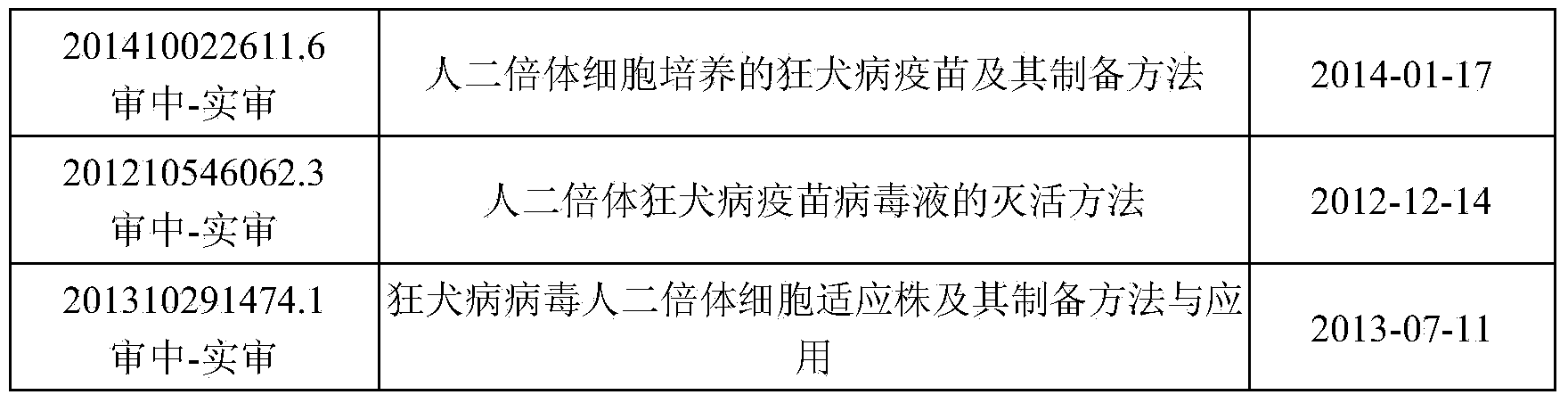
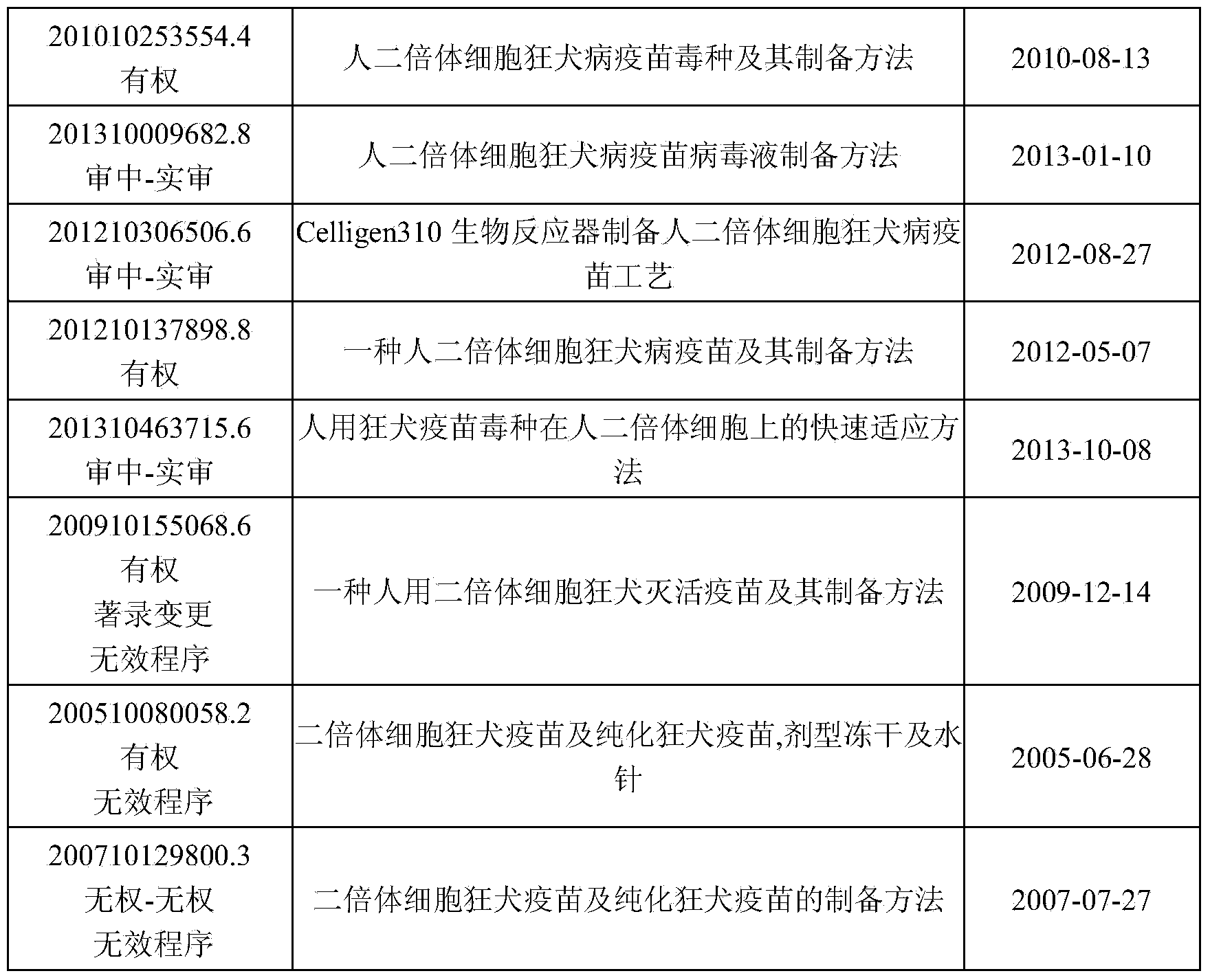
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Rabies virus strain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
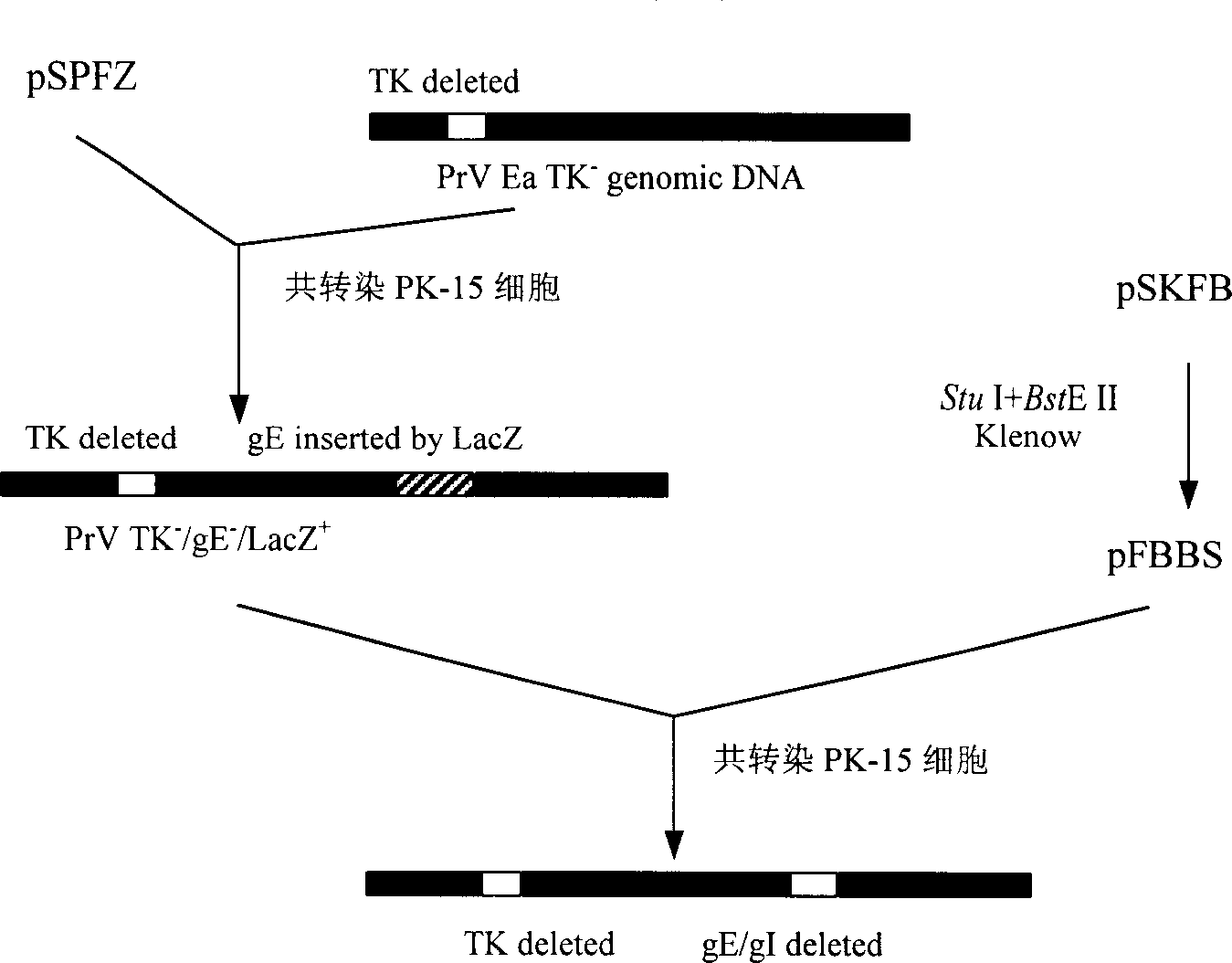
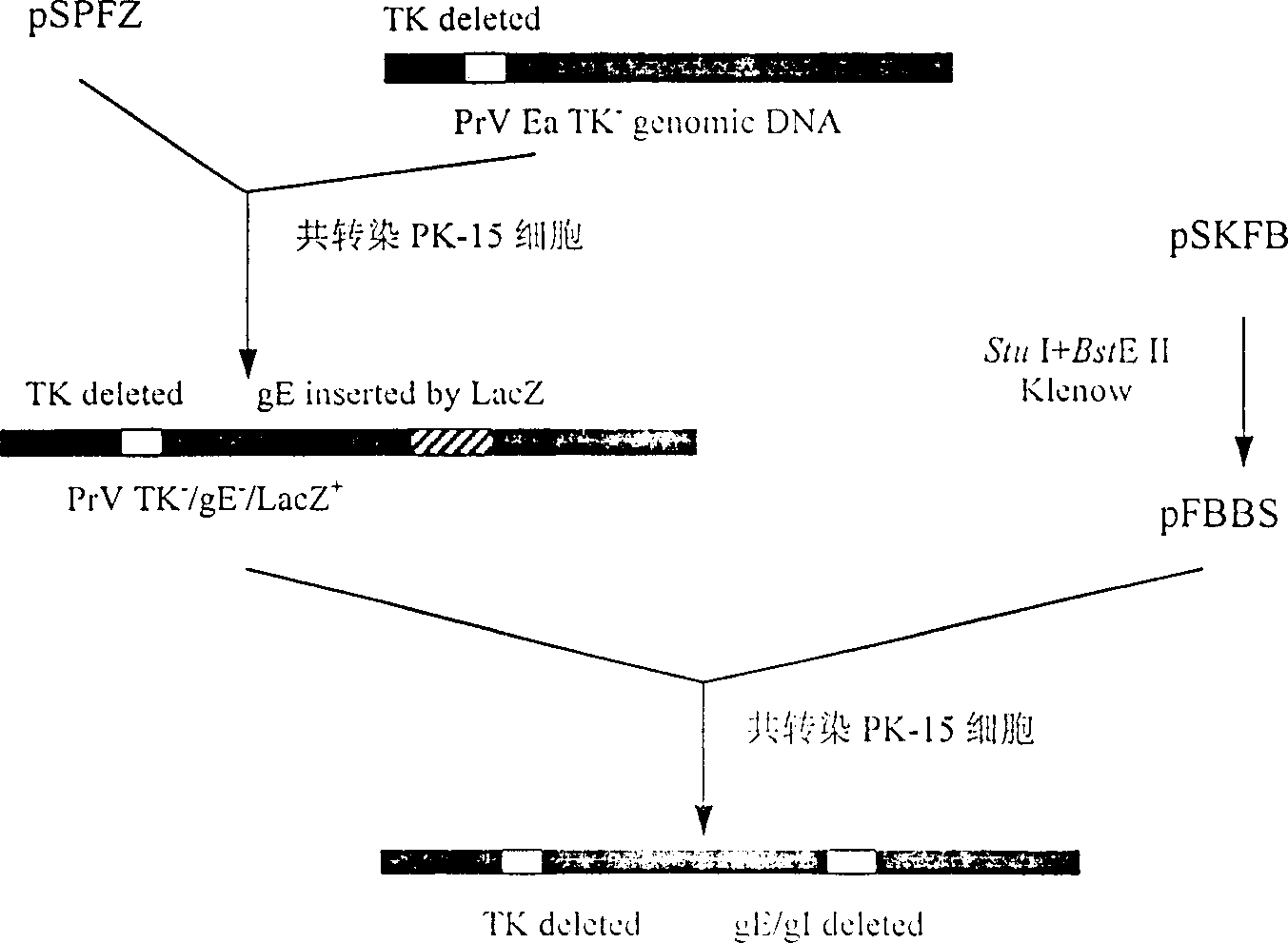
Pseudorabies TK*/gE*/gI* gene dificiency mark live vaccine and preparation method thereof
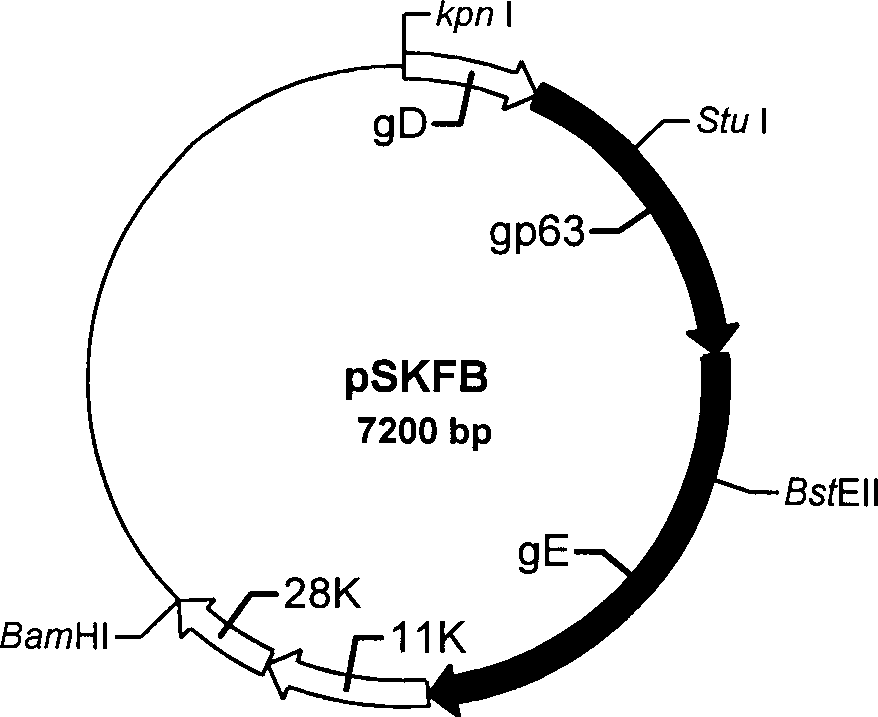
The present invention discloses a construction of three-gene defected recombinant pseudorabies virus (PrV) strain, vaccine prepared by said strain, method for constructing said strain and method for preparing said vaccine. The described recombinant pseudorabies virus strain defects genes of TK.gE ang gI, and contains no exogenous gene. The described vacine belongs to the freeze-dried live vaccine made up by virus liquid containing said invention and gelatin. Said invented live vaccine can be inoculated on the piglet, slaughter pig and sow with farrow, and can obtain obious immune effect. Said vaccine has good biological safety, and can be used for preventing and curing pseudorabies.
Owner:HUAZHONG AGRI UNIV
Human diploid cell rabies vaccine virus seed and preparation method thereof
ActiveCN102093983ASafeEffectiveMicroorganism based processesViruses/bacteriophagesBiotechnologyRabies
The invention relates to the field of biotechnology, in particular to a virus seed for producing vaccines for preventing human rabies by utilizing human diploid cells (KMB17) and a preparation method thereof. In the invention, a rabies fixed virus CTN-1V5 strain is continuously subcultured in the human diploid cells (KMB17), and a terminal dilution method is used for screening viruses with highertiter, thereby obtaining a rabies virus strain which is suitable for the human diploid cells (KMB17) and has good immunogenicity and heredity stability, and culturing a rabies vaccine virus seed (CTN-DK strain) capable of efficiently reproducing in the human diploid cells (KMB17). By using the virus seed for producing a human diploid