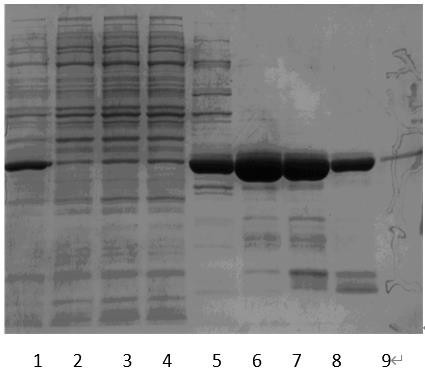
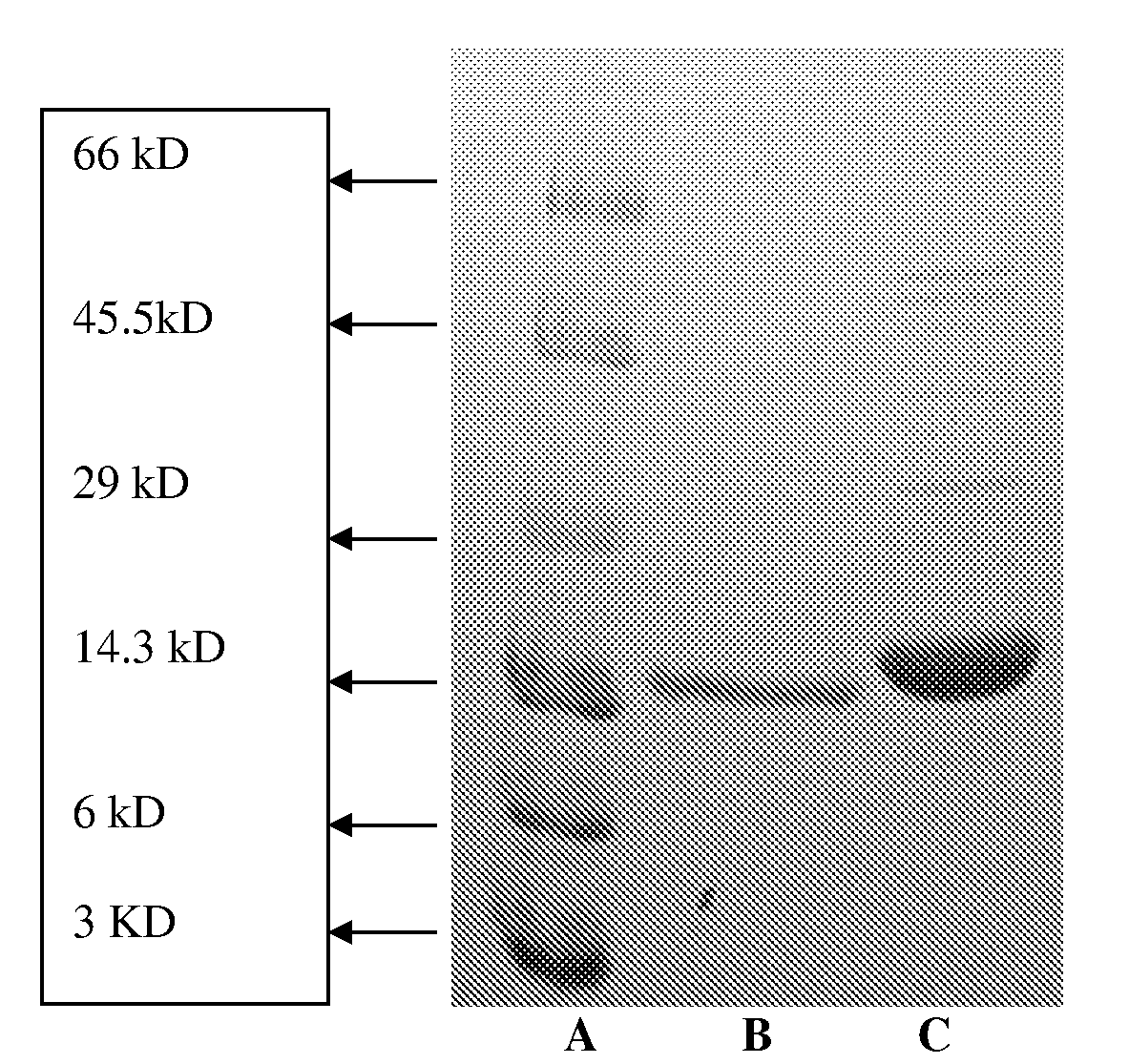
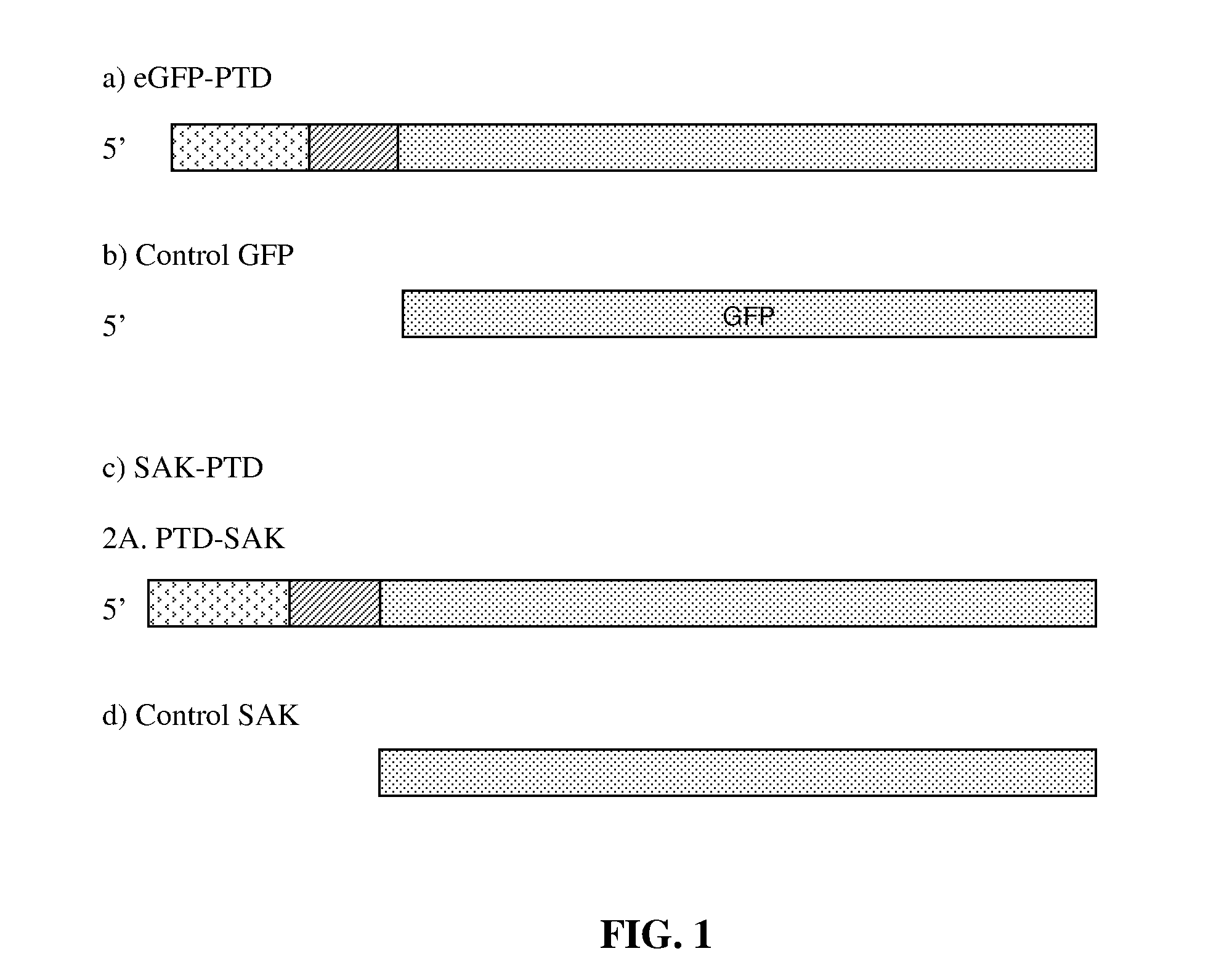
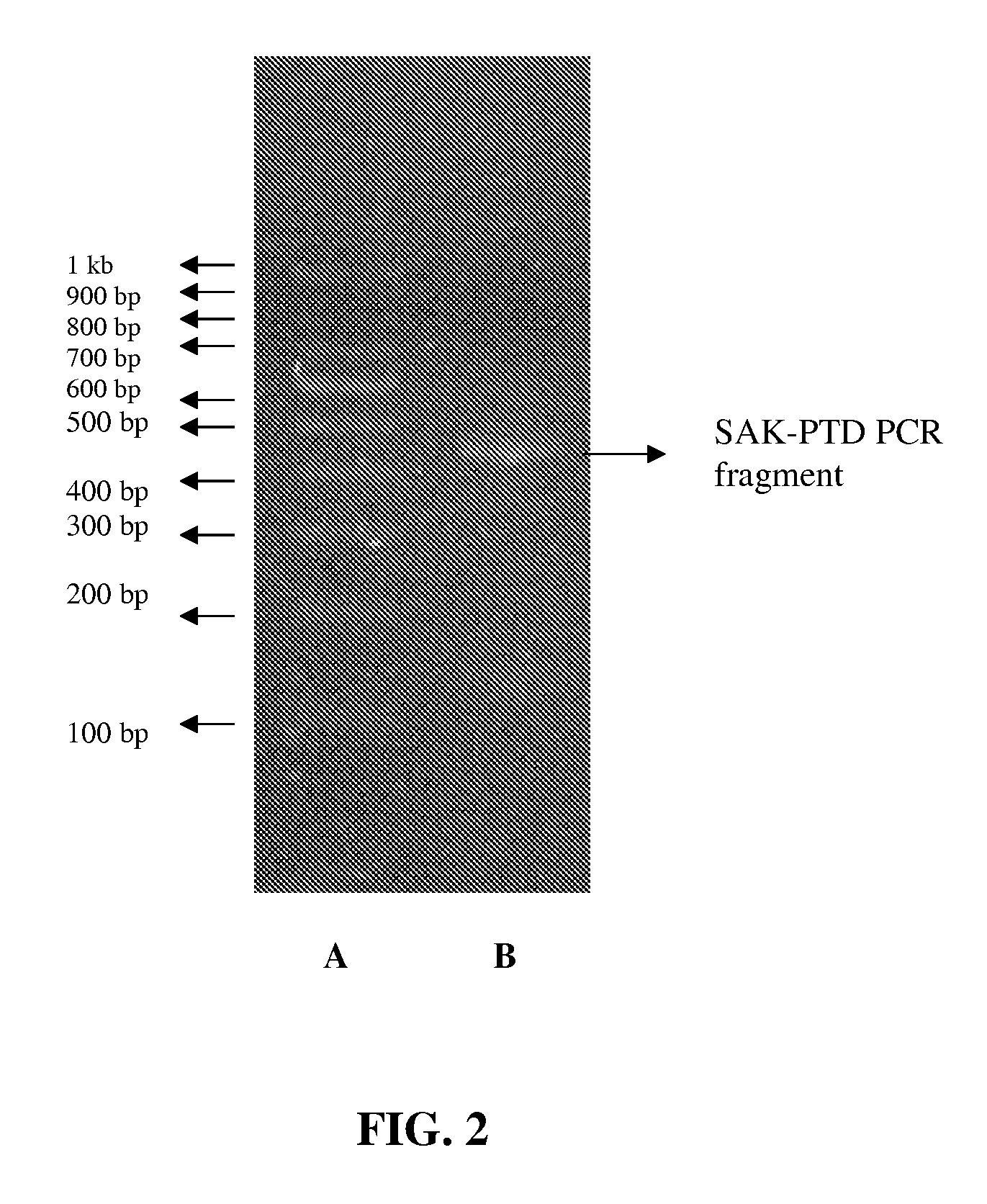
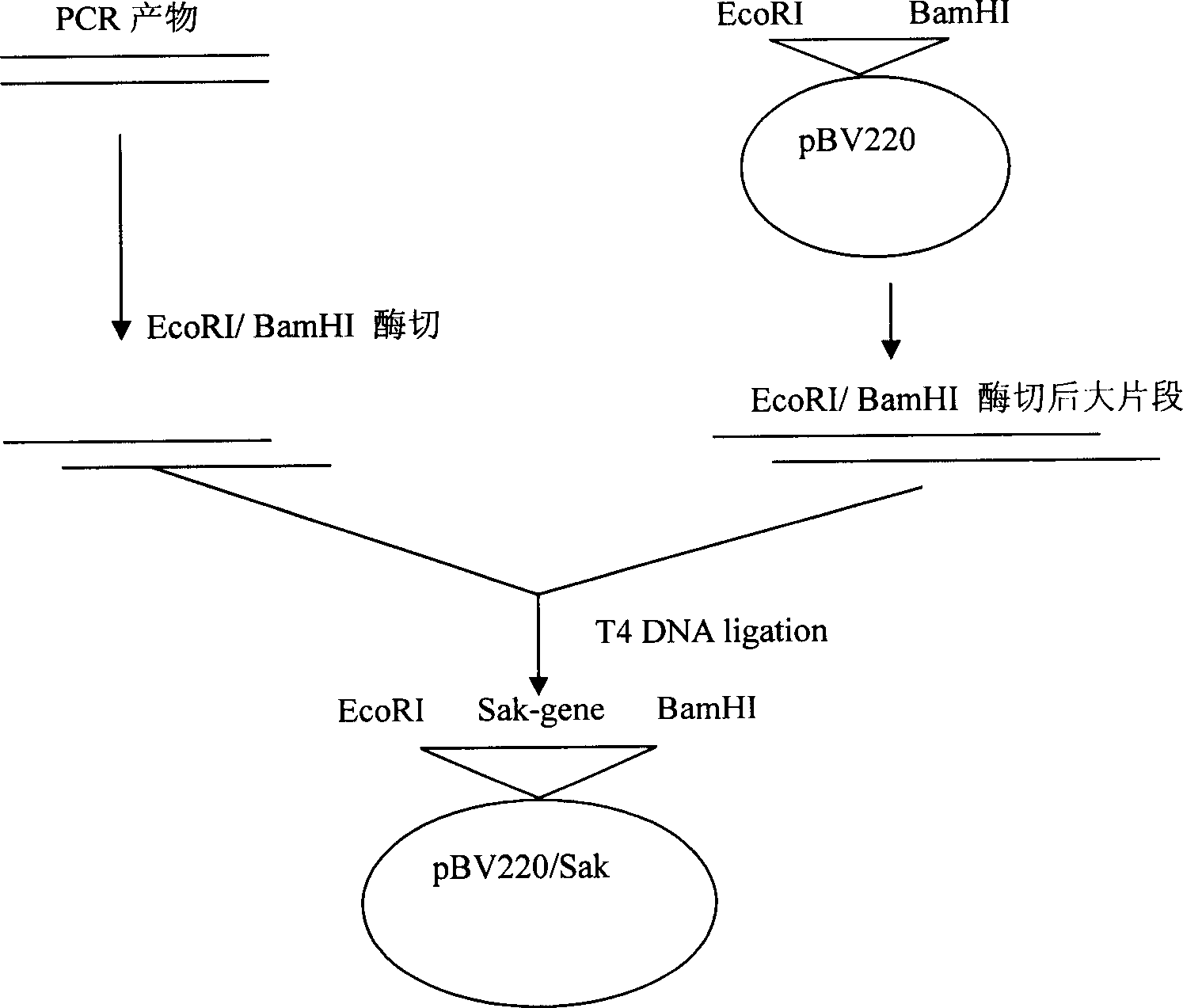
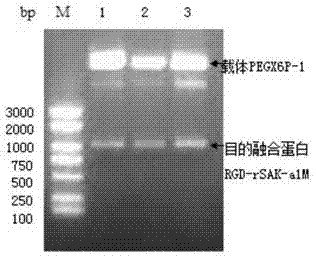
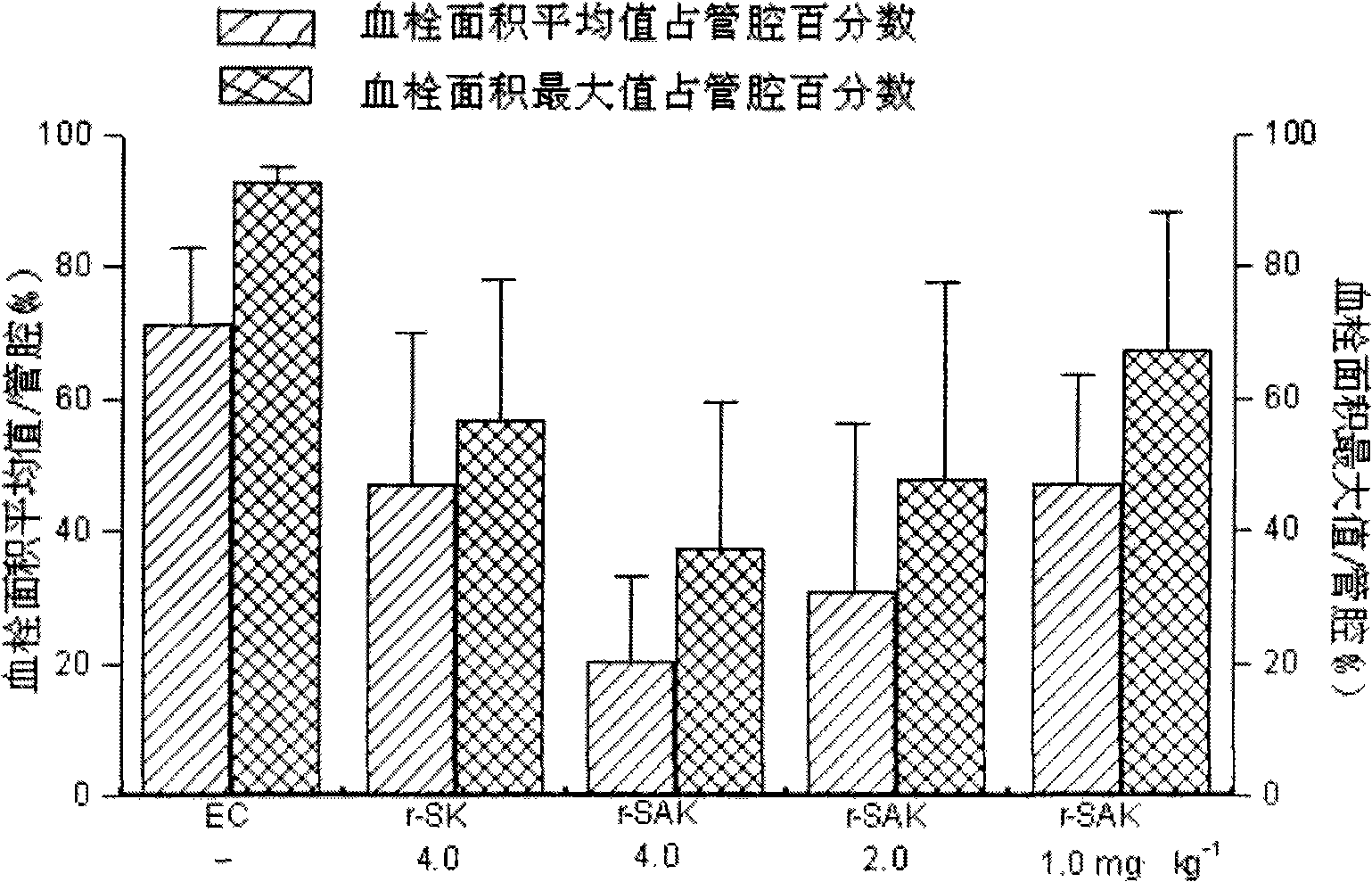
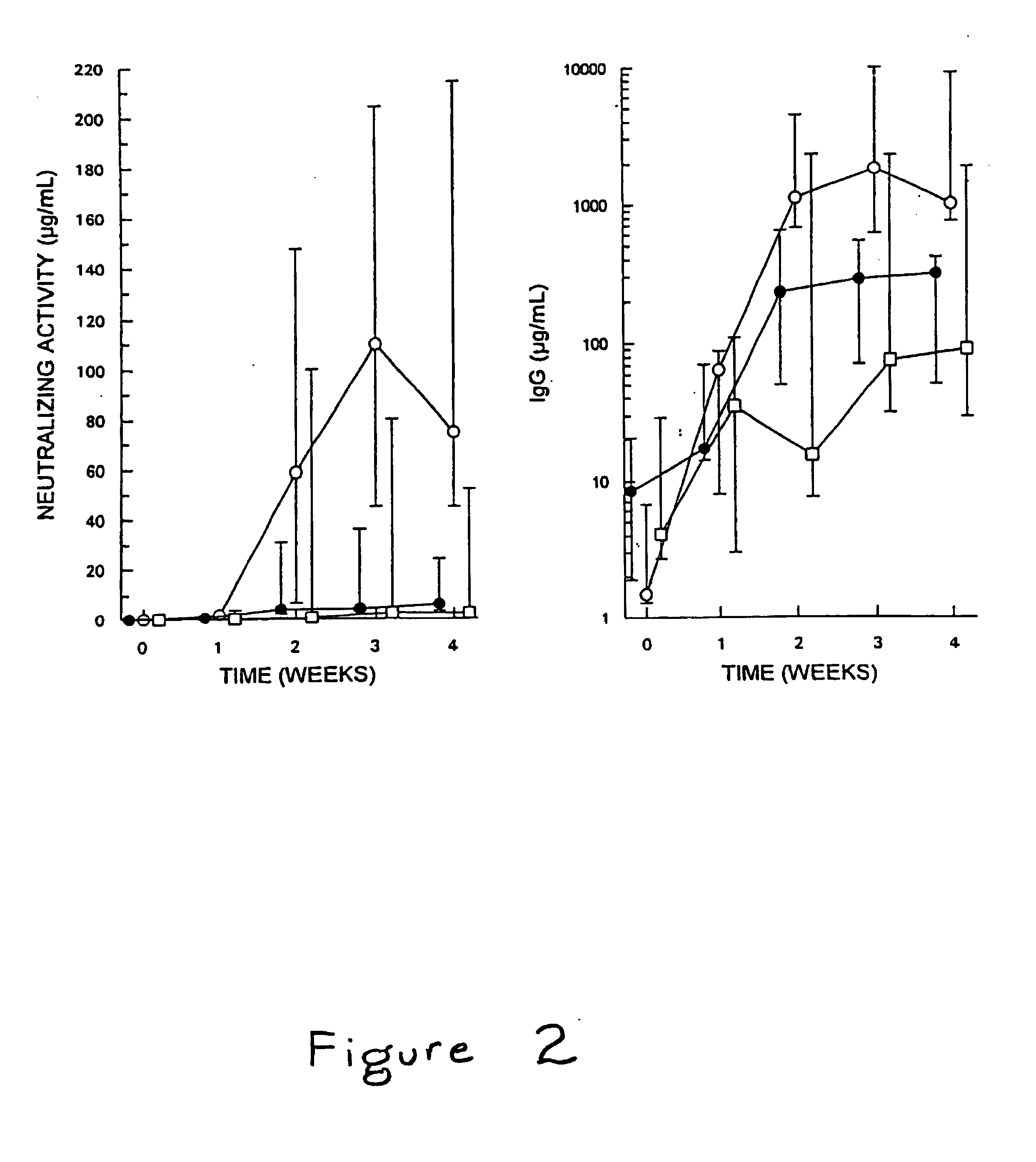
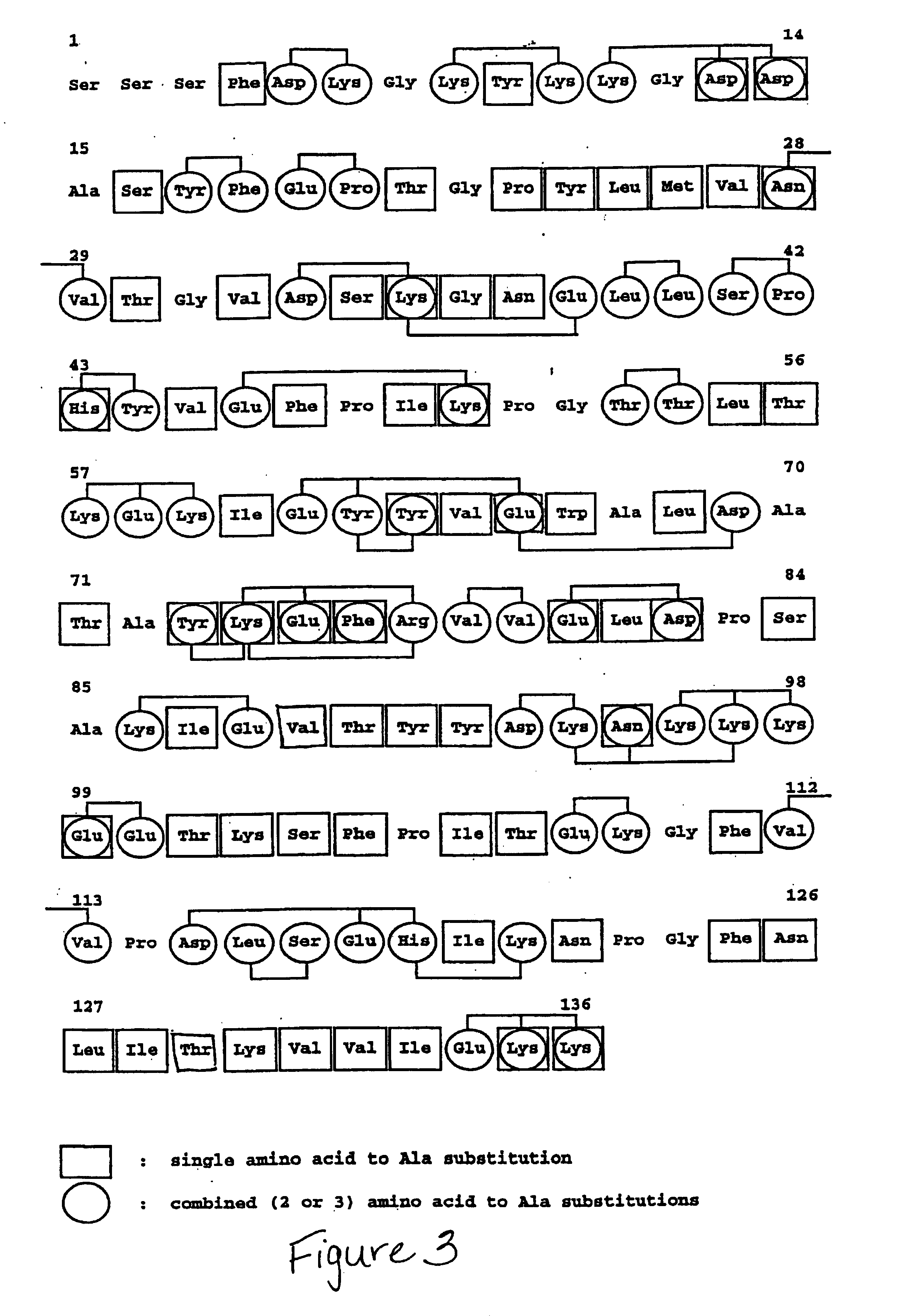
