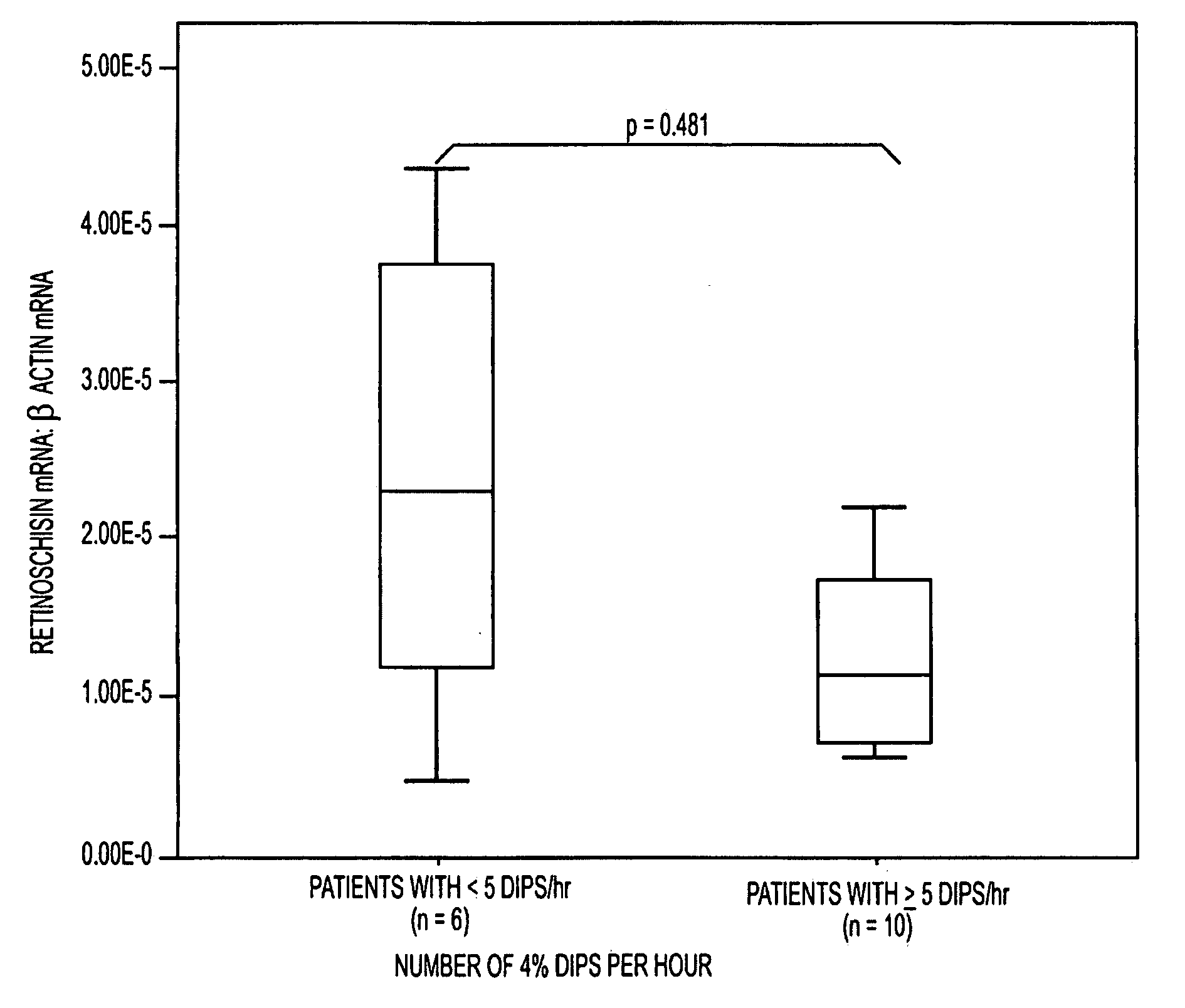
The present invention provides circulating biomarkers for conditions associated with metabolic syndrome, including diabetes mellitus, hypertension and congestive heart failure. The biomarkers include plasma DNA, neuron-specific enolase, 11β-hydroxysteroid dehydrogenase, rhodopsin, retinoschisin, RPE65 and cardiac troponin T. Methods and kits for detecting these biomarkers in the prediction, monitoring and diagnosing of disease are provided, particularly for determining mRNA levels thereof in a subject's blood.
Owner:KING'S COLLEGE LONDON
Alpha enolase-directed diagnostics and therapeutics for cancer and chemotherapeutic drug resistance
InactiveUS20070077583A1High sensitivityHigh expressionOrganic active ingredientsBiocideAlpha-enolaseInduction chemotherapy
Disclosed are methods for detecting a neoplasm and / or chemotherapeutic drug resistance or angiogenic potential in neoplastic cells by detecting an increase in the expression of α-enolase in such cells, or in the case of metastatic potential on the surface of such cells, as compared to the level of expression of α-enolase protein in a normal or non-MDR neoplastic cell or on the surface of a non-metastatic neoplastic cell. In addition, methods and a composition are disclosed for increasing the sensitivity of a neoplasm to a chemotherapeutic drug treatment regime, for inhibiting angiogenesis and metastatic potential in chemotherapeutic drug resistant or neoplastic cells, and for inducing apoptosis in chemotherapeutic drug resistant or neoplastic cells.
Owner:AURELIUM BIOPHARMA
Marker joint detection model for diagnosis of lung cancer
ActiveCN110376378AAccurate diagnosisImprove diagnostic accuracyMaterial analysisAlpha-enolaseCarcinoembryonic antigen
