Polypeptide vaccine for resisting multiple hypotype avian influenza viruses and preparation method thereof
An avian influenza virus and polypeptide vaccine technology, which is applied in the directions of antiviral agents, chemical instruments and methods, and medical preparations containing active ingredients, etc., to achieve the effects of long validity period, convenient transportation and storage, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
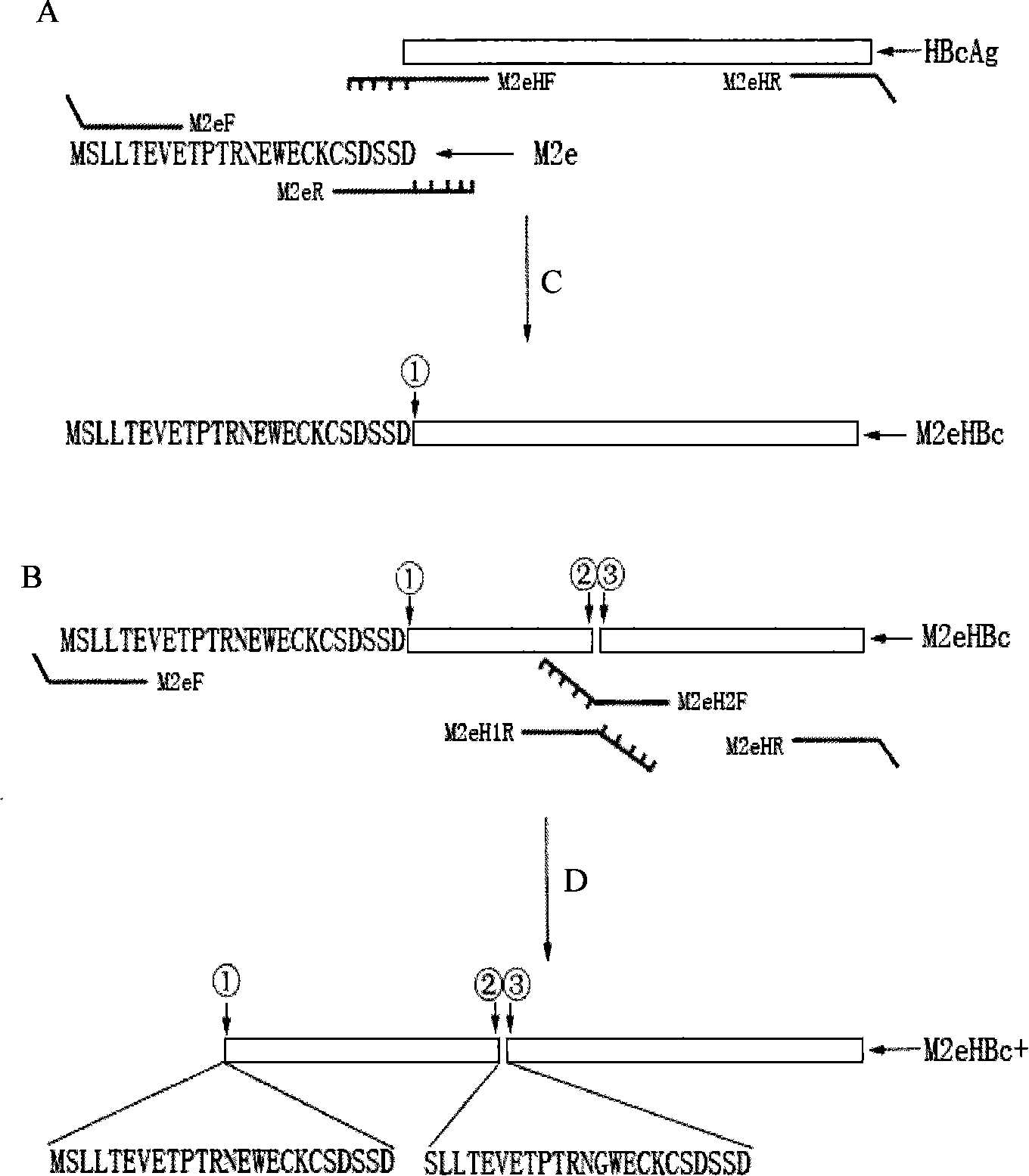
[0032] Example 1: Obtaining of M2e Fragment and HBcAg Fusion Gene
[0033] The OE-PCR (overlap extension PCR) method was used to construct the fusion gene, and the following primers were synthesized:
[0034] M2eF: 5′-GGCGTCGACATGAGTCTTTCTAACCGAGG-3′
[0035] M2eR: 5′-GTCAATGTCAGGATCACTTGAATCGCTG-3′
[0036] M2eHF: 5′-AGTGATCCTGACATTGACCCGTATAAAG-3′
[0037] M2eH 1R: 5′-CCAACCGTTACGGGTTGGGGGTTTCCACTTCGGTCAGCAGGCTTGCTGGGTCTTCCAAATTAC-3′
[0038] M2eH2F: 5′-ACCCGTAACGGTTGGGAATGCCGTTGCAGCGATAGCAGCGATTCCAGGGAATTAGTAGTCAG-3′
[0039] M2eHR: 5′-GGCAAGCTTCTAACATTGAGATTCCCGAG-3′
[0040]In the primers, 18 bases are complementary between M2eR and M2eHF, and 15 bases are complementary between M2eH1R and M2eH2F. Primer M2eF has a restriction site Sal I, and M2eHR is designed with a restriction site HindIII, so that the restriction fragment can be ligated into the prokaryotic expression vector pMALc2x.