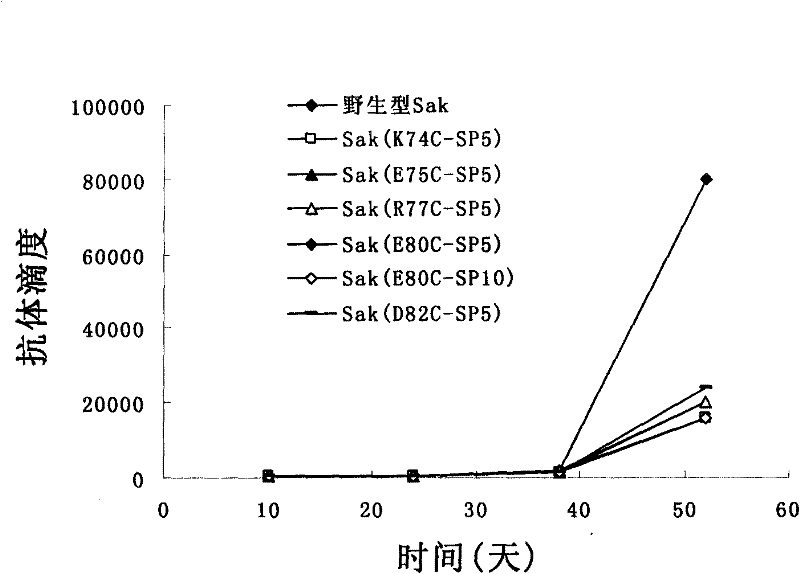
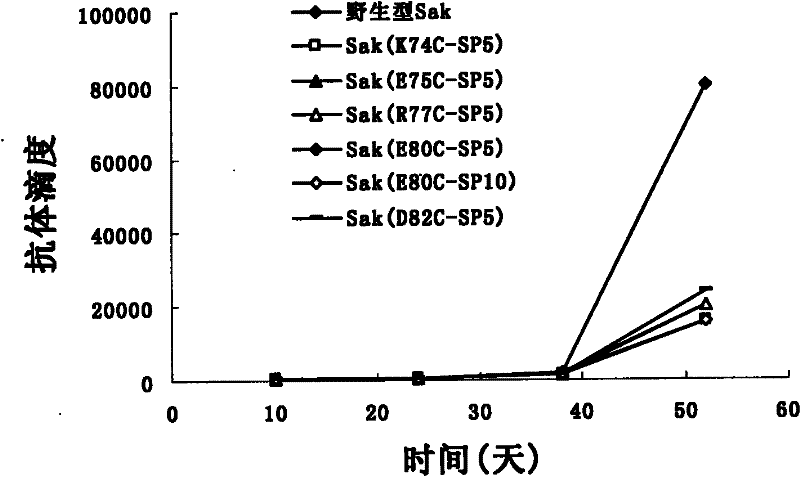
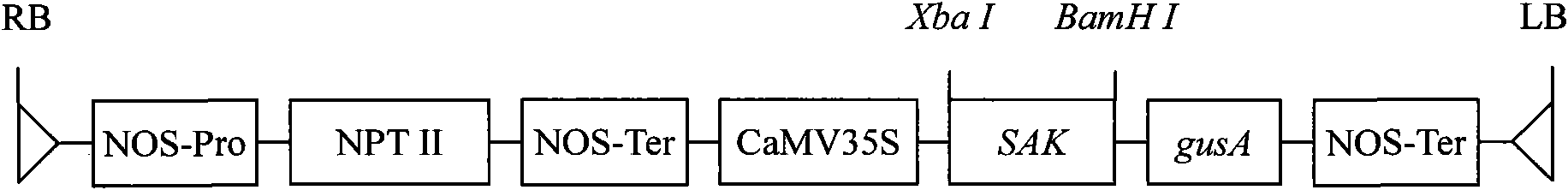
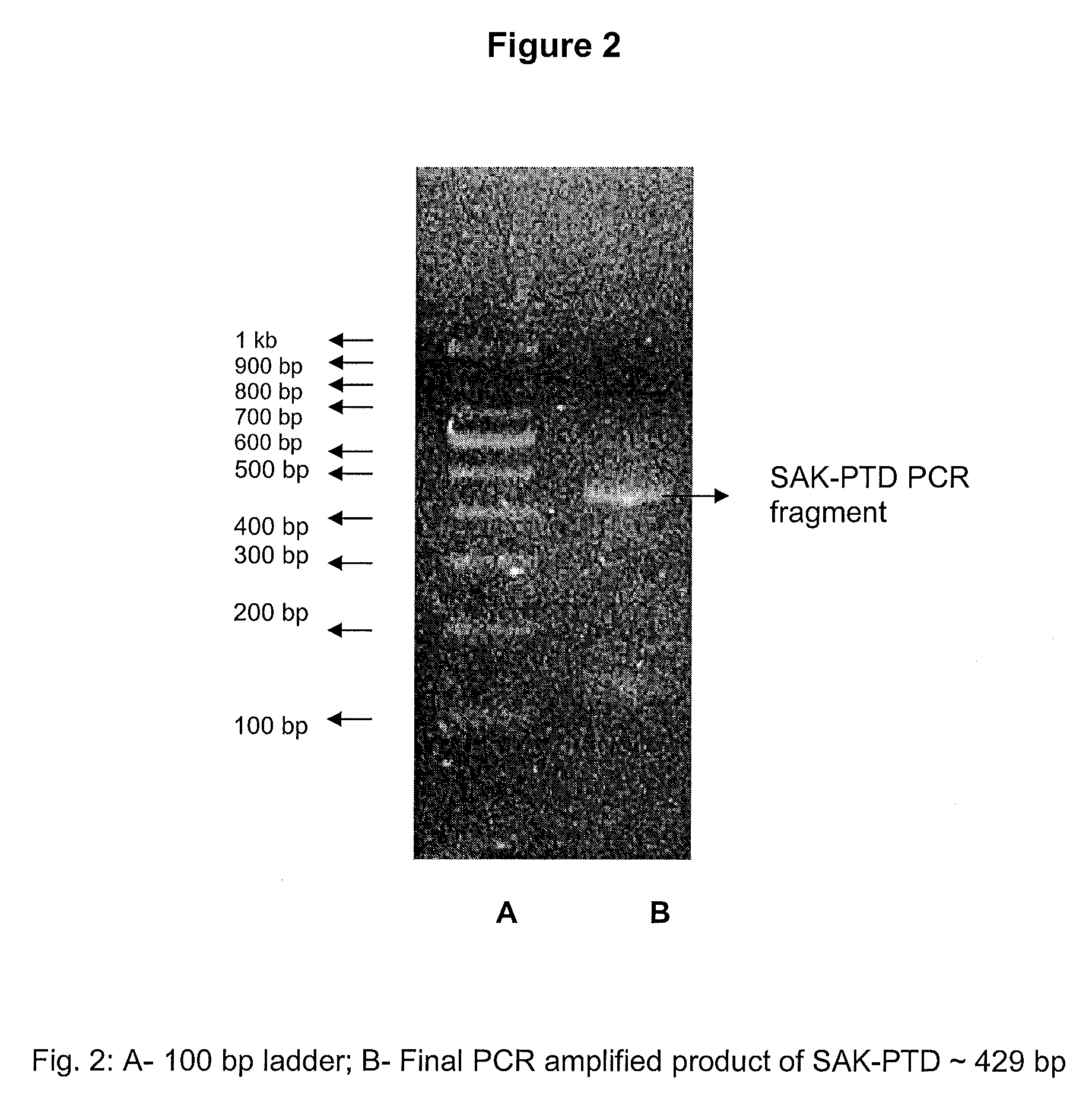
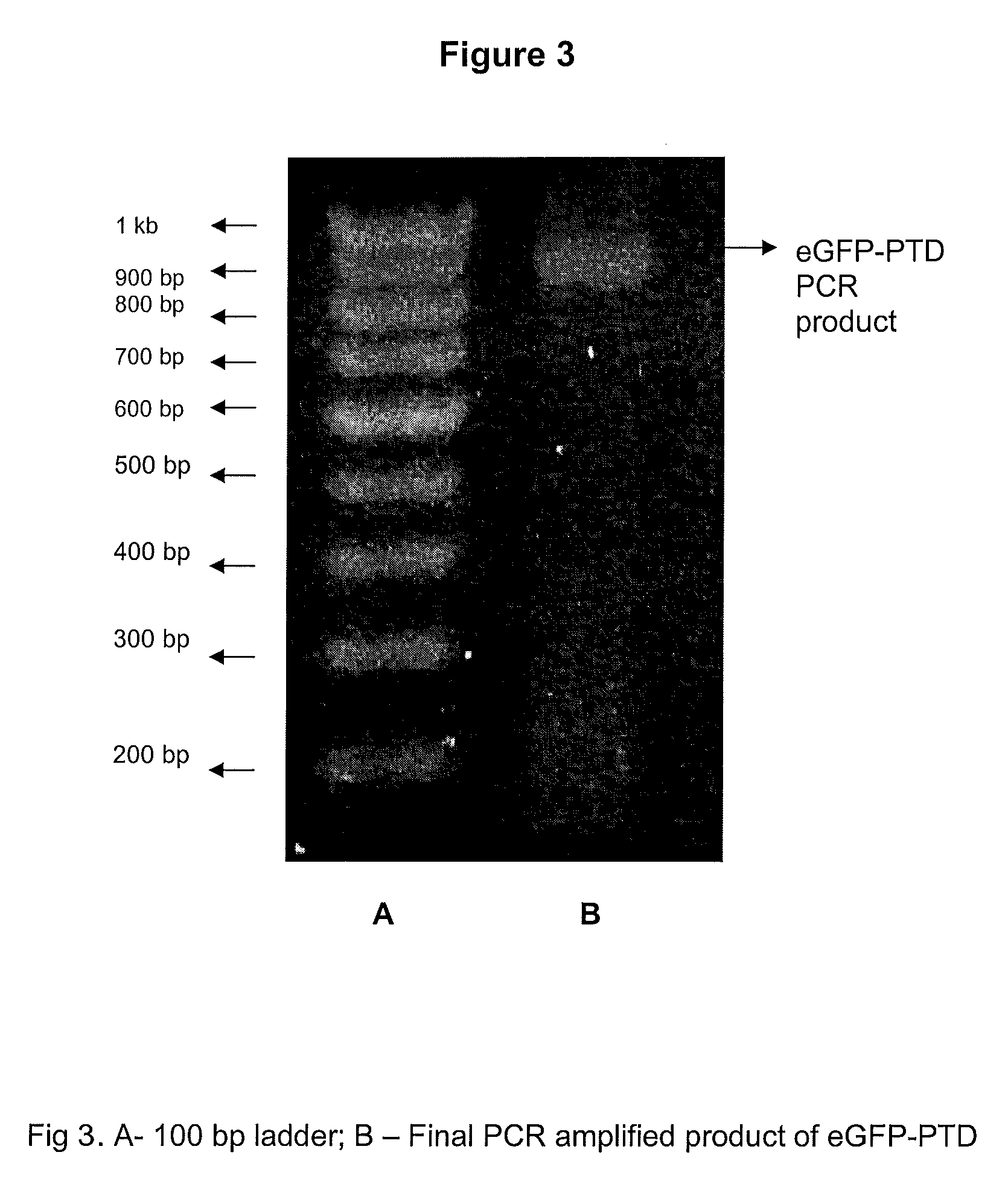
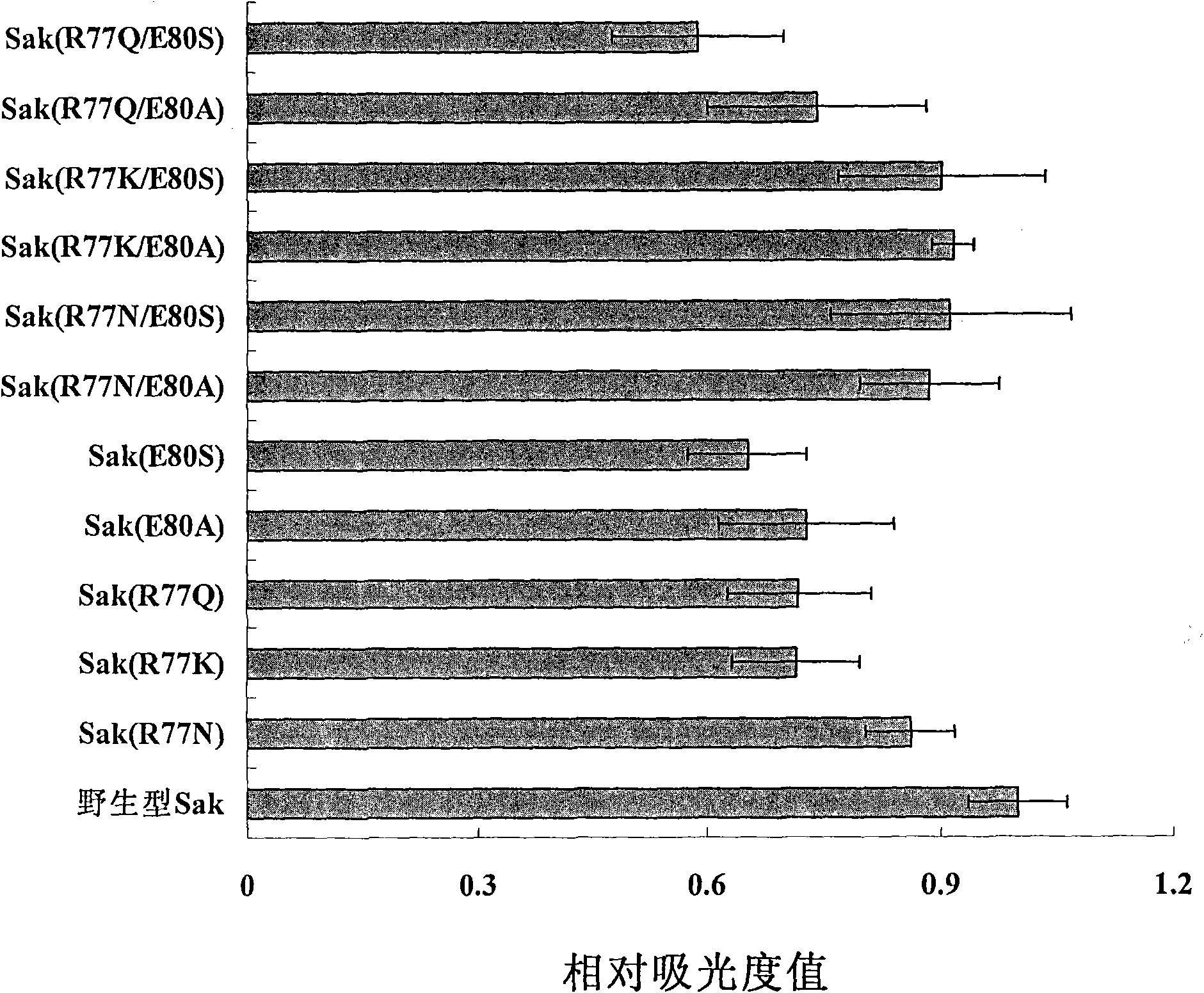
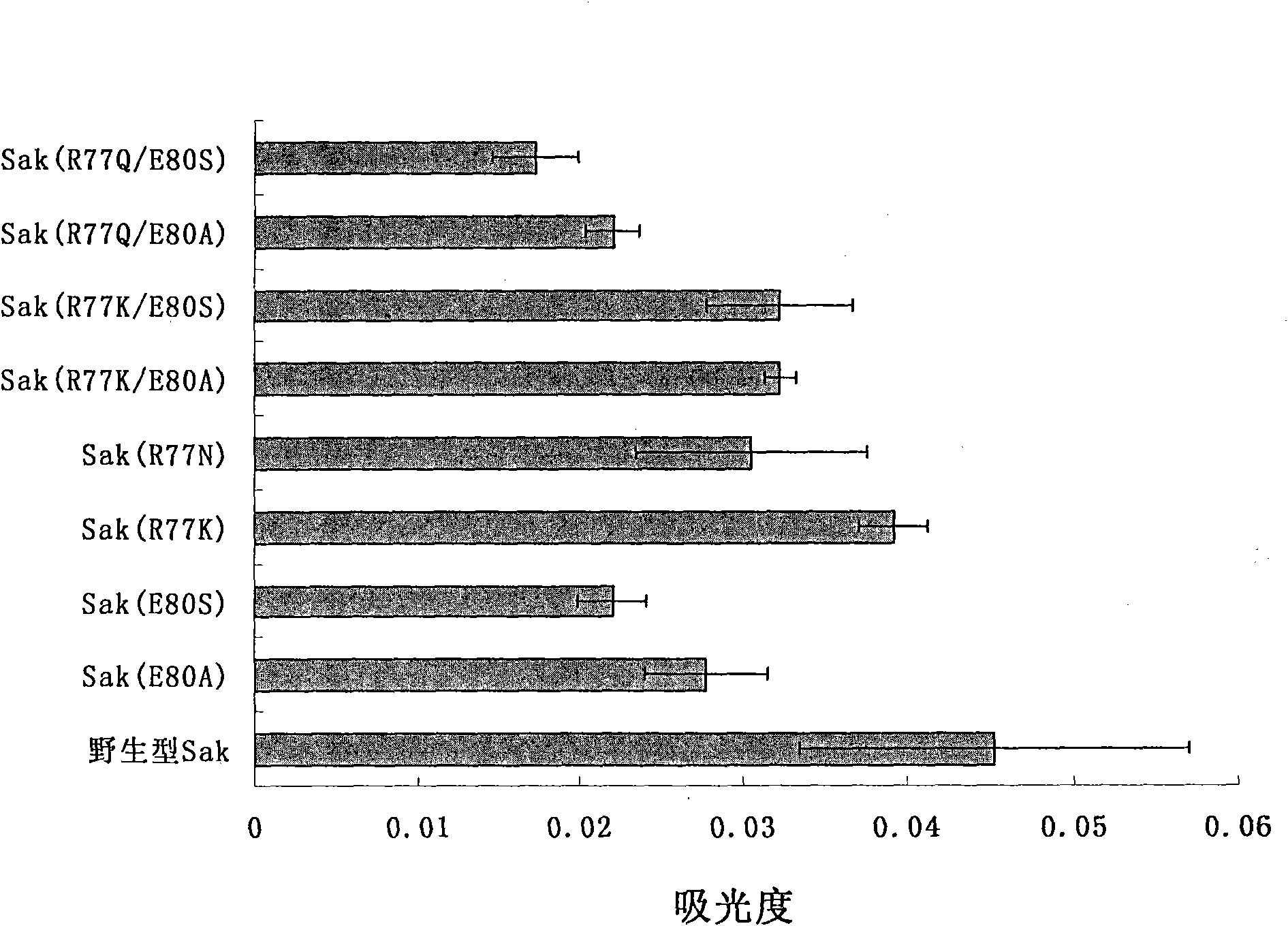
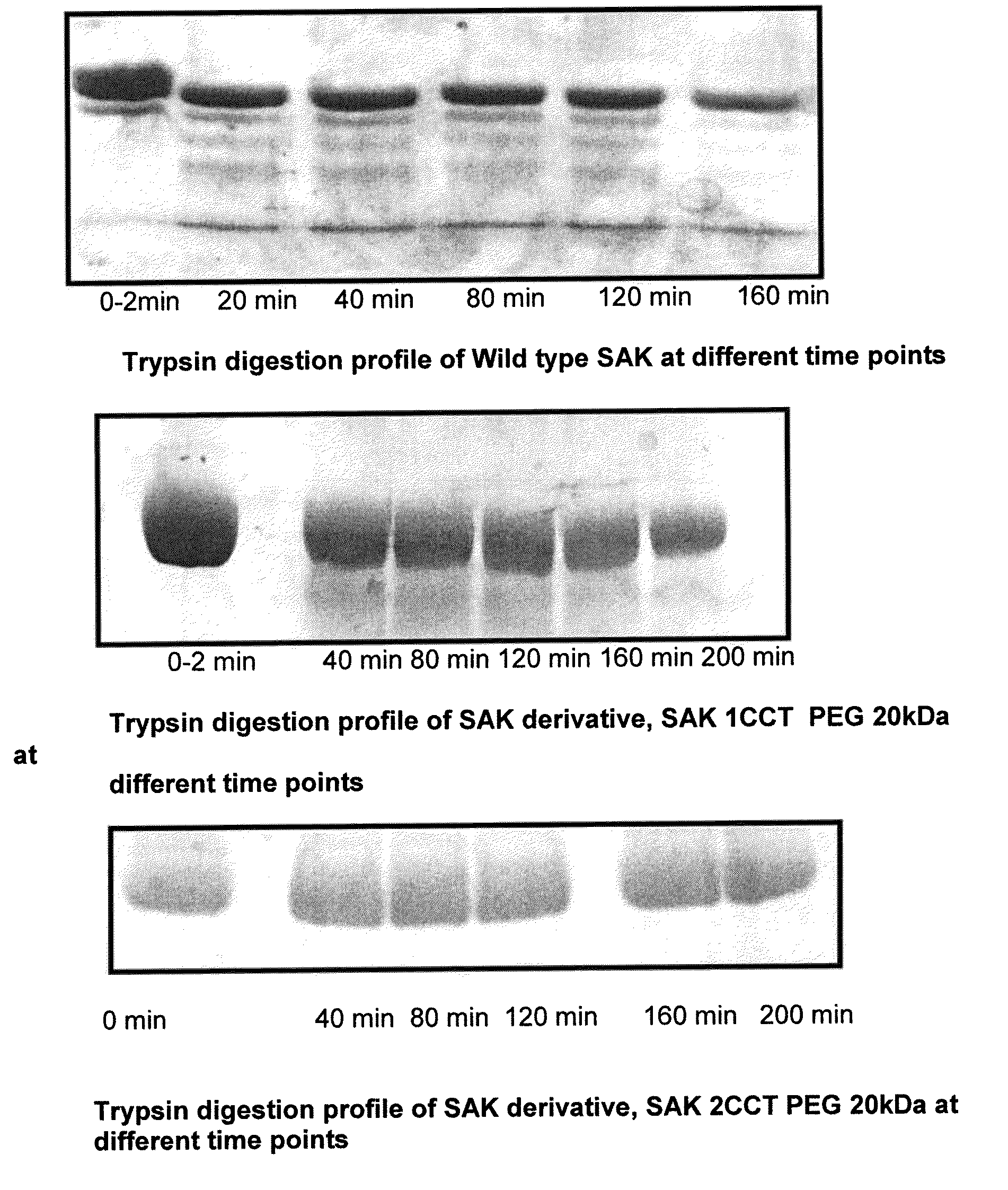
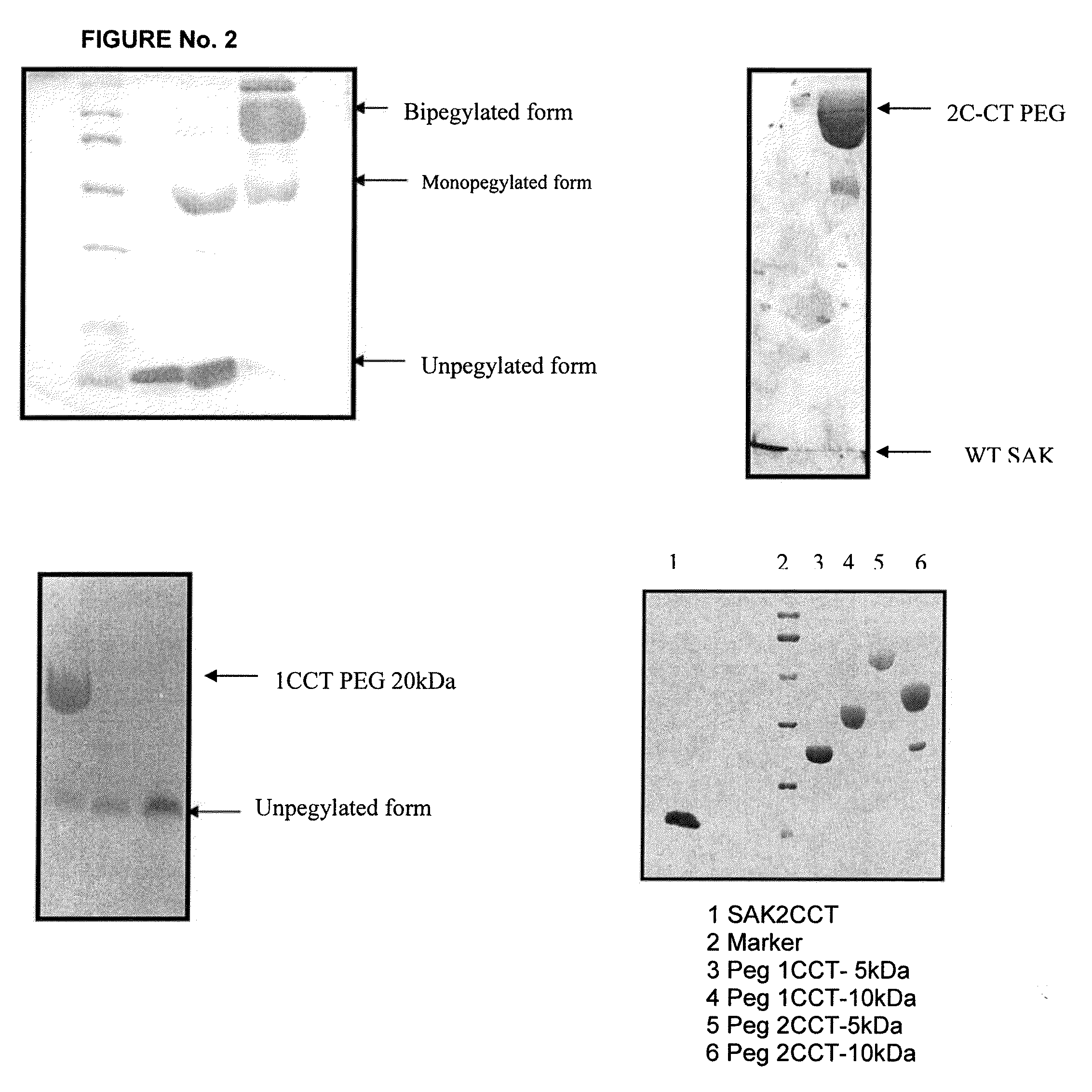
