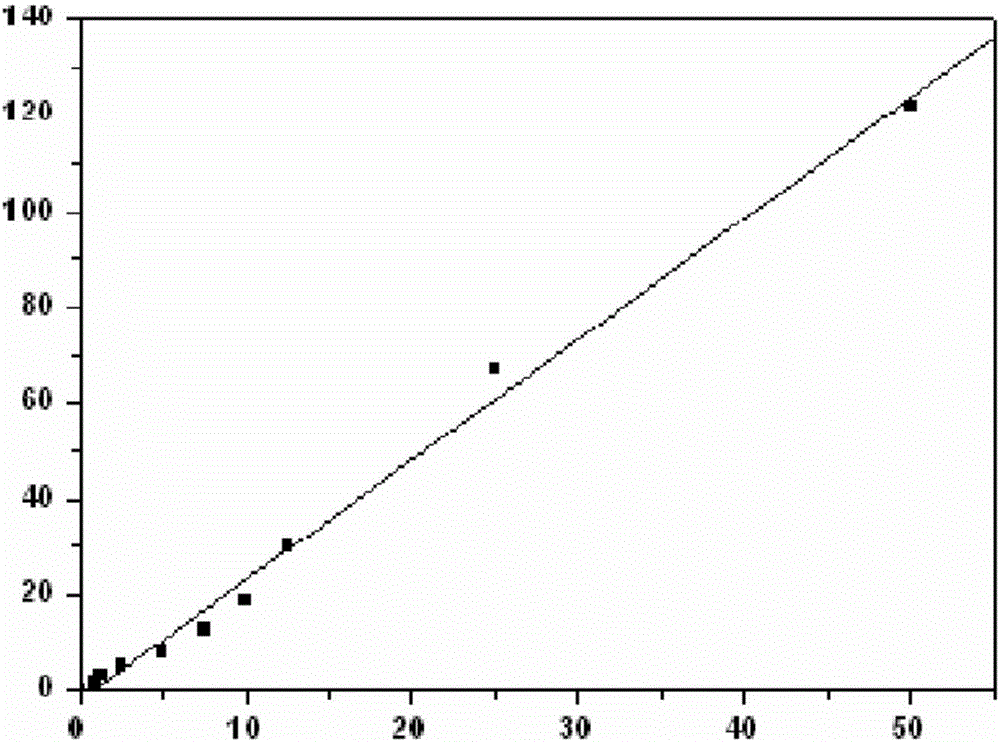
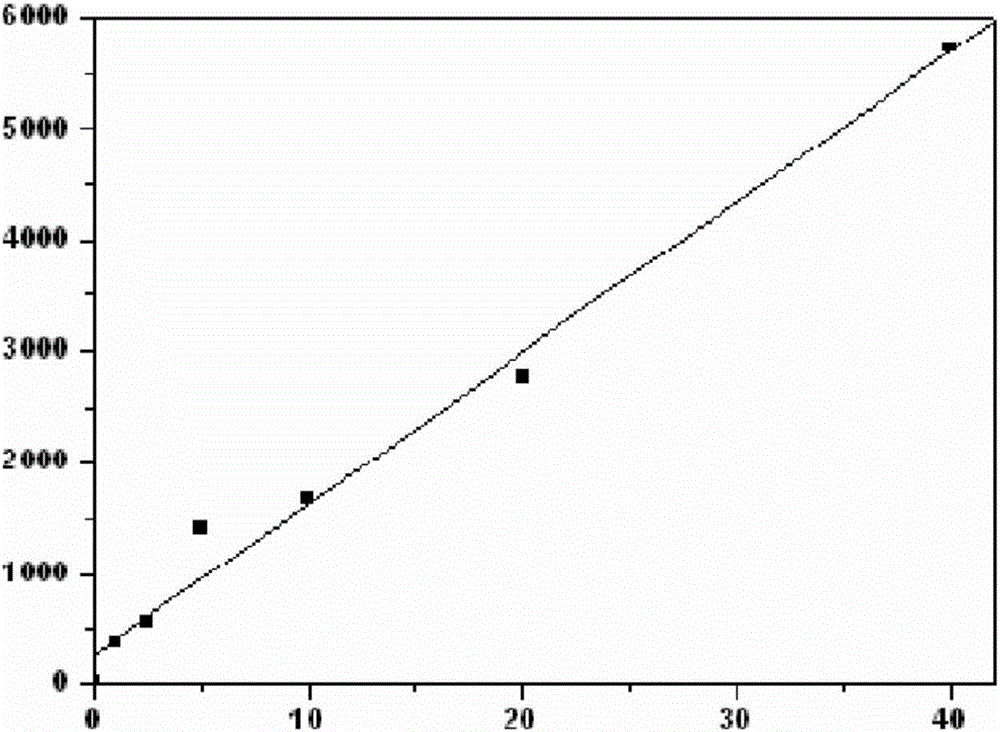
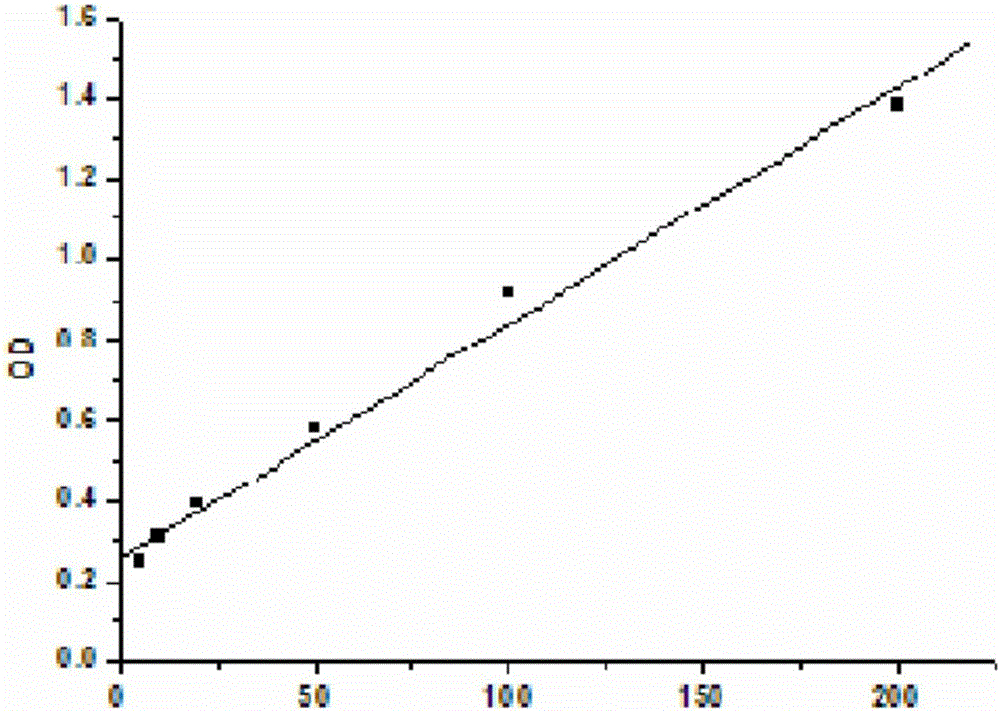
Immunological marker detection method, reagent, and detection kit
A marker and anti-immune technology, which is applied in the field of medical testing, can solve problems such as difficult automation, and achieve the effects of improving detection efficiency, high accuracy, and simplifying operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1. Preparation of DCP Magnetic Particle Separation Immunofluorescence Analysis Detection Reagent
[0090] 1. Preparation of DCP antigen
[0091] (1) Construction of pColdⅡ-DCP expression vector
[0092] Design primers and synthesize the DCP gene with reference to the gene sequence of human DCP in GenBank. After PCR amplification, 1% agarose gel electrophoresis was used to detect the DCP amplification product, and the target band was excised, and the target fragment was recovered using a gel recovery kit. The prokaryotic expression vector pColdⅡ was ligated with restriction endonucleases NdeⅠ and HindⅢ, transformed into E. coli E.coli.DH5α for amplification, and then transferred into E.coli.BL21 (DE3) competent cells, inoculated In LB solid medium containing 100 mg / L ampicillin (Amp), the plasmid was extracted, identified by NdeI and HindIII enzyme digestion and sequenced. The recombinant plasmid pColdⅡ-DCP was double digested with 5'NdeⅠand 3'HindⅢ, and two sp...
Embodiment 2
[0149] Example 2. Preparation of AFP-L3 Magnetic Particle Separation Immunofluorescence Analysis Detection Reagent
[0150] 1. Preparation of AFP-L3 antigen
[0151] (1) Construction of pColdⅡ-AFP-L3 expression vector