The invention discloses a marker joint detection model for diagnosis of the lung cancer. According to the invention, a carcinoembryonic antigen, a cancer antigen 125, an annexin A1 antibody and an alpha-enolase antibody are used as markers and are applied to preparation of a product for the diagnosis or assisted diagnosis of the lung cancer. According to the invention, the good optimization modelbeing optimal diagnosis combination of four kinds of biomarkers (the CEA, CA125, annexin A1 antibody and and alpha-enolase antibody) is established, wherein the e good optimization model has the better diagnosis capability superior to the single traditional marker diagnosis. The marker joint detection model as a novel method for lung cancer diagnosis has advantages of high sensitivity and specificity and has the important significance in accurate diagnosis of the lung cancer.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
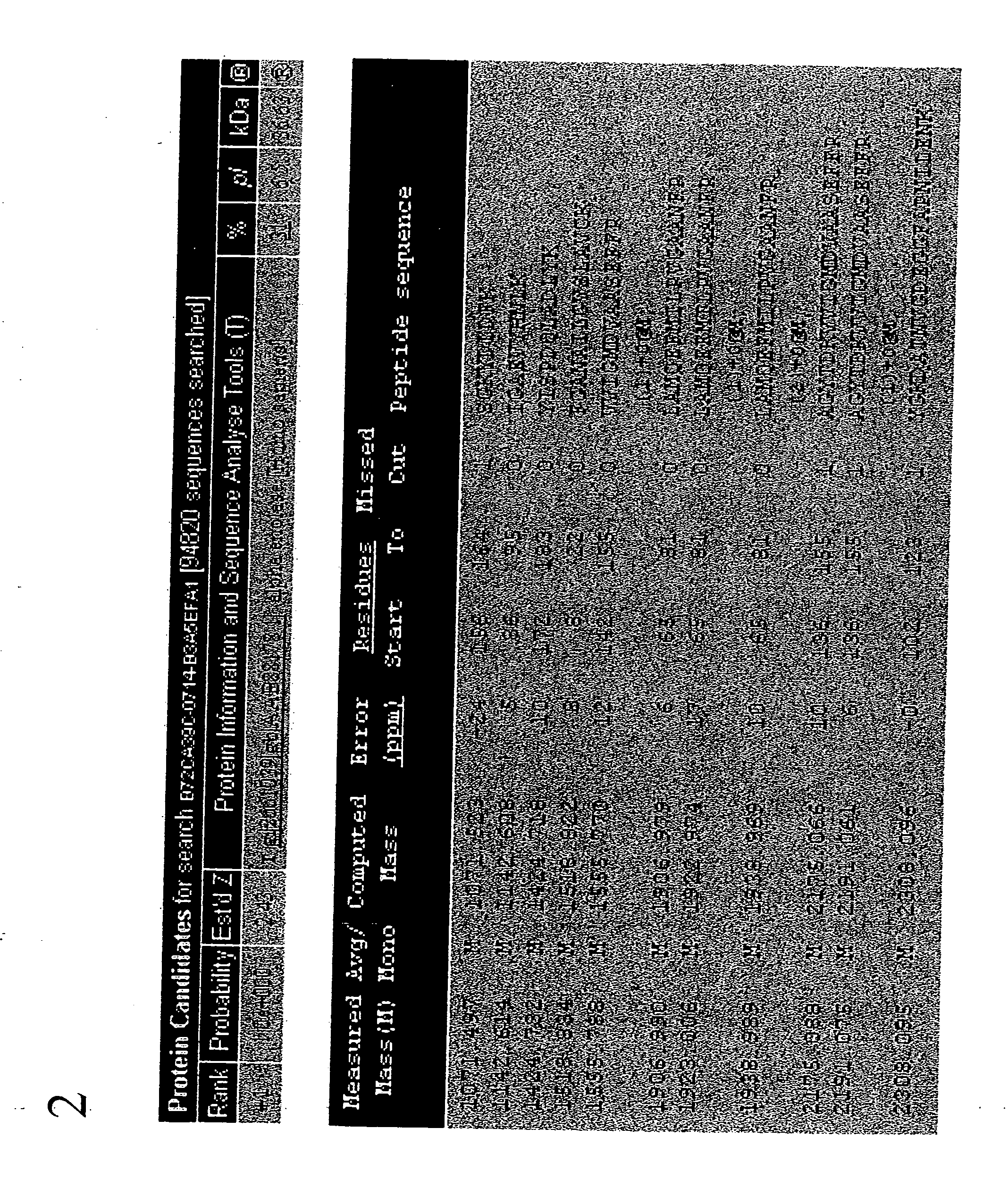
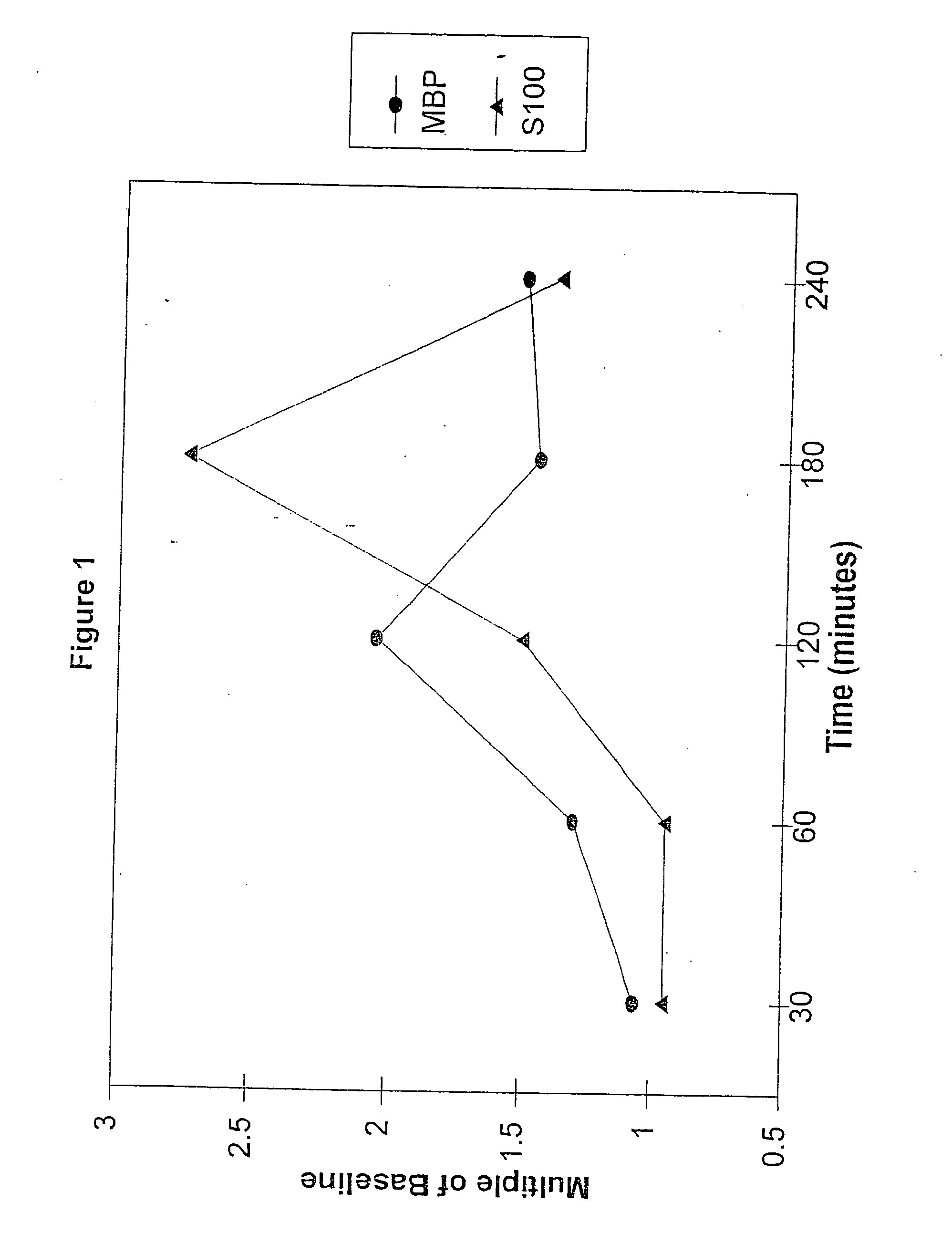
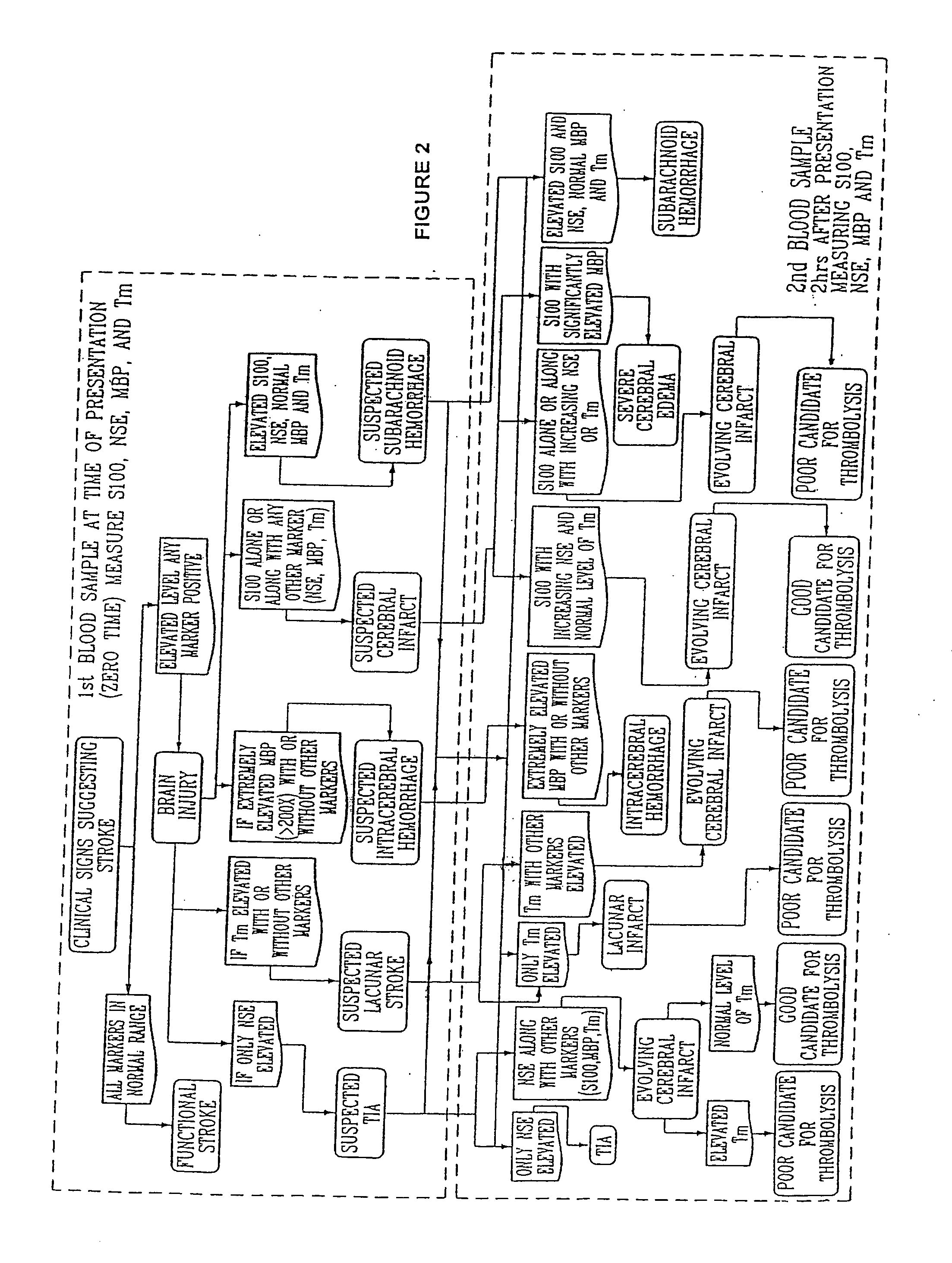
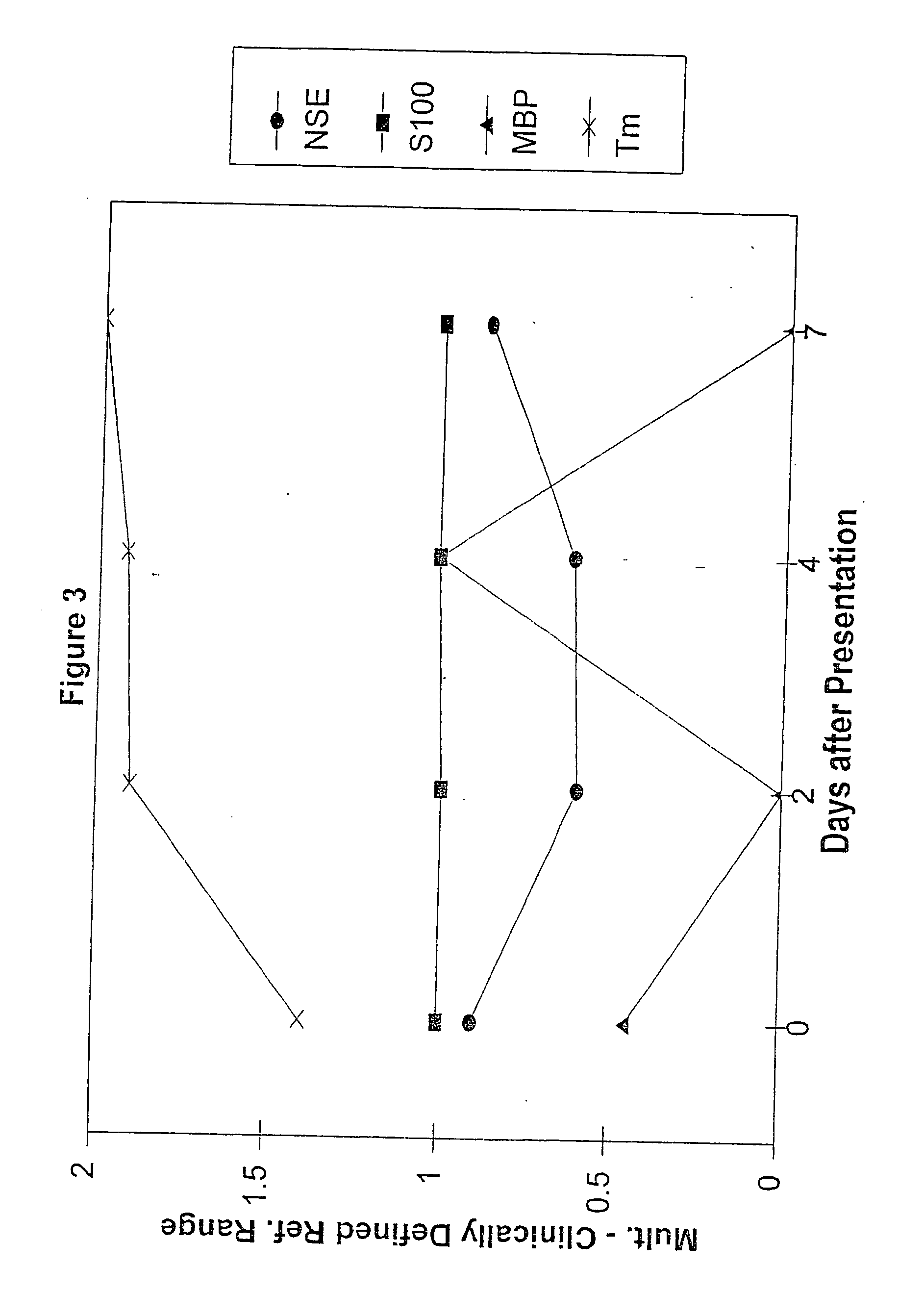
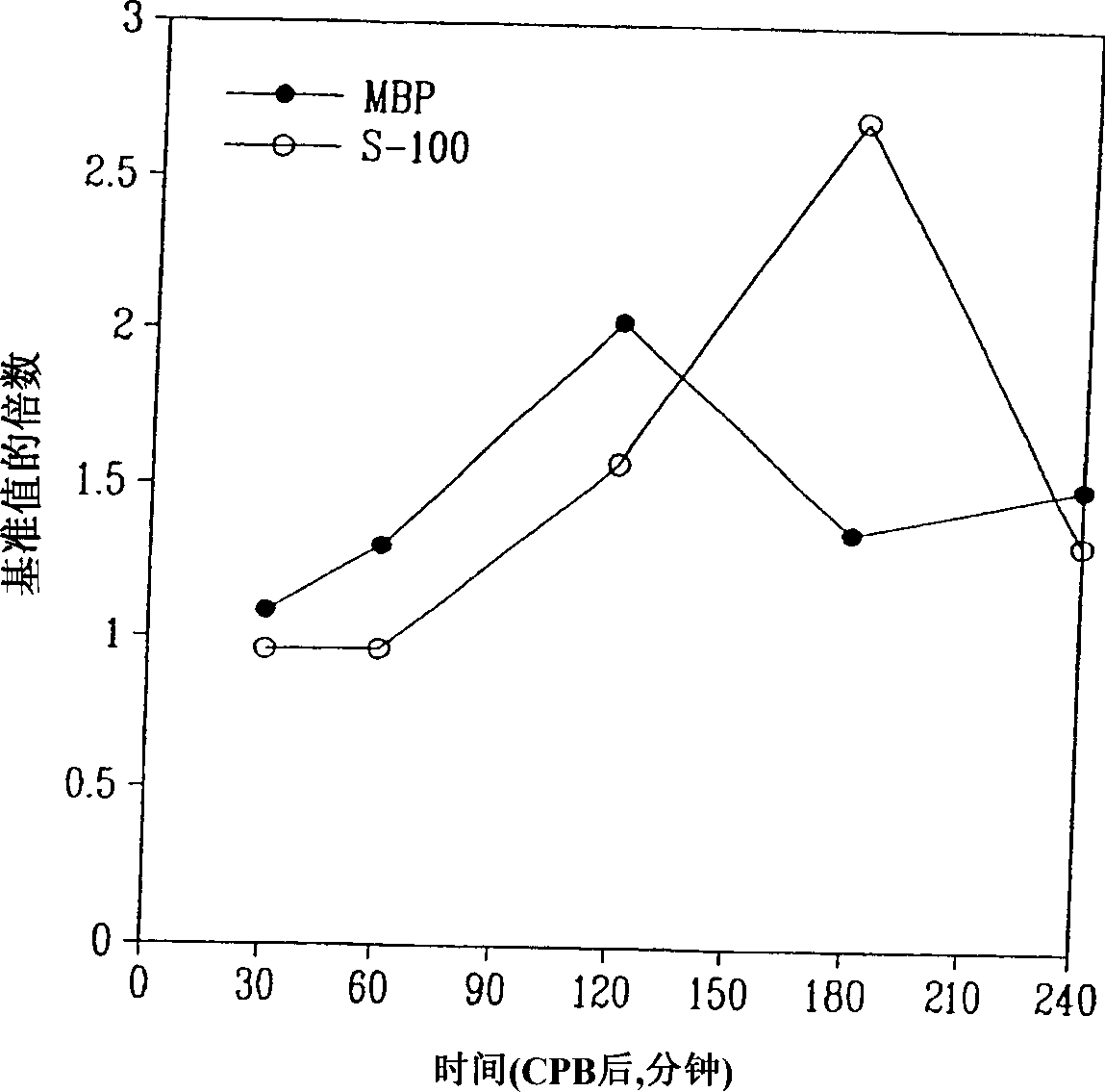
Method for diagnosing and distinguishing stroke and diagnostic devices for use therein
InactiveUS20050136496A1Improve patient selectionMicrobiological testing/measurementSnake antigen ingredientsThrombusS100 protein
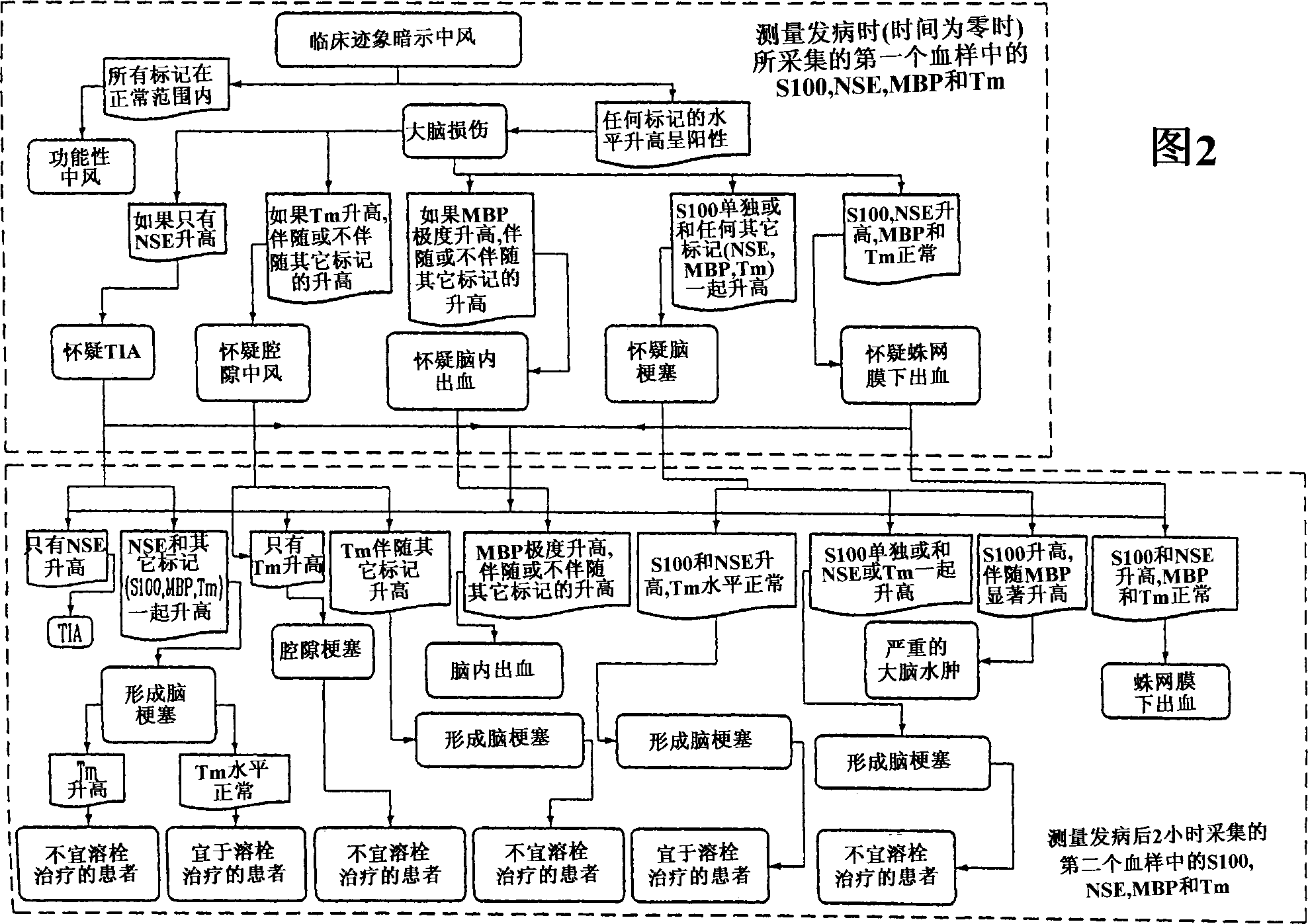
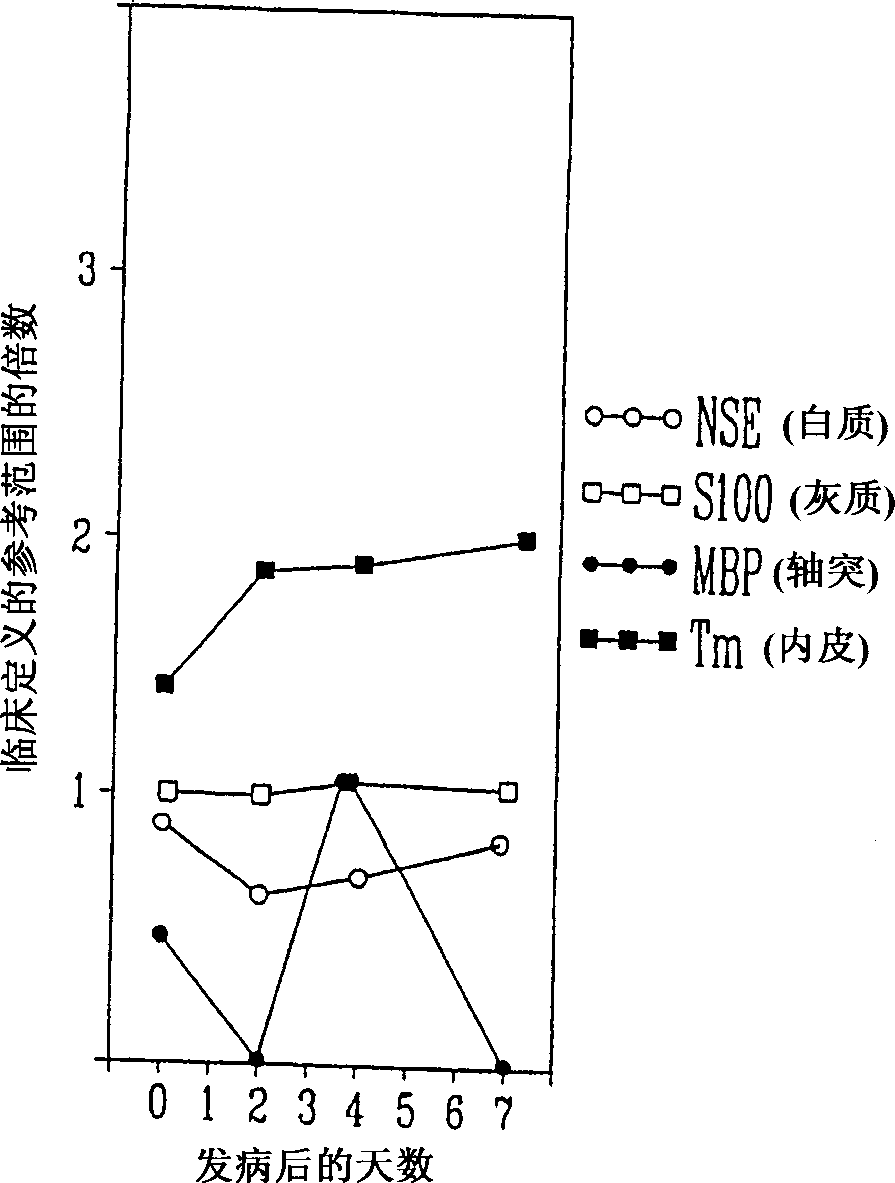
A method for determining whether a subject has had a stroke and, if so, the type of stroke which includes analyzing the subject's body fluid for at least four selected markers of stroke, namely, myelin basic protein, S100 protein, neuronal specific enolase and a brain endothelial membrane protein such as thrombomodulin or a similar molecule. The data obtained from the analyses provide information as to the type of stroke, the onset of occurrence and the extent of brain damage and allow a physician to determine quickly the type of treatment required by the subject.
Owner:SKYE PHARMATECH
Streptococcus suis type 2 three-component subunit vaccine and use
ActiveCN101412984AImproving immunogenicityImprove the immunityAntibacterial agentsBacterial antigen ingredientsEscherichia coliRecombinant escherichia coli