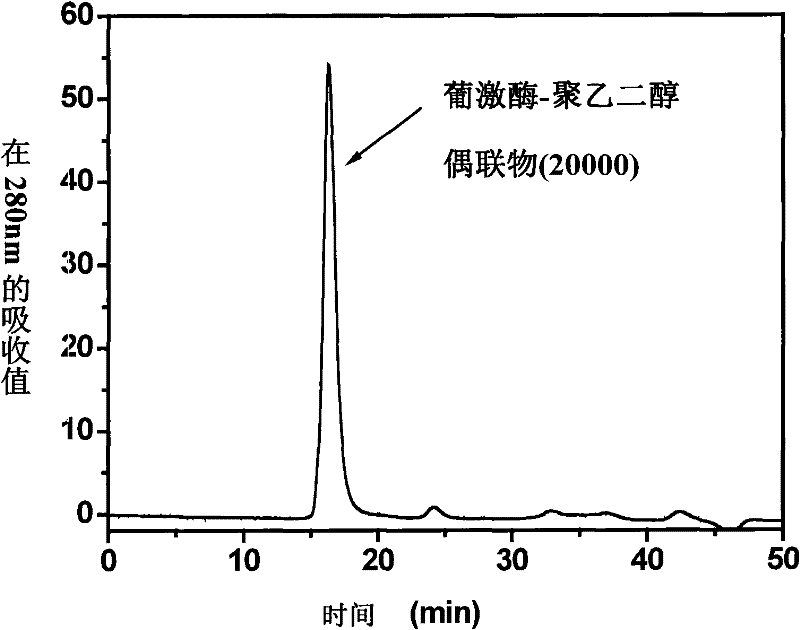
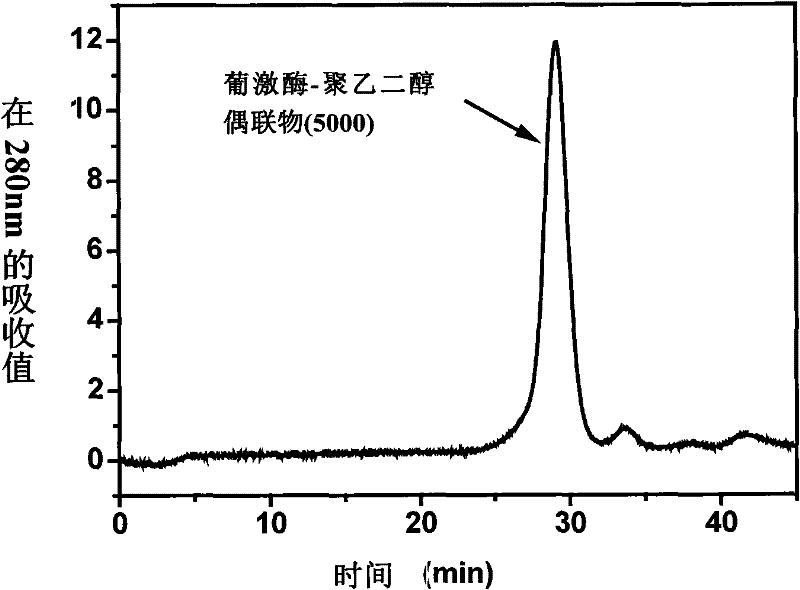
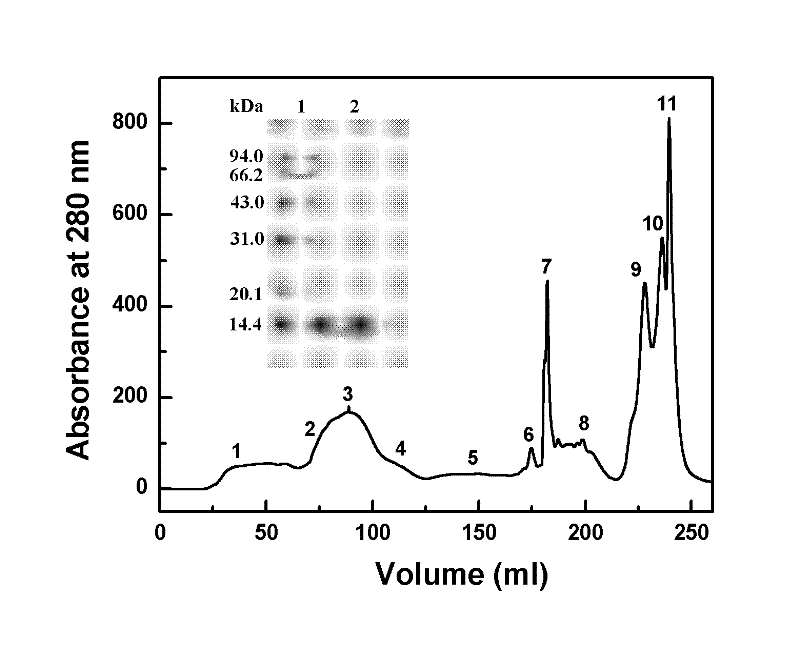
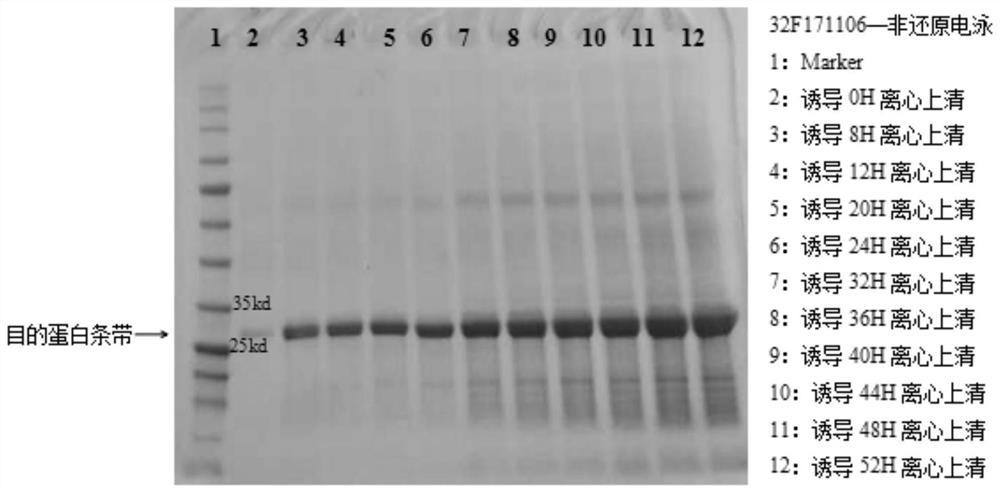
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Staphylokinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Staphylokinase (SAK; also known as staphylococcal fibrinolysin or Müller's factor) is a protein produced by Staphylococcus aureus. It contains 136 amino acid residues and has a molecular mass of 15kDa. Synthesis of staphylokinase occurs in late exponential phase. It is similar to streptokinase.
Reformed lyophylization preparation of recombinant staphylokinase (r-Sak), its preparing method and application
InactiveCN1446912AReduce manufacturing costBacteriaPeptide/protein ingredientsFreeze-dryingPhosphate
A freeze-dried preparation of recombinant glucokinase in the form free dried powder, injection, lipoplasm, or microcapsule for thrombolytic purpose contains the glucokinase with mutation of amino acids at positions 7, 3 and 43, and one or more of mannitol, phosphate, EDTA and sodium chloride. Its preparing process is also disclosed.