cell (KMB17) rabies vaccine, the risk caused by residual heterogonous DNA (deoxyribonucleic acid) in the vaccine which is currently used at home can be effectively avoided, and the safety and practicability of the rabies vaccine in China are further improved, thus the invention has great social and economic benefits.
Owner:ZHEJIANG PUKANG BIOTECH
Method for producing rabies vaccine for human
The invention relates to a method for producing a rabies vaccine for human, comprising the following steps of: culturing human diploid cell lines by adopting a linear amplification technique of three or more levels of bioreactors; after the human diploid cell line in each level of bioreactor reaches 106 / ml, carrying out the vaccination on the next level of bioreactor; after the human diploid cell line in the last level of bioreactor grows on a microcarrier until the density reaches 106 / ml, vaccinating rabies virus strains; propagating viruses on cells by vaccinating the rabies virus strains,harvesting virus stock solutions, and inactivating, concentrating and purifying the harvested virus stock solutions to obtain the rabies vaccine for human. By using the linear amplification techniqueof the bioreactor to culture the human diploid cell lines, the cells do not contain exogenous pollution factors and tumorigenicity, and the residual DNA of the cells has no danger, and the rabies vaccine for human has the advantages of good immunizing effect and high safety and meets the requirement of large-scale industrial production of the rabies vaccine.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD +1
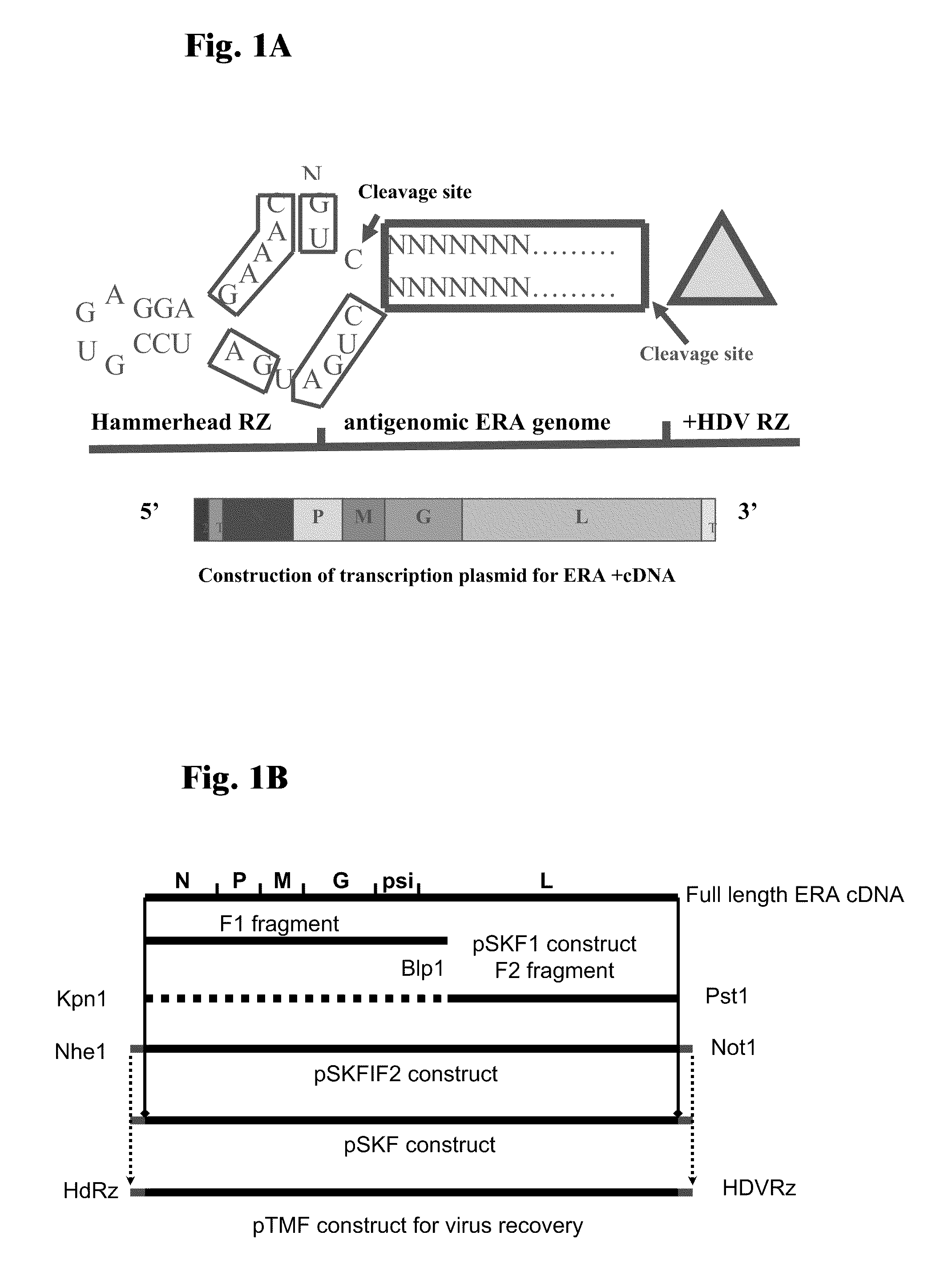
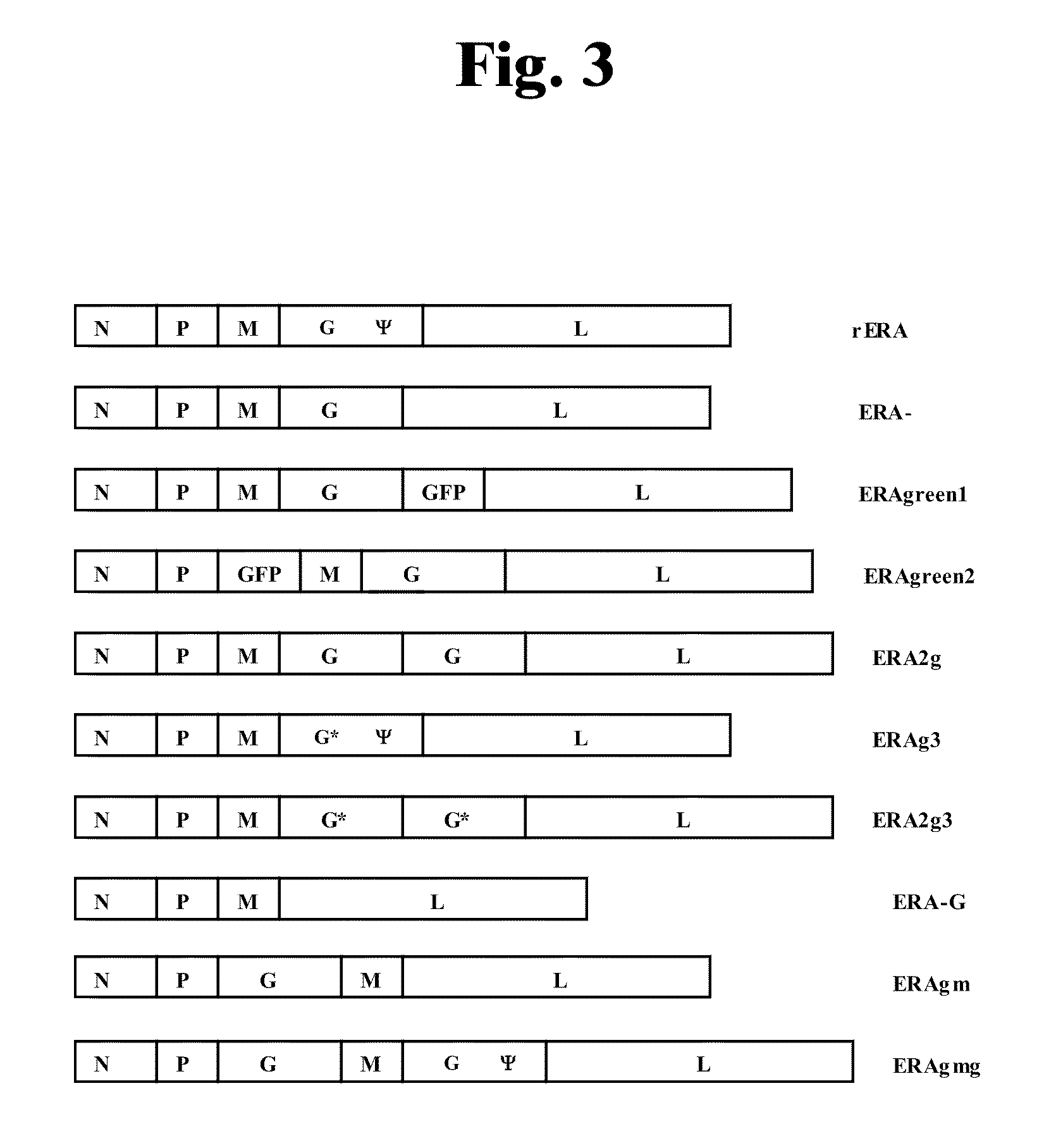
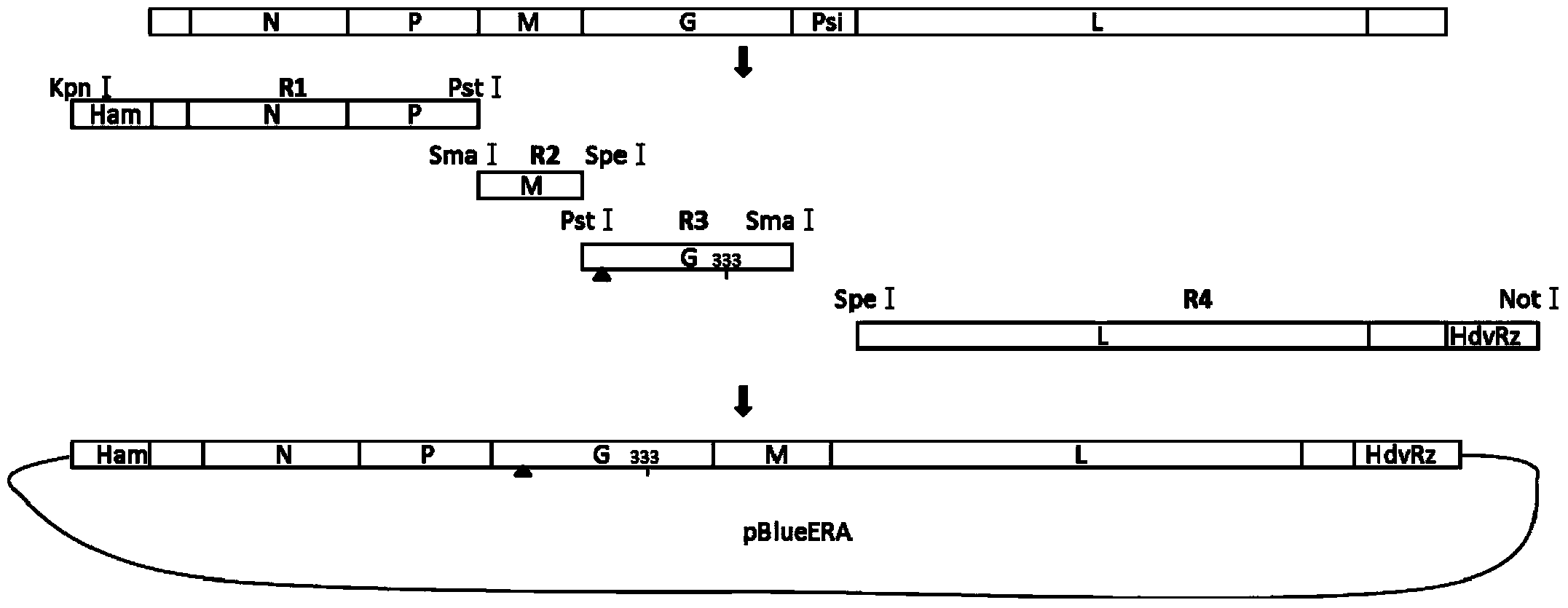
Rabies Virus Vector Systems and Compositions and Methods Thereof
ActiveUS20080274130A1Facilitated virus recoveryEfficient expressionSsRNA viruses negative-sensePeptide/protein ingredientsVector systemRabies virus strain
