Recombinant rabies virus carrying two genes G and gene VP2 and application of recombinant rabies virus
A rabies virus and gene technology, applied to recombinant rabies virus and its application fields, can solve the problems of attenuated strain mutation, harm to dogs and wild animals, and immune failure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Screening of CPV epidemic strains
[0061] 1. Process and extract nucleic acid from suspected CPV samples collected clinically, and perform PCR amplification.
[0062] (1) See Table 1 for primer sequences
[0063] CPV detection primers CPV1 / CPV2 refer to the literature of Liu Zhonghua et al. (2003). By comparing the VP2 gene sequence with accession number M74849 published in GenBank, two pairs of primers VP2.F / VP2-1.R and VP2-2.F / VP2.R were designed with Primer Premier 5.0 (PREMIER Biosoft International) software.
[0064] Table 1
[0065]
[0066] (2) Utilize primer CPV1 / CPV2, carry out PCR amplification according to the system shown in Table 2:
[0067] Table 2
[0068]
[0069] (3) Take the positive samples detected by PCR with primers CPV1 / CPV2, and perform PCR amplification with primers VP2.F / VP2-1.R and VP2-2.F / VP2.R respectively. The system is shown in Table 3.
[0070] table 3
[0071]
[0072](4) According to the instruction manual of t...
Embodiment 2
[0078] Example 2 Cloning of CPV strain VP2 gene into HEP-Flury
[0079] 1. Construct the recombinant plasmid pHEP-Flury (VP2), see the strategy figure 2 shown.
[0080] (1) Utilize primer VP2.F / VP2.R (see Table 1) to amplify the VP2 gene (sequence shown in SEQ ID NO.1) of the CPV strain selected in the embodiment 1, VP2.F and VP2.R The primers are expected to amplify with a length of 1755bp (the amplification reaction system is shown in Table 5), and respectively introduce BsiWI and NheI restriction sites.
[0081] table 5
[0082]
[0083] Add the above reagents in turn to the PCR tube.
[0084] The PCR reaction conditions were: 95°C for 5min; 25 cycles of 94°C for 30s, 52°C for 30s, and 68°C for 2min; and finally extended at 68°C for 10min.
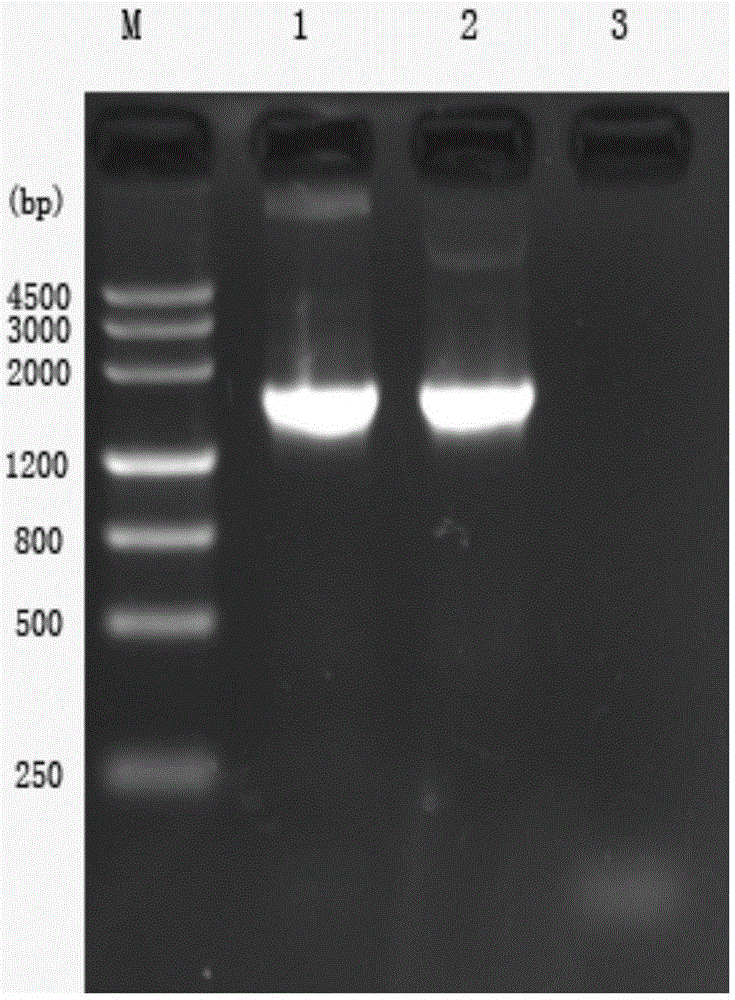
[0085] (2) After the reaction, 5 μL of PCR products were taken for electrophoresis detection on 1.0% agarose gel. Obtain a specific DNA electrophoresis band, the size is about 1755bp, which is consistent with the experiment exp...
Embodiment 3
[0099] Example 3 Construction of recombinant plasmid pHEP-Flury (dG-VP2) using recombinant plasmid pHEP-Flury (VP2)
[0100] 1. Use the recombinant plasmid pHEP-Flury (VP2) constructed in Example 2 to construct pHEP-Flury (dG-VP2). For the construction scheme, see Figure 5 .
[0101] (1) Using the pHEP-Flury (VP2) plasmid as a template, using the upstream primer pDV2.F (the sequence is 5'-CGG GGTACC ATGGTTCCTCAGGTTCT-3') and downstream primer pDV2.R (sequence: 5'-GCCTGCAGTTTTATTCCATATTATTC-3') for PCR amplification.
[0102] In the upstream primer pDV2.F, the sequence of the underlined part is the enzyme cutting site of Acc65I, which is the homologous enzyme with BsiWI. The sequence below Acc65I is the initiation codon ATG of the ORF box of the G gene.
[0103] The position of the downstream primer in the ORF box of VP2 gene is 281-305, and the position of PstI in the ORF box of VP2 gene is 269-274.
[0104] The pair of primers used the pHEP-Flury (VP2) plasmid as a temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com