The present invention belongs to the technical field of murrain vaccine preparation, and in particular relates to a Streptococcus suis type 2 trimaceral subunit vaccine and a preparation method thereof. The key technology is that recombinant Escherichia coli such as Escherichia coli BL21 / pET-28a-1036N, Escherichia coli BL21 / pET-28a-0197 and Escherichia coli BL21 / pET-28a-enolase is prepared, which can excrete and express Streptococcus suis type 2 antigen albumen 1036N,0197 and enolase, and which is preserved as CCTCC NO M208147, CCTCC NO M208146 and CCTCC NO M208148 in China Center for Type Culture Collection respectively. The present invention also discloses a preparation method and a use suitable for the trimaceral subunit vaccine of Streptococcus suis type 2.
Owner:HUAZHONG AGRI UNIV
Double-antibody sandwich enzymelinked immunosorbent detection kit and preparation method thereof
ActiveCN102435748AStrong specificityHigh detection sensitivityMaterial analysisCreatine kinaseAntiendomysial antibodies
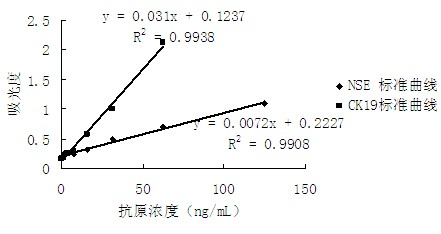
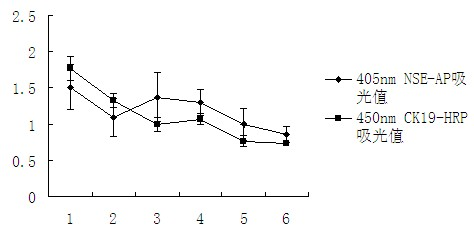
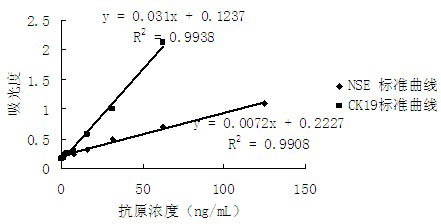
The invention discloses a double-antibody sandwich enzymelinked immunosorbent detection kit and a preparation method of the double-antibody sandwich enzymelinked immunosorbent detection kit. The detection kit comprises a perforated plate coated with neuron-specific enolase (NSE) monoclonal antibodies and CK 19 (creatine kinase 19) monoclonal antibodies, NSE labeled by horse radish peroxidase (HRP), CK 19 labeled by alkaline phosphatase, chromogenic substrate tetramethylbenzidine (TMB), p-nitrophenyl disodium phosphate (PNPP), disodium hydrogen phosphate and substrate stop solution. The double-antibody sandwich enzymelinked immunosorbent detection kit has the advantages of being simple and convenient, sensitive, stable, good in repeatability and the like, and has practical value.
Owner:GUANGDONG PHARMA UNIV
Chemiluminescent immunodetection kit for neuronspecific enolase and preparation method of chemiluminescent immunodetection kit