Owner:BEIJING YILING BIOENG
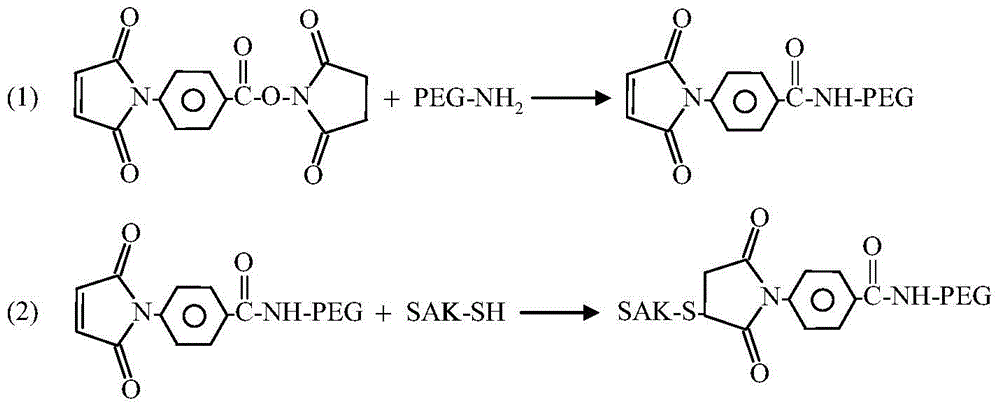
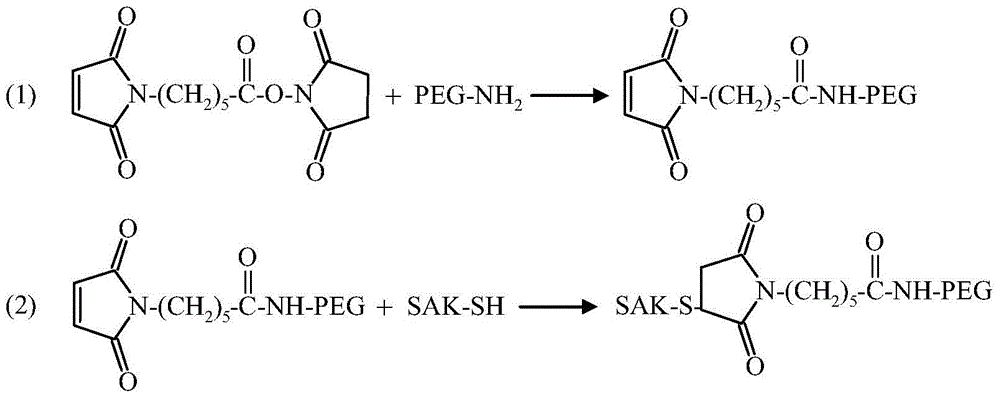
Method used for selective modification of protein carbon terminal carboxyl groups with polyethylene glycol
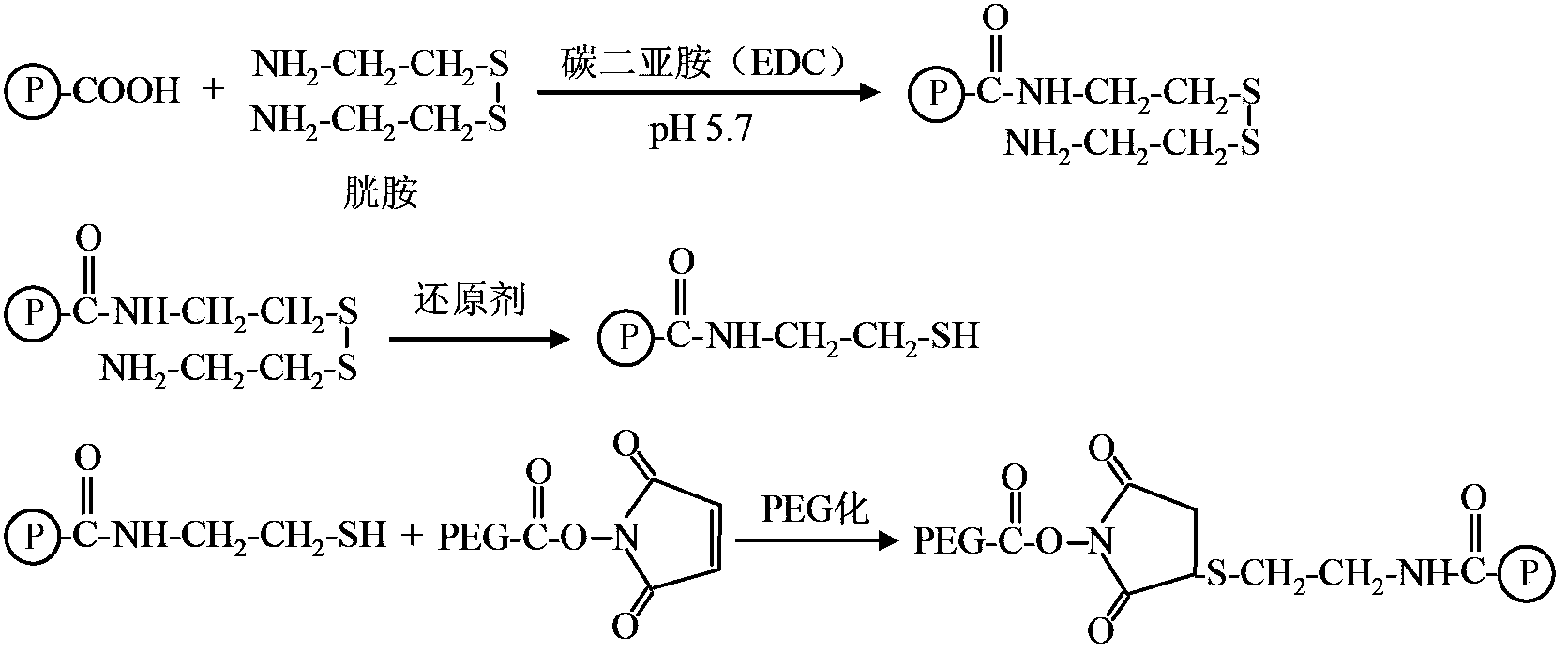
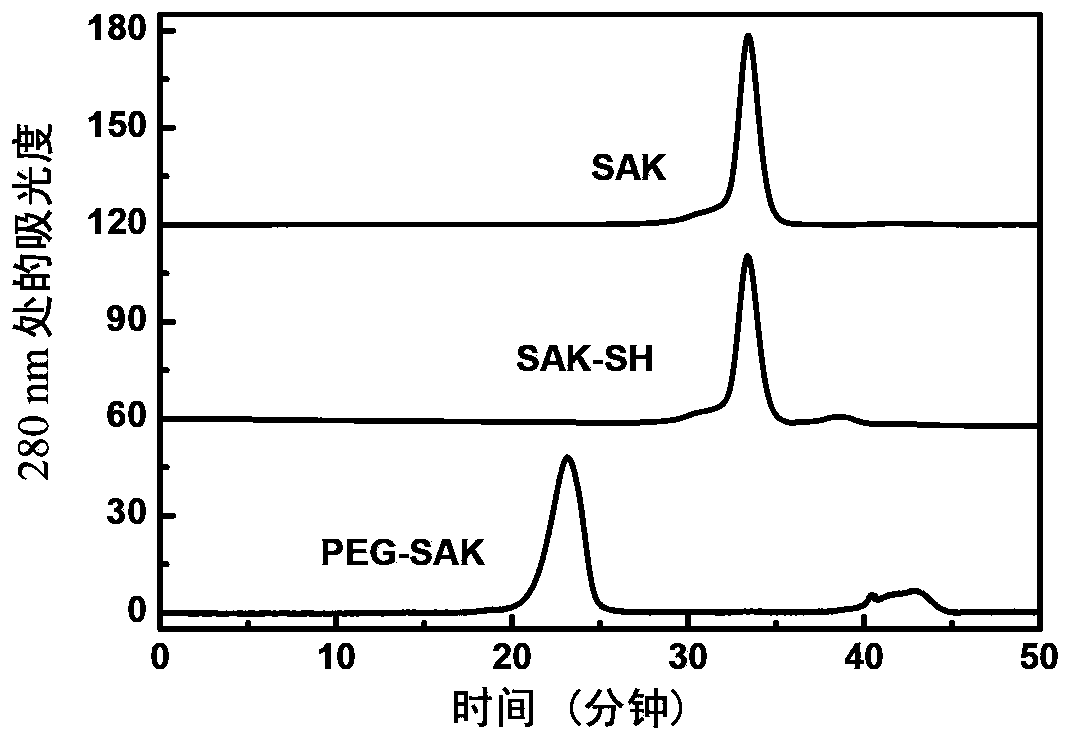
The invention relates to a polyethylene glycol (PEG) modification method. The method is capable of realizing selective modification of carbon terminal carboxyl groups of proteins and polypeptides, and each protein molecule and polypeptide molecule is connected with one PEG molecule. According to the method, staphylokinase is used as a model protein. The polyethylene glycol (PEG) modification method comprises following main steps: (1) under slightly acidic conditions (pH 5.0), carboxyl groups of staphylokinase and amino groups of cystamine are connected; and (2) reduction of disulfide bonds of cystamine is realized using a reducing agent, and generated sulfhydryl groups are reacted with methoxy polyethylene glycol-maleinimide (mPEG-Mal). pKa value of the carbon terminal carboxyl groups of staphylokinase is 2.1 to 2.4, pKa value of side chain carboxyl groups of aspartic acid is 3.7 to 4.0, and pKa value of side chain carboxyl groups of glutamic acid is 4.2 to 4.5, so that under the conditions with a pH value of 5.0, PEG is capable of realizing selective modification of the carbon terminal carboxyl groups of staphylokinase. The method is mainly used for PEG selective modification of the carbon terminal carboxyl groups of the proteins and the polypeptides; a novel PEG modification method is provided; and an advantage of the method is that uniformity of modification sites of the PEG modification product is excellent.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Purification and preparation process of recombinant staphylokinase mutant
InactiveCN113416722AIncrease ionic strengthChange the state of adsorptionBacteriaHydrolasesSpecific adsorptionProtein target
The invention provides a purification and preparation process of a recombinant staphylokinase mutant. The purification and preparation process comprises the following steps of: step 1, homogenizing engineering bacteria and treating homogenate; step 2, capturing recombinant protein; and step 3, refining by ion-exchange chromatography. The capturing of the recombinant protein in the step 2 comprises the following steps of: loading an engineering bacterium cell homogenate treatment solution to a nickel affinity column, eluting a target protein by changing the concentration of imidazole, and simultaneously adding 0.3 mol / L NaCl to reduce non-specific adsorption caused by the charge effect. The method is good in repeatability and high in yield, staphylokinase with the purity close to 100% can be obtained, and the method is suitable for large-scale production in the pharmaceutical industry.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD +1
Novel thrombolytic molecules and a process therefor
ActiveUS20100015123A1Promote absorptionDissolve thrombusPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein moleculesCerebrovascular disorder
New thrombolytic protein molecules such as recombinant staphylokinase or streptokinase, urokinase, tissue plasminogen activator and the like, and suitable variants thereof, for targeting to brain tissue or any other tissue by either fusing to, or by synthesizing the candidate thrombolytic molecule(s) with a protein sequence comprising a strong amphipathic alpha helix containing protein transduction domain. Thrombolytic protein molecule(s) so engineered with the protein transduction domain is useful for enhanced uptake of such protein thrombolytic molecule(s) across the cell membranes and tissues including the blood brain barrier and find their use in the treatment of vascular thrombosis including cerebrovascular disorders caused by cerebral thrombosis or cerebral haemorrhage when used a as a therapeutic. The design and processes for cloning, expression, purification and protein transduction of such proteins across cell membranes.
Owner:BHARAT BIOTECH INTERNATIONAL
Low pyrogen staphylokinase and its preparation method
This invention belongs to the biology project pharmacy filed, which provides a Staphylokinase (SAK) with low pyrogen and it's preparing method. The Staphylokinase has low pyrogen, high purify and strong activity, which is completely fit for the medicinal demand. This invention includes the following steps: a breaking up the material containing with the Staphylokinase and distilling the floating liquid; b. sieving the product from step a with the DEAD gelatin rob, then removing salt and condensing; c. sieving the product form step b with CM gelatin rob, collecting the sample apex, condensing the washing liquid and transforming the medium; d. sieving the product from step c with the Q gelatin rob to obtain the Staphylokinase. The Staphylokinase of this invention has good bolt dissolving effect, and it is safe and efficient for users..
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
RGD-recombinatn staphylokinase-human alpha microglobulin fusion protein, and preparation method and application thereof
InactiveCN104845949AMaintain thrombolytic activityLittle changePeptide/protein ingredientsGenetic material ingredientsEscherichia coliVaccine Immunogenicity
The invention provides an RGD-recombinatn staphylokinase-human alpha microglobulin fusion protein, and a preparation method and an application thereof. The amino acid sequence of the RGD-recombinatn staphylokinase-human alpha microglobulin fusion protein is represented by SEQ ID NO.12. The preparation method of the fusion protein comprises the following steps: obtaining a target gene fusion fragment through adopting an overlap extension PCR technology; inserting the target gene fusion fragment into an expression vector to construct a recombinant expression plasmid; transforming the constructed recombinant expression plasmid into Escherichia coli, and allowing the recombinant expression plasmid to highly express in the Escherichia coli; and carrying out fusion protein separation and purification. Compared with present products of same kind, the fusion protein has the advantages of efficient thrombolysis and anti-coagulating activity and low immunogenicity. The fusion protein also can overcome the disadvantages of activity decrease and insoluble expression of some present RGD-recombinant staphylokinases.