[0041] The fusion gene construction of the M2e fragment connected to the 5' end...
Embodiment 2
[0043] Embodiment 2: the prokaryotic expression vector construction of M2eHBc+ fusion gene
[0044] After the M2eHBc+ fusion gene fragment was digested by Sal I and HindIII, it was ligated with the prokaryotic expression vector pMALc2x purified by the same digestion, and the ligation product was transformed into DH5α. The white clone picked from the resistance plate was digested and identified by sequencing. The correct clones were named pMALc2x-M2eHBc+, respectively.
Embodiment 3
[0045] Example 3: Expression, identification and purification of M2eHBc+ fusion protein
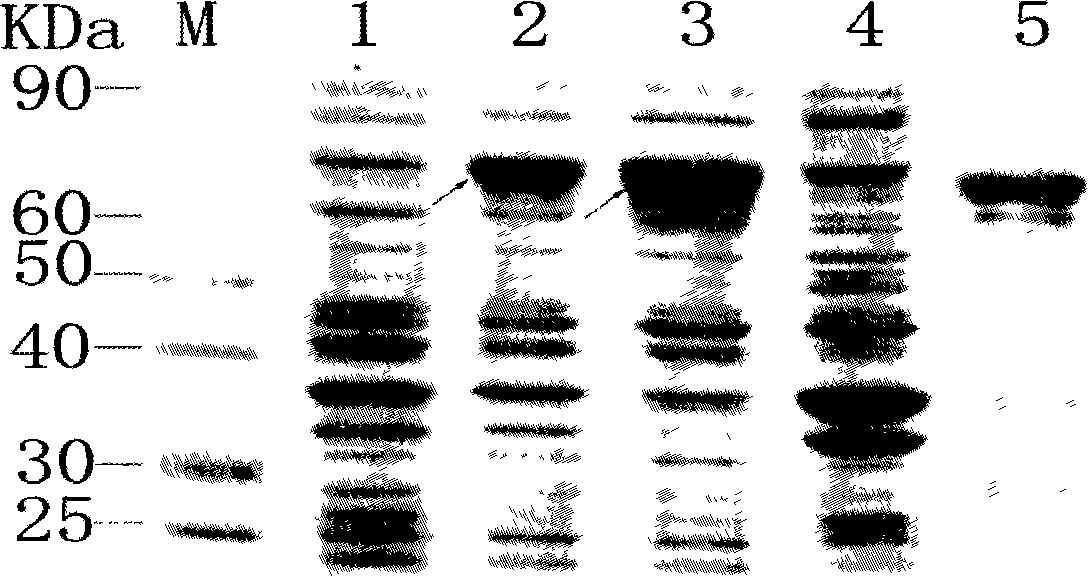
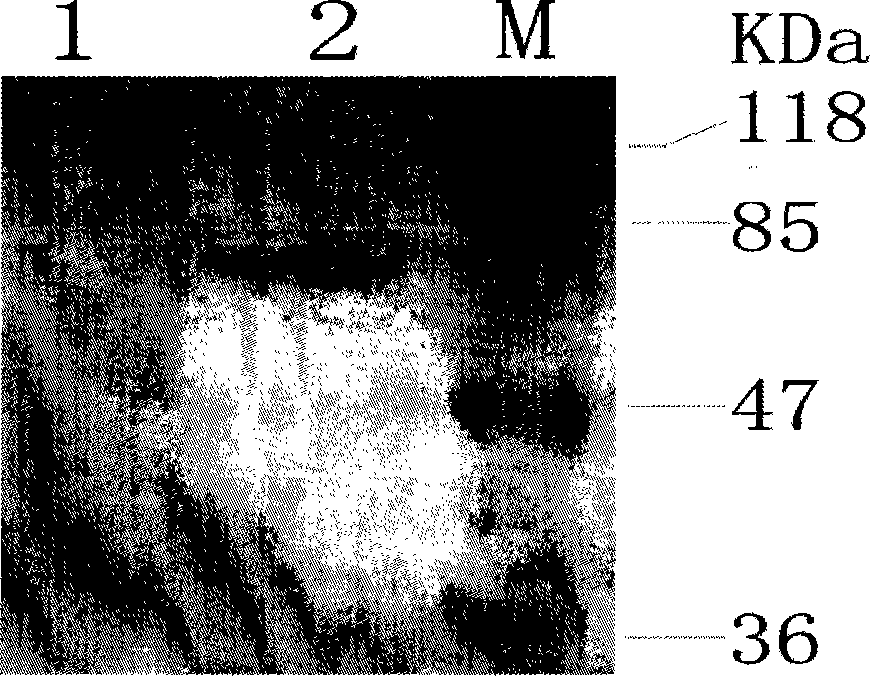
[0046] Transform the expression strain BL21(DE3) with the correctly sequenced prokaryotic expression vector pMALc2x-M2eHBc+, and inoculate the clones identified as positive in the pMAL special medium added with ampicillin and resistance, and culture with shaking until OD 600 = about 0.6, add IPTG to a final concentration of 0.8mmol / L to induce fusion gene expression. The induction conditions are: temperature 37°C, rotation speed 160r / min, induction 4h. After the induction, the bacterial cells were collected by centrifugation, and the protein expression was detected by SDS-PAGE electrophoresis (for the protein expression results, see figure 2 ). SDS-PAGE electrophoresis was performed on the bacterial protein induced by IPTG and the uninduced bacterial control. After the electrophoresis, the protein was transferred to a nylon membrane, and the rabbit anti-H5N1M2 protein antibody was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com