InactiveCN109187971AImprove accuracyImprove anti-interference abilityChemiluminescene/bioluminescenceDisease diagnosisMonoclonal antibodyStreptavidin
The invention provides a chemiluminescent immunodetection kit for a neuronspecific enolase and a preparation method of the chemiluminescent immunodetection kit and belongs to the technical field of in-vitro detection. The kit comprises a streptavidin magnetic particle suspension, a neuronspecific enolase monoclonal antibody marked by a chemiluminescent marker and a neuronspecific enolase monoclonal antibody marked by a coupling marker. The invention provides the preparation method of the chemiluminescent immunodetection kit for the neuronspecific enolase. The chemiluminescent immunodetection kit for the neuronspecific enolase, provided by the invention, is capable of automatically measuring a sample and directly giving a numerical value, so that human operation errors are reduced; in addition, unmanned operation is realized, so that the time required by clinical detection is shortened; meanwhile, the detection precision is relatively high; and a reagent and an instrument form a sealedsystem, so that small system errors are caused.
Owner:DIRUI MEDICAL TECH CO LTD
Method for diagnosing and distinguishing stroke
InactiveCN1339108AConfirm progressIncreased risk of bleedingMicrobiological testing/measurementEnzymologyS100 proteinRisk stroke
A method for determining whether a subject has had a stroke and, if so, the type of stroke which includes analyzing the subject's body fluid for at least four selected markers of stroke, namely myelin basic protein, S100 protein, neuronal specific enolase and a brain endothelial membrane protein such as thrombomodulin or a similar molecule. The data obtained from the analyses provide information as to the type of stroke, the onset of occurrence and the extent of brain damage and allow a physician to determine quickly the type of treatment required by the subject.
