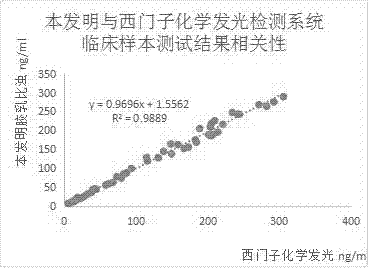
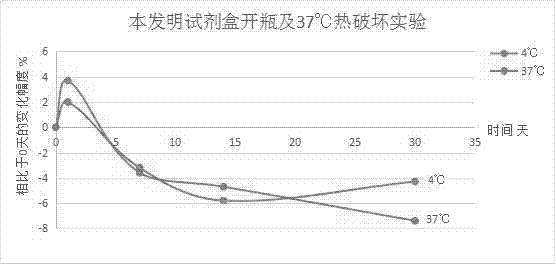
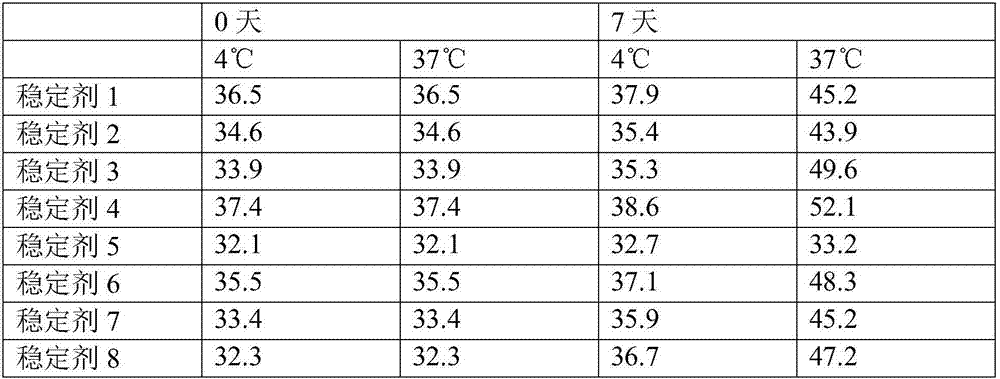
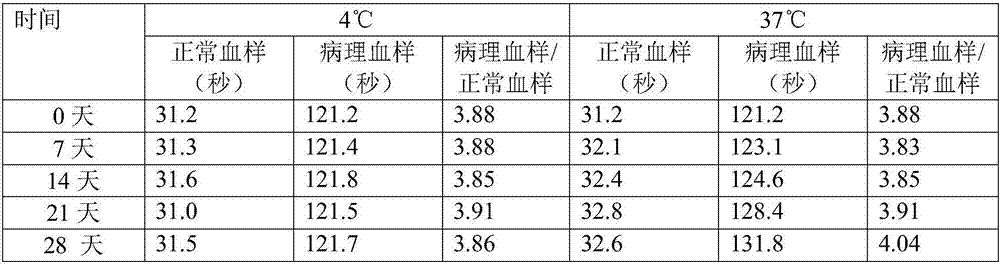
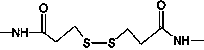Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
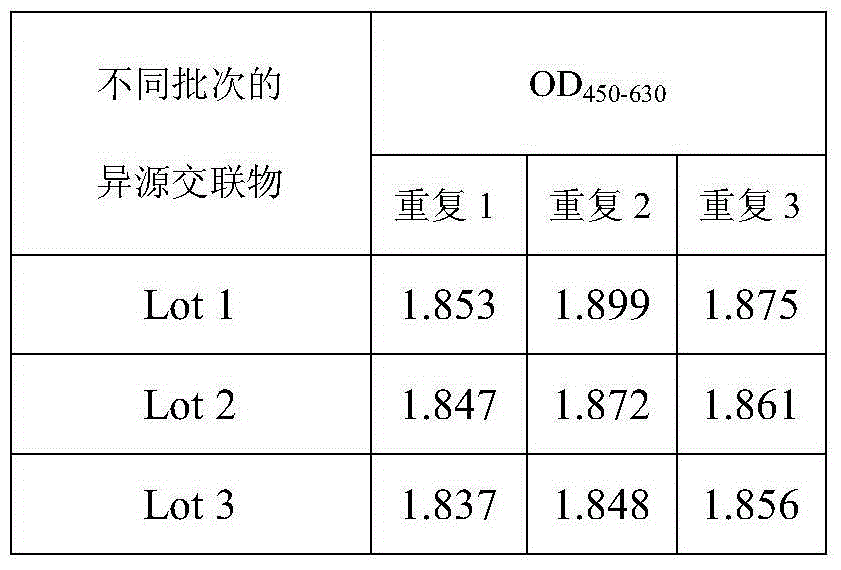
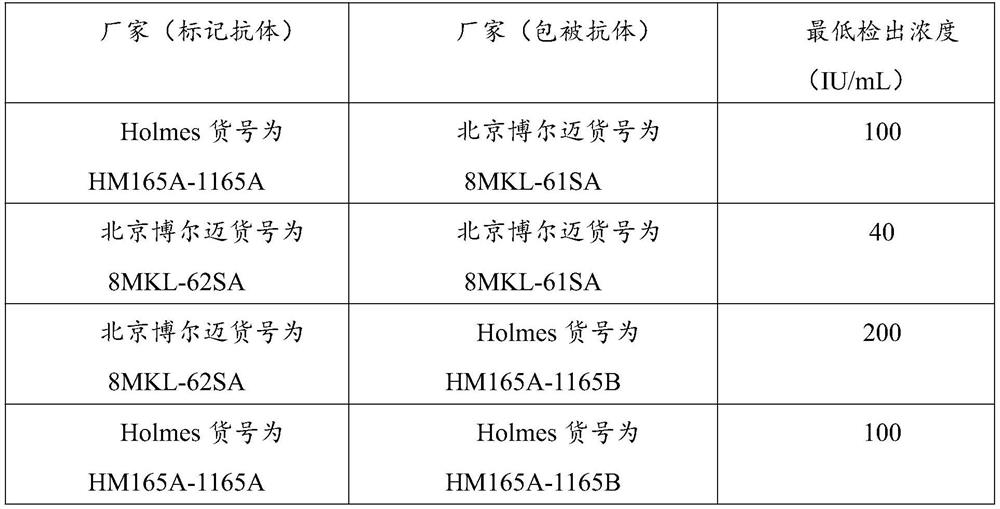
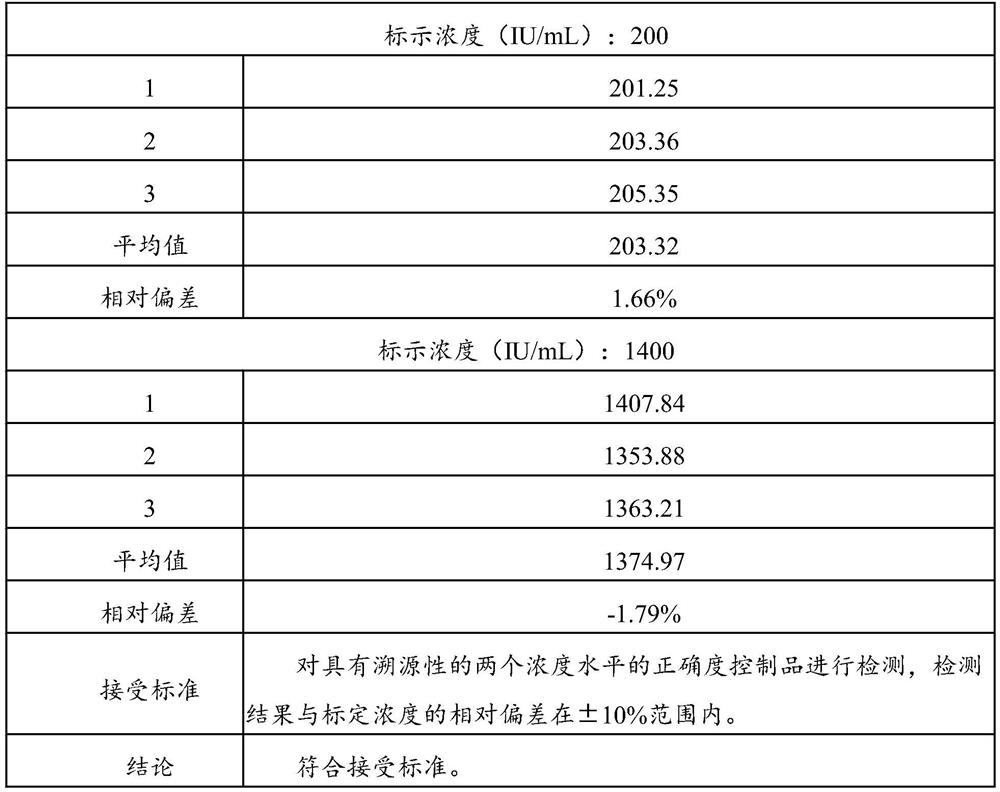
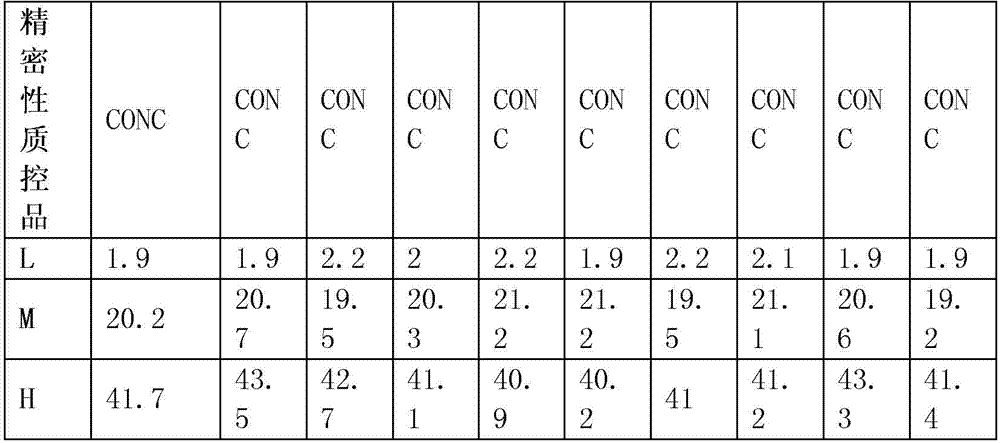
84results about How to "Small difference between batches" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
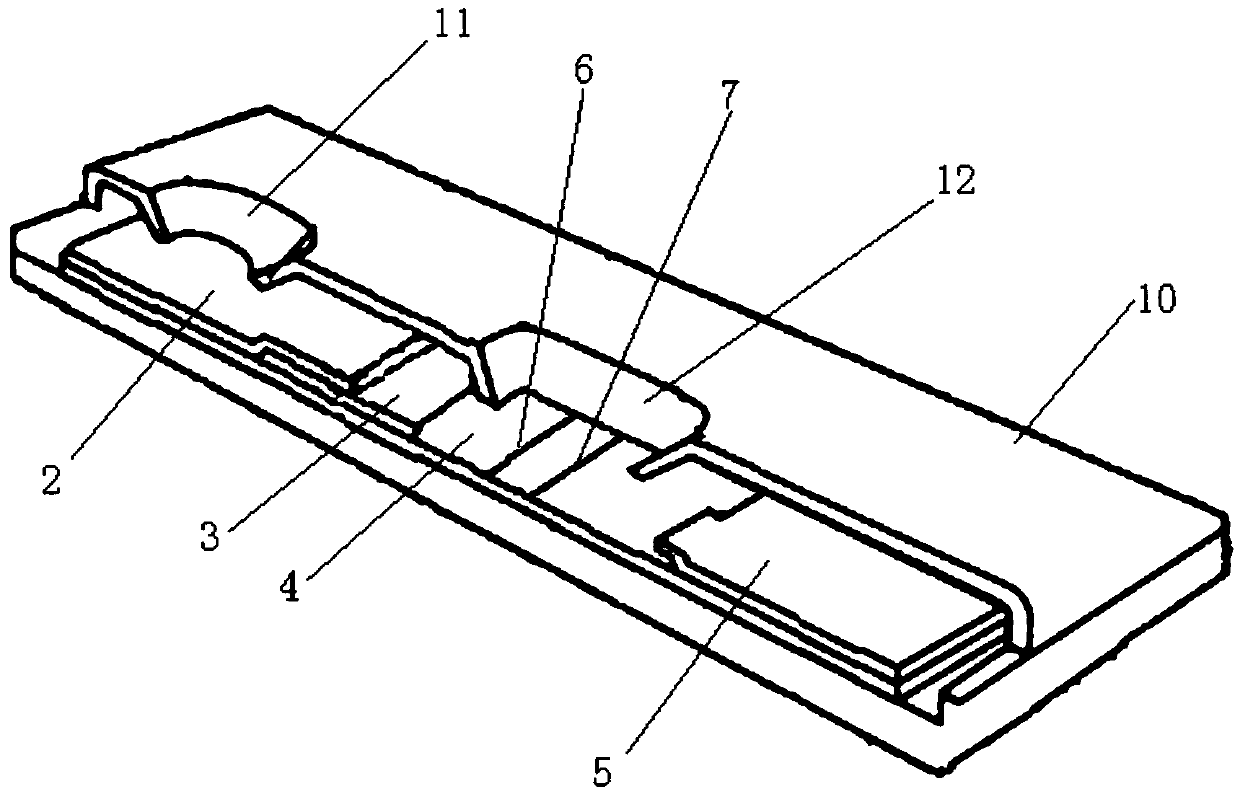
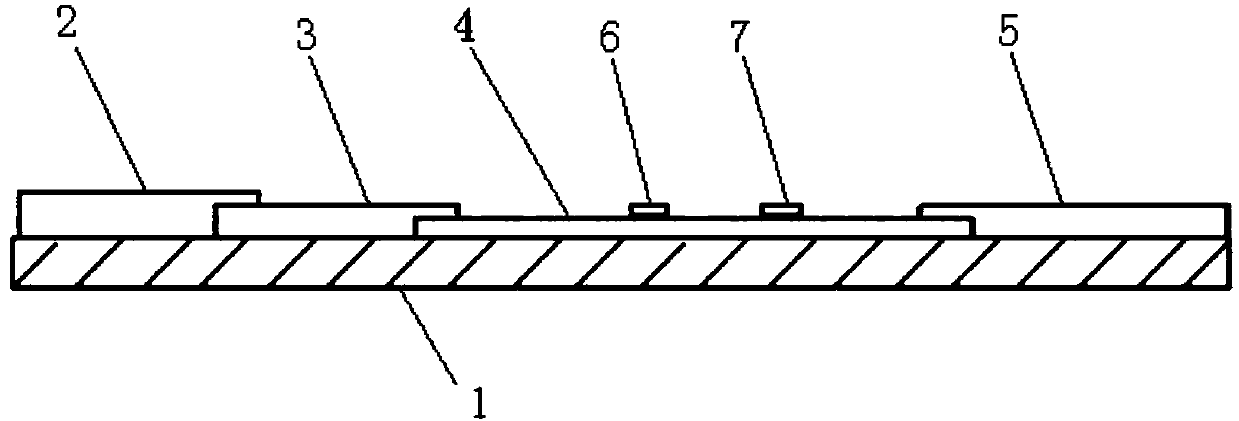
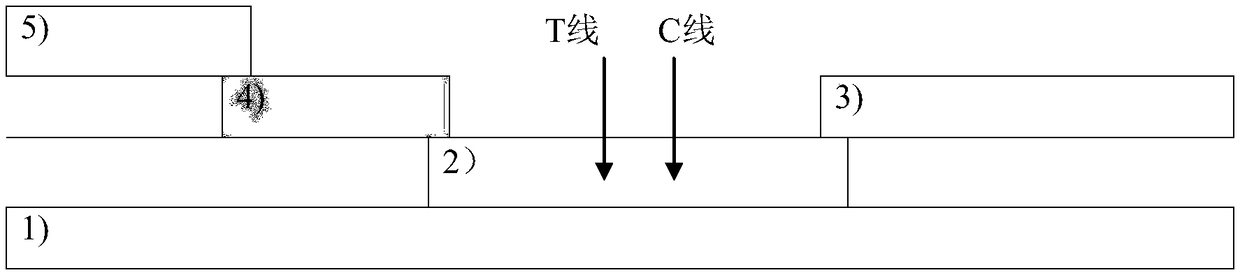
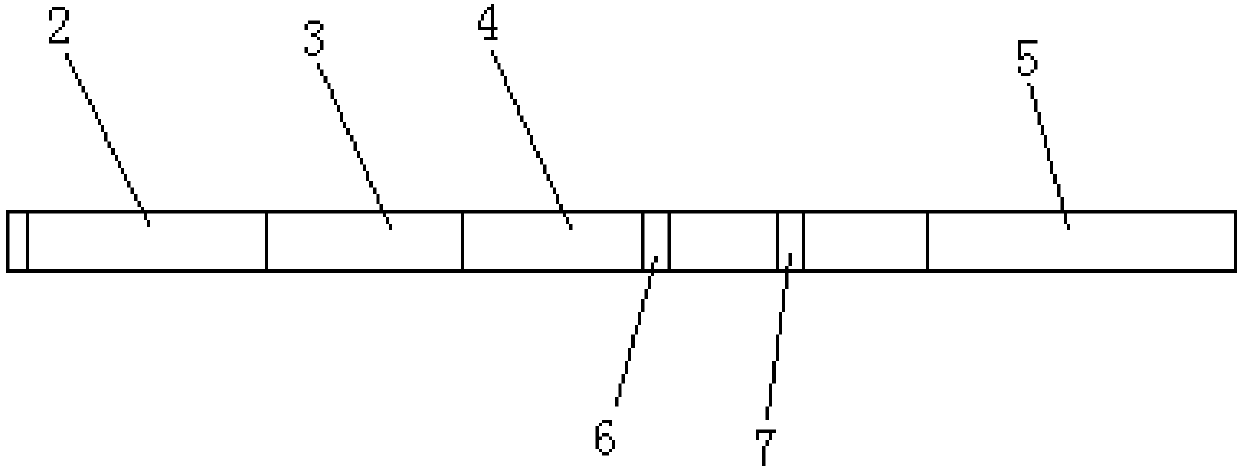
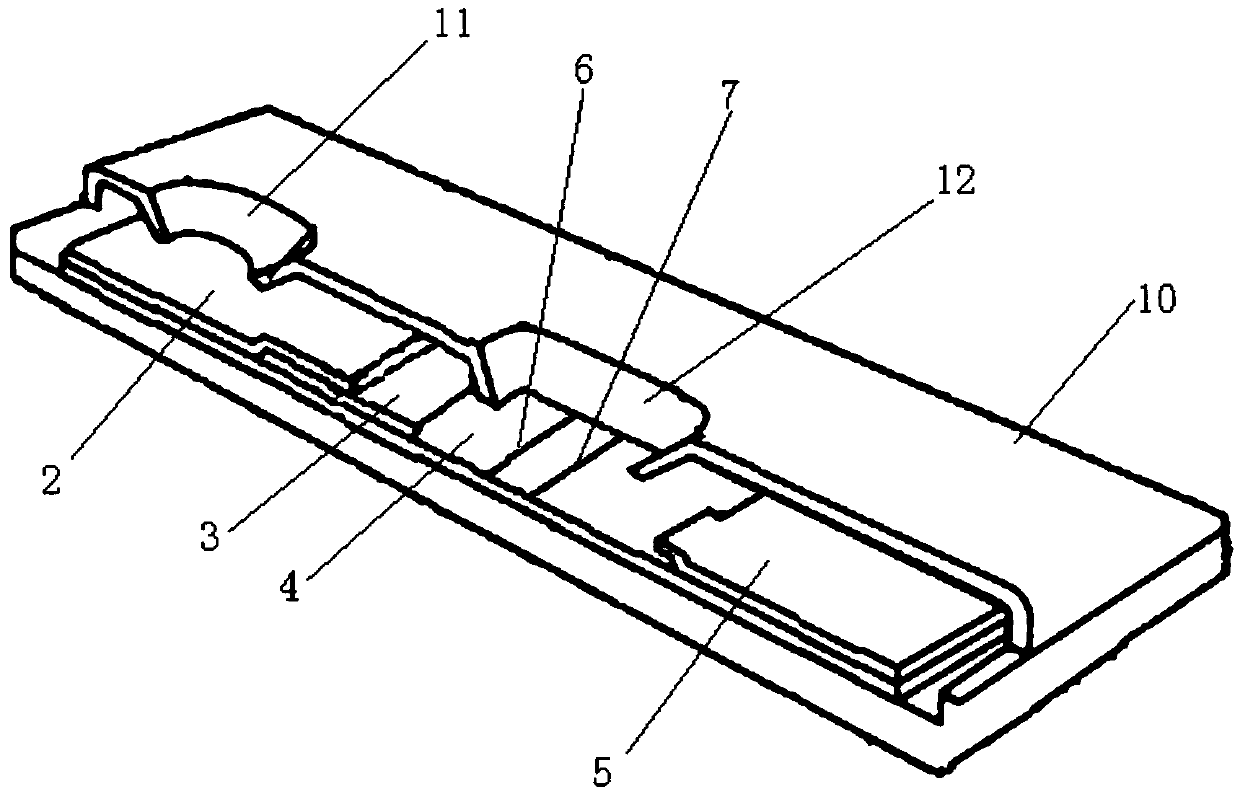
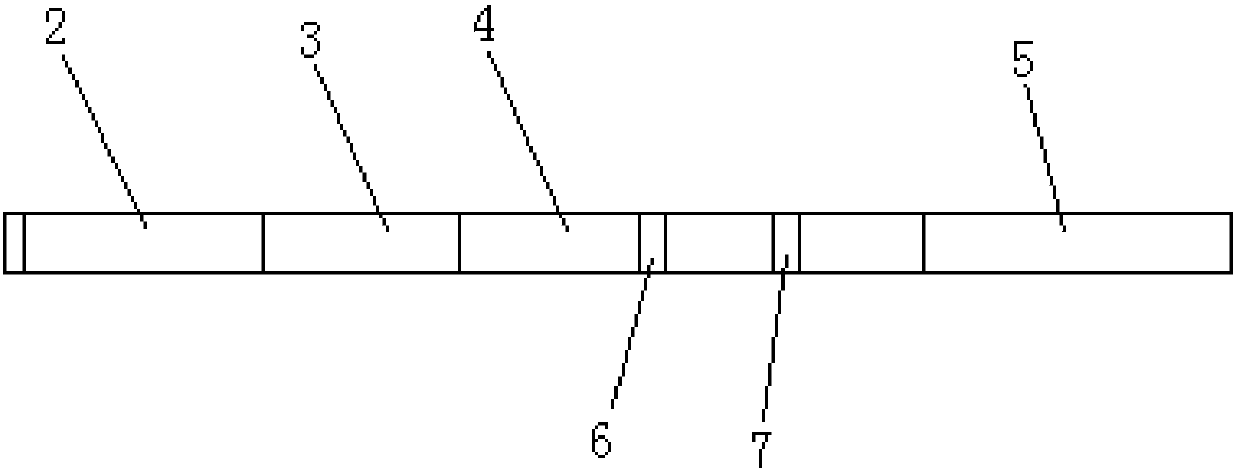
Time resolution immunochromatographic test strip for quantitatively detecting pepsinogen I as well as preparation method of time resolution immunochromatographic test strip
ActiveCN104422772AHigh sensitivitySmall difference between batchesDisease diagnosisPepsinPepsinogen I
The invention relates to the field of the clinic immunological detection, and in particular to a time resolution immunochromatographic test strip for quantitatively detecting pepsinogen I. The test strip comprises a plastic snap shell, a soleplate as well as a sample pad, a conjugate pad, a coating film and a piece of water absorption paper, which are sequentially adhered onto the soleplate in a staggering manner. The conjugate pad is coated with a pepsinogen I monoclonal antibody I marked by rare-earth ion microspheres; the coating film is coated with a detection band and a quality control band, a pepsinogen I monoclonal antibody II for recognizing different epitope is fixed on the detection band, and a rabbit anti-mouse IgG antibody is fixed on the quality control band. The invention also discloses a preparation method of the test strip. The two-antibody antigen sandwiched measuring technology and a time resolution immunochromatograhic technology are introduced to the detection of the pepsinogen I, and the single-person quantitative detection of the pepsinogen I is realized by combining a fluorescent detector; moreover, the sensitivity is high, the intra difference and inter difference are small, and great convenience is provided for the clinical use.
