Method of purifying therapeutic proteins
a technology of therapeutic proteins and purification methods, applied in the field of purification methods of therapeutic proteins, can solve the problems of insufficient blood clotting, risk of haemorrhage, and destabilisation of therapeutic proteins in solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Fibrinogen Through HEA, PPA and MEP Hydrophobic Charge Induction Chromatographic (HCIC) Resin
[0167]Human pooled plasma cryoprecipitate was used as the starting material (i.e., fibrinogen-containing feedstock). Briefly, the pooled plasma cryoprecipitate was solubilised in an extraction buffer containing 20 mM Tri-sodium citrate, 200 mM epsilon-amino caproic acid (ε-ACA), 60 IU / mL heparin and 500 mM NaCl (pH 7.2±2) at 31±2° C. for 30 minutes (1 g cryoprecipitate per 4 g of buffer). Aluminium hydroxide 2% (w / w) was then added to the solubilised cryoprecipitate at a concentration of 25% (w / w). After which the aluminium hydroxide gel was removed by either centrifugation or depth filtration and the fibrinogen-containing supernatant was recovered for further chromatographic purification through a HCIC chromatographic resin.
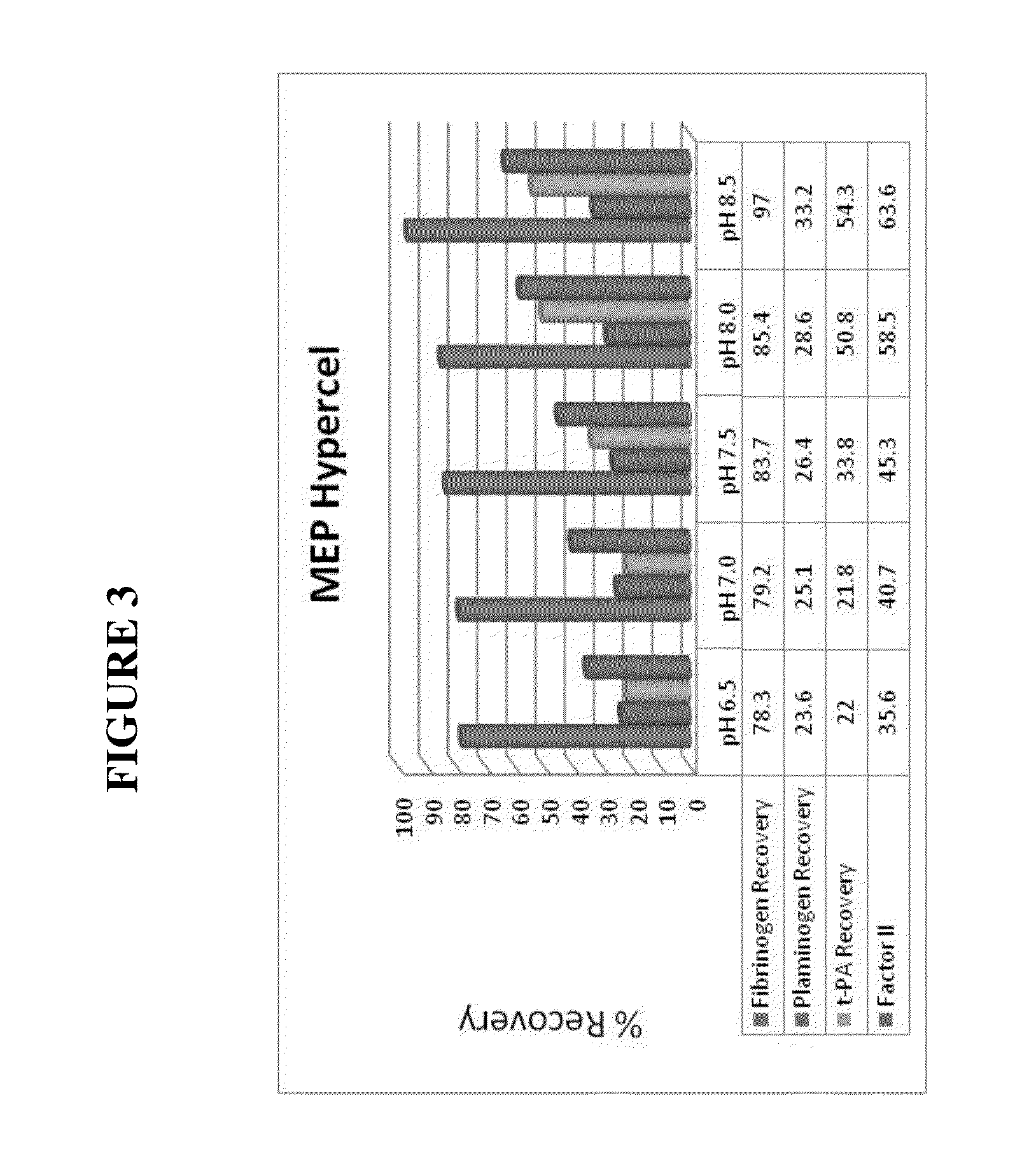
[0168]The fibrinogen-containing supernatant was applied to chromatography columns that were packed with 1.8 mL of either HEA, PPA or MEP Hypercel resin. ...
