Pseudorabies/porcine parvovirus infection combined inactivate vaccine and suspension culture preparation method
A technology of porcine pseudorabies virus and dual inactivated vaccines, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problem of increasing the number of artificial and animal stress, large area occupied by spinner bottle culture, and affecting the use effect of vaccines, etc. problems, to achieve the effect of reducing the number of animal stress, reducing the number of immunizations, and large batches of antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 porcine pseudorabies virus suspension culture antigen
[0030] 1.1 Porcine pseudorabies virus gE gene deletion strain (HNX-12 strain)
[0031] 1.1.1 Preparation of virus seeds for production
[0032] 1.1.1.1 Breeding of poisonous seeds
[0033] The suspension type BHK-21 cells were inoculated into cell shaker flasks, cultured on a shaker at 37°C and 120r / min, the cell viability was over 95%, and the cell density was 4-6×10 6 Seed cells per ml were added to the inoculation bottle, mixed well, sampled and counted, and transferred to the bioreactor. Dissolved oxygen (DO) electrodes, pH electrodes, and temperature electrodes were calibrated before the bioreactor was inoculated with cells, and the tank was autoclaved. According to the culture volume, pump 70% of the culture medium in advance, and set the best culture conditions: temperature 37°C, inoculation density 5~7×10 5 cells / ml, rotation speed 50-60r / min, pH value 7.2-7.4, dissolved ...
Embodiment 2
[0078] Embodiment 2 Porcine pseudorabies / porcine parvovirus disease dual inactivated vaccine
[0079] 2 Vaccine preparation