Owner:CHONGQING MEDICAL UNIVERSITY
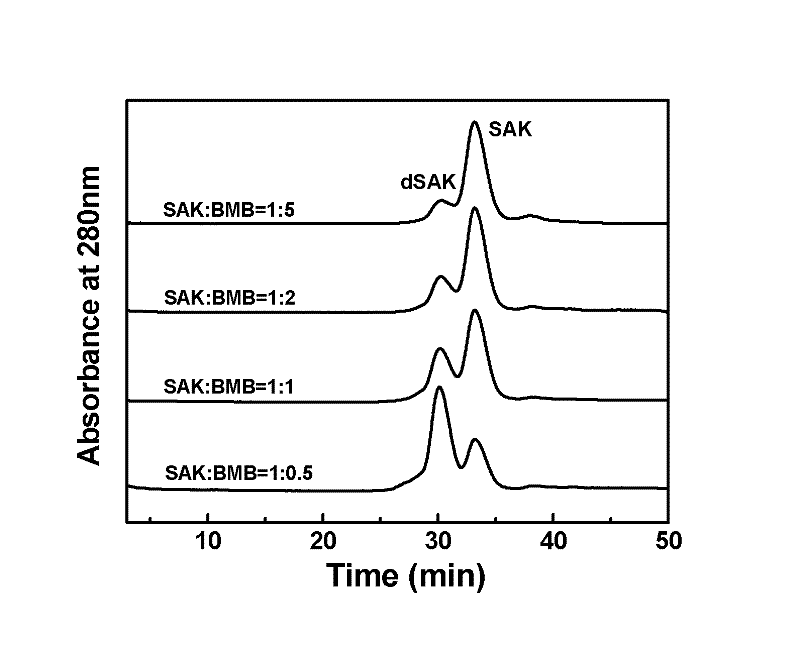
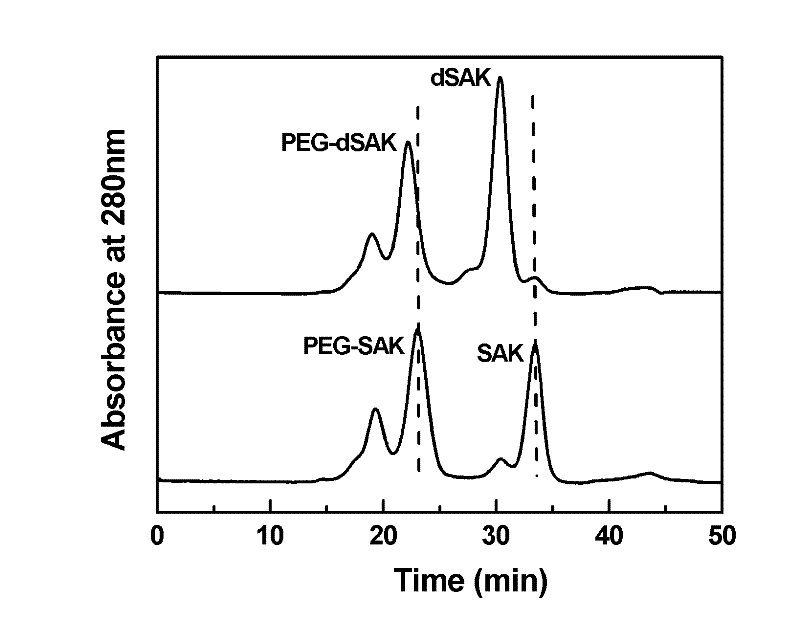
Preparation method of recombinant staphylokinase dimer and site-specific modificaton of polyethylene glycol
The invention discloses a staphylokinase mutant, wherein cysteine is introduced to the C-end of the staphylokinase mutant. Compared with the wild type staphylokinase, the C-end of the mutant has three more amino acids, namely glycine-glycine-cysteine (Gly-Gly-Cys). The invention also discloses a preparation method of the dimer of the staphylokinase mutant. The dimerization method of the staphylokinase is that the two maleimide groups of the homotype bifunctional binder 1,4-dimaleimide butane and the cysteine at the C-end of the staphylokinase are utilized to form a stable covalent bond. The invention also discloses a preparation method for realizing the N-end site-specific modificaton of polyethylene glycol by using the dimer of the staphylokinase mutant. Under special conditions, methoxy polyethylene glycol-propionaldehyde is connected with one N-end of the staphylokinase dimer molecule through the covalent bond. The biological activity of the polyethylene glycol modified product of the staphylokinase dimer is higher than that of the modified product of the staphylokinase monomer.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
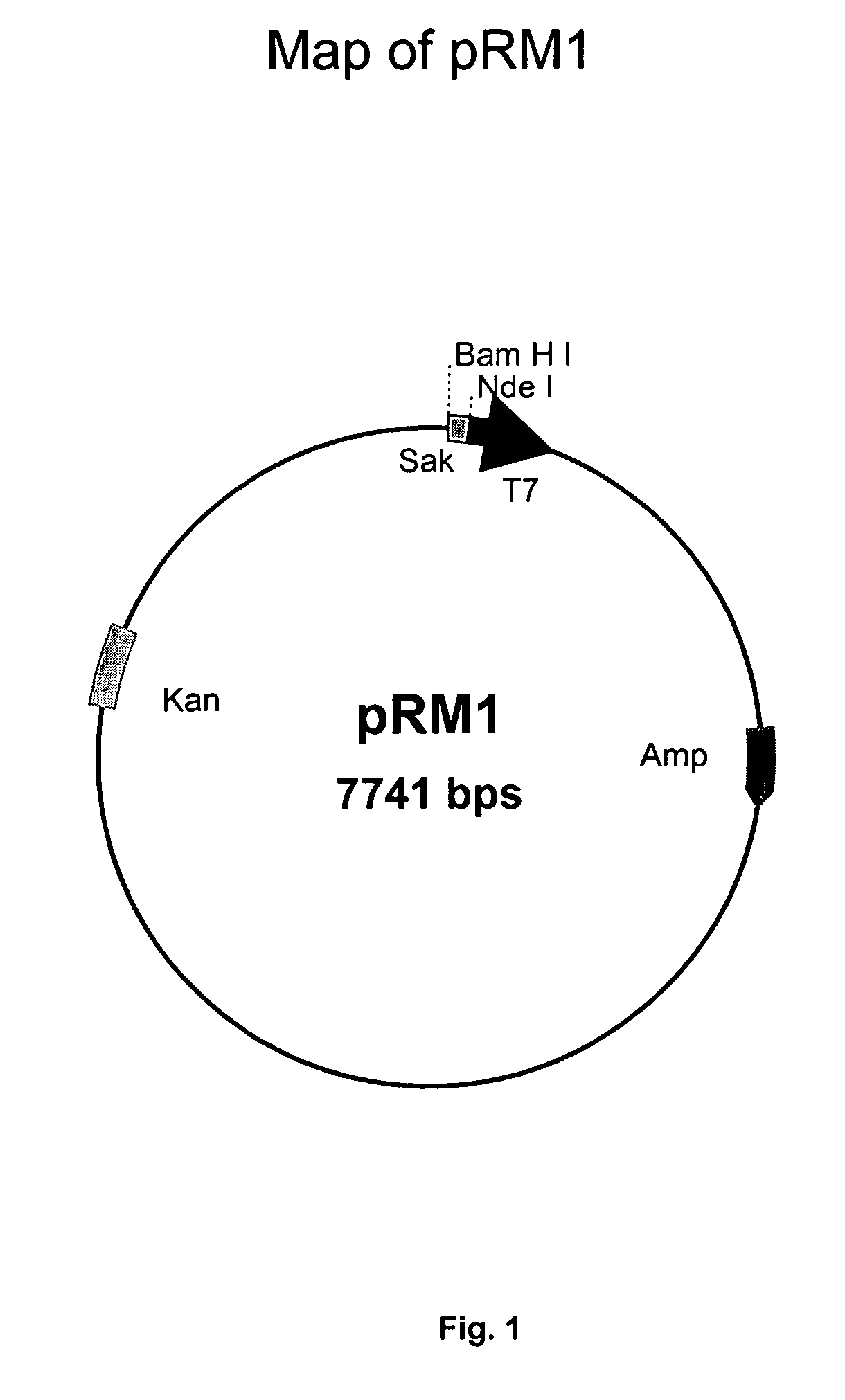
Staphylokinase and expression vector thereof
ActiveCN101314769AStrong thrombolytic effectExcellent thrombolytic activityBacteriaPeptide/protein ingredientsDiseaseTotal protein
The invention discloses a staphylokinase and an expression vector thereof. The staphylokinase is polypeptide with amino acid sequence shown in SEQ ID No: 1, and the gene coding sequence thereof is shown in SEQ ID No: 2. The invention also discloses an expression vector containing a staphylokinase gene. The thrombolysis of the staphylokinase has strong effect, so that the staphylokinase can be used for preparing medicine for remedying cardio-cerebrovascular diseases, in particular to medicine for remedying myocardial infarction and other thrombotic diseases; the thrombolysis activity of the staphylokinase is high, and the activity rate thereof is 4.0*10<4>AU / mg; in addition, the expression amount of the expression vector of the aphylokinase is high, and the expression amount of the aphylokinase accounts for more than 45 percent of total protein of thallus.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Staphylokinase derivatives with polyethyleneglycol
Methods for the identification, production and use of staphylokinase derivatives characterized by a reduced immunogenicity after administration in patients. The derivatives of the invention are obtained by preparing a DNA fragment comprising at least the part of the coding sequence of staphylokinase that provides for its biological activity; performing in vitro site-directed mutagenesis on the DNA fragment to replace one or more codons for wild-type amino acids by a codon for another amino acid; cloning the mutated DNA fragment in a suitable vector; transforming or transfecting a suitable host cell with the vector; culturing the host cell under conditions suitable for expressing the DNA fragment; and purifying the expressed staphylokinase derivative to homogeneity. Preferably the DNA fragment is a 453 bp EcoRI-HindIII fragment of the plasmid pMEX602sakB, (pMEX.SakSTAR), the in vitro site-directed mutagenesis is performed by spliced overlap extension polymerase chain reaction and the mutated DNA fragment is expressed in E. coli strain TG1 or WK6. The invention also relates to pharmaceutical compositions comprising at least one of the staphylokinase derivatives according to the invention together with a suitable excipient, for treatment of arterial thrombosis.
Owner:DESIRE JOSE COLLEN +1
Pegylated staphylokinase mutant, and preparation method and application thereof
InactiveCN102212512ALow immunogenicityFibrinolytic activity retentionBacteriaPeptide/protein ingredientsEscherichia coliHalf-life
The invention discloses a pegylated staphylokinase mutant. The pegylated staphylokinase mutant is prepared by the following steps of: performing site directed mutagenesis on any one of hydrophilic amino acids in the 71st to 87th site amino acid sequences of wild type staphylokinase; substituting cysteine for the hydrophilic amino acid; performing high-efficiency expression in escherichia coli; purifying a staphylokinase mutant by chromatography; and performing site directed modification on the cysteine in the staphylokinase mutant by using methoxy maleimide polyethylene glycol to obtain the pegylated staphylokinase mutant. In the prepared pegylated staphylokinase mutant, the immunogenicity is obviously reduced; in vivo plasma half-life is obviously prolonged; medicament effect is durable; and fibrinolytic activity of dissolving thrombus is basically remained. The pegylated staphylokinase mutant can be used as a thrombolytic therapy medicament for a thromboembolic disease.
Owner:HEBEI NORMAL UNIV
Method for culturing staphylokinase transgenic tomato
InactiveCN101914569AHas thrombolytic activityHydrolasesMicrobiological testing/measurementStaphylokinaseExpression vector
The invention discloses a method for culturing a staphylokinase (SAK) transgenic tomato, comprising the following steps: (1) building a recombinant plant expression vector containing an SAK encoding gene; and (2) converting a tomato plant into the SAK transgenic tomato by the built recombinant plant expression vector. The method of the invention can help successfully obtain the SAK transgenic tomato plant; and a solusphere method proves that SAK protein expressed by the transgenic plant has thrombolytic activity, thus laying the foundation for large-scale production of SAK by taking the tomato as a bioreactor.
Owner:BEIJING FORESTRY UNIVERSITY
Chimeric fusion proteins
ActiveUS8968728B2Promote absorptionDissolve thrombusPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein moleculesCerebrovascular disorder
Owner:BHARAT BIOTECH INTERNATIONAL
Low-immunogenicity staphylokinase mutant and preparation method and use thereof
InactiveCN102108351ALower levelSimple methodPeptide/protein ingredientsMicroorganism based processesEscherichia coliDisease
The invention discloses a protein structure of a low-immunogenicity recombinant staphylokinase mutant and a preparation method thereof. The invention relates to the molecular structure of the low-immunogenicity recombinant staphylokinase mutant. The preparation method comprises: directly obtaining the expression plasmid of the recombinant staphylokinase mutant by a polymerase chain reaction (PCR)site-directed mutagenesis technique; transforming Escherichia coli; performing fermentation culture; inducing expression; breaking thalli; centrifuging; collecting supernate; obtaining pure protein by continuous three-step chromatographic purification; and subjecting the pure protein to freeze drying to obtain the product. The obtained staphylokinase mutant has much lower immunogenicity, retains fibrinolysis activity for dissolving thrombi and can be used in preparation of medicaments for treating thromboembolic diseases.
Owner:HEBEI NORMAL UNIV
Mutants of Staphylokinase Carrying Amino and Carboxy-Terminal Extensions for Polyethylene Glycol Conjugation
InactiveUS20100221236A1Improve high temperature stabilityImprove stabilityBacteriaPeptide/protein ingredientsHalf-lifePolyethylene glycol
The present invention relates to the development of new derivatives of a bacterial plasminogen activator, Staphylokinase (SAK), having one or more amino acid residues with single or multiple cysteines at the amino and / or carboxy terminal ends and their conjugation with PEG (Polyethylene Glycol), resulting in new Staphylokinase derivatives that display altered oligomeric states, enhanced thermal and protease stability and extended plasma half-life. Also included is the cloning and expression in a suitable bacterial host; purification of Staphylokinase derivatives to homogeneity and their chemical modification by integrating a PEG molecule to create new biologically active Staphylokinases having higher protein stability and improved in vivo plasma half life, that may enhance the clinical potential of Staphylokinase in thrombolytic therapy for the treatment of cardiovascular diseases.