[0152] Refer to the gene sequence of human AFP-L3 in GenBank to design primers and synthesize the AFP-L3 gene. After PCR amplification, 1% agarose gel electrophoresis was used to detect the AFP-L3 amplification product. Recycle the target fragment. The prokaryotic expression vector pColdⅡ was ligated with restriction endonucleases NdeⅠ and HindⅢ, transformed into E. coli E.coli.DH5α for amplification, and then transferred into E.coli.BL21 (DE3) competent cells, inoculated In LB solid medium containing 100 mg / L ampicillin (Amp), the plasmid was extracted, identified by NdeI and HindIII enzyme digestion and sequenced. The recombinant plasmid pColdⅡ-AFP-L3 was double digested with 5'NdeⅠ and 3'HindⅢ, and two specific bands appeared, whose positions were consist...
Embodiment 3
[0195] Example 3. Preparation of GP73 Magnetic Particle Separation Immunofluorescence Analysis Detection Reagent
[0196] 1. Preparation of GP73 antigen
[0197] (1) Construction of pColdⅡ-GP73 expression vector
[0198] Design primers with reference to the human GP73 gene sequence in GenBank and synthesize the GP73 gene. After PCR amplification, 1% agarose gel electrophoresis was used to detect the GP73 amplification product, and the target band was excised, and the target fragment was recovered using a gel recovery kit. The prokaryotic expression vector pColdⅡ was ligated with restriction endonucleases NdeⅠ and HindⅢ, transformed into E. coli E.coli.DH5α for amplification, and then transferred into E.coli.BL21 (DE3) competent cells, inoculated In LB solid medium containing 100 mg / L ampicillin (Amp), the plasmid was extracted, identified by NdeI and HindIII enzyme digestion and sequenced. The recombinant plasmid pColdⅡ-GP73 was digested with 5'NdeⅠand 3'HindⅢ, and two speci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com