Owner:SYN X PHARMA
Homogeneous phase multi-index fluorescence/chemiluminescence measuring method and application thereof
InactiveCN101995396ALess serumShort detection timeChemiluminescene/bioluminescenceFluorescence/phosphorescenceCritical illnessFluorescence
The invention belongs to the field of blood testing, and relates to a homogeneous phase multi-index fluorescence / chemiluminescence measuring method and application thereof. The method comprises the steps of: fixing a plurality of specificity recognition monoclonal antibodies on a polystyrene microsphere, and reacting with a plurality of labeled antibody cores modified by haptens after combining with carcinoembryonic antigens, cell keratin fragments 19, neuron specificity enolase and other multiple markers; reacting with horse radish peroxidase modified by corresponding hapten antibodies, alkaline phosphatase and quantum dots, and measuring a plurality of markers on the same instrument by combining with detections of chemiluminescence, fluorescence and the like. The method has the advantages of small blood serum consumption, rapid detection time, low diagnosis cost and simple operation, is suitable for detecting a plurality of popularized indexes simultaneously, and can provide auxiliary judgment basis for the early diagnosis of critical illnesses of tumor and the like.
Owner:FUDAN UNIV
Chemiluminescence immunoassay kit for detecting neuronspecific enolase
ActiveCN109187973AStrong specificityWide detection linear rangeChemiluminescene/bioluminescenceDisease diagnosisEdta plasmaCapture antibody
The invention provides a chemiluminescence immunoassay kit for detecting neuronspecific enolase, and belongs to the technical field of in vitro diagnosis. The kit A comprises a calibrator, a magneticparticle reaction liquid Ra1, a trace binder reaction liquid Ra2 and a capture antibody reaction liquid Ra3. The kit B comprises a calibrator, a magnetic particle reaction liquid Rb1, a trace binder reaction liquid Rb2 and a project dilution reaction liquid Rb3. The kit 3 comprises a calibrator, a magnetic particle reaction liquid Rc1 and a trace binder reaction liquid Rc2. The kit has wide detection linear range, high sensitivity and good specificity, is an automatic chemiluminescence instrument mated reagent, and is applicable to quantitative detection of NSE in serum and plasma. According to the kit, consistent detection on serum and EDTA plasma samples can be realized by adding a proper amount of Mg<2+> ions, and the serum and plasma measured value related coefficient r is more than 0.975.
Owner:DIRUI MEDICAL TECH CO LTD
Enolase peptide conjugate vaccines against staphylococcus aureus
InactiveUS20140030287A1Sufficient immunogenicityAnimal cellsBacterial antigen ingredientsStaphylococcus cohniiADAMTS Proteins
The present invention relates to peptides of the enolase protein from Staphylococcus aureus as well as nucleic acid and nucleic acid sequence homologues encoding the peptides. The present invention also relates to a composition, particularly a S. aureus vaccine, comprising one or more of the enolase peptides described herein or a fragment, derivative or variant thereof capable of generating an immune response that induces a protective antibody response or opsonophagocytic activity of human neutrophils for S. aureus. The present invention also encompasses methods of treating and / or reducing the likelihood of a Staphylococcus infection by administering a composition of the invention.
Owner:MERCK SHARP & DOHME CORP
Brain specific exosome based diagnostics and extracorporeal therapies
InactiveUS20200171084A1Reliable and inexpensive and portable and rapid and simple approachMinimally invasive, inexpensive, portable, and reliableCell receptors/surface-antigens/surface-determinantsImmunoglobulins against animals/humansNeural cellPhosphorylation
Disclosed are methods, compositions, devices, and kits for the isolation of brain-specific exosomes. Specifically, methods, compositions, devices, and kits comprising an isolated brain-specific extracellular vesicle or exosome joined to a first binding agent that is specific for tau, β-amyloid, S100 β, neuron-specific enolase, glycoprotein A2B5, CD133, NQ01, synaptophysin, neuronal nuclei, MAB1569, polysialic acid-neural cell adhesion molecule (PSA-NCAM), or neurogenic differentiation 1 (NeuroD or Beta2), or glycosylated or phosphorylated forms of these molecules, are provided.
Owner:EXOSOME SCI
Extraction method and application of component group of traditional Chinese medicine extract for treating hemorrhagic stroke
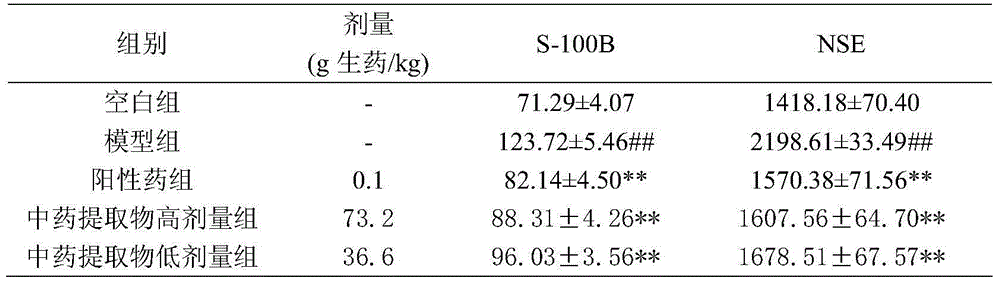
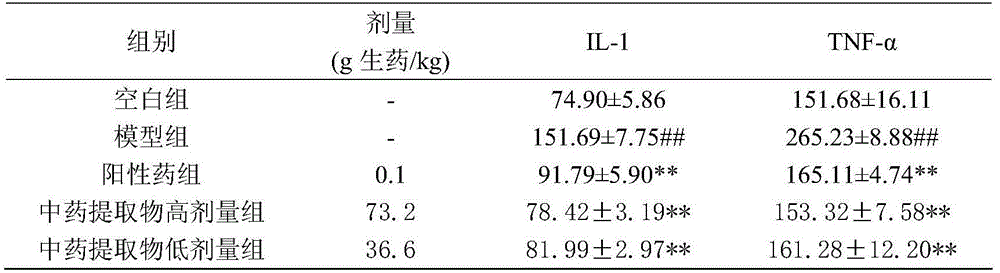
The invention discloses an extraction method and an application of a component group of a traditional Chinese medicine extract for treating hemorrhagic stroke. The extraction method comprises the following steps: after crushing rheum officinale, cortex moutan, red peony roots, dried radix rehmanniae and pseudo-ginseng in a certain weight ratio, adding water to decoct and extract; after extracting an extraction liquid by using dichloromethane, extracting a water layer by using n-butyl alcohol; and recovering and drying the n-butyl alcohol extraction liquid to obtain the component group of the traditional Chinese medicine extract. Analyzed by HPLC-MS, the traditional Chinese medicine extract is prepared from 33 components: paeoniflorins, tannins, paeonosides, anthraquinones, stilbenes, saponins and iridoid glycosides and can be used for remarkably reducing levels of neuronspecific enolase of essential hypertension male rats, S-100beta proteins, IL-1, TNF-alpha and plasma fibrinogen and can be used for preparing the medicine for treating hemorrhagic stroke.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for the detection of renal damage
InactiveUS20120220480A1Damage causedFailure causedLibrary screeningDisease diagnosisAlpha-enolaseFetuin b
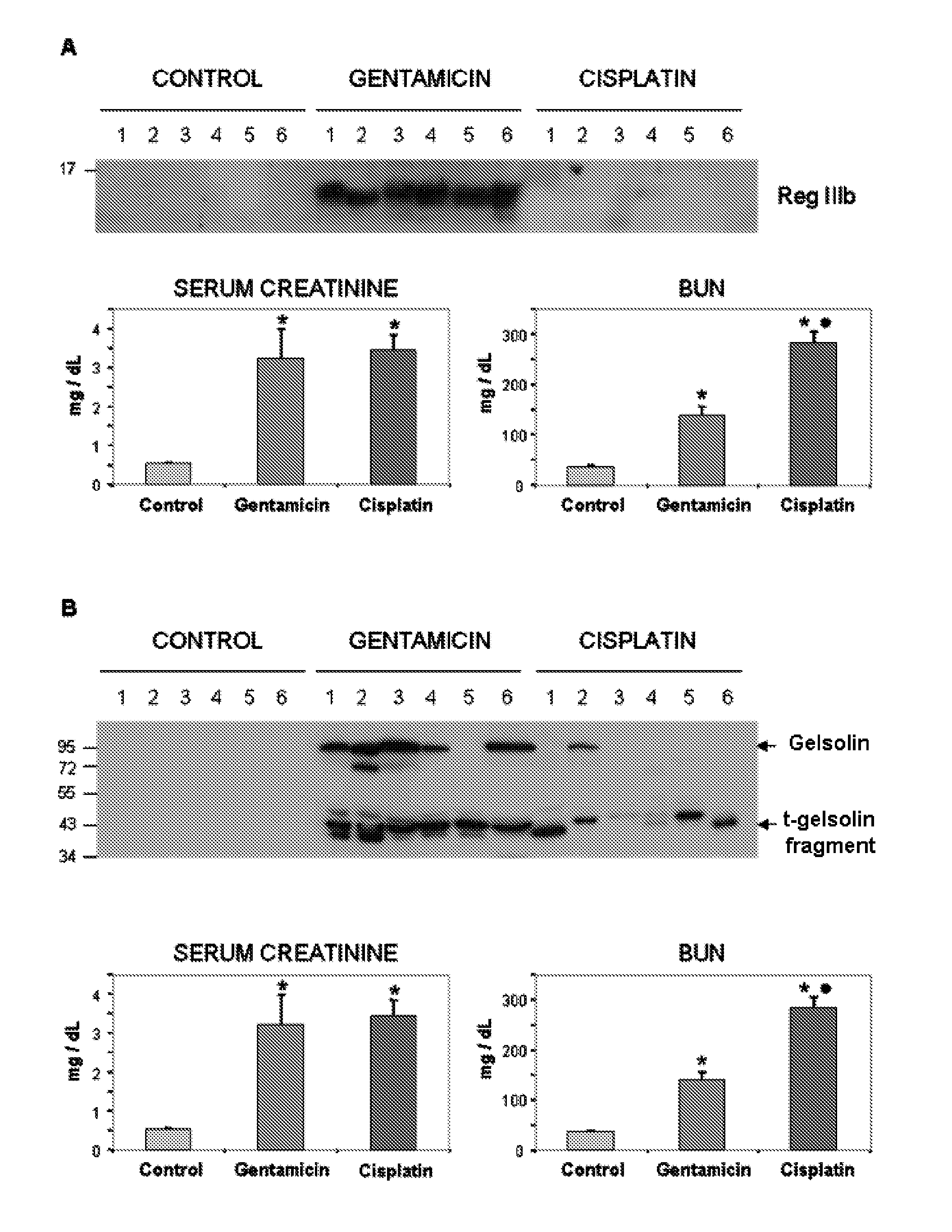
The invention relates to a method for determining the presence of renal damage in an individual and also to a method for detecting one or several proteins selected from the list comprising Reg3B, fetuin B, Ras-related GTP-binding protein A serine protease inhibitor A3L, subunit 1 of COP9, gamma subunit of ATP synthase, gelsolin, ribonuclease UK114, aminoacylase 1A, alpha-enolase, keratin 5, parvalbumin alpha, ribonuclease 4 or serine protease inhibitor A3K. The renal damage may be acute renal failure. Said renal pathologies may be caused by the administration of a nephrotoxic agent, wherein the nephrotoxic agent may be an aminoglycoside antibiotic such as gentamicin, or cisplatin. The invention also provides means to differentiate the renal damage or renal failure induced by gentamicin from that induced by cisplatin, through the biochemical analysis of the urinary level of Reg3B and / or gelsolin, or fragments thereof.