Rabies Virus compositions and methods are provided. The full-length sequence of Rabies Virus strain Evelyn-Rokitnicki-Abelseth (ERA) is disclosed. A reverse genetics system for producing recombinant ERA virus and derivatives thereof is provided, along with compositions including ERA and / or ERA derivative strain viruses, nucleic acids and / or proteins. In some instances, the compositions are immunogenic compositions useful for the pre- or post-exposure treatment of Rabies Virus.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
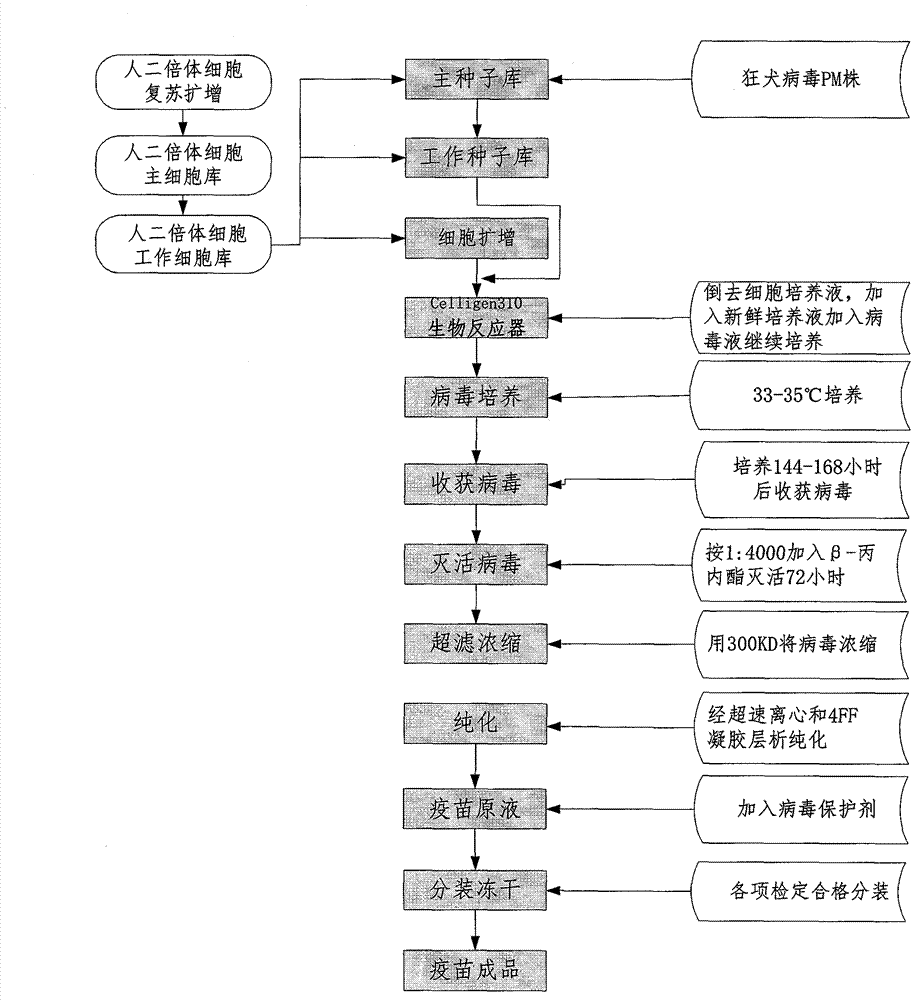
Process for preparing human diploid cell rabies vaccine through Celligen310 bioreactor
InactiveCN103083654ASimple purification processSimple preparation processPowder deliveryAntiviralsRabies virus strainTGE VACCINE
The invention belongs to the field of biological products, in particular to a process for preparing rabies vaccine by culturing human diploid cells WI-38 and MRC-5 (called as human diploid cells as follows) as toxigenic cells through a Celligen310 bioreactor and taking a rabies virus PM strain as a virus seed, wherein the finished vaccine is prepared by comprising the steps of resuscitating human diploid cells, culturing and proliferating the human diploid cells, inoculating the virus seed of the rabies virus PM strain onto the human diploid cells, domesticating, inoculating and culturing virus seeds, collecting virus filtrate, inactivating, purifying, concentrating, adding a protective solution and the like. Verification indexes of the vaccine meet standards of Chinese Pharmacopoeia of 2010 version. The process is characterized in that the human diploid cells are cultured and prepared as matrix cells by using the Celligen310 bioreactor; therefore, exogenous pollution factors and tumorigenicity of animal passage cells can be avoided. As the inoculated virus strain is the rabies virus PM strain in the preparation process of the vaccine, the immune effect is better than that of the traditional vaccine. The vaccine prepared by using the process has the advantages of being high in purity, good in immune effect and high in safety.