Owner:无锡市江原实业技贸有限公司
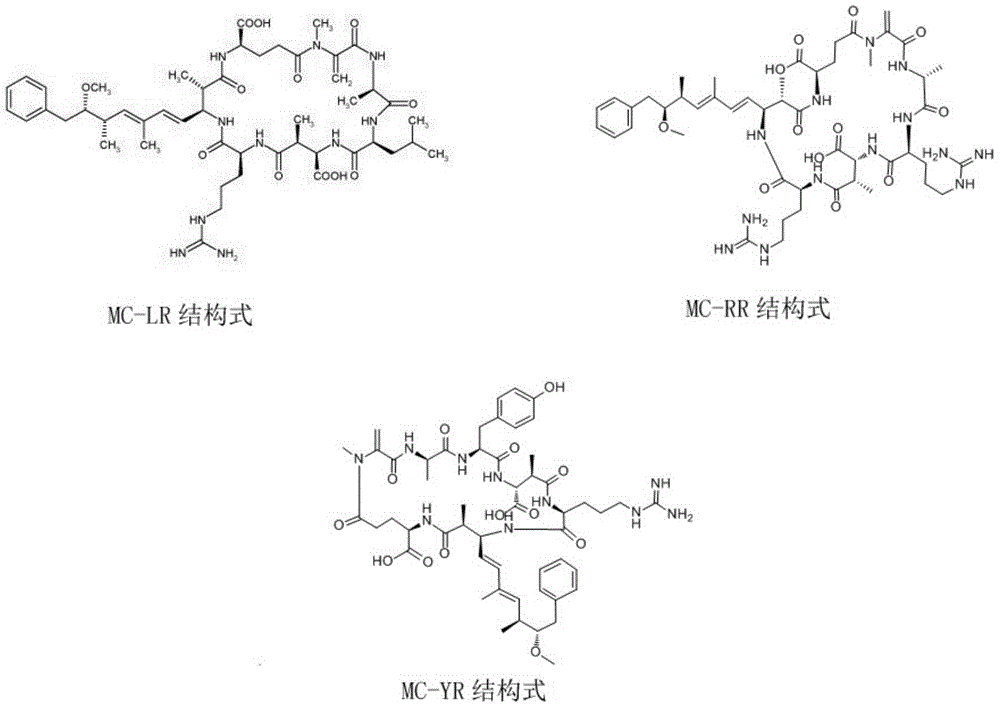
Fluorescent quantitative test paper strip for simultaneously detecting algal toxins MC-LR/RR/YR and preparation method and application of fluorescent quantitative test paper strip
InactiveCN104655837AHigh sensitivityIntra-batch variation is smallFluorescence/phosphorescenceQuality controlToxin
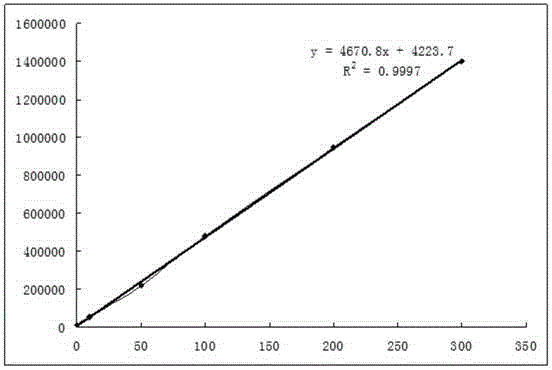
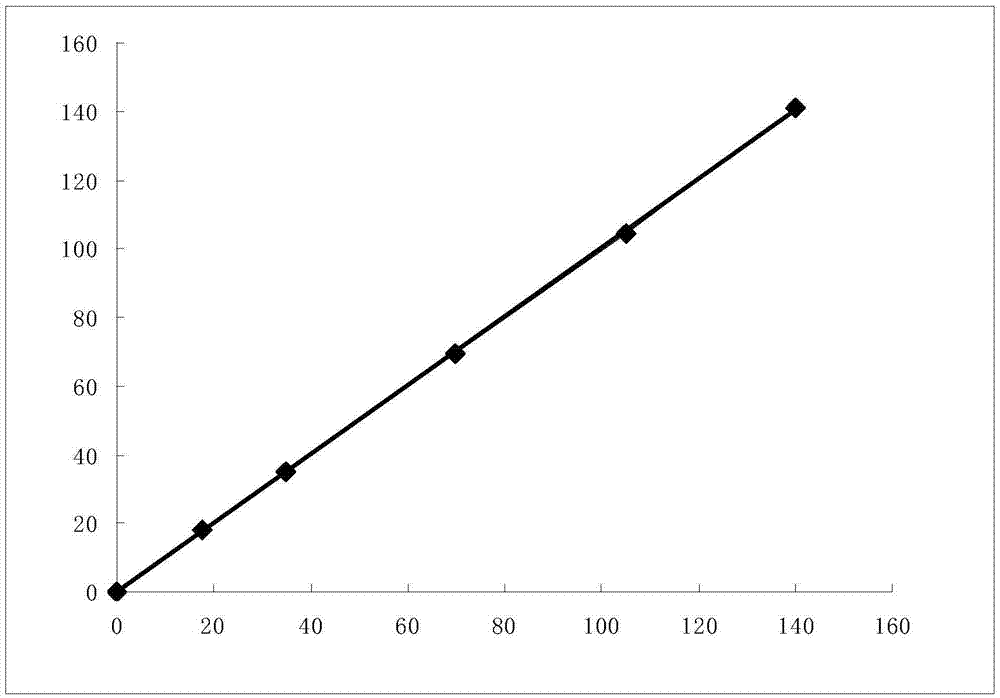
The invention discloses a fluorescent quantitative test paper strip for simultaneously detecting algal toxins MC-LR / RR / YR in a water body and a preparation method and application of the fluorescent quantitative test paper strip. The preparation method comprises the following steps: firstly, marking specific antibodies and rabbit IgG of three algal toxins by using fluorescent microspheres, and drying the three algal toxins on a fluorescent combining pad; sequentially drawing detection lines T1, T2 and T3 of conjugate of the three algal toxins and bovine serum protein and a goat anti-rabbit quality control line C on an NC membrane; finally assembling the test paper strip, namely, sequentially lapping and adhering a sample pad, a fluorescent marker conjugate pad, a nitrocellulose membrane with the three detection lines (T1, T2 and T3 lines) and one quality control line (line C), and a piece of water absorbing paper to a PVC hard board, after the components are assembled, shearing so as to obtain the test paper strip, and finally packaging the test paper strip in a plastic shell. The detection sensitivity on the three algal toxins, of the test paper strip disclosed by the invention, is up to 0.05ng / ml, the quantitative linear range of the test paper strip is 0.1-10ng / ml, the sample needs no pretreatment, the detection time is only 6 minutes, and rapid quantitative detection on the three common algal toxins in water bodies can be achieved, so that the test paper strip has very high practical values.