example 2
Level of Impurities in a Fibrinogen Solution Reduced Through HEA Hypercel
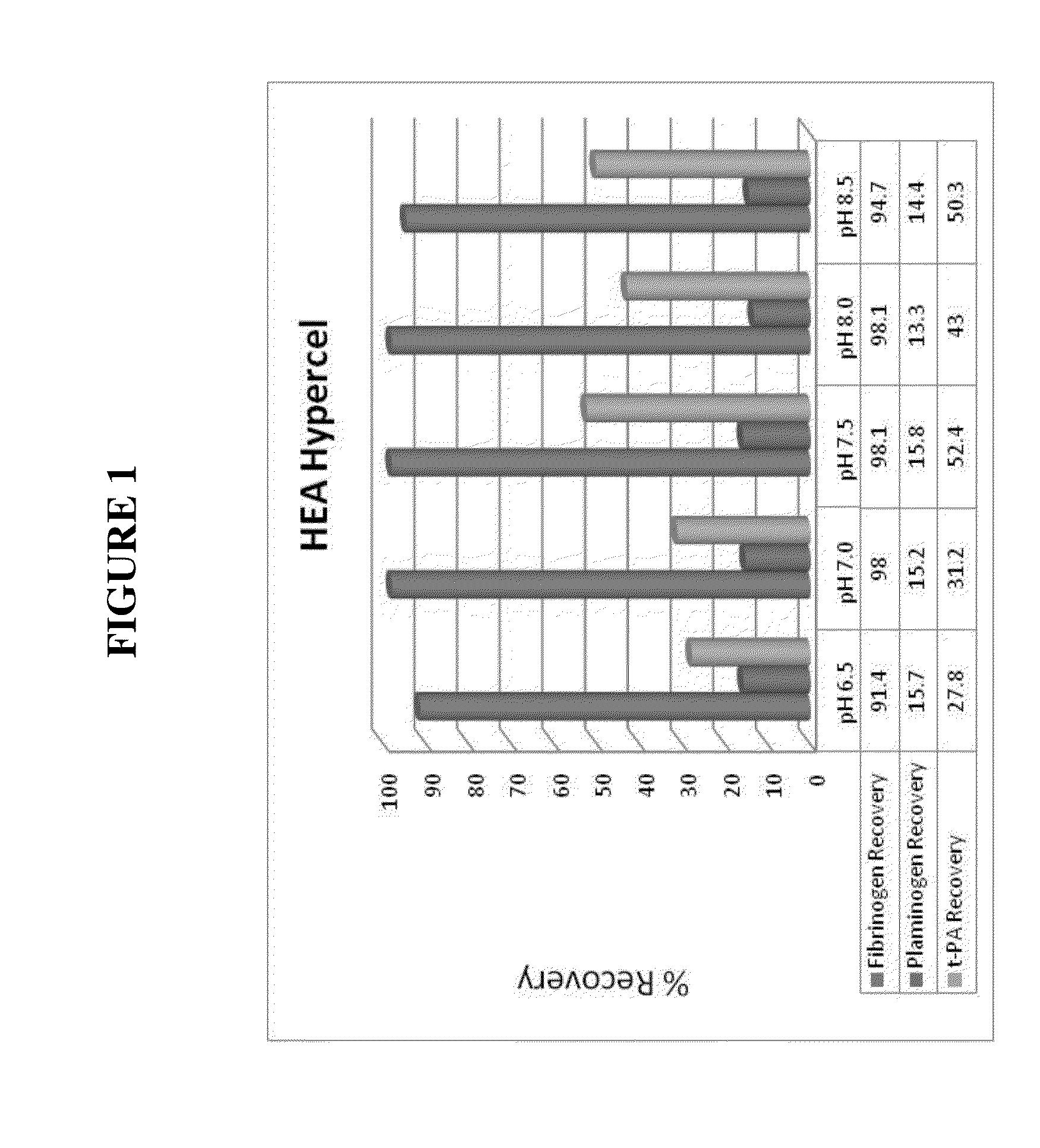
[0170]Approximately 48.5 mL of fibrinogen containing solubilised cryoprecipitate prepared according to Example 1 was applied onto a 5 mL HEA Hypercel column which was pre-equilibrated in 25 mM Tris at either pH 6.5, 7.0, 7.5, 8.0 or 8.5. HCIC purification was performed in negative mode with respect to fibrinogen, in which fibrinogen was allowed to drop-through in the unbound flow-through fraction, whilst t-PA, plasminogen and Factor II remained bound to the resin. FIG. 4a shows step recovery of fibrinogen, plasminogen, t-PA and Factor II during post-chromatographic purification using HEA Hypercel. The results show that pH had little or no effect on the binding of plasminogen and Factor II to the HCIC resin, whereas the binding of t-PA to the resin appeared to be most effective at the lower pH range of 6.5-7.0. Recovery of fibrinogen was greater than 90% in the drop-through fraction under different pH conditions...
example 3
Level of Impurities in a Fibrinogen Solution Purified Through HEA Hypercel
[0172]Approximately 500 mL of the fibrinogen-containing supernatant obtained post alhydrogel adsorption step generated in accordance with Example 1 was loaded onto an XK 16 / 30 column packed with 36 mL of HEA Hypercel resin, pre-equilibrated in 25 mM Tris pH 7.0. The drop-through fraction was collected for fibrinogen, plasminogen, t-PA, and Factor II testing. A summary of the results is provided in Table 1 below.
TABLE 1FibrinogenVolumeProteinby ClaussPlasminogent-PAFactor II(mL)(mg / mL)(mg / mL)(ng / mL)(ρg / mL)(U / mL)Fibrinogen50021.816.053172.01716.50.00097supernatantDrop-through54017.613.215655.5706.70.00025fraction% recovery in87.2%89.1%31.8%44.5%27.8%drop-through fraction
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com