Owner:万里明
Rabies virus vector systems and compositions and methods thereof
ActiveUS7863041B2Promote recoveryEfficient expressionSsRNA viruses negative-senseViral antigen ingredientsVector systemRabies virus strain
Rabies Virus compositions and methods are provided. The full-length sequence of Rabies Virus strain Evelyn-Rokitnicki-Abelseth (ERA) is disclosed. A reverse genetics system for producing recombinant ERA virus and derivatives thereof is provided, along with compositions including ERA and / or ERA derivative strain viruses, nucleic acids and / or proteins. In some instances, the compositions are immunogenic compositions useful for the pre- or post-exposure treatment of Rabies Virus.
Owner:UNITED STATES OF AMERICA
Rabies virus SNK-CTN strain and application thereof
InactiveCN104357406AReduce contentReduce extractionMicroorganism based processesAntiviralsRabies virus strainDiploid cells
The invention relates to the field of microbes and biological pharmacy, and particularly relates to an adapted strain of a rabies virus strain (a CTN-1V strain) in a diploid cell MRC-5 strain. The preparation method of the rabies virus strain comprises the following steps: 1) taking MRC-5 cells in a cell bank for resuscitation, cultivating for 3 days for forming single layer cells, pouring away a cell culture fluid in an MRC-5 cell culture flask, adopting a 0.25% of trypsin digestive juice for digestion, dispersing into uniform cells, inoculating a rabies virus CTN-1V5 strain suspension according to the dosage of 0.01-1.0 MOI, cultivating at 37 DEG C for 2-3 days, replacing the suspension with a maintenance medium containing 2-4% of bovine serum, cultivating at 33-35 DEG C for 3-5 days to obtain a virus liquid, and cryopreserving at -70 DEG C to prepare a virus suspension; 2) adopting the method, and continuously subculturing for 30-32 generations on MRC-5 cells. The preservation code of the rabies vaccine virus strain (an SNK-CTN strain) is CGCC No8887.
Owner:SHINAIKE JIANGSU BIOLOGICAL PHARMA
Rabies live vaccine and method for preparing rabies live vaccine for oral administration
ActiveCN103877570AImprove efficiencyQuality improvementAntiviralsPharmaceutical non-active ingredientsOral medicationRabies virus strain
The invention discloses a rabies live vaccine and a method for preparing the rabies live vaccine for oral administration. The rabies live vaccine comprises a rabies virus ERAg3m. A strain of the rabies virus ERAg3m is obtained by using a reverse genetic method for transforming a rabies virus strain ERA and has high safety. Therefore, the immune efficacy of the rabies live vaccine containing the rabies ERAg3m virus can be further improved.
Owner:北京中联康生物科技股份有限公司
Raccoon Poxvirus Expressing Rabies Glycoproteins
ActiveUS20090010963A1Effective immunizationEffective immunizingSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininViral glycoprotein
The present invention relates to recombinant raccoon poxvirus vectors that express the rabies virus glycoprotein gene at the hemagglutinin (ha) locus of the poxvirus genome or express the glycoprotein gene of the same or different rabies strains at the thymidine kinase (tk) and the hemagglutinin (ha) loci of the poxvirus genome, and their use as adjuvant-free vaccines. The raccoon poxvirus vector comprises the nucleic acid molecules encoding the glycoprotein of a Challenge Virus Standard rabies strain inserted and expressed at the tk locus of the poxvirus genome and of a Pasteur-Paris rabies strain inserted and expressed at the ha locus of the poxvirus genome. The vaccine may optionally contain a mixture of additional feline and canine antigens for immunization of animals. Also disclosed are methods for inducing an immune response to rabies in a mammal by administering to the mammal an effective immunizing amount of the vaccine of the invention.
Owner:ELANCO US INC
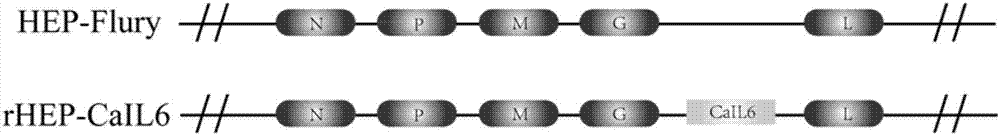
Recombinant rabies virus carrying interleukin 6 gene and application thereof
InactiveCN106967691AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainEukaryotic plasmids
The invention discloses a recombinant rabies virus rHEP-CaIL6 carrying an immune enhancement factor interleukin (IL) 6 gene and application thereof. The recombinant virus takes rabies virus HEP-Flury strain as the skeleton, the IL6 gene of canine is inserted to a position between the G and L gene of HEP-Flury to obtain a recombinant plasmid pHEP-CaIL6, and finally saving screening is carried out to obtain the recombinant rabies virus strain rHEP-CaIL6. The recombinant virus carries the immune enhancement factor, can enhance the immune response and induce the production of higher rabies virus neutralizing antibody, thus better protecting the body from resisting the attack of lethal rabies virus, also can produce a neutralizing antibody with protective ability at a low dose, and lowers the cost of canine vaccines. Moreover, the IL6 gene is recombined into the rabies virus, thus achieving stable expression of IL6 protein, also avoiding the overexpression thereof, and overcoming the defect that excessive IL6 can cause pathological injury.
Owner:SOUTH CHINA AGRI UNIV
Recombinant rabies virus carrying deoptimized M gene and two G genes
ActiveCN107201371AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainRecombinant virus vaccine
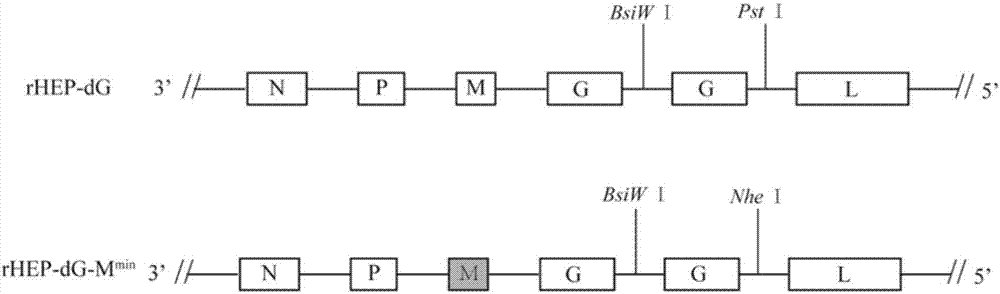
The invention discloses a recombinant rabies virus carrying a deoptimized M gene and two G genes. Firstly, a reading frame part of the M gene is deoptimized, wherein the sequence after deoptimization is as shown in SEQ ID NO.2; secondly, a rabies virus HEP-Flury strain is taken as a skeleton, an M gene in the HEP-Flury is replaced by the deoptimized M gene, and an additional rabies virus G gene is inserted to obtain recombinant rabies virus pHEP-dG-Mmin plasmid carrying the deoptimized M gene and two G genes; finally, rescuing and screening are conducted to obtain a recombinant rabies virus strain rHEP-dG-Mmin. The recombinant virus has higher virus titer, and the cost of canine vaccine can be reduced. Under the condition of multiplicity of infection, G protein with higher level can be expressed, virus replication and transcription of each structural gene are increased, and the recombinant virus has the potential of serving as a rabies vaccine candidate strain.
Owner:SOUTH CHINA AGRI UNIV
Pseudorabies TK*/gE*/gI* gene dificiency mark live vaccine and preparation method thereof
The present invention discloses a construction of three-gene defected recombinant pseudorabies virus (PrV) strain, vaccine prepared by said strain, method for constructing said strain and method for preparing said vaccine. The described recombinant pseudorabies virus strain defects genes of TK.gE ang gI, and contains no exogenous gene. The described vacine belongs to the freeze-dried live vaccine made up by virus liquid containing said invention and gelatin. Said invented live vaccine can be inoculated on the piglet, slaughter pig and sow with farrow, and can obtain obious immune effect. Said vaccine has good biological safety, and can be used for preventing and curing pseudorabies.