Owner:南京微测生物科技有限公司
Test kit for measuring content of retinol conjugated protein in serum of human body and preparation method of test kit
InactiveCN106932589AImprove stabilityStrong specificityBiological testingVitamin A RetinolLatex particle
The invention provides a test kit for measuring the content of retinol conjugated protein in serum of a human body and a preparation method of the test kit. The volume ratio of a reagent R1 and a reagent R2 is 3:1. The R1 comprises 0.01-4%w / w of emulsifiers, 0.1-2%w / w of sodium chloride, 0.05-2%w / w of sensitizers, 0.02%w / w of preservative and 30-100mmol / L of buffer solutions. The R2 comprises 0.1-0.5%w / w of latex particle combined with a retinol conjugated protein antibody, 0.1-1.0%w / w of bovine serum albumin, 0.02-2%w / w of Tween 20, 0.02-2%w / w of stabilizers 2, 0.02-2%w / w of protein protective agents, 0.02%w / w of preservative and 20-50mmol / L of buffer solutions. The test kit has wide linearity range, high sensitivity and stability and good clinical application prospect.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
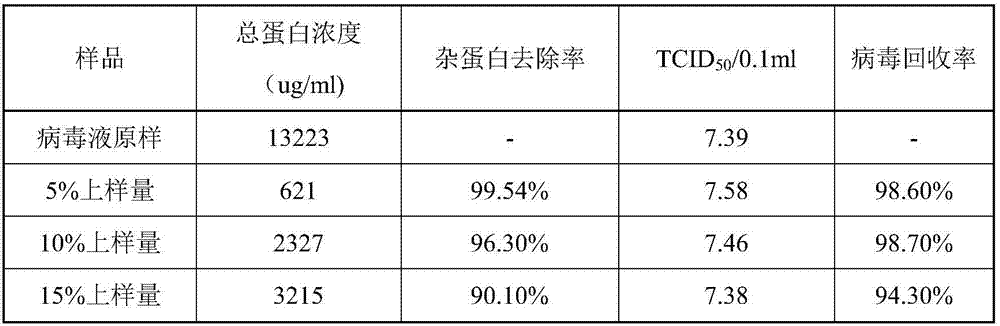
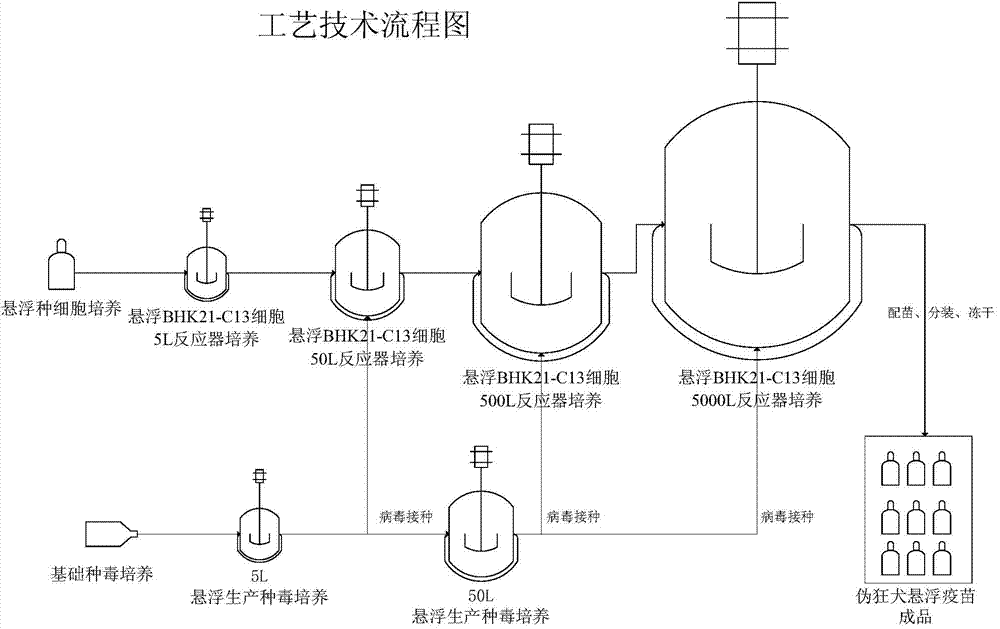
Process for preparing veterinary rabies inactivated and freeze-dried vaccine through suspension culture cell
ActiveCN102228686AQuality improvementSmall difference between batchesInactivation/attenuationAntiviralsAntigenHamster
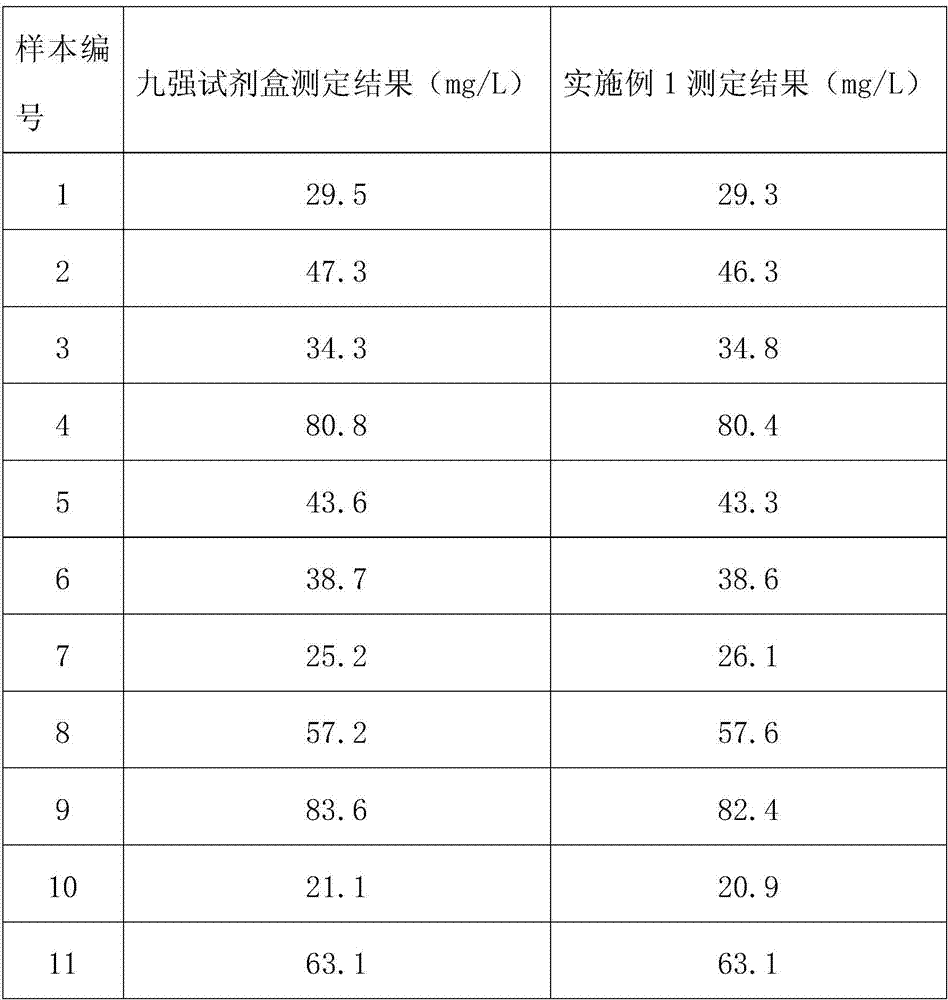
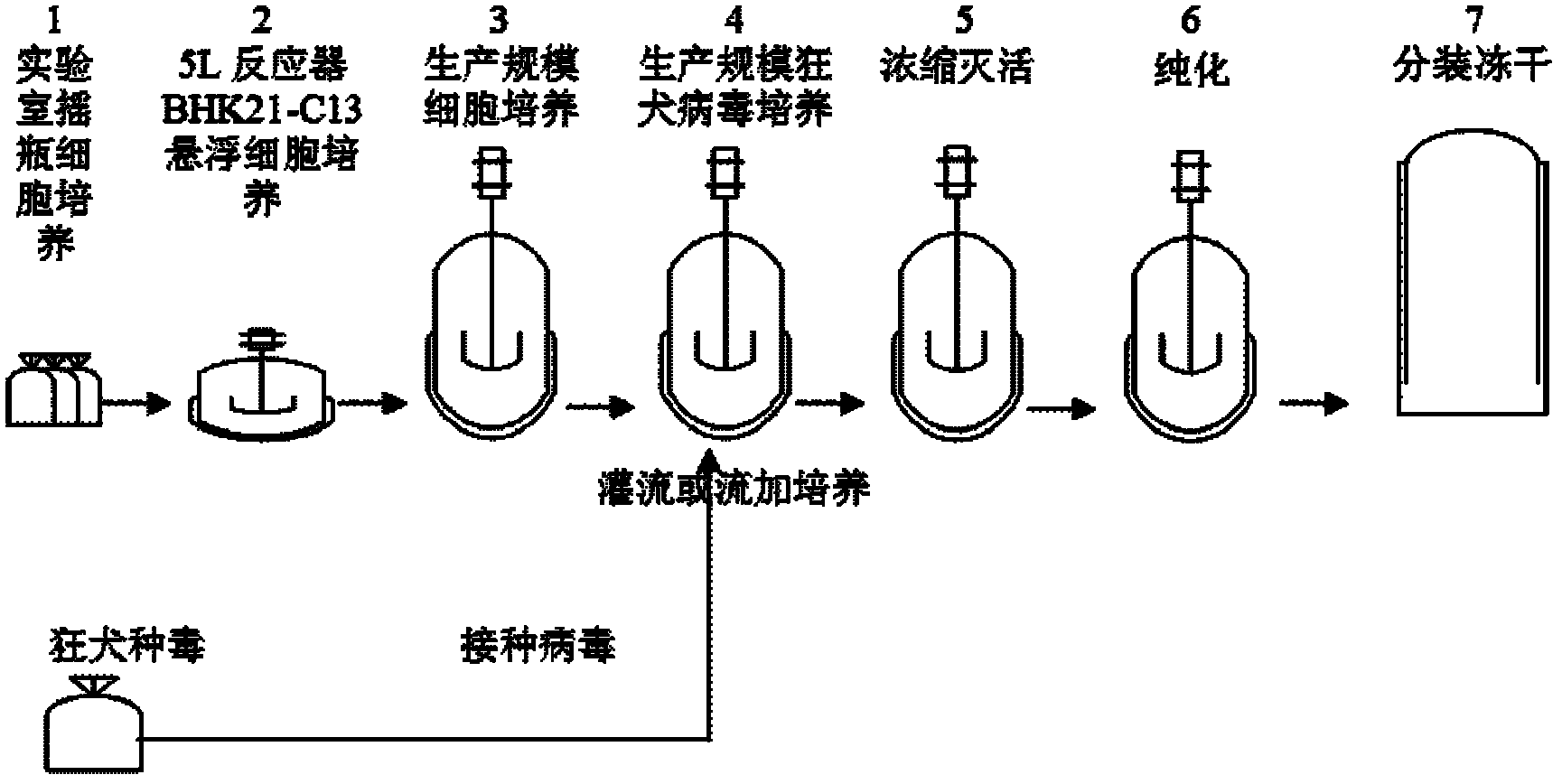
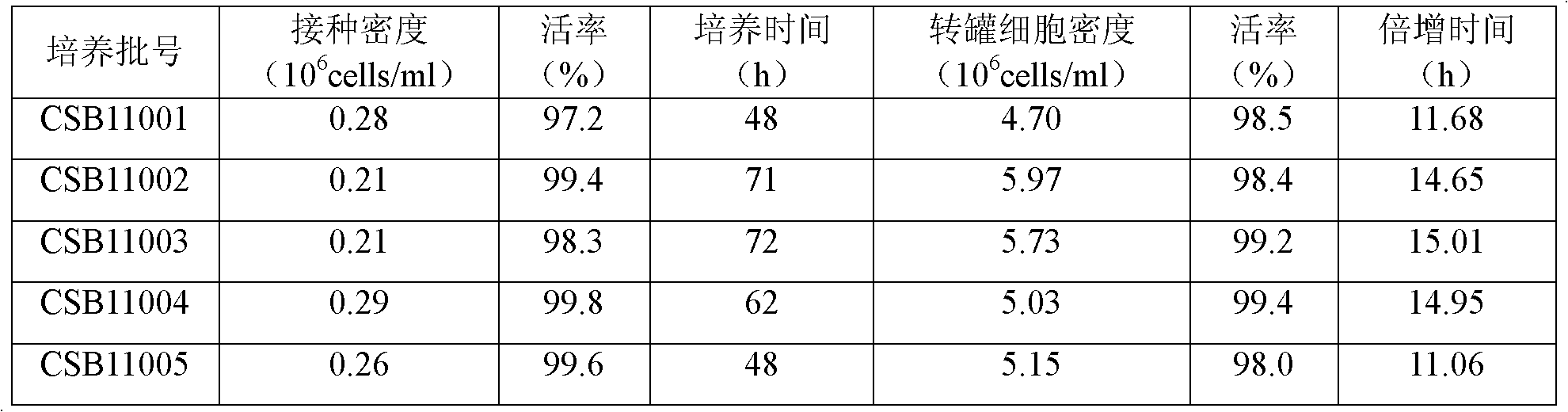
The invention relates to a process for preparing a veterinary rabies inactivated vaccine, in particular to a process for preparing a veterinary rabies inactivated and freeze-dried vaccine through a suspension culture cell. The method has the key point that: a bioreactor is used for large-scale suspension culture of baby hamster kidney BHK21-C13 cells and rabies virus is inoculated, and the rabiesvirus is subjected to mass propagation through a fed-batch and perfusion technology, so that a high-concentration and high-titer rabies antigen is obtained and concentrated, inactivated and purified to prepare the veterinary rabies inactivated vaccine; and thus, technical problems such as complexity, low antigen content, low effectiveness, large dosage, poor batch-to-batch variation and the like existing in the conventional process are solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
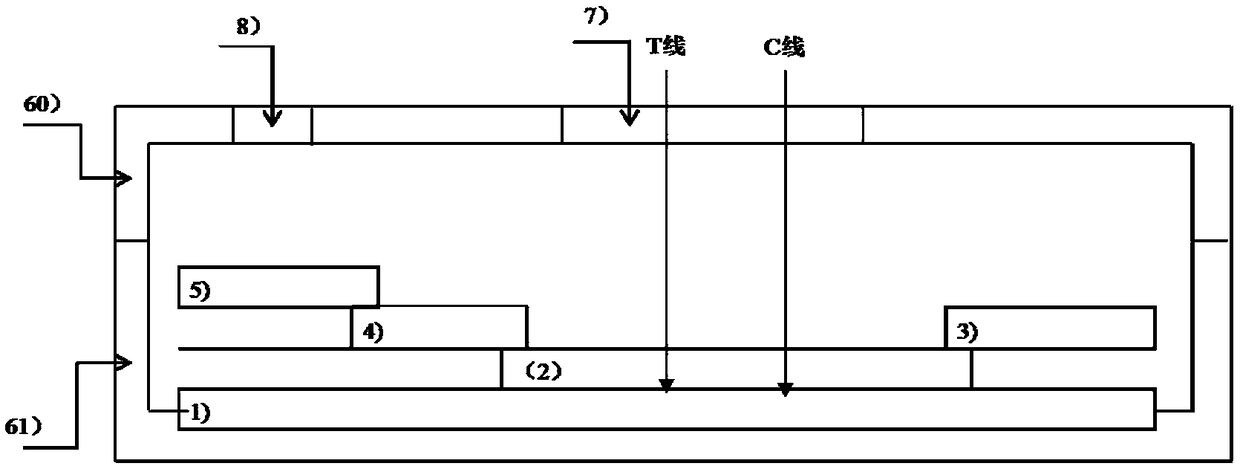
Rapid quantitative detection card for canine distemper virus antibody and using method
The invention discloses a rapid quantitative detection card for a canine distemper virus antibody and a using method. The rapid quantitative detection card comprises a detection card shell and a teststrip assembled in the detection card shell. The test strip comprising a plastic base plate with pressure-sensitive adhesive. A sample pad, a marker pad, a nitrocellulose membrane and absorbent paperare sequentially pasted on the base plate. The marker pad is composed of a carrier base layer and a marker, wherein the marker is a membrane formed by spraying the carrier base layer with lanthanide fluorescent detection microspheres and lanthanide fluorescent quality control microspheres. The part, coated with a canine distemper virus H protein antigen, of the nitrocellulose membrane is a detection line. The part, coated with an anti-Chicken IgY antibody, of the nitrocellulose membrane is a quality control line. The marker is fluorescent detection microspheres marked with a canine distemper virus structural protein H protein recombinant antigen and fluorescent quality control microspheres marked with the anti-Chicken IgY antibody. By the adoption of the rapid quantitative detection card,on-site rapid quantitative determination of the canine distemper virus antibody can be achieved, and the practical value and the promotional value are higher.
