Preparation method for living-vector vaccine against rabies, product thereof and application thereof
A live carrier vaccine and rabies technology, which is applied in botany equipment and methods, biochemical equipment and methods, vaccines, etc., can solve the problems that there are no rabies live carrier vaccines on the market, and achieve short production cycle and good immune effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 rabies live vector vaccine
[0068] The method of this example can be used to prepare a replication-defective adenovirus rabies live vector vaccine for direct vaccination of animals. Including the following process, refer to Figure 4 :
[0069] 1. Preparation of target gene
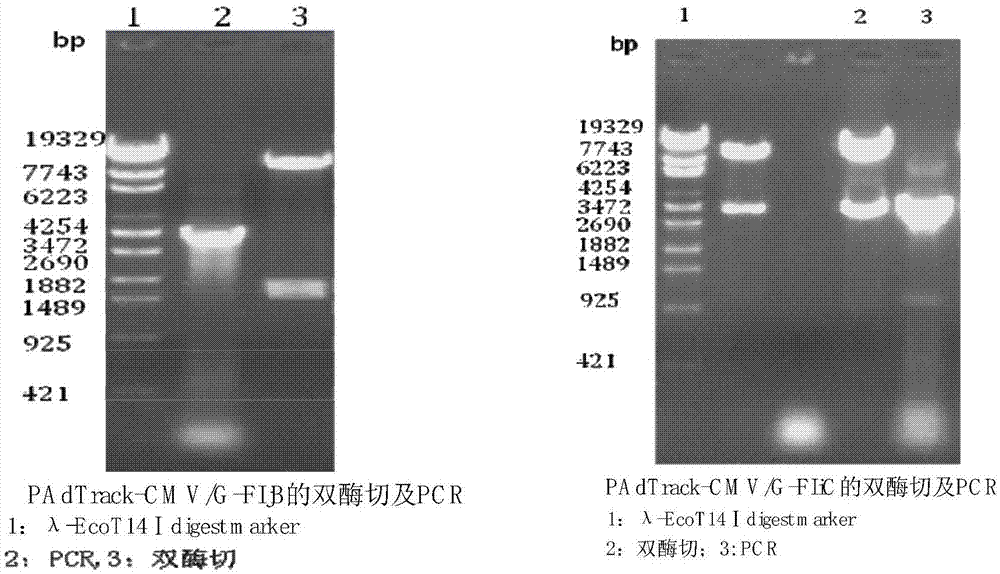
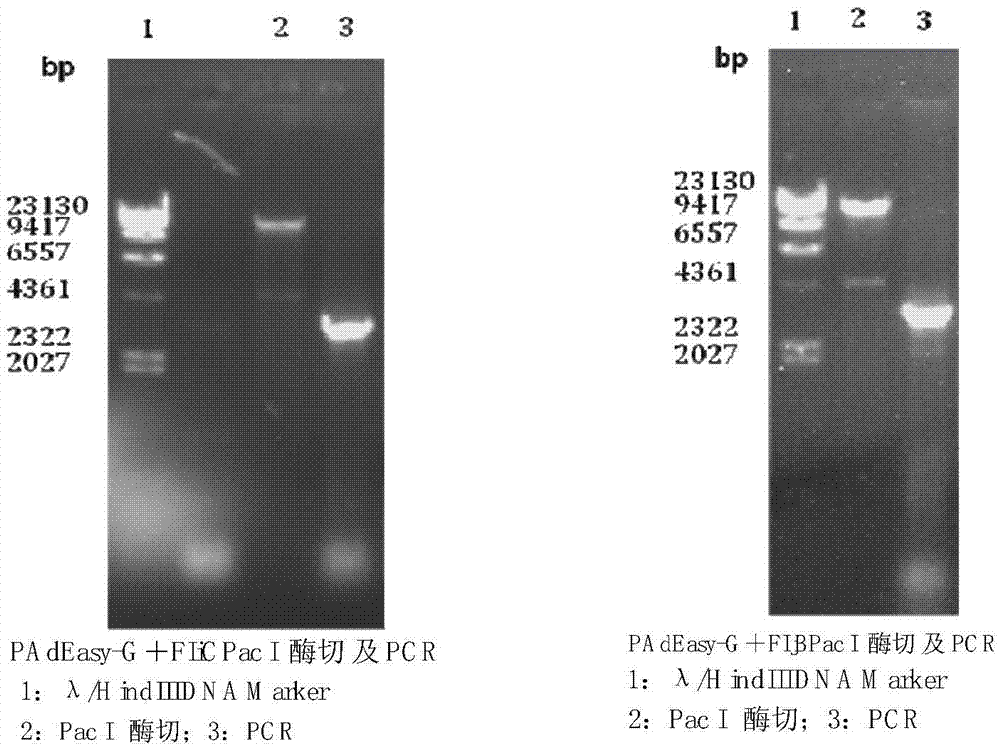
[0070] Design primers, amplify rabies virus (Rabies Virus, RV) antigenic protein G gene and bacterial flagellin FIjB gene or FIiC gene by the method for PCR; Designed antigenic protein gene G and bacterial flagellin FIjB gene or FIiC gene amplification The primers are:
[0071] GF: 5'GCAGATCTGCCACCATGGTTCCTCAAAGCTCTTTTG3' (contains Bgl Ⅱ restriction site)
[0072] GR: 5'GCGTCGACTCACAGTCTGATCTCACC 3' (contains Sal Ⅰ restriction site)
[0073] FIjBF: 5'ATTGTCGACGCCACCATGGCACAAGTAATCAACACT 3' (contains Sal Ⅰ site)
[0074] FIjBR: 5'GCTCTAGATTAACGTAACAGAGACAGC 3' (contains Xba Ⅰ restriction site)
[0075] FIiCF: 5'ATTGTCGACGCCACCATGGCACAAGTCATTAATACA 3' (contains...