Owner:HUAZHONG AGRI UNIV
High immunogenicity rabies virus glycoprotein, its preparation method and application
InactiveCN102964433AAvoid failureImproving immunogenicityAntiviralsDepsipeptidesViral glycoproteinRabies virus strain
The invention discloses a high immunogenicity rabies virus glycoprotein and its preparation method as well as application. The preparation method consists of: performing artificial synthesis to acquire an all dominant neutralization epitope-containing and codon optimized rabies virus glycoprotein gene, then taking a recombinant adenovirus, a recombinant baculovirus or a slow virus as an expression vector, and performing high-efficiency expression in a mammalian cell line, an insect cell line or a silkworm expression system, thus obtaining the rabies virus glycoprotein that has high immunogenicity to domestic epidemic rabies virus strains currently. And the high immunogenicity rabies virus glycoprotein is used for immunoprophylaxis of animal rabies.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Construction of recombinant rabies virus of double-expression G gene and biological property analysis thereof
InactiveCN101768575AImmunogenicHigh and Antibody LevelsMicroorganism based processesAntiviralsBiological propertyRabies virus strain
The invention provides a recombinant rabies virus strain with a G gene additionally inserted in a genome, a method for constructing the recombinant rabies virus strain and application thereof. The recombinant rabies virus strain constructed by the method has high safety and immunogenicity, and is suitable for preparing vaccines for inactivating rabies virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Pseudorabies virus JS-2012 infectious clone plasmid and construction method and application
ActiveCN106939320AViral antigen ingredientsMicrobiological testing/measurementReverse geneticsPlasmid
The invention provides a pseudorabies virus JS-2012 infectious clone plasmid and a construction method and application; the JS-2012 infectious clone plasmid is constructed on the basis of newly appeared PRV variant JS-2012 in China by insertion of a pBeloBAC11 vector sequence in the downstream of gG gene termination codon of JS-2012 by use of a loxP-Cre enzyme system, a galK positive and negative screening system is used for genetic sequence operation of the JS-2012 infectious clone plasmid to establish a variant JS-2012 reverse genetic operating system, and a technical platform is provided for deep research of PRV variant genetic variation mechanisms, virulence enhancement and virus latent infection mechanisms, vaccine development and the like.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Herpesviridae strain
ActiveCN105200015AHighly toxicPhysical and chemical properties meetMicroorganism based processesViruses/bacteriophagesWater bathsHigh resistance
The invention discloses a herpesviridae strain which is characterized in that a preservation number of the herpesviridae strain is CCTCC (China Center For Type Culture Collection) NO:V201314; a PRV / HN2012 strain is inherited to the seventh generation, the seventh-generation virus is subjected to determination of a virus titer TCID50 (Tissue Culture Infectious Dose), and final result shows that the TCID50 is 10<9.0>; the PRV / HN2012 strain is inoculated to mice, and after the inoculated virus is challenged for 24h, the mice in a PRV / HN2012 strain infected group are found with neurologic symptoms; after the inoculated virus is challenged for 48h, all the mice are found to die, experimental results show that the strain has high toxicity; based on the results, the research of physicochemical properties of the PRV / HN2012 strain indicates that the strain is sensitive to analytically-pure chloroform and belongs to a virus with an envelope; with higher resistance to acid and alkali, the strain can be deactivated after being treated with hydrochloric acid with the pH value of 3.0 and NaOH with the pH value of 11.0; with higher resistance to heat, the strain can be deactivated after being treated in a water bath with 56DEG C for 1h; the strain is sensitive to trypsin, so that the influence of ultraviolet rays on the infectivity of the strain is not obvious after the strain is treated by the ultraviolet rays for 30min.
Owner:HENAN AGRICULTURAL UNIVERSITY
Construction and culture methods of recombinant rabies virus
InactiveCN104651322AImproving immunogenicityHigh and Antibody LevelsMicroorganism based processesViruses/bacteriophagesNucleotideRabies virus strain
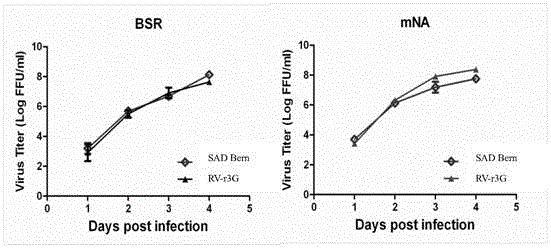
The invention provides a recombinant rabies virus strain. A nucleotide sequence as shown in SEQ ID NO.1 is formed by inserting two rabies virus G genes into a rabies virus genome which is obtained by reverse genetic operation of a whole genome sequence of a SAD Bern rabies virus. The constructed recombinant rabies virus has better security and immunogenicity and is suitable for preparing an inactivated rabies vaccine.
Owner:山东华宏生物工程有限公司
Pseudorabies virus gene deleted strain, inactivated vaccine for porcine pseudorabies, and preparation method and application of inactivated vaccine
PendingCN110846285AHigh viral titerGreat stress responseViral antigen ingredientsVirus peptidesRabiesAdjuvant
The invention discloses a pseudorabies virus gene deleted strain, and a preparation method of an inactivated vaccine for porcine pseudorabies. The gene deleted strain is a pseudorabies virus gE gene deleted strain, which is constructed through reading frame frameshift mutation caused by deletion of a plurality of basic groups from a wild-type pseudorabies virus (PRV) strain gE gene sequence. The preparation method includes the following steps: constructing the pseudorabies virus gE gene deleted strain; domesticating a porcine testicular cell and subjecting the same to suspension culture; inoculating the porcine testicular cell with the pseudorabies virus gE gene deleted strain, and harvesting a cell culture when 80-90% of the cell has lesions to obtain cell venom containing supernatant; taking the supernatant to measure the valence, and inactivating the qualified cell venom in a sterilization container; and mixing the inactivated cell venom with an adjuvant to obtain the inactivated vaccine for the porcine pseudorabies. The prepared inactivated vaccine for the porcine pseudorabies has high immunogenicity, long immunization period, and no side effects after immunization, is safe andreliable, and can effectively prevent infections of pseudorabies virus epidemic strains.
Owner:SHANGHAI ACAD OF AGRI SCI +1
Recombinant rabies viruses in which canine distemper virus main immune genes are embedded and application of recombinant rabies viruses
InactiveCN109943576ALow costMicroorganism based processesAntiviralsRabies virus strainCanine distemper virus CDV
The invention discloses recombinant rabies viruses BNSP-CDV-F and BNSP-CDV-H in which canine distemper virus main immune genes are embedded. The recombinant viruses take a rabies virus strain SAD-B19as a framework, CDV-F and CDV-H genes shown in SEQ ID NO.1 and SEQ ID NO.2 are separately inserted between an N gene and P gene of a recombinant plasmid SAD-19 full-length cDNA, and finally recombinant rabies virus strains BNSP-CDV-F and BNSP-CDV-H are saved through a reverse genetic operation technology. The recombinant viruses BNSP-CDV-F and BNSP-CDV-H can express fusion protein and hemagglutinin protein of canine distemper viruses; after a vaccine prepared by mixing the recombinant viruses is used for immunizing animals, a great number of anti-rabies virus antibodies and main immune gene antibodies resistant to the canine distemper viruses can be induced and generated. The cost of the vaccine can also be reduced, and the recombinant viruses BNSP-CDV-F and BNSP-CDV-H have a good application and popularization prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
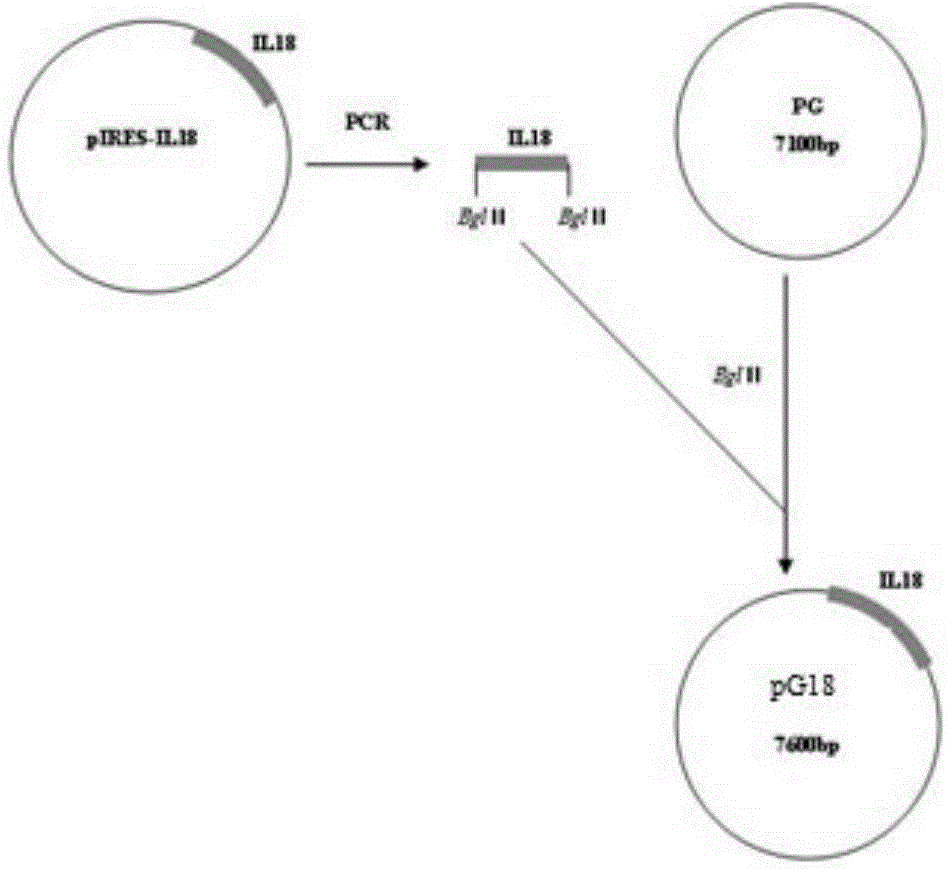
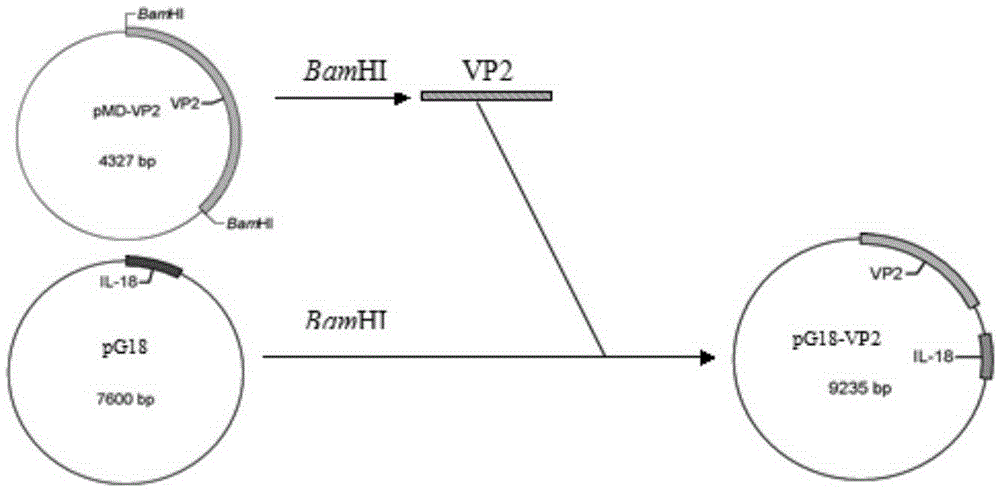
Recombinant porcine pseudorabies virus strain expressing PPV VP2 gene and porcine IL-18 gene
InactiveCN105368796ANon-toxicNo pollution in the processMicroorganism based processesViruses/bacteriophagesPositive controlRabies virus strain
The invention discloses a recombinant porcine pseudorabies virus strain expressing a PPV VP2 gene and a porcine IL-18 gene. The recombinant porcine pseudorabies virus (Herpesviridae) strain is numbered as CCTCC NO.V201347. The recombinant porcine pseudorabies virus PGVP218 established by the recombinant porcine pseudorabies virus strain and an IL-18-free recombinant pseudorabies virus rPRV-VP2 established in a laboratory respectively immunize rats, meanwhile a DMEM culture solution is used as a negative control and a PPV HB98 vaccine and a PRV inactivated vaccine are used as a positive control group to immunize the rats, and the immunogenicity of the recombinant virus is studied through PPV specific antibody detection, a PRV antibody neutralization test, peripheral blood T lymphocyte subpopulation determination and a attacking protection test. Results show that the recombinant virus PGVP218 is safe and effective to the rats, can induce the rats to produce specific PPV and PRV antibodies, and the number of CD4+ / CD8+T lymphocyte subpopulations is increased.