Owner:杭州微瑞科技有限公司
Method for large-scale production of porcine pseudorabies inactivated vaccine
ActiveCN107267466AHigh purityLess side effectsViral antigen ingredientsInactivation/attenuationMolecular sieveAdjuvant
The invention belongs to the technical field of vaccines and relates to a method for large-scale production of a porcine pseudorabies inactivated vaccine. The method comprises preparing a virus solution of porcine pseudorabies (XF-1 strain), carrying out continuous flow centrifugation, hollow fiber column clarification filtration, hollow fiber column ultrafiltration concentration and Sepharose 4FF molecular sieve gel chromatography purification treatment to obtain purified porcine pseudorabies viruses, adding a formaldehyde solution having a final concentration of 0.4% (v / v) into the purified porcine pseudorabies viruses, carrying out inactivation at 37 DEG C for 48h, and carrying out emulsification with a 201 adjuvant to obtain the porcine pseudorabies inactivated vaccine. The porcine pseudorabies inactivated vaccine can well prevent highly pathogenic mutant pseudorabies prevailing in the market.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Liquid ready-to-use prothrombin time detection reagent
InactiveCN107356768AOvercome the difference between bottlesOvercome the defect of large batch differenceBiological testingTissue factorCholesterol
The invention discloses a liquid ready-to-use prothrombin time detection reagent, which includes a buffer, a synthetic phospholipid, a recombinant rabbit tissue factor, a surfactant and a stabilizer. The synthetic phospholipid is composed of phosphatidylserine, phosphatidylcholine and Cholesterol composition. The present invention uses rabbit recombinant factors and synthetic phospholipids to prepare prothrombin time detection reagents by selecting synthetic phospholipid components and optimizing stabilizers. It does not need to be reconstituted during use and can be used immediately after opening the bottle. The reagent overcomes the problem of difficult-to-control batch-to-batch variation of existing prothrombin time detection reagents and has high sensitivity, good stability, small batch-to-batch variation, easy quality control, and easy production.
Owner:NINGBO ACCUTECH BIOSCI LTD
Detection card for serotype O foot and mouth disease virus antibody and preparation method of detection card
InactiveCN105606819AQuick checkHigh sensitivityBiological material analysisBiological testingAntigenQuality control
The invention discloses a detection card for a serotype O foot and mouth disease virus antibody and a preparation method of the detection card. The detection card comprises a detection card body assembled in a shell. The detection card body comprises a base plate. A sample pad, a marking pad, a nitrocellulose membrane and water absorption paper are sequentially arranged on the base plate. A serotype O foot and mouth disease antigen coats the nitrocellulose membrane to serve as a detection line, and an anti-chicken antibody produced in goat coats the nitrocellulose membrane to serve as a quality control line. A sample adding hole corresponding to the sample pad is formed in the shell. Window holes corresponding to the detection line and the quality control line on the nitrocellulose membrane are formed in the shell. The detection card is high in sensitivity, small in detection error in a batch and detection error between batches, good in repeatability, stable in performance, capable of fast detecting the serotype O foot and mouth disease virus antibody on site, and high in practical value.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Purification technology applicable to mass production of human-used avian influenza vaccine
The invention belongs to the field of biotechnology and relates to the purification technology for avian influenza viruses, which is applicable to the mass production of human-used avian influenza vaccines. The purification technology is characterized in that: the avian influenza viruses are inoculated on a 9 to 11 days chicken embryo; the influenza viruses are cultured for 68 to 72 hours; and the allantoic fluid of the chicken embryo is sequentially collected, coarsely filtered, centrifugated with a continuous flow, ultrafiltered, condensed, centrifugated in a sucrose density gradient to be purified. The technology has the advantages of simple operation, small difference between batches, stable quality, high yield, low ovalbumin content and the like.
Owner:DALIAN ALEPH BIOMEDICAL
Method for rapidly detecting CP4-EPSPS in transgenic plant
InactiveCN106885907AEasy to manufactureEase of mass productionBiological testingNitrocelluloseCarbon label
The invention relates to a method for rapidly detecting CP4-EPSPS in transgenic plants, comprising the following steps: step 1, preparation of colloidal carbon; step 2, preparation of colloidal carbon-labeled antibodies suitable for pH conditions; step 3, antibody coating to The reaction membrane is used as the T line and the C line of the reaction membrane coated with the secondary antibody; step 4, preparation of colloidal carbon test strips; step 5, extracting protein from the plant to be tested, and preparing protein extract; step 6, testing the test strip The paper strip is inserted into the protein extract solution to react; Step 7, after an appropriate reaction time, judge the result with the naked eye. The present invention adopts the colloidal carbon pad labeled with polyclonal antibody E01, the nitrocellulose membrane coated with polyclonal antibody E01 and the nitrocellulose membrane coated with goat anti-rabbit IgG to make detection test strips; The principle of immunochromatography is to form antigen-antibody complexes to make the results visible to the naked eye.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI +1
Production method of porcine transmissible gastroenteritis virus by utilizing bioreactor
ActiveCN102660510AQuality improvementIncrease productionMicroorganism based processesViruses/bacteriophagesVultureTiter
The invention provides a production method of porcine transmissible gastroenteritis virus by utilizing a bioreactor. The method comprises the following steps: 1) preparing monolayer subculture cells; 2) preparing virus seed for porcine transmissible gastroenteritis virus production; 3) preparing a cell suspension from the monolayer subculture cells prepared in the step 1), and inoculating into a bioreactor to carry out adsorption culture on the subculture cells in a microcarrier in the bioreactor; 4) inoculating the virus seed prepared in the step 2) at the inoculation amount of 2 to 5 percent when the subculture cells grow to 80-90 percent of the microcarrier, the empty bead rate is lower than 5 percent, the full bead rate is more than 80 percent and the cell count is over 3-5*10<6> per mL, and performing virus adsorption culture; and 5) harvesting virus fluid when over 80 percent of the subculture cells on the microcarrier have pathological changes. The method can be used for solving the problems of low production efficiency, unstable product quality and low virus titer, so that the unit vulture titer of the virus can be improved by 5 to 10 times, the quality and yield of vaccine can be comprehensively improved, and the safety of vaccine is improved.
Owner:兆丰华生物科技(南京)有限公司 +1
Porcine reproductive and respiratory syndrome antibody detecting card and preparation method thereof
The invention discloses a porcine reproductive and respiratory syndrome antibody detecting card and a preparation method thereof. The porcine reproductive and respiratory syndrome antibody detecting card comprises a detecting card body assembled in a shell, wherein the detecting card body comprises a bottom plate; a sample pad, a label pad, a nitrocellulose film and absorbent paper are sequentially arranged on the bottom plate; the nitrocellulose film is coated with porcine reproductive and respiratory syndrome antigens serving as a detection line, and coated with goat anti-chicken antibodies serving as a quality control line; a sample adding hole is formed in the shell and corresponds to the sample pad; and window holes are formed and correspond to the detection line and the quality control line on the nitrocellulose film. The porcine reproductive and respiratory syndrome antibody detecting card is high in sensitivity, small in detection intra-batch difference and inter-batch difference, good in repeatability and stable in performance, can quickly detect porcine reproductive and respiratory syndrome antibodies in situ, and has high practical value.
Owner:成都微瑞生物科技有限公司
Detection card for quickly quantitative detection of echinococcosis antibody in serum
The invention discloses a detection card for quickly quantitative detection of echinococcosis antibody in serum. The detection card includes a detection card case and a test paper strip assembled therein. The test paper strip includes a plastic base board provided with pressure-sensitive adhesive, wherein a sample pad, a marker pad, a nitrocellulose membrane and water absorption paper are successively adhered to the base board. The marker pad is composed of a carrier base layer and a marker. The marker is a film formed by spray-coating the carrier base layer with lanthanum-series fluorescencedetection microspheres and lanthanum-series fluorescence quality control microspheres. The nitrocellulose membrane is coated with an echinococcosis recombinant antigen to form a detection line and rabbit-anti-chicken IgY antibody to form a quality control line. The marker includes the fluorescence detection microspheres labeled by the echinococcosis recombinant antigen and the fluorescence qualitycontrol microspheres labeled by the chicken IgY antibody. The detection card can achieve quick quantitative detection of the echinococcosis antibody in situ and has great practical and application values.