Owner:UNIV DE SALAMANCA
Synthesizing process of cis-dihydro jasmine keto-acid methyl ester
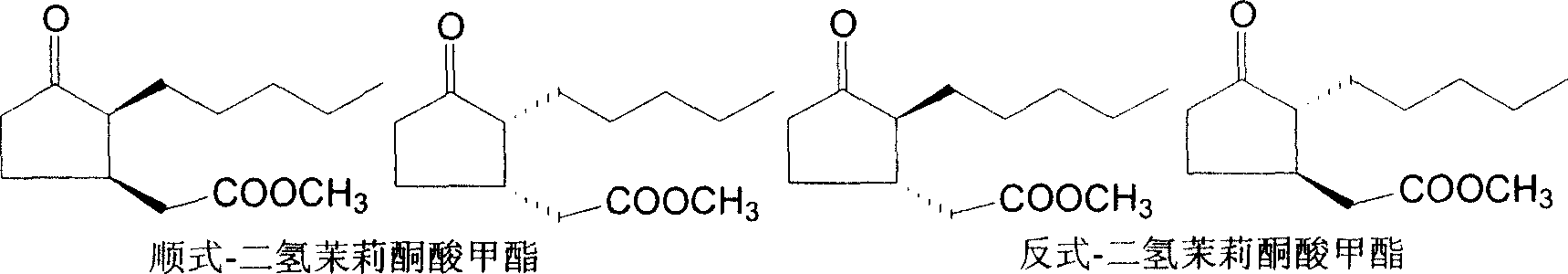
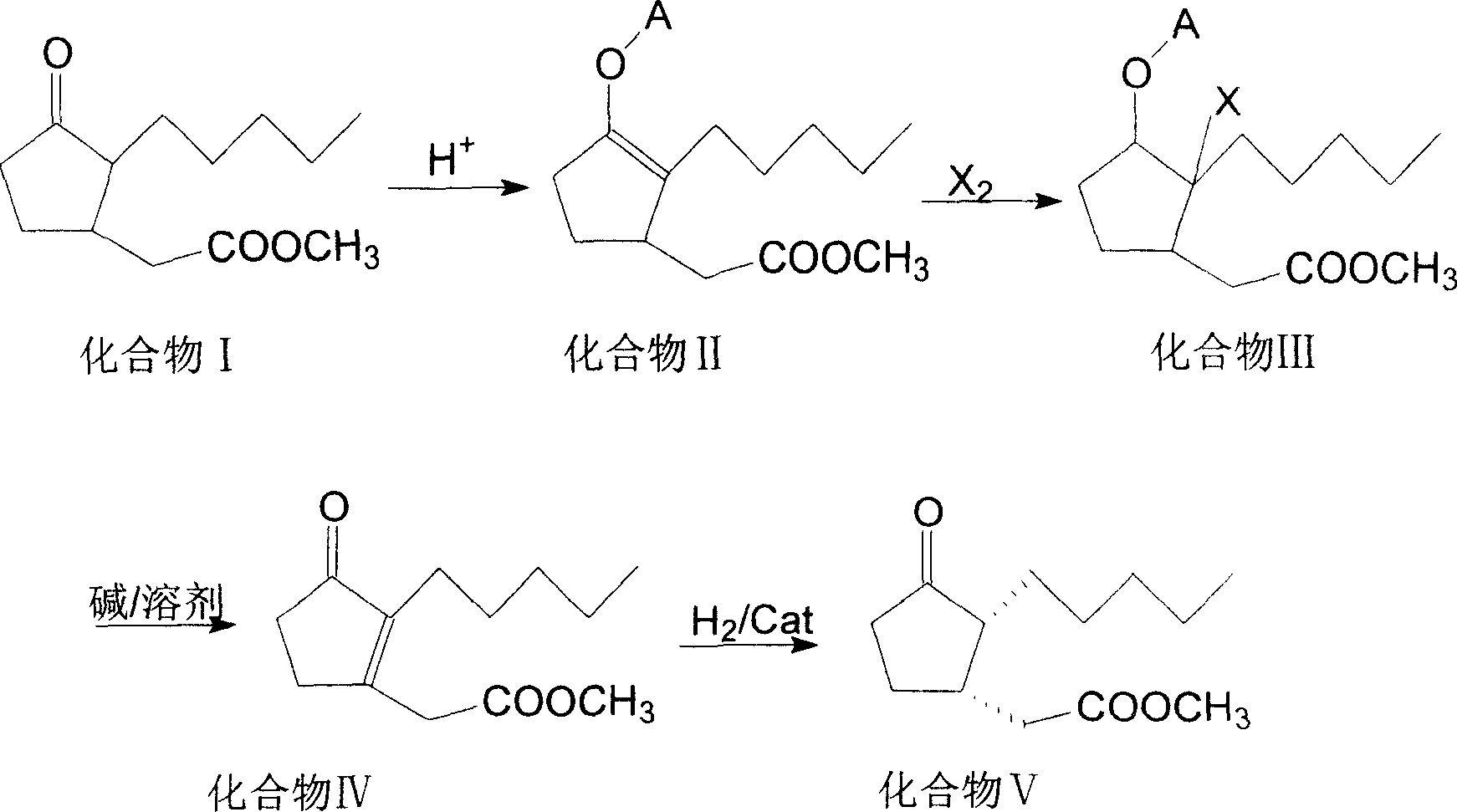
ActiveCN100999466AIncrease contentEasy to operateOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsSolvent free
This invention involves a cis-dihydrojasmone acid methyl ester synthetic method. The existing multi-step synthesis method is low yield and product content. This invention takes ordinary dihydrojasmone acid methyl ester as raw material, by enolase reaction and halogenation reaction, to dehalogenate to prepare double bonds jasmonate methyl ester; finally, under the solvent-free state through hydrogenation to obtain cis-dihydrojasmone acid methyl ester.
Owner:ZHEJIANG NHU CO LTD
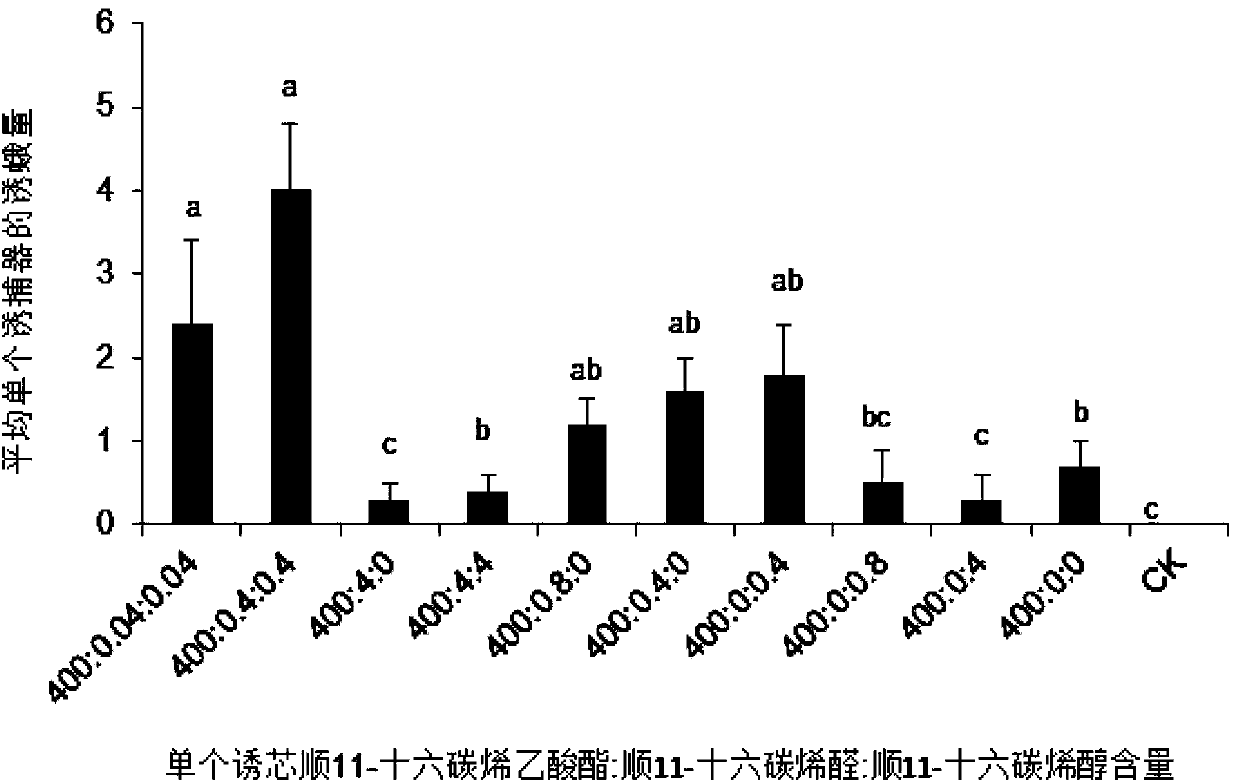
Sex pheromone composition of orthaga olivacea warre and lure core thereof
The present invention relates to a sex pheromone composition of orthaga olivacea warre, and a lure core thereof. The sex pheromone composition is characterized in that the sex pheromone composition comprises cis-11-hexadecenyl acetate, trace amounts of cis-11-hexadecenoic enolase and cis-11-hexadecenal. Antioxidants between 1% to 10% of the total component by mass and anti-ultraviolet stabilizers between 1% to 10% of the total component by mass are added into the prepared composition. A releaser can be prepared froma chlorobutyl rubber or a PVC capillary tube. The present invention firstly discovers and identifies that lures of the sex pheromone of orthaga olivacea warre are cis-11-hexadecenyl acetate, trace amounts of cis-11-hexadecenoic enolase and cis-11-hexadecenal, and concludes that each lure core has luring effects on male orthaga olivacea warreat at a dosage of 10 to 4000 micrograms, and the optimal dosage of each lure core is 1000 micrograms. According to the present invention, the sex pheromone composition of orthaga olivacea warre combined with a trapping device can effectively booby a large number of male orthaga olivacea warre, and can be applied to monitoring, prevention and controling of orthaga olivacea warre populations. The sex pheromone composition plays an important role in orthaga olivacea warre trapping and forecasting.