Owner:HENAN AGRICULTURAL UNIVERSITY
Human diploid cell rabies vaccine virus seed and preparation method thereof
ActiveCN102093983BIncrease the cultivation areaLarge batch sizeMicroorganism based processesViruses/bacteriophagesBiotechnologyRabies
The invention relates to the field of biotechnology, in particular to a virus seed for producing vaccines for preventing human rabies by utilizing human diploid cells (KMB17) and a preparation method thereof. In the invention, a rabies fixed virus CTN-1V5 strain is continuously subcultured in the human diploid cells (KMB17), and a terminal dilution method is used for screening viruses with highertiter, thereby obtaining a rabies virus strain which is suitable for the human diploid cells (KMB17) and has good immunogenicity and heredity stability, and culturing a rabies vaccine virus seed (CTN-DK strain) capable of efficiently reproducing in the human diploid cells (KMB17). By using the virus seed for producing a human diploid cell (KMB17) rabies vaccine, the risk caused by residual heterogonous DNA (deoxyribonucleic acid) in the vaccine which is currently used at home can be effectively avoided, and the safety and practicability of the rabies vaccine in China are further improved, thus the invention has great social and economic benefits.
Owner:ZHEJIANG PUKANG BIOTECH
Raccoon poxvirus expressing rabies glycoproteins
ActiveUS8795681B2Improve securityEasy doseSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininViral glycoprotein
The present invention relates to recombinant raccoon poxvirus vectors that express the rabies virus glycoprotein gene at the hemagglutinin (ha) locus of the poxvirus genome or express the glycoprotein gene of the same or different rabies strains at the thymidine kinase (tk) and the hemagglutinin (ha) loci of the poxvirus genome, and their use as adjuvant-free vaccines. The raccoon poxvirus vector comprises the nucleic acid molecules encoding the glycoprotein of a Challenge Virus Standard rabies strain inserted and expressed at the tk locus of the poxvirus genome and of a Pasteur-Paris rabies strain inserted and expressed at the ha locus of the poxvirus genome. The vaccine may optionally contain a mixture of additional feline and canine antigens for immunization of animals. Also disclosed are methods for inducing an immune response to rabies in a mammal by administering to the mammal an effective immunizing amount of the vaccine of the invention.
Owner:ELANCO US INC
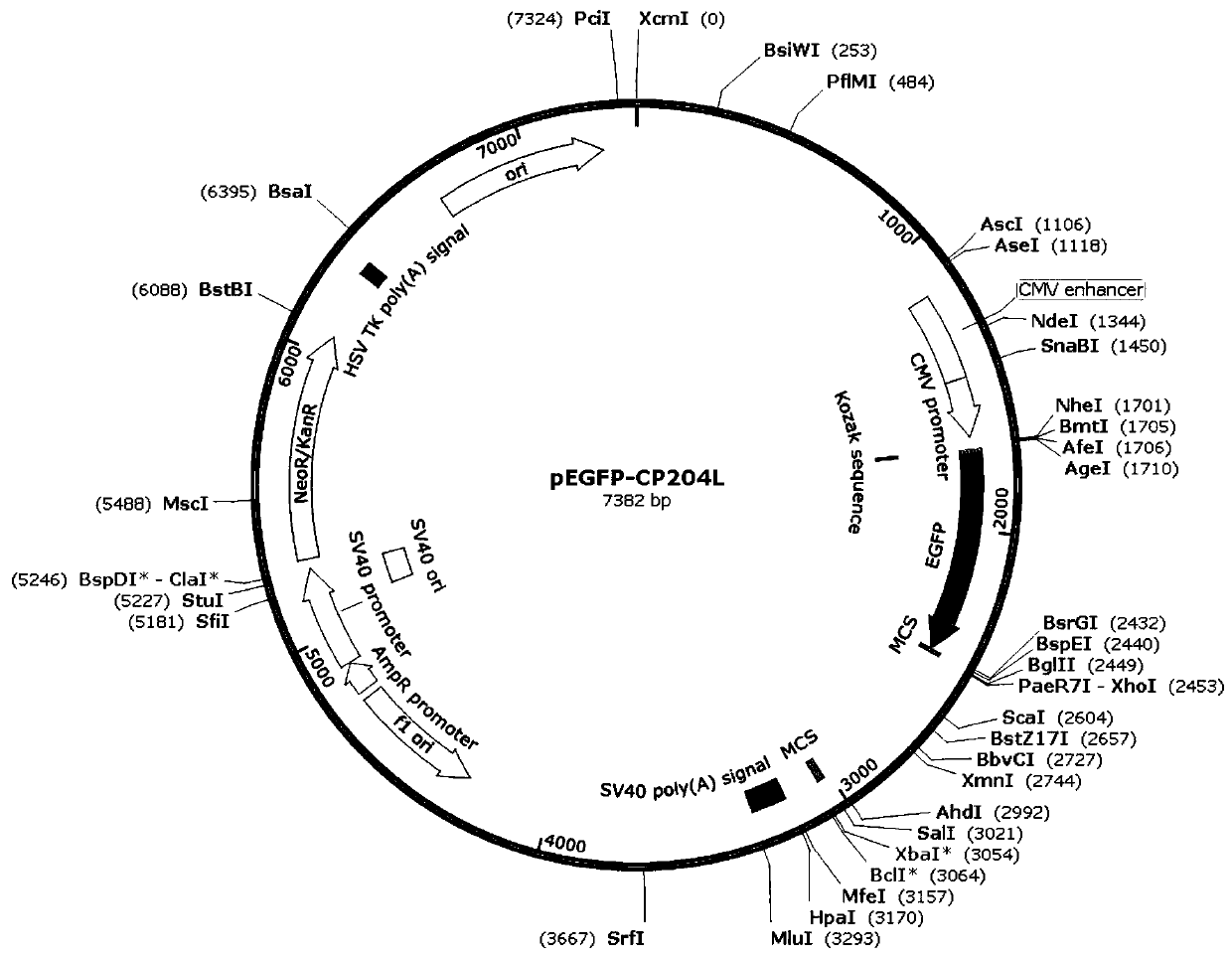
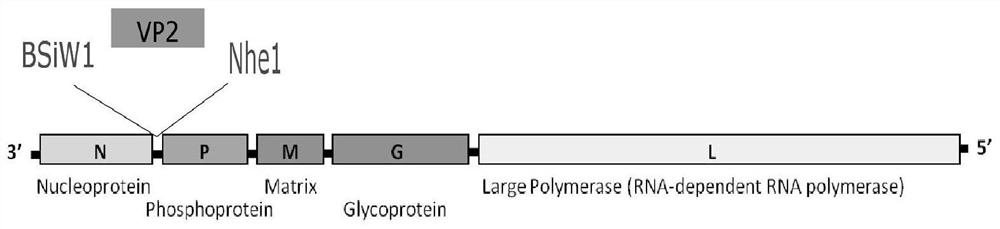
Construction method of PRV gE/gI dual-gene deletion strains for expressing ASFV P30 protein
InactiveCN111471657AAvoid infectionEasy to makeVirus peptidesAntiviralsRabies virus strainVirus strain
The invention discloses a construction method of PRV gE / gI dual-gene deletion strains for expressing ASFV P30 protein and belongs to the biopharmaceutical field. The construction method specially comprises the following steps of S1, preparing a reagent; S2, preparing cloned plasmids; S3, constructing a PRV eukaryon transfer plasmid pEGFP-CP204L; S4, performing cotransfection on DNA of PRV and pEGFP-CP204L plasmids. P30 protein gene (CP204L) fragments are synthesized, the transfer plasmid pEGFP-CP204L is constructed, a classical homologous recombination manner is adopted for inserting an ASFV CP204L gene into a PRV genome to replace gI and gE virulence genes, and recombinant pseudorabies virus strains rXJgE / gI-CP204L for constructing co-expression P30 protein can be used for preventing ASFVand PRV infection, and the cloned plasmids are simple to prepare, low in cost and suitable for industrial production.