Owner:杭州微瑞科技有限公司
Test strip capable of quantificationally detecting content of c-reactive protein in peripheral blood and preparation method thereof
PendingCN109270274AImprove stabilityHigh luminous intensityBiological testingQuality controlMonoclonal antibody
The invention relates to a test strip capable of quantificationally detecting content of c-reactive protein in peripheral blood. The test strip comprises a sample cushion, a quantum cushion, a nitrocellulose membrane, water absorbing paper and a lining plate, the quantum cushion wraps c-reactive protein antibodies marked by carbon quantum dots, and a detecting line and a quality control line are successively arranged on the nitrocellulose membrane from the sample cushion side to the water absorbing paper side; the detecting line wraps c-reactive protein recognizing monoclonal antibodies, and the quality control line wraps goat anti mouse IgG. The test strip is simple in structure and can quickly and accurately detect CRP in the peripheral blood quantificationally, the detection of the teststrip is high in sensitivity and stability and good in specificity, the detecting time is shorter than 15 min, and the diagnostic efficiency and the work efficiency of detecting operators can be greatly improved.
Owner:重庆新赛亚生物科技有限公司
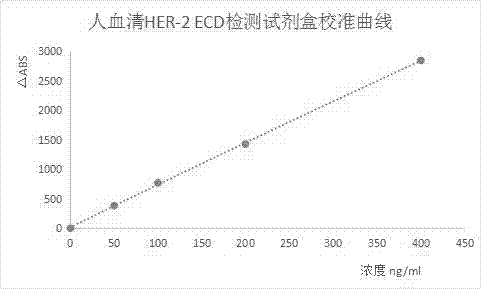
Kit for testing concentration level of HER-2 ECD in human serum
InactiveCN106970226AGive full play to the synergistic effectAvoiding the False Positive ProblemMaterial analysisSerum igeSerum samples
The invention relates to a kit for testing concentration level of epidermal growth factor receptor-2- extracellular region protein (hereinafter referred to as HER-2 ECD) in the human serum. The technical problem to be solved is to provide a kit for testing the concentration level of HER-2 ECD in the human serum, which is applicable to a full automatic biochemical analyzer and a specific protein apparatus; the kit consists of three associated parts which are reagent R1, reagent R2 and a calibration product Cal; after calibration on a specific instrument, above reagent combination is directly applied to test a clinic serum sample, and the acquired result is the concentration level of HER-2 ECD in the human serum. Compared with a chemiluminiscence method, the result has high consistence, the cost is lower, and the kit is more suitable for the application and popularization of clinical detection. The technical scheme can provide a kit for testing the concentration level of HER-2 ECD in the human serum, which is good in both affinity of antibody and specificity, high in method sensitivity, and good in stability.
Owner:童坤
Liquid ready-to-use activated partial thromboplastin time detection reagent
InactiveCN107356769AImprove stabilitySmall difference between batchesBiological testingCholesterolReady to use
The invention discloses a liquid ready-to-use detection reagent for activated partial thromboplastin time, which comprises a buffer, a synthetic phospholipid, an activator, a stabilizer and phenol, and the synthetic phospholipid is composed of phosphatidylserine, phosphatidylcholine, phospholipid Ethanolamine and cholesterol composition, the activator is ellagic acid. The detection reagent of the invention has good stability, small difference between batches, easy quality control during production, and high sensitivity.
Owner:NINGBO ACCUTECH BIOSCI LTD
Quality control object for substituting patients' positive blood
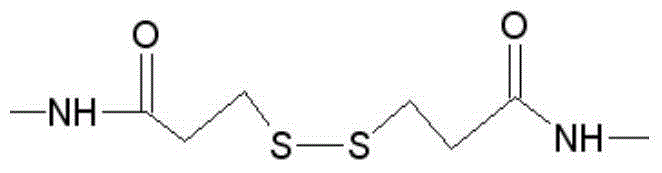
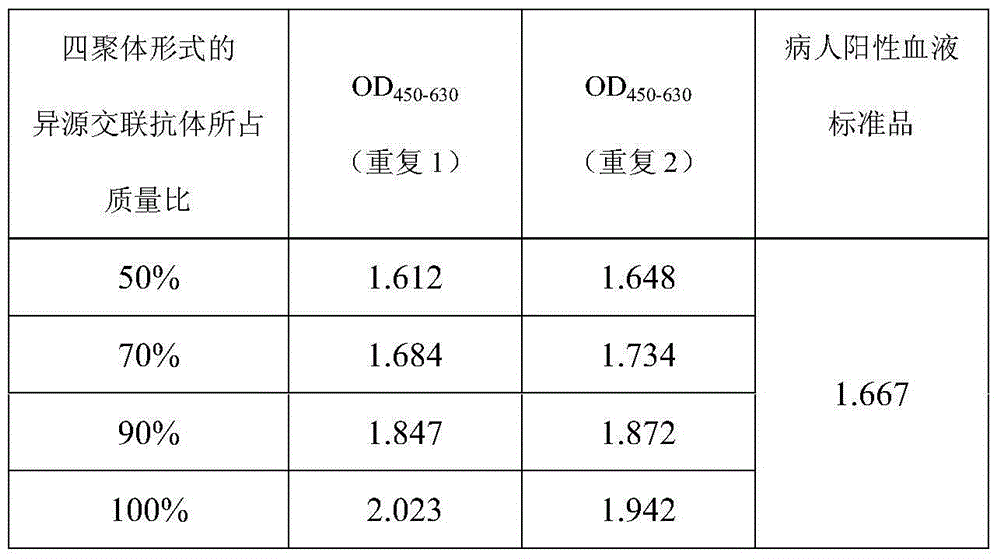
ActiveCN103383393AImprove stabilityEasy to produceMaterial analysisAgainst vector-borne diseasesCross-linkVirology
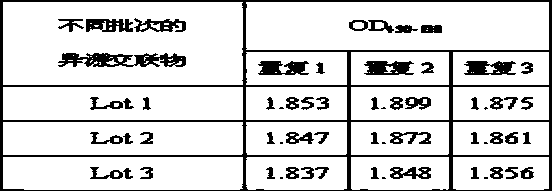
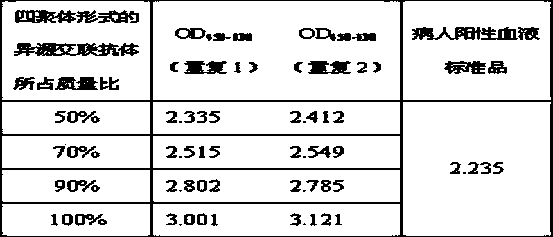
The invention provides a heterologous cross-linking object containing heterologous cross-linking antibody in a tetramer form, the heterologous cross-linking object is obtained from cross-linking reactions between two heterologous antibodies, the mass ratio of the heterologous cross-linking antibody in a tetramer form in the heterologous cross-linking object is preferably not less than 50%, the heterologous cross-linking object can be taken as a substituent of a pathogen antibody in the patients' positive blood, and can be applied to a immunity detection kit as a quality control object to substitute the patients' positive blood after being diluted by a diluting liquid. The heterologous cross-linking object has the advantages of simple production and preparation, low cost, and very good stability and safety. Furthermore, qualities of different batches of quality control objects are stable, so detection errors can be effectively reduced.
Owner:GENCLONN BIOTECH HANGZHOU
Needle milling production process of powdery cell culture medium
InactiveCN106222127ARealize continuous productionSmall footprintCulture processCell culture mediaCell culture mediaSmall footprint
The invention discloses a needle milling production process of a powdery cell culture medium. The process includes the steps of: 1) weighing raw materials according to types for later use; 2) pre-mixing the weighed raw materials in the step 1); 3) grinding and pulverizing the pre-mixed raw materials by means of a needle-type grinding miller; 4) final-mixing the pulverized materials in the step 3); and 5) packaging the final-mixed product. The process can achieve continuous production of the cell culture medium, wherein the productivity of the process is equal to that of a process with a large-size ball miller. The process is less in occupied area of equipment, saves production cost and production time, is high in productivity, is less in batch difference and has stable product quality.
Owner:TIANXINHE SUZHOU BIOTECH CO LTD
Quick quantitative determination card for canine parvovirus antibody and use method thereof
The invention discloses a quick quantitative determination card for a canine parvovirus antibody and a use method thereof. The quick quantitative determination card comprises a detection card casing and a test strip assembled in the detection card casing, wherein the test strip comprises a plastic bottom plate with pressure sensitive adhesive, and a sample pad, a marker pad, a nitrocellulose membrane and absorbent paper are sequentially bonded to the bottom plate; the marker pad comprises a carrier base layer and a marker; the marker is a layer of membrane which is formed by spraying lanthanide-series fluorescence detection microspheres and lanthanide-series fluorescence quality control microspheres on the carrier base layer; the nitrocellulose membrane shows a detection line when being coated with a canine parvovirus recombinant antigen and shows a quality control line when being coated with a rabbit anti-chicken lgY antibody; the marker is fluorescence detection microspheres marked with a canine parvovirus structural protein VP2 recombinant antigen and fluorescence quality control microspheres marked with a chicken lgY antibody. The quick quantitative determination card disclosedby the invention can achieve in-site quick quantitative detection on the canine parvovirus antigen and has higher practical value and popularization value.
Owner:杭州微瑞科技有限公司
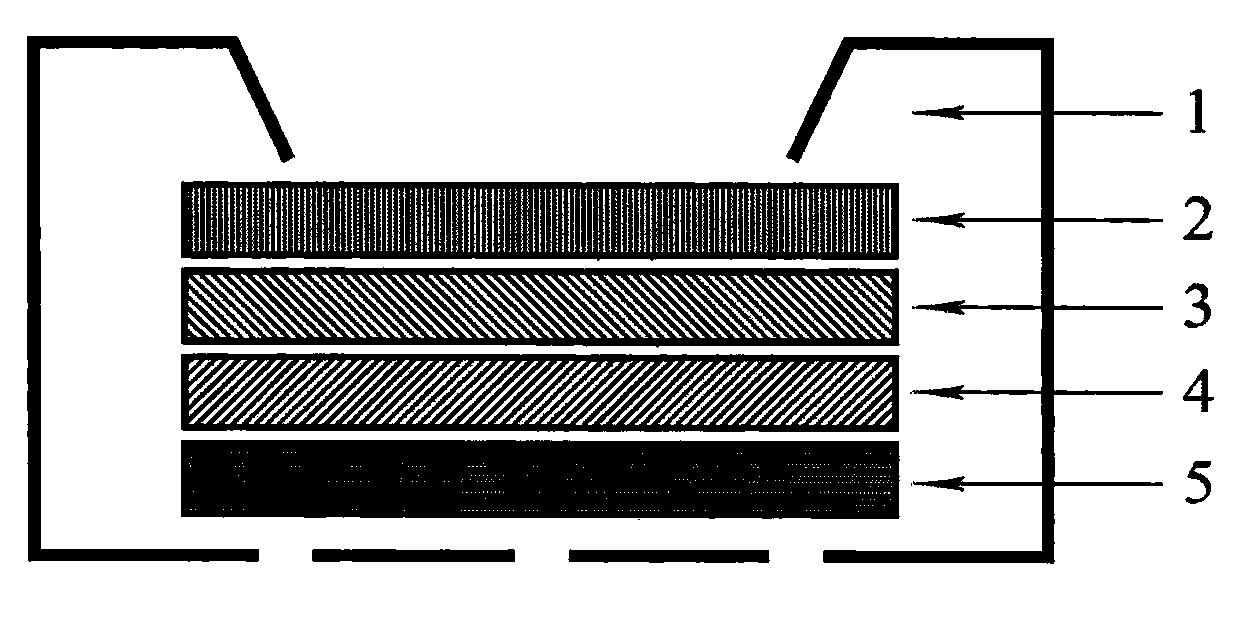
Aptamer percolated biochip and preparation method thereof
InactiveCN103439493AHigh affinityHigh degree of automationMaterial analysisNitrocelluloseReverse osmosis
The invention relates to an aptamer percolated biochip and a preparation method thereof. The preparation method comprises the following steps: arranging a window on a casing of the biochip; arranging a nitrocellulose membrane, a reverse osmosis layer, a water absorbing layer and a leakage preventing layer in the casing of the biochip from top to bottom; fixing an aptamer molecule for a target substance on the surface of the nitrocellulose membrane, wherein the aptamer molecule is RNA (Ribose Nucleic Acid), DNA (Deoxyribose Nucleic Acid) or modified RNA or DNA. According to the aptamer percolated biochip and the preparation method thereof disclosed by the invention, the defects of limited detection range, low sensitivity, weak specificity, easiness for deterioration, complex process, long reaction time and the like existing in the prior art are overcome; DNA molecules which are specifically combined with target molecules are screened from mass in-vitro single stranded oligonucleotide libraries through SELEX screening and are amplified greatly to obtain an aptamer; in addition, a sample to be detected can quickly pass through a micropore of the nitrocellulose membrane by the percolated biochip; the target molecule of the percolated biochip can be quickly combined with the aptamer on the membrane, so that the reaction time can be greatly shortened; higher sensitivity and specificity are obtained.