Owner:NINGBO CITY COLLEGE OF VOCATIONAL TECH +1
Nerve specificity olefinic alcohol enzyme chemiluminescence immune analysis determination reagent kit and preparing method thereof
ActiveCN101377499AHigh sensitivityRapid clinical diagnosisChemiluminescene/bioluminescenceAntigenDisease
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for nerve specific enolase and a preparation method thereof. The kit of the invention comprises 1) nerve specific enolase calibrators, 2) solid-phase vectors which are coated by nerve specific enolase monoclonal antibodies, 3) nerve specific enolase monoclonal antibody antigens which are marked by enzyme and 4) chemiluminescent substrates which are acted by the enzyme. The invention also provides a preparation method of the kit, which comprises the steps: preparing the calibrators, coating the vectors, marking the antibodies, packaging, and the like. The kit of the invention has the advantages of convenience, rapidness, sensitivity, stability, and the like. The invention can provide good technical support for the early detection, the detection of disease progress and the judgment of healing effect of some diseases such as small cell lung carcinoma (SCLC), neuroblastoma, brain damage, and the like.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Compositions comprising ENO1 and their use in methods of treating obesity or overweight and reducing weight gain
The invention provides a method for reducing or preventing body weight gain in a subject comprising administering to the subject enolase 1 (Eno1). The invention also provides methods of treating obesity, and of reducing body weight in a subject afflicted with an overweight condition, comprising administering to the subject enolase 1 (Eno1). In certain embodiments, the body weight gain, obesity oroverweight condition is caused by a therapeutic treatment, such as a diabetic drug. In certain methods of the invention, the Eno1 is delivered to muscle.
Owner:BERG
Mycoplasma bovis enolase and new applications of coding gene thereof
InactiveCN102533940ACtiveImmunogenicMicrobiological testing/measurementMicroorganism based processesDiseaseMycoplasma bovis Antibody
The invention discloses mycoplasma bovis enolase and new applications of a coding gene thereof. Specifically, the invention discloses the mycoplasma bovis enolase and applications of the coding gene of the mycoplasma bovis enolase in preparing reagents for detecting plasminogen and detecting or diagnosing infections caused by mycoplasma bovis. According to the mycoplasma bovis enolase disclosed by the invention, an enolase protein is obtained through prokaryotic expression, and soluble expression is successfully performed on the protein through regulating induction conditions, and further, through recombinant enolase protein and plasminogen ligand experiments and enolase protein and mycoplasma bovis antibody Western blot experiments, the protein is finally proved to have the binding activity and the immunogenicity of the plasminogen, therefore, the protein can be used for detecting the plasminogen and detecting or diagnosing the infections or diseases caused by the mycoplasma bovis. Moreover, a new path is also opened up for researching the adhesion mechanism and the pathogenicity of the mycoplasma bovis and developing vaccines in future.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Anti-alpha-enolase I antibodies for diagnosis and treatment of alpha-enolase I-associated diseases