Owner:SICHUAN AGRI UNIV
Recombinant rabies virus of chimeric canine parvovirus VP2 gene and application of recombinant rabies virus
PendingCN112852759ASsRNA viruses negative-senseViral antigen ingredientsRabies virus strainChimera Protein
The invention provides a recombinant rabies virus of a chimeric canine parvovirus VP2 gene, and belongs to the technical field of immunology. The recombinant rabies virus takes a rabies vaccine candidate strain SAD-B19 as a skeleton, and the VP2 gene of a CPV-2a strain currently popular in China is inserted into an SAD-B19 genome; the recombinant rabies virus strain of the chimeric VP2 protein is obtained through rescue, after immunization, high-level anti-rabies virus antibodies and anti-canine parvovirus VP2 antibodies can be induced to be generated, and the vaccine is low in cost, safe and effective.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Rabies vaccine virus screening method and preparation method of rabies vaccine
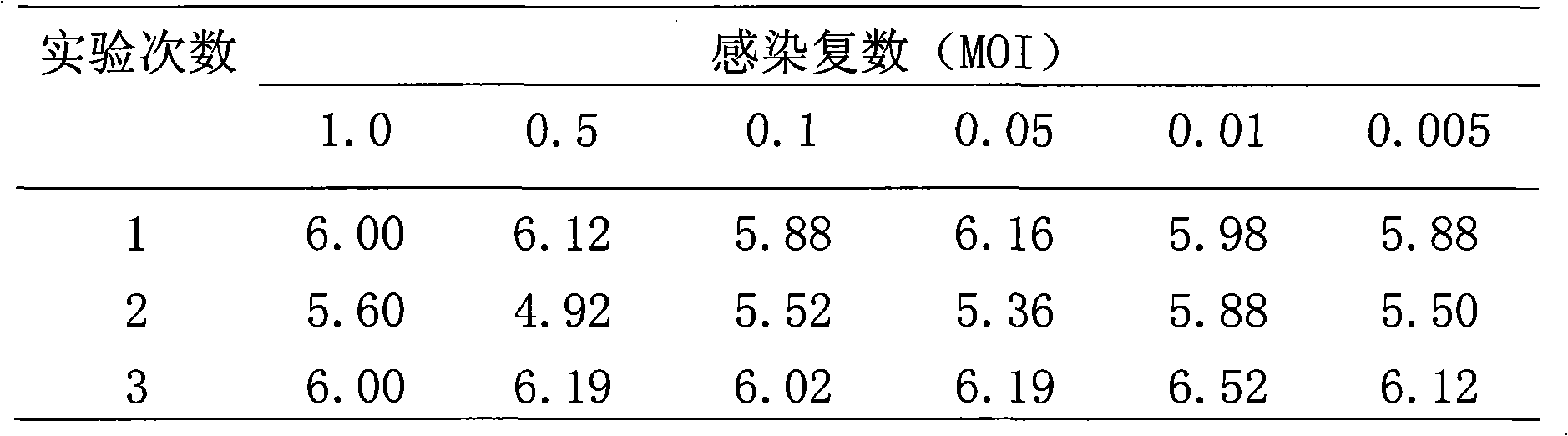
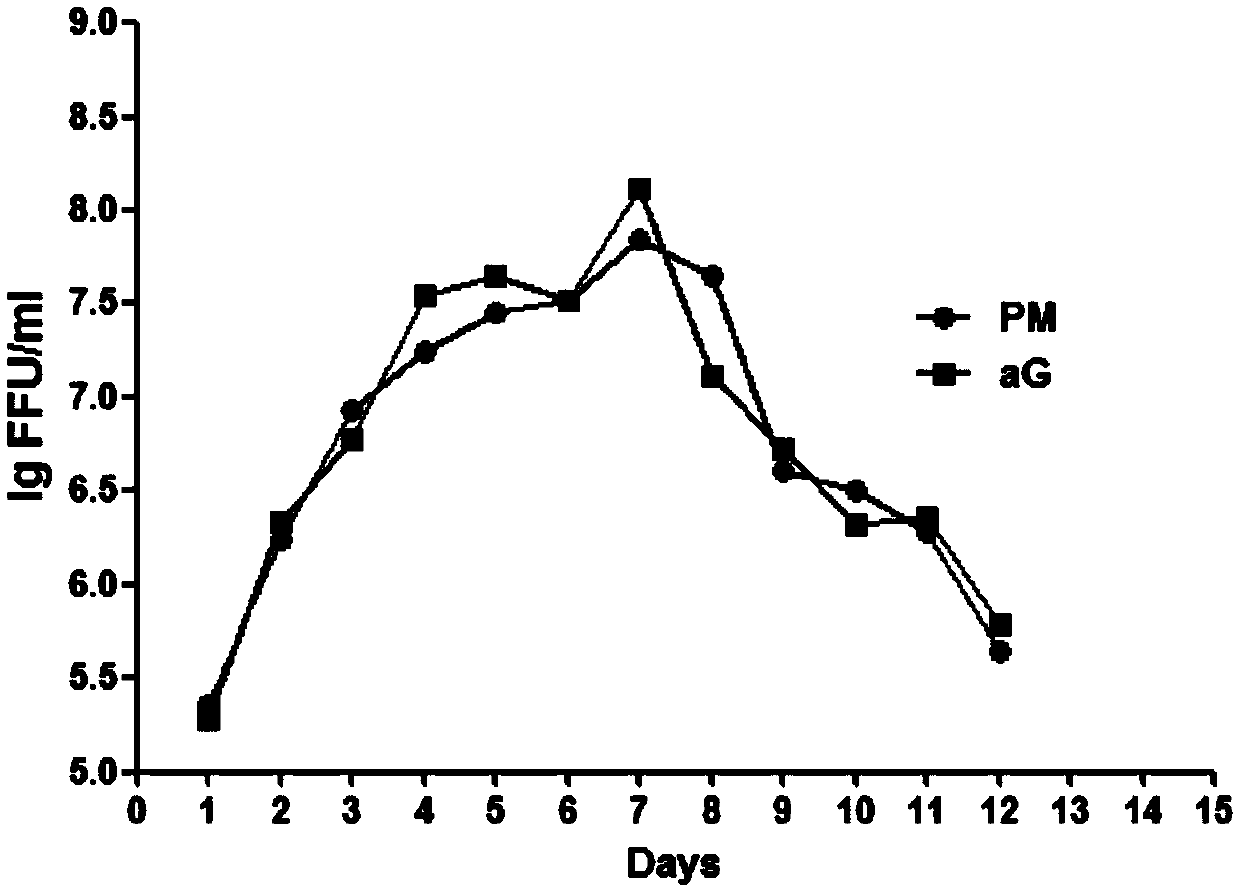
ActiveCN109666653AIncrease productivityHigh yieldSsRNA viruses negative-senseViral antigen ingredientsVaccine ProductionScreening method
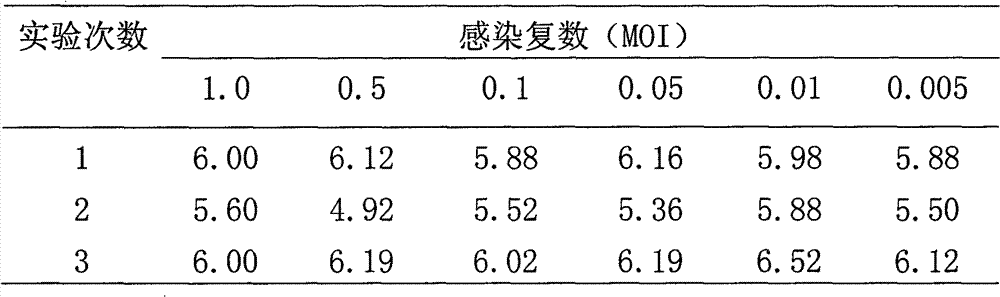
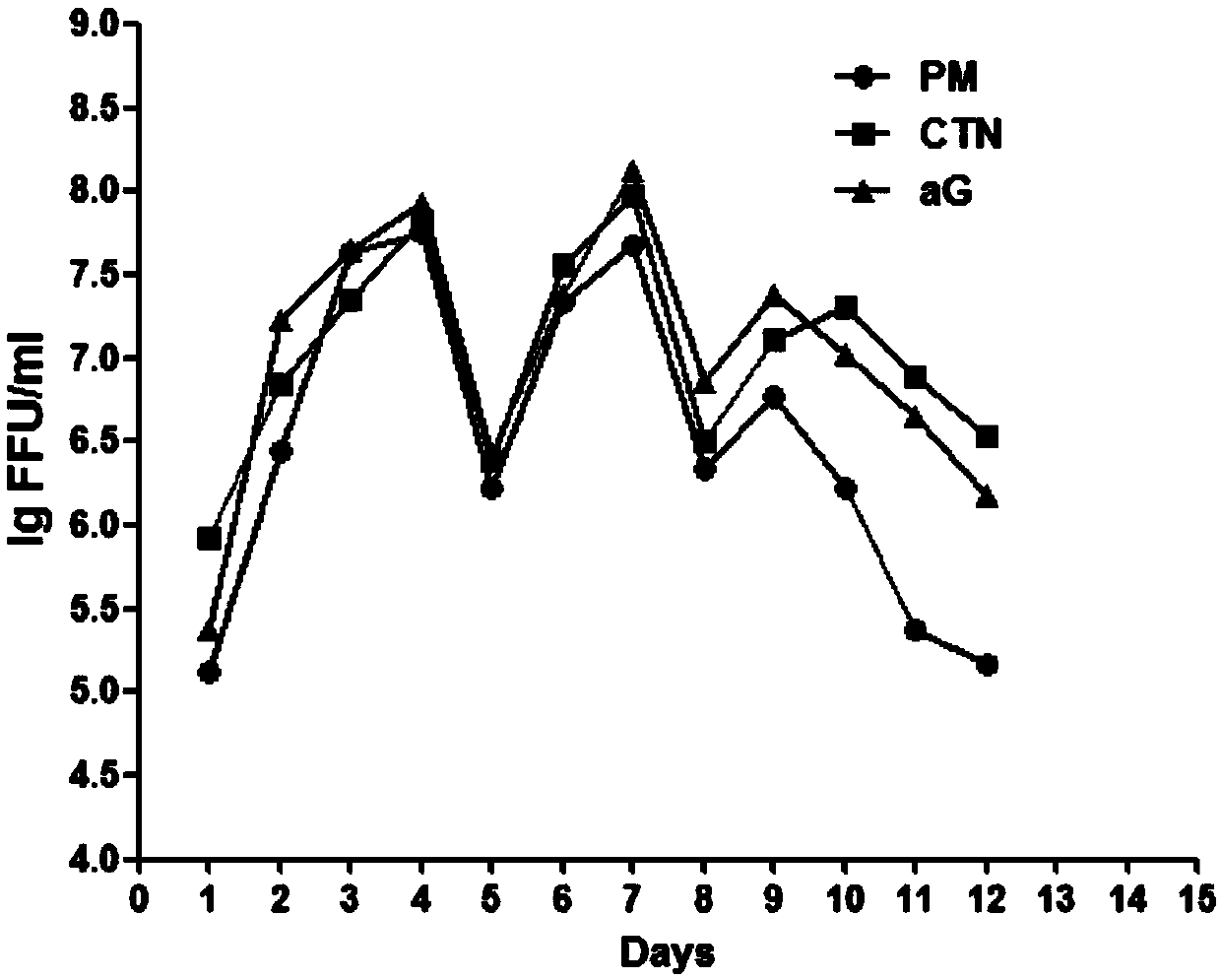
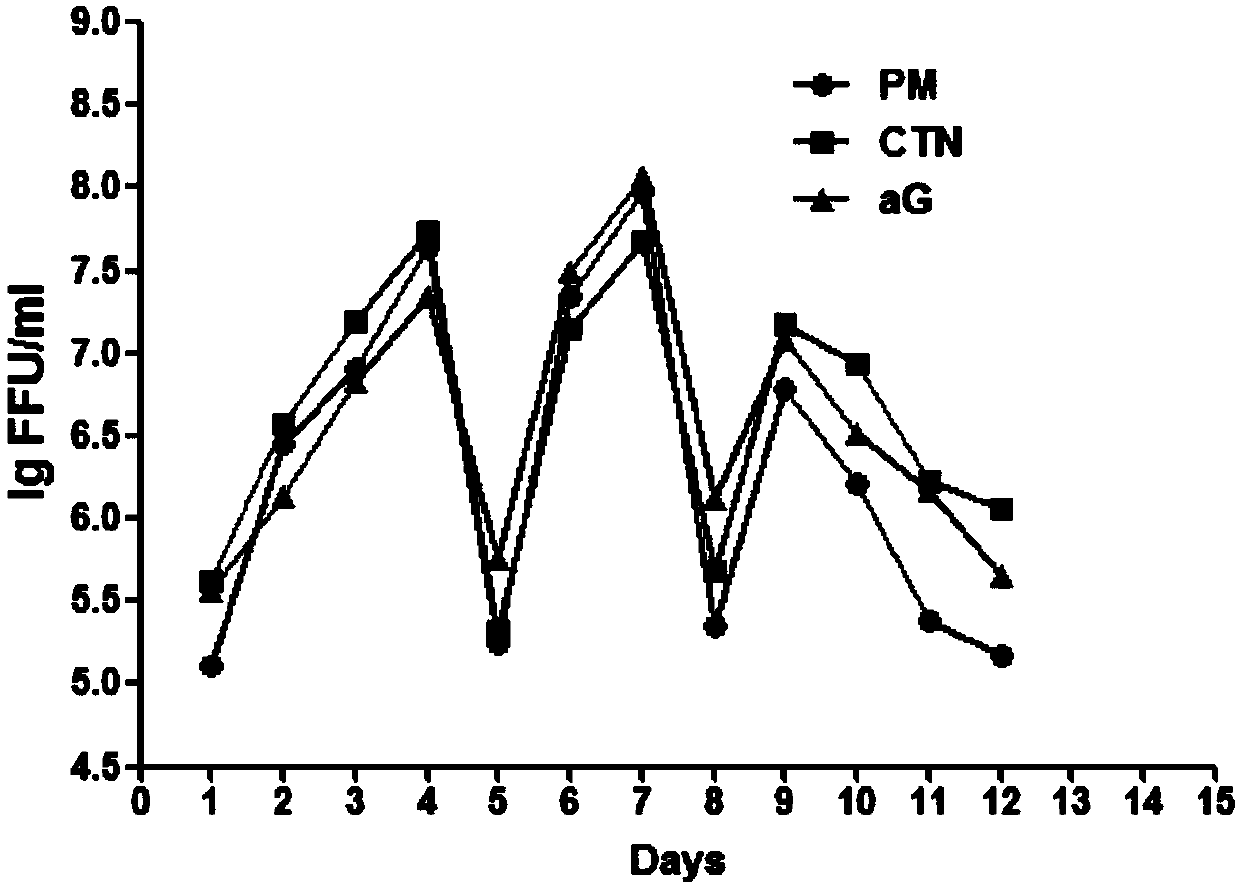
The invention relates to the technical field of biological medicines, in particular to a rabies vaccine virus screening method and a preparation method of a rabies vaccine. The screening method comprises the step that rabies virus strains are culture in the way that ultrahigh MOI and ultralow MOI are alternatively circulated. Viruses can quickly adapt to cells for six or eight passages, the virustiter is raised to 7.51 gFFU / ml from 4.61 gFFU / ml, the passage adaptation cycle of the virus strains is obviously shortened, the virus yield and the vaccine production efficiency are greatly improved,and the preparation method is suitable for scale production of the rabies vaccine.
Owner:吉林惠康生物药业有限公司
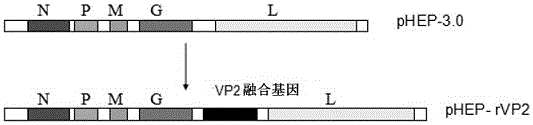
VP2 fusion gene, recombinant rabies virus rHEP-rVP2 strain and construction method and application of strain
ActiveCN106497951ALow costImproving immunogenicityAntiviralsViruses/bacteriophagesRabies virus strainMicrobacterium
The invention provides a VP2 fusion gene, a recombinant rabies virus rHEP-rVP2 strain and a construction method and application of the strain, and belongs to the field of microbial immunology. The sequence of the VP2 fusion gene is as shown in SEQ ID NO:1. The recombinant rabies virus rHEP-rVP2 strain is obtained by inserting the VP2 fusion gene between a G gene and an L gene of a genome of a HEP-Flury strain. The construction method of the recombinant rabies virus strain rHEP-rVP2 includes the steps that the VP2 fusion gene is inserted in full-length cDNA of the HEP-Flury strain to construct recombinant plasmids, a host cell is subjected to transfection, a virus is rescued, and the rHEP-rVP2 strain is obtained. The recombinant rabies virus rHEP-rVP2 strain has virus titer similar to that of a parent strain, has good immunogenicity, can generate a high-level antibody for the rabies virus and the canine parvovirus after immunizing, and can serve as a bivalent vaccine candidate strain.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
A strain of porcine pseudorabies virus
ActiveCN105200015BHighly toxicStrong resistanceMicroorganism based processesViruses/bacteriophagesWater bathsHigh resistance
The invention discloses a porcine pseudorabies virus strain, which is characterized in that: porcine pseudorabies virus (Herpesviridae) strain, CCTCC NO: V201314. The present invention passes the PRV / HN2012 strain to the 7th generation, and carries out the virus titer TCID to the 7th generation virus 50 The results of the determination of TCID 50 for 10 9.0 . The PRV / HN2012 strain was inoculated into mice. After 24 hours of challenge, the mice in the PRV / HN2012 strain-infected group developed neurological symptoms. After 48 hours of challenge, all the mice died, indicating that the strain has strong toxicity. Based on this, through the research on the physical and chemical characteristics of PRV / HN2012 strain, the results show that the strain is sensitive to analytical pure chloroform and belongs to enveloped virus; it has strong resistance to acid and alkali, hydrochloric acid at pH 3.0, and It was inactivated by NaOH treatment for 1 h; it had strong resistance to heat and could be inactivated by treatment in a water bath at 56°C for 1 h; it was sensitive to trypsin and had no obvious effect on its infectivity after being treated with ultraviolet light for 30 min.