[0080] 2.1 Preparation of aqueous phase Mix the two inactivated virus liquids (porcine pseudorabies virus: porcine parvovirus) uniformly at a ratio of 1:1 (volume ratio). Among them, the virus content of porcine pseudorabies virus before inactivation should be ≥10 8.0 TCID50 / ml, the porcine parvovirus content before inactivation should be ≥10 7.0 TCID 50 / ml or virus hemagglutination (HA) value ≥ 2 10 .
[0081] 2.2 Emulsification: Mix the water phase and the adjuvant according to the ratio (mass ratio) of 1:1 and then emulsify. Take a sample, draw 10.0ml of vaccine into a centrifuge tube, centrifuge at 3000r / min for 15 minutes, and the water precipitated at the bottom of the tube should not exceed 0.5ml.
[0082] 2.3 Subpackaging: Quantitatively subpackage the emulsified vaccines, crimp and label.
Embodiment 3
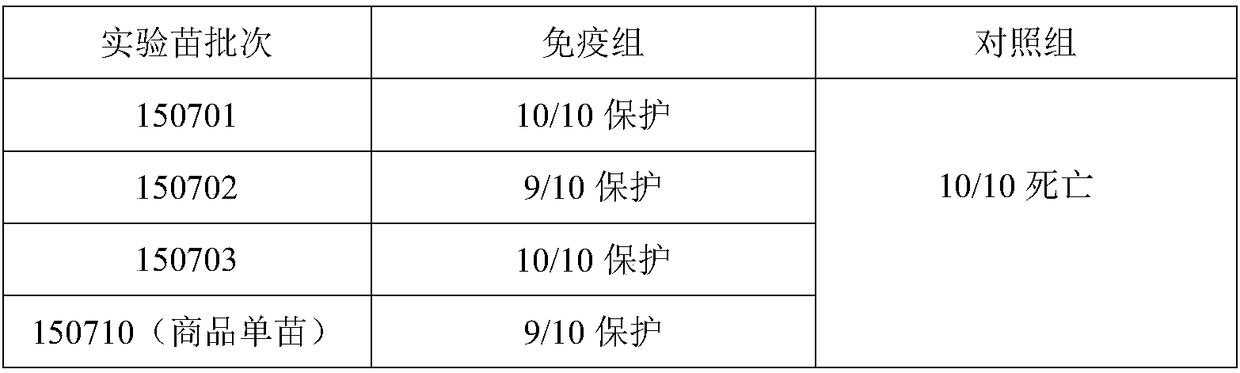
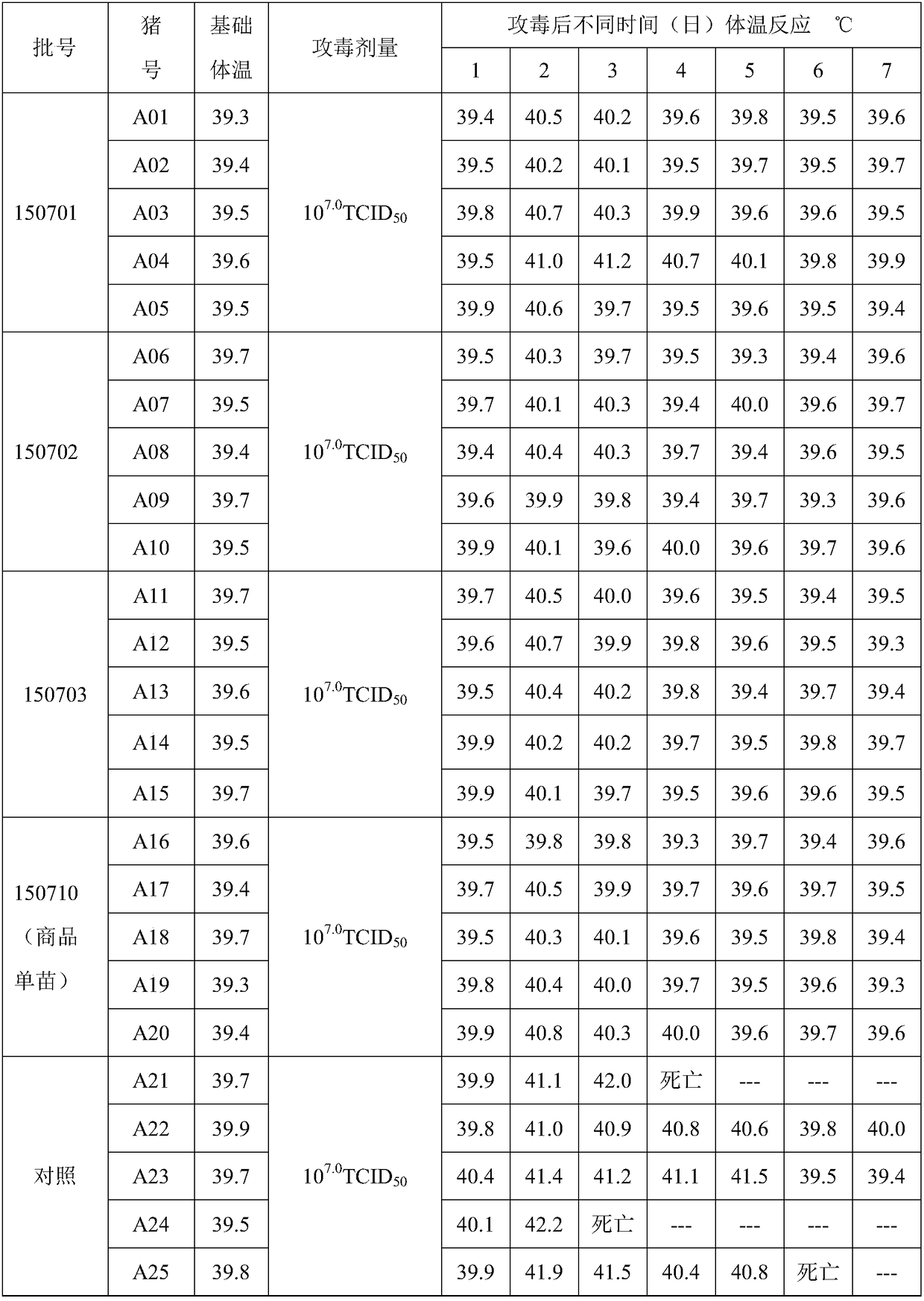
[0083] The toxicity determination of embodiment 3 antigen
[0084] 3 Porcine pseudorabies virus TCID 50 Determination of
[0085] 3.1.1 The virus was serially diluted 10 times with DMEM maintenance solution (containing 2% newborn bovine serum), and 10 -5 、10 -6 、10 -7 、10 -8 、10 -9 5 dilutions, each dilution was inoculated in a 96-well micro-cell culture plate in a vertical row with a total of 8 wells, and 8 wells of cell control were set at the same time, 100 μl per well.
[0086] 3.1.2 Add 100 μl of BHK-21 cell suspension to each well.
[0087] 3.1.3 5% CO at 37°C 2 Cultivate and observe in the incubator for 4 to 6 days. When 80% of the cells are lesioned (cells aggregate and bulge, the outline of the cells is blurred, round and shrink, and finally fall off and disintegrate), it is judged as infection, record the number of cells with lesion, and calculate the TCID according to the Reed-Muench method 50 . The results are shown in Table 1 below. The experimental data ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com