Owner:NANJING POTOMAC BIO TECH
Heterogenous conjugate and application thereof to RV (rubella virus) assay
ActiveCN104650236AEasy to produceLow costHybrid immunoglobulinsMaterial analysisHeterologousRubella virus antibody
The invention provides a heterogenous conjugate. The heterogenous conjugate comprises a tetramer shaped heterogenous crosslinking antibody formed by mouse anti-RV (rubella virus) McAb and healthy human IgG (immunoglobulin G) which is not infected with RV, wherein the antibody can serve as a substitute of an RV antibody in the positive blood of patients and can be used in immunoassay kits as a quality control product replacing the positive blood of patients after being diluted in a diluent. The heterogenous conjugate is simple to produce and prepare, is low in cost, has very good safety and stability at the same time and can conduce to reducing the assay errors.
Owner:GENCLONN BIOTECH HANGZHOU
Reagent or kit for magnetic particle chemiluminescence detection of salivary liquefied carbohydrate chain antigen and application of reagent or kit
PendingCN112946255AImprove luminous efficiencyHigh strengthChemiluminescene/bioluminescenceDisease diagnosisAntigenHigh concentration
The invention provides a reagent or a kit for magnetic particle chemiluminescence detection of salivary liquefied carbohydrate chain antigen and application of the reagent or the kit, and belongs to the technical field of in-vitro diagnostic reagents. The reagent comprises a magnetic particle reagent and an acridinium ester reagent, wherein the magnetic particle reagent is composed of 2-8 [mu] g / ml of a magnetic particle labeled KL-6 antibody 1, 20%-30% of newborn bovine serum, 0.01%-0.1% of a preservative, 20%-30% of glycerin, 0.01%-0.1% of Tween 20, 0.1%-1% of BSA and 50%-70% of a PBS buffer solution, and the acridinium ester reagent is composed of 6-10 [mu] g / ml of an acridinium ester labeled KL-6 antibody 2, 10%-40% of glycerin, 0.5%-5% of BSA and 60%-90% of a PBS buffer solution. The KL-6 detection kit has the characteristics of high sensitivity, high precision and high accuracy, has excellent anti-interference capability, and does not have an obvious HOOK effect under a high-concentration sample of 40000IU / mL.
Owner:HUNAN YONGHE YANGGUANG SCI & TECH
Plate-type chemiluminescence detection kit of cytokeratin 19-fragment and preparation method
InactiveCN107462721AEasy to produceGood repeatabilityChemiluminescene/bioluminescenceBiological testingAntigenBiotin
The invention relates to a plate-type chemiluminescence detection kit of a cytokeratin 19-fragment. The kit comprises the following reagents: biotin-marked CYFRA21-1 antibody solution, an elisa plate coated by anti-biotin, horse-radish-peroxidase-marked CYFRA21-1 antibody solution, a CYFRA21-1 calibration product A-F, concentrated washing liquor, substrate solution A, and substrate solution B. The main design idea of the detection kit is that the detection specificity of the kit is stabilized by introducing a biotin system with the anti-biotin on the basis of chemiluminescence immune assay, and the detection sensitivity and detection time are greatly increased. A high-temperature oscillatory-type anti-biotin coating technology is improved, so the usage amount of antibodies / antigens is reduced, the production flow of the reagents is simplified, and the production cycle is shortened.
Owner:JIANGSU FLON BIOTECH
A plate-type chemiluminescence-method detection kit for a carbohydrate antigen 50 and a preparing method
InactiveCN107490695AHigh sensitivityReduce usageChemiluminescene/bioluminescenceBiological testingAntigenEnzyme
The invention relates to a plate-type chemiluminescence-method detection kit for a carbohydrate antigen 50. Agents of the kit include a biotin-marked CA50 antibody solution, an enzyme linked immunosorbent assay plate coated with an antibiotin antibody, a CA50 antibody solution marked by horseradish peroxidase, CA50 calibrating materials A-F, a concentrated washing solution, a substrate liquid A and a substrate liquid B. According to a main design thought of the kit, an antibiotin antibody-biotin system is introduced based on chemiluminescence immune assay, detection specificity of the kit is stabilized, detection sensitivity is greatly increased and detection time is reduced. A high-temperature oscillating type antibiotin antibody coating process is improved, thus reducing the using amount of the antibody / antigen, simplifying agent production procedures and shortening the production period.
Owner:JIANGSU FLON BIOTECH
Large-scale production method for veterinary pseudorabies attenuated live vaccines
ActiveCN104740627ASolve outputSolve efficiency problemsMicroorganism based processesAntiviralsVirology
The invention discloses a large-scale production method for veterinary pseudorabies attenuated live vaccines. The method comprises the steps of culturing pseudorabies virus attenuated strains by using a cell suspension process so that the density of BHK21-C13 cells subjected to suspension culture in a reactor is more than or equal to 2*10<6> cells per milliliter, then culturing with a virus maintenance solution containing pseudorabies suspended virus seeds, and performing clarification, seedling, packaging and freeze-drying on the harvested pseudorabies attenuated virus solution to obtain the veterinary pseudorabies attenuated live vaccines. By adopting the method, mass multiplication of pseudorabies attenuated viruses can be realized, the titer of viruses and the yield of antigen are greatly improved, process automation is realized, the quality of the vaccines is improved, the density of the BHK21-C13 cells can be improved, the optimal biochemical condition of cell growth or virus multiplication can be automatically monitored, the titer of viruses is improved, large-scale production of the process is relatively easy, and the vaccines are stable in quality and low in batch difference and have broad production and application prospect.
Owner:LIAONING YIKANG BIOLOGICAL CORP LTD
Card for rapidly and quantitatively detecting pseudorabies virus antibody, and use method thereof
The invention discloses a card for rapidly and quantitatively detecting a pseudorabies virus antibody, and a use method thereof. The card comprises a detection card shell and a test strip assembled inthe detection card shell; the test strip comprises a plastic bottom plate with pressure-sensitive adhesive; a sample pad, a marker pad, a nitrocellulose film and water-absorbing paper are adhered tothe bottom plate sequentially; the marker pad consists of a carrier base layer and a marker; the marker is one layer of film formed by spray-coating the carrier base layer with a lanthanide fluorescence detection microsphere and a lanthanide fluorescence quality control microsphere; the nitrocellulose film is coated with a pseudorabies virus recombinant antigen serving as a detection line and coated with a rabbit anti-chicken IgY antibody serving as a quality control line; and the marker is a fluorescence detection microsphere marked with pseudorabies virus structure protein gB and gI (or gE)recombinant antigens and a fluorescence quality control microsphere marked with a chicken IgY antibody. The card can detect the pseudorabies antibody on site rapidly and quantitatively, and has higherpractical value and popularization value.
Owner:杭州微瑞科技有限公司
Time-resolved fluorescence immunochromatographic test strip and kit for detection of cTnI, and preparation methods thereof
ActiveCN108254563AQuick checkReliable test resultsBiological testingFluorescence/phosphorescenceNitrocelluloseMicrosphere
The invention discloses a time-resolved fluorescence immunochromatographic test strip and kit for detection of cTnI, and preparation methods thereof. The test strip comprises a bottom liner and further comprises a sample pad, a conjugate pad, a coating membrane and absorbent paper successively arranged on the bottom liner, wherein the conjugate pad is coated with a cTnI monoclonal detection antibody labeled by fluorescence microspheres; the coating membrane comprises a test zone and a control zone arranged in parallel and spaced apart from each other; the test zone is coated with a cTnI monoclonal capture antibody capable of recognizing a single epitope, and the control zone is coated with a goat anti-mouse IgG antibody; and the coating membrane a nitrocellulose membrane bonded with a polymer, and the polymer is a material having light transmittance of 10% or below under irradiation of light with wavelengths of less than 450 nm and light transmittance of 95% or more under irradiation of light with wavelengths of 500 nm or more. The test strip of the invention can realize quick and quantitative detection, and is accurate and reliable in test results and high in sensitivity; the preparation method is simple in process and suitable for large-scale production; and the test strip has positive significance to quantitative detection of cTnI.