Owner:HENAN AGRICULTURAL UNIVERSITY
Reverse cross-multistage nerve tracing method
PendingCN114196704ARestoration of infection spreading activityRealize the purpose of reverse multi-level markingSsRNA viruses negative-senseVirus peptidesInfected cellRabies virus strain
The invention discloses a reverse cross-multistage nerve tracing method, which comprises the following steps of: constructing a transgenic animal capable of expressing G protein of rabies virus, infecting the transgenic animal by using a defective rabies virus strain losing the G protein, and providing the G protein for the defective rabies virus strain losing the G protein by using the infected cells of the transgenic animal. The infection transmission activity is recovered, and the purpose of reverse cross-multistage nerve tracing is achieved. The method is more excellent in neuron marking efficiency and effect, and compared with PRV neurons, the neuron toxicity is smaller, the genome length is shorter, and primate animals can be infected, so that subsequent research on neuron morphology, namely functions, is more facilitated, and the requirements of subsequent brain connection group research are better met.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for producing rabies vaccine for human
The invention relates to a method for producing a rabies vaccine for human, comprising the following steps of: culturing human diploid cell lines by adopting a linear amplification technique of three or more levels of bioreactors; after the human diploid cell line in each level of bioreactor reaches 106 / ml, carrying out the vaccination on the next level of bioreactor; after the human diploid cell line in the last level of bioreactor grows on a microcarrier until the density reaches 106 / ml, vaccinating rabies virus strains; propagating viruses on cells by vaccinating the rabies virus strains, harvesting virus stock solutions, and inactivating, concentrating and purifying the harvested virus stock solutions to obtain the rabies vaccine for human. By using the linear amplification technique of the bioreactor to culture the human diploid cell lines, the cells do not contain exogenous pollution factors and tumorigenicity, and the residual DNA of the cells has no danger, and the rabies vaccine for human has the advantages of good immunizing effect and high safety and meets the requirement of large-scale industrial production of the rabies vaccine.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD +1
Rabies viruses CTN strain complete genome infectious cDNA cloning, and preparation and use thereof
InactiveCN101475943BPreserve weak toxicitySafeMicroorganism based processesAntiviralsRabies virus strainCdna cloning
The present invention provides a rabies virus CTN strain whole genome cDNA infectious clone, as well as a method of using reverse genetics technology to save the active rabies virus. The present inventive method includes: using the single restriction enzyme sites in the CTN strain of rabies virus full-length genome to divide the full-length virus into four segments to amplify, simultaneously using the green fluorescent protein gene to substitute 423bp base at the pseudo-gene point, cloning in the pVAX-R expression vector, to construct a recombinant full-length plasmid of the virus genome; performing PCR amplification of nucleoprotein, phosphoprotein, glycoprotein and transcription large protein gene sequence of the CTN strain of the rabies virus, and cloning the amplified fragment into a vector pVAX1, to constitute four auxiliary plasmids in the reverse heredity system; and then using the five plasmids to jointly transfect cells, to rescue the rabies virus. The present invention successfully saves the rabies virus, and has a great significance in the study of the pathogenic mechanism of rabies virus, development of new vaccines, viral vectors and the like areas.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com