The invention relates to antibodies against α-enolase I, their pharmaceutical compositions and diagnosis and treatment uses. Particularly, the invention provides polyclonal anti-α-enolase I antibodies and monoclonal single-chain variable fragment (scFv) anti-α-enolase antibodies, pharmaceutical compositions containing the same and their uses in uses in diagnosis and treatment of cancers, autoimmune disorders, ischemia and bacterial infection.
Owner:TAIPEI MEDICAL UNIV
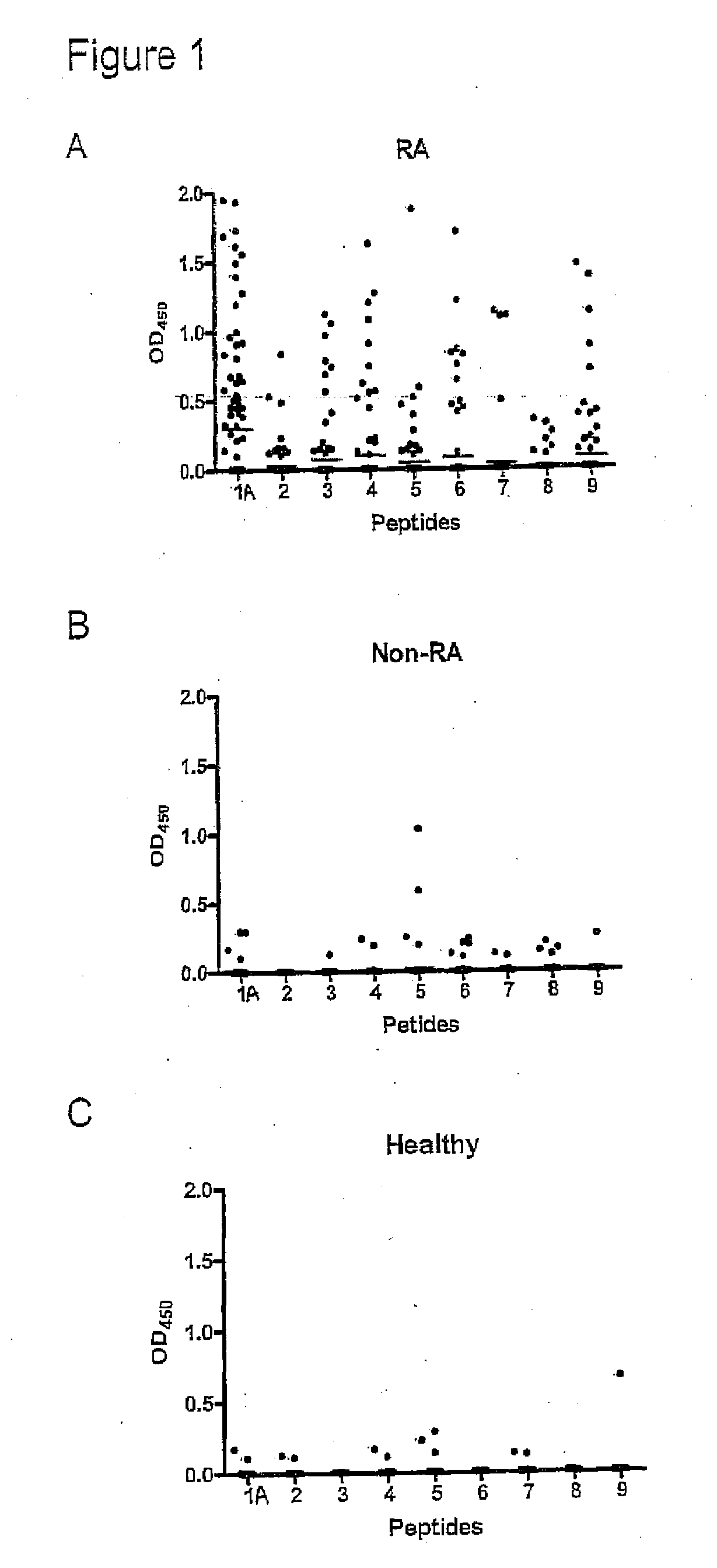
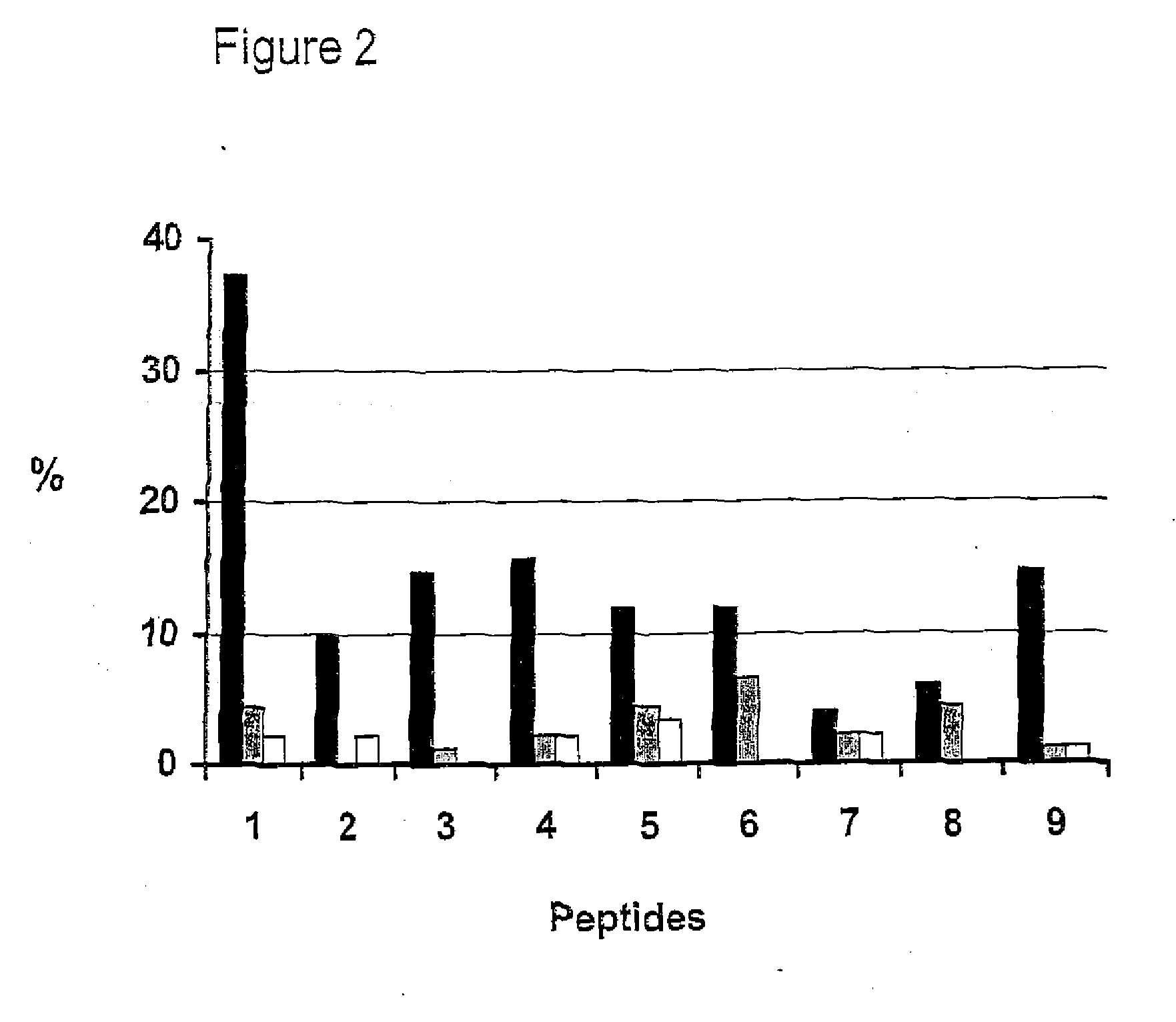
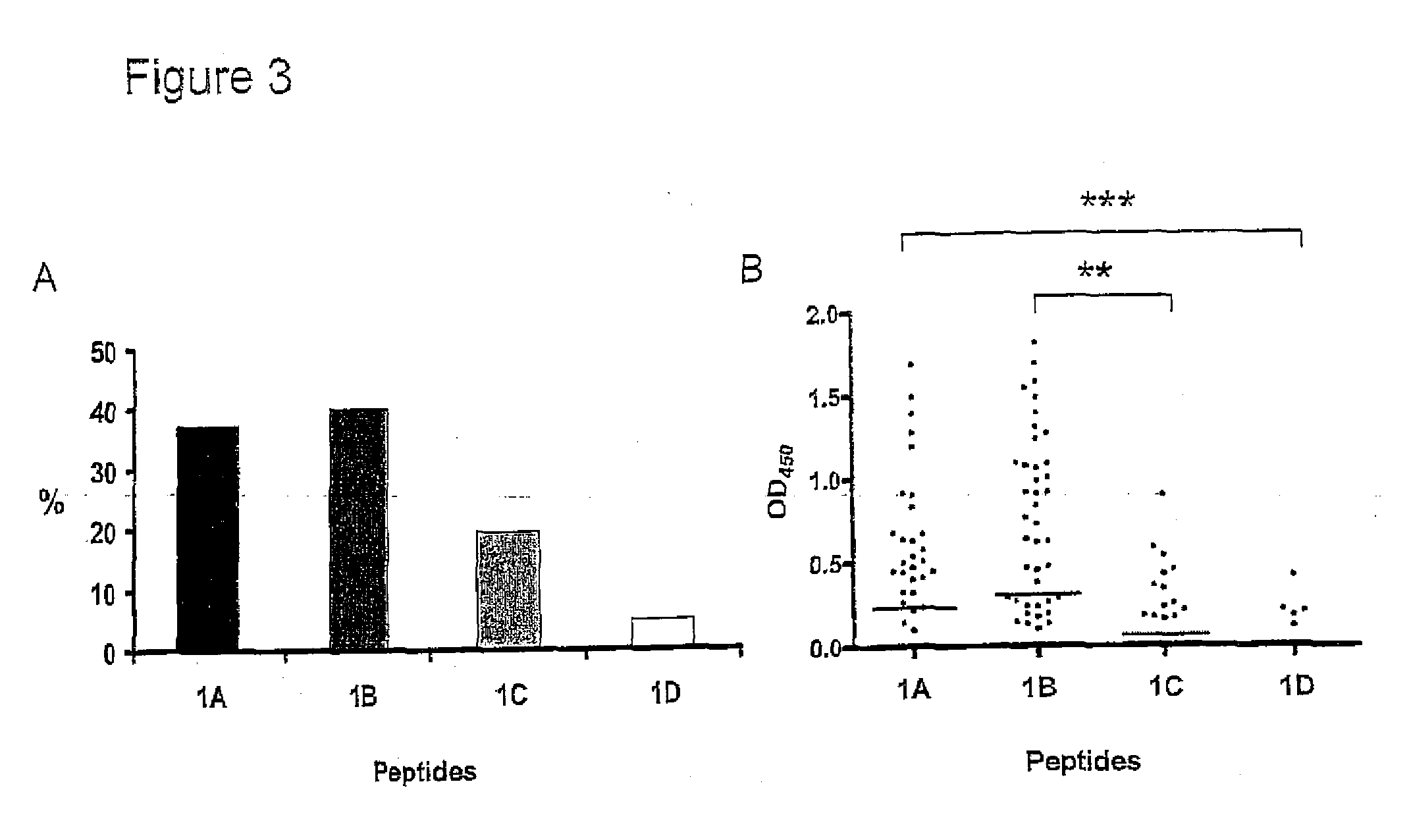
Methods For Diagnosing Rheumatoid Arthritis
The present invention relates to methods of diagnosing, prognosing, preventing or delaying onset of or treating rheumatoid arthritis, methods of distinguishing between different types or stages of rheumatoid arthritis, of identifying an individual at risk of developing rheumatoid arthritis, and of monitoring efficacy of a treatment regime in an individual being treated for rheumatoid arthritis, using a citrullinated enolase peptide to detect or capture antibodies associated with rheumatoid arthritis.
Owner:IMPERIAL INNOVATIONS LTD
Taenia multiceps enolase TmENO recombinant protein with immunizing protection
ActiveCN103131719AHigh purityHigh expression yieldAntiparasitic agentsAntibody medical ingredientsEscherichia coliProtective antigen
The invention relates to taenia multiceps protective antigen enolase TmENO recombinant protein with immunizing protection and a prepared method and application thereof. Amino acid sequence of the recombinant protein is GenBank. Amino acid sequence of ORF sequence encoding of accession number is SEQ No.5. Gene sequence of encoding gene sequence according to escherichia coli prefered codons to optimize is SEQ No.6. The recombinant protein can obviously reduce the number of cysts parasitized in sheep brains, and immunizing protection is achieved. Related experiments show that high title antibodies can be produced after animals are immunized by the recombinant protein, and the stronger immunizing protection can be created to taenia multiceps egg attack infection. The TmENO recombinant protein is a candidate target of coenurosis vaccine development and can be used for immunoprophylaxis of the coenurosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for identification and purification of intracellular enolase
InactiveCN102492769ASimple and fast operationShort experiment cycleMicrobiological testing/measurementLyasesStainingElectrophoresis
The invention belongs to a method for identification and purification of intracellular enolase. The method comprises the method for identifying and purifying the intracellular enolase. The method comprises the following steps that: 0.5 g (wet weight) of collected bacteria or cells are suspended by using 0.5 ml of a PBS buffer; treatments of electrophoresis and gel stripping are performed; the resulting gel sheet is subjected to activity staining; a small amount purification treatment is performed by a dialysis method to obtain the enolase; and the like. With the present invention, the intracellular enolase can be intuitionally identified and purified, and the method has characteristics of simple operation, short experimental period, low price, economy and practicality.
Owner:TIANJIN YAOYU BIOLOGICAL TECH
Methods and pharmaceutical compositions for the treatment of autoimmune diseases
InactiveUS20140086895A1Good curative effectLow toxicityPeptide/protein ingredientsAntipyreticDiseaseAutoimmune disease
The present invention relates to methods and pharmaceutical compositions for the treatment of autoimmune diseases, especially rheumatoid arthritis. More particularly, the present invention relates to an a-enolase polypeptide for use in the prophylactic treatment of an autoimmune disease in a subject in need thereof.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com