Owner:RAYBIOTECH INC GUANGZHOU
Adiponectin monoclonal antibody, antibody pair and preparation method and application thereof
ActiveCN112574306AHigh sensitivityHigh degree of purityImmunoglobulins against hormonesBiological testingImmuno detectionGene
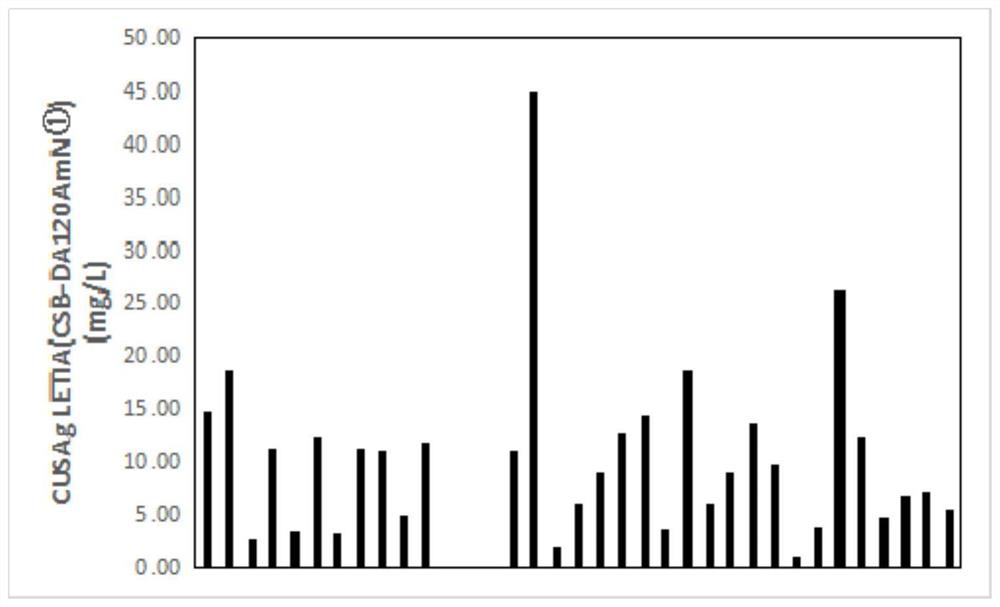
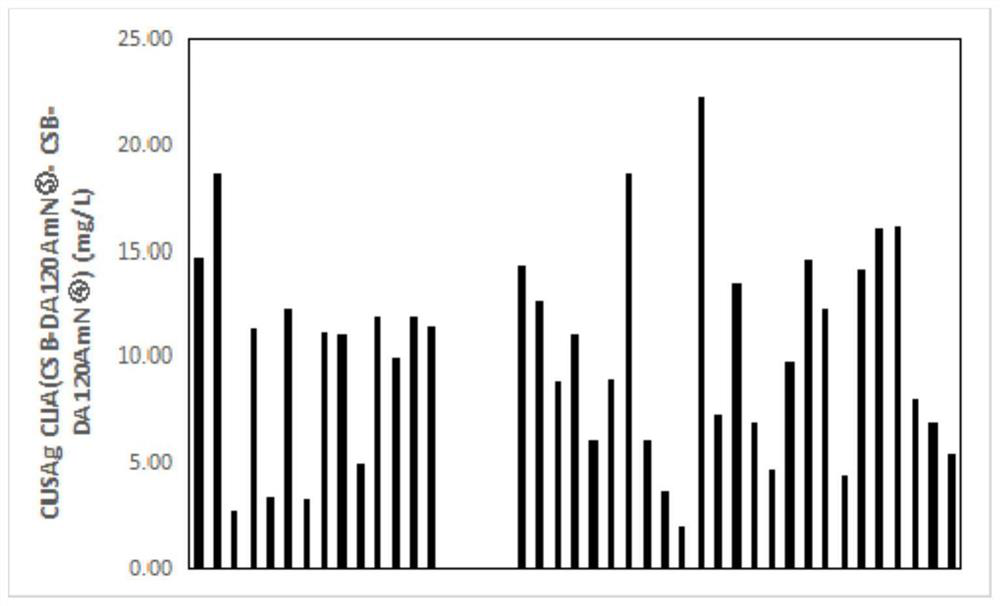
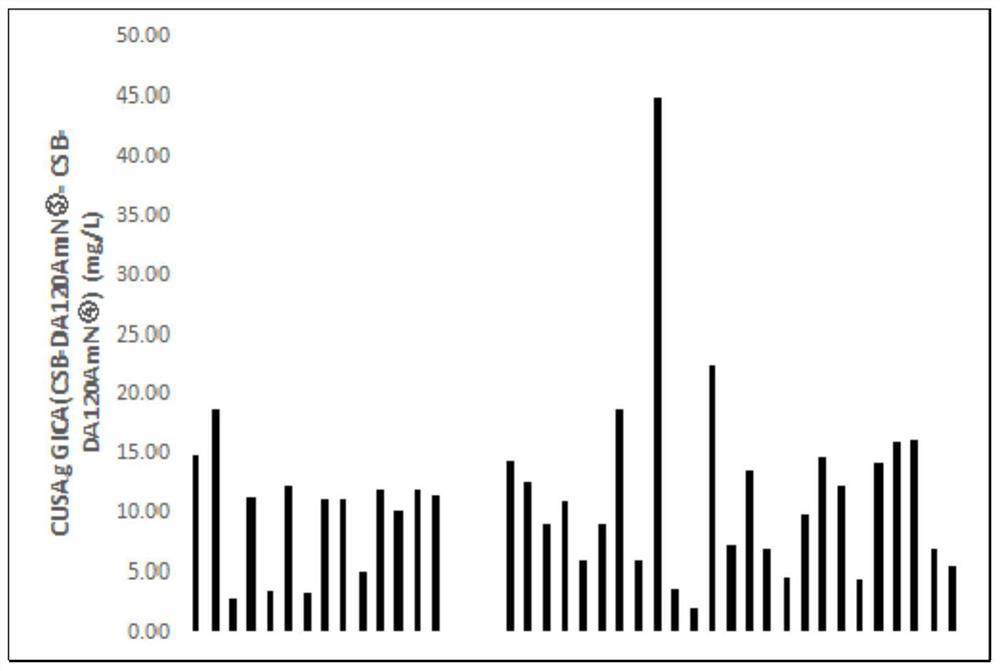
The invention relates to an adiponectin monoclonal antibody and an antibody pair, the adiponectin monoclonal antibody has three kinds, and the three kinds of adiponectin monoclonal antibodies are respectively CSB-DA120AmN (1), CSB-DA120AmN (3) and CSB-DA120AmN (4); and the antibody pair is CSB-DA120AmN (3)-CSB-DA120AmN (4). The invention also provides a preparation method of the adiponectin monoclonal antibody and the antibody pair, and application of the adiponectin monoclonal antibody and the antibody pair in latex enhanced turbidimetry and double-antibody sandwich method immunodetection products, or in preparation of reagents, medicaments or kits for detecting adiponectin. Through gene recombination, the adiponectin monoclonal antibody and antibody pair with more excellent performance are obtained, and the adiponectin monoclonal antibody and antibody pair are stable, efficient, small in batch-to-batch difference and suitable for serving as antibody raw materials of diagnostic reagents.
Owner:CUSABIO TECH LLC
Preparation method of up-conversion fluorescent material with controllable grain diameter
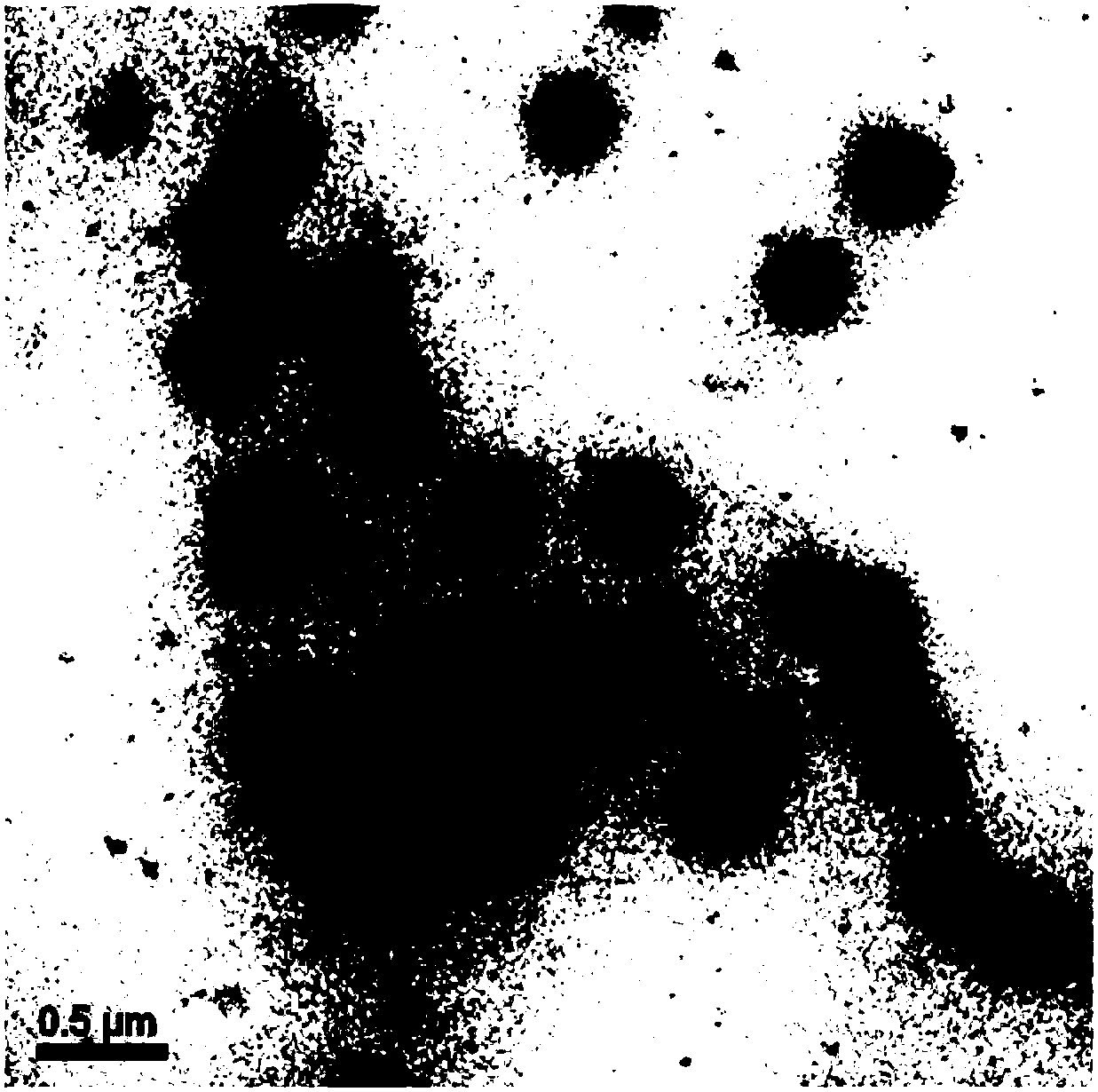
PendingCN108441219AImprove solubilityLarge particle sizeNanotechnologyLuminescent compositionsFluorescenceRare earth
The invention discloses a preparation method of an up-conversion fluorescent material with a controllable grain diameter. The preparation method comprises the following steps: step 1, adding rare earth chloride or a rare earth acetic acid compound into a container; then adding oleic acid and heating to obtain a clarified and transparent rare earth-oleic acid precursor; adding sodium hydroxide andammonium fluoride into an organic solvent with a low boiling point so as to obtain a clarified and transparent sodium fluoride precursor; step 2, adding octadecene into the rare earth-oleic acid precursor in step 1 and heating; step 3, cooling the rare earth-oleic acid precursor in step 2; adding the sodium fluoride precursor into the rare earth-oleic acid precursor; raising the temperature of a reactant under the protection of argon gas and carrying out heat insulation and reaction; after the reaction is finished, cooling a reaction mother solution; then centrifuging to precipitate a productin the solution; then washing and precipitating the washed product; dissolving the product in the organic solvent and storing or drying and storing.
Owner:康永生物技术有限公司
Rabies virus rapid antibody quantitative detection card and application method thereof
The invention discloses a rabies virus rapid antibody quantitative detection card and an application method thereof. The rabies virus rapid antibody quantitative detection card comprises a detection card housing and a test strip assembled in the detection card housing, wherein the test strip comprises a plastic baseplate with a pressure-sensitive adhesive; a sample pad, a marker pad, a nitrocellulose membrane and absorbent paper are sequentially bonded on the baseplate; the marker pad consists of a carrier base layer and a marker; the marker is a membrane formed by spraying lanthanide fluorescence detection microspheres and lanthanide fluorescence quality control microspheres onto the carrier base layer; the nitrocellulose membrane is coated with a rabies virus recombinant antigen as a detection line and coated with a rabbit anti-chicken IgY antigen as a quality control line; and the marker is fluorescence detection microspheres marked with a rabies virus structural protein G recombinant antigen and fluorescence quality control microspheres marked with a chicken IgY antibody. The rabies virus rapid antibody quantitative detection card can realize field rapid quantitative detectionof a rabies virus antibody and has a high practical value and popularization value.
Owner:杭州微瑞科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com