Embodiment 2
[0114] The immune efficacy experimental animal test data of embodiment 2 rabies live carrier vaccines
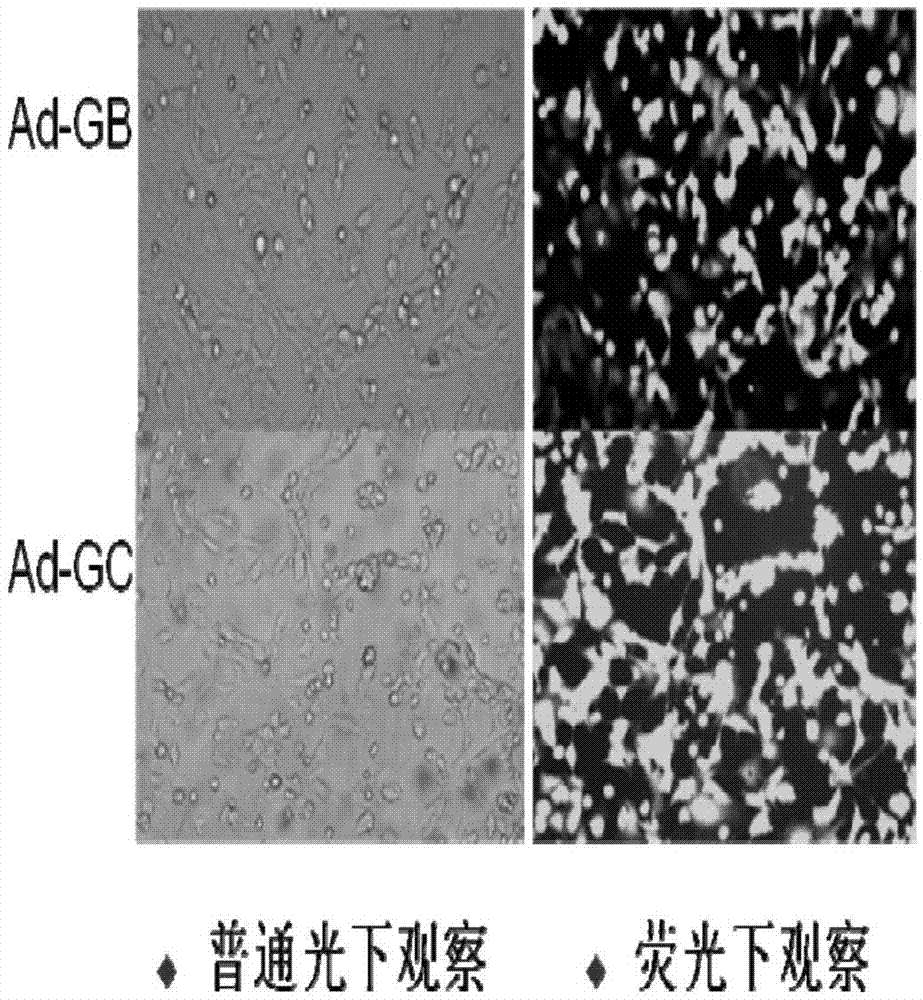
[0115] Three recombinant adenovirus live vectors constructed in Example 1, Ad-RV-G, Ad-RV-G-FljB, and Ad-RV-G-FliC, were used to immunize 6-8-week-old Balb / c on days 0 and 14, respectively. Blood was collected from the mice at 7, 14, 21, 28, 35, and 42 days after immunization, and the neutralizing antibody was measured by RFFIT method. The results are as follows: Figure 5 shown. The results showed that the neutralizing antibodies of the three recombinant adenoviruses all began to increase after the second immunization, and the level of neutralizing antibodies stimulated by the recombinant adenovirus Ad-RV-G-FljB was the highest, while the recombinant adenovirus Ad-RV-G The level of neutralizing antibody produced by -FliC was lower, but higher than that of Ad-RV-G. The results showed that the recombinant adenovirus co-expressed with flagellin FljB or FliC and rabies virus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com