Owner:SINGH SATISH +1
Glucokinase preparing process
ActiveCN100564522CComply with medicinal requirementsHigh activityEnzymesEscherichia coliFusion Protein Expression
The invention discloses a method for expressing and preparing staphylokinase (Staphylokinase, SAK), comprising: 1. Construction of staphylokinase fusion protein expression engineering bacteria: constructing a recombinant plasmid containing the gene sequence of SEQ NO.1, and introducing the recombinant plasmid into Escherichia coli to obtain fermentation strains. 2. The preparation method includes: a. fermenting and expressing a soluble fusion protein having an amino acid sequence such as SEQ NO.2 by engineering bacteria; b. absorbing the fusion protein in step a with a nickel ion affinity gel column to extract the fusion protein; c. Dilute the fusion protein in step b with enterokinase, dilute appropriately, pass through the gel column in step b, and collect the permeate; d, concentrate the permeate through ultrafiltration in step c, and pass through the Q gel column to obtain the staphylokinase stock solution . The method of the invention has simple and reliable steps, and is especially suitable for large-scale production. The staphylokinase obtained by the method of the invention has high purity, high specific activity and good thrombolytic activity; it provides a new way for producing medicinal staphylokinase.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Polyethyleneglycol-staphylokinase conjugate as well as preparing method and application thereof
InactiveCN102451471AAffect biological activityRetain biological activityPeptide/protein ingredientsPharmaceutical non-active ingredientsMonomethoxypolyethylene glycolHalf-life
The invention relates to the field of biological pharmacy, in particular to a polyethyleneglycol-staphylokinase conjugate as well as a preparing method and application thereof. The conjugate is characterized in that the N end of staphylokinase is connected with a polyethyleneglycol covalence conjugate, wherein the polyethyleneglycol covalence is connected to the N end of the staphylokinase through polyethyleneglycol modifying agents, the polyethyleneglycol modifying agents optimally adopt methoxy polyethyleneglycol aldehyde modifying agents, the molecular weight of the polyethyleneglycol modifying agents is optimally 1000 to 40000, and the polyethyleneglycol modifying agents comprise line type and branching type structures. The polyethyleneglycol-staphylokinase conjugate provided by the invention has the advantages that the in-vivo half-life period can be greatly prolonged, simultaneously, the biological activity of the original protein can also be completely maintained, and the greatapplication potential can be realized in the preparation of cardiovascular and cerebrovascular disease treatment medicine.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of recombinant staphylokinase dimer and site-specific modificaton of polyethylene glycol
The invention discloses a staphylokinase mutant, wherein cysteine is introduced to the C-end of the staphylokinase mutant. Compared with the wild type staphylokinase, the C-end of the mutant has three more amino acids, namely glycine-glycine-cysteine (Gly-Gly-Cys). The invention also discloses a preparation method of the dimer of the staphylokinase mutant. The dimerization method of the staphylokinase is that the two maleimide groups of the homotype bifunctional binder 1,4-dimaleimide butane and the cysteine at the C-end of the staphylokinase are utilized to form a stable covalent bond. The invention also discloses a preparation method for realizing the N-end site-specific modificaton of polyethylene glycol by using the dimer of the staphylokinase mutant. Under special conditions, methoxypolyethylene glycol-propionaldehyde is connected with one N-end of the staphylokinase dimer molecule through the covalent bond. The biological activity of the polyethylene glycol modified product of the staphylokinase dimer is higher than that of the modified product of the staphylokinase monomer.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Injection for treating thrombus
InactiveCN101401932ASpecific thrombolysisImprove solubilityPeptide/protein ingredientsBlood disorderDiseaseClinical efficacy
The invention relates to an injection liquid for the treatment of thrombosis, which consists of radix salviae miltiorrhizae injection, chymotrypsin, and staphylokinase, wherein each 5 milliliters of the injection liquid contains 1.8 to 2.5 milliliters of the radix salviae miltiorrhizae injection, 0.8 to 1.2 milligrams of the chymotrypsin, and 80,000 to 150, 000 IU of the staphylokinase. The injection liquid is mainly used for the treatments of thrombosis and vascular occlusion of heart, brain and every part of a whole body, and the main adaptation diseases are cardio-cerebral ischemia, myocardial infarction, cerebral infarction, pulmonary infarction, venous thrombosis, arterial thrombosis, and femoral head necrosis. The clinical efficacy of the injection liquid is significant, the total effective rate of treatment reaches 89 percent, and the cure rate is 76.5 percent.
Owner:山西长安血液血管病研究所
Method for oxygen regulated production of recombinant staphylokinase
InactiveUS7524644B2Efficient and economicalOverall system can be economically viableSugar derivativesBacteriaEscherichia coliNucleotide
The present invention relates to a nucleotide sequence of expression cassette OXY-1 of SEQ ID No. 1, a modified staphylokinase SAK-2 gene of SEQ ID No. 2, a peptide sequence of modified staphylokinase SAK-2 gene, of SEQ ID No. 3, three plasmids having International Deposition Nos. BPL-0019, BPL-0020, and BPL-0021, and their corresponding three recombinant E. Coli; also invention relates to a process for over-producing staphylokinase and its analogues by modulating level of oxygen of its growth medium in a host system, and lastly, a method of dissolving blood clot in a subject in need thereof.
Owner:COUNCIL OF SCI & IND RES
Mutant of recombined glucokinase for anti blood platelet collecting and low immunogencity
InactiveCN1286974CInhibit aggregationLow immunogenicityPeptide/protein ingredientsEnzymesEscherichia coliFreeze-drying
The invention relates to an anti-bloodpatelet aggregation, low-immunogenicity recombinant staphylokinase mutant and its preparing method. The invention analyses the structures of recombinant staphylokinase monomer and micro-lumbrokinase-staphylokinase-micro- lumbrokinase compound crystal, and designs a novel molecular structure of staphylokinase mutant, which is recombined with a prokaryotic expression carrier pLY-4 after constructing mutant gene by using PCR fixed-point mutation, converting Escherichia coli, and screening high-expression engineering bacteria; by fermenting and amplifying, crushing the bacteria, centrifuging and collecting, then making two-step purification, and freeze-drying and obtaining the product, which basically maintains fibrinolytic activity of wild staphylokinase, can inhibit the bloodpatelet aggregation and has remarkable function of preventing and curing blood acid and its immunogenicity is remarkably reduced.
Owner:FUDAN UNIV
Pegylation modification of staphylokinase epitope and application
InactiveCN106511985ALow immunogenicityAdequate thrombolytic activityPeptide/protein ingredientsPharmaceutical non-active ingredientsEpitopeGenetic engineering
The invention belongs to the technical field of genetic engineering and particularly relates to pegylated staphylokinase and preparation and application thereof. The pegylated staphylokinase structurally comprises polyethylene glycol and mutated staphylokinase which are coupled with each other. Compared with current similar products, the pegylated staphylokinase has the advantages that the immunogenicity of natural staphylokinase is effectively reduced, and sufficient thrombolytic activity is maintained.
Owner:CHONGQING MEDICAL UNIVERSITY
Low-immunogenicity staphylokinase mutant and preparation method and use thereof
InactiveCN102108351BLower levelSimple methodPeptide/protein ingredientsMicroorganism based processesEscherichia coliFreeze-drying
The invention discloses a protein structure of a low-immunogenicity recombinant staphylokinase mutant and a preparation method thereof. The invention relates to the molecular structure of the low-immunogenicity recombinant staphylokinase mutant. The preparation method comprises: directly obtaining the expression plasmid of the recombinant staphylokinase mutant by a polymerase chain reaction (PCR)site-directed mutagenesis technique; transforming Escherichia coli; performing fermentation culture; inducing expression; breaking thalli; centrifuging; collecting supernate; obtaining pure protein by continuous three-step chromatographic purification; and subjecting the pure protein to freeze drying to obtain the product. The obtained staphylokinase mutant has much lower immunogenicity, retains fibrinolysis activity for dissolving thrombi and can be used in preparation of medicaments for treating thromboembolic diseases.
Owner:HEBEI NORMAL UNIV
Low pyrogen staphylokinase and its preparation method
ActiveCN100469871CHigh purityHigh activityHydrolasesPeptide/protein ingredientsPharmacyBiochemical engineering
This invention belongs to the biology project pharmacy filed, which provides a Staphylokinase (SAK) with low pyrogen and it's preparing method. The Staphylokinase has low pyrogen, high purify and strong activity, which is completely fit for the medicinal demand. This invention includes the following steps: a breaking up the material containing with the Staphylokinase and distilling the floating liquid; b. sieving the product from step a with the DEAD gelatin rob, then removing salt and condensing; c. sieving the product form step b with CM gelatin rob, collecting the sample apex, condensing the washing liquid and transforming the medium; d. sieving the product from step c with the Q gelatin rob to obtain the Staphylokinase. The Staphylokinase of this invention has good bolt dissolving effect, and it is safe and efficient for users..
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Method and Application of Heat Treatment to Improve Biological Activity of Pegylated Staphylokinase
The invention relates to the field of biological medicines, in particular to a heat treatment method for improving the biological activity of polyethylene glycol (PEG) staphylokinase. The heat treatment method comprises the steps that (1) PEG staphylokinase is heated by a water bath; PEG staphylokinase is subjected to ice bath treatment immediately after heating stop; (2) the selected heating temperature is 50-80 DEG C; the optimal heating temperature is 70 DEG C; and (3) the selected heating time is 1-6h; and the optimal heating time is 2h. The heat treatment method can improve the biological activity of PEG staphylokinase.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Injection for treating thrombus
InactiveCN106511986ASpecific thrombolysisImprove solubilityPeptide/protein ingredientsPharmaceutical delivery mechanismClinical efficacyThrombus
An injection for treating thrombus is prepared from a radix salviae miltiorrhizae injection, chymotrypsin and staphylokinase, wherein every 5 mL of the injection contains 1.8-2.5 mL of radix salviae miltiorrhizae injection, 0.8-1.2 mg of chymotrypsin and 80, 000-150, 000 IU of staphylokinase. The injection is mainly used for treating thrombus and vascular occlusion of the heart, the brain and all body parts, is mainly applicable to symptoms including insufficient blood supply to heart and brain, myocardial infarction, cerebral infarction, pulmonary infarction, venous thrombosis, arterial thrombosis and femoral head necrosis, and is remarkable in clinical efficacy; the total effective rate reaches 89%, and the cure rate is 76.5%.
Owner:威海恒基伟业信息科技发展有限公司
Method for preparing recombinant human octoplasmin
ActiveCN114480353AEfficient removalHigh purityPeptidasesAgainst vector-borne diseasesBiotechnologyZymogen
The invention provides a method for preparing recombinant human octoplasmin, and belongs to the technical field of biological medicine. The method comprises the following steps: (1) preparing staphylokinase subjected to enzyme digestion: taking recombinant staphylokinase for enzyme digestion, and separating and purifying an enzyme digestion substance to obtain staphylokinase subjected to enzyme digestion; wherein the amino acid sequence of the staphylokinase subjected to enzyme digestion is as shown in SEQ ID NO: 3; and (2) preparing the recombinant plasmin: adding staphylokinase subjected to enzyme digestion into the recombinant plasminogen for enzyme digestion, and separating and purifying an enzyme digestion substance to obtain the recombinant plasmin. It is found for the first time that the impurity A in the recombinant plasmin can be effectively removed through the method, the purity of the recombinant plasmin is improved, and potential stability and safety risks of drugs in clinical application are eliminated. The method disclosed by the invention is low in cost, simple to operate and suitable for industrial production.
Owner:JINGZE PHARMA (HEFEI) CO LTD +2
rgd-recombinant staphylokinase-human alpha microglobulin fusion protein and its preparation and application
InactiveCN104845949BImprove aggregation effectInhibit aggregationPeptide/protein ingredientsGenetic material ingredientsEscherichia coliKinase activity
Owner:CHONGQING MEDICAL UNIVERSITY
Method for oxygen regulated production of recombinant staphylokinase
InactiveUS20050019862A1Efficient and economicalEconomically viableSugar derivativesBacteriaEscherichia coliNucleotide
The present invention relates to a nucleotide sequence of expression cassette OXY-1 of SEQ ID No. 1, a modified staphylokinase SAK-2 gene of SEQ ID No. 2, a peptide sequence of modified staphylokinase SAK-2 gene, of SEQ ID No. 3, three plasmids having International Deposition Nos. BPL-0019, BPL-0020, and BPL-0021, and their corresponding three recombinant E. Coli; also invention relates to a process for over-producing staphylokase and its analogues by modulating level of oxygen of its growth medium in a host system, and lastly, a method of dissolving blood clot in a subject in need thereof.
Owner:COUNCIL OF SCI & IND RES
Staphylokinase and expression vector thereof
ActiveCN101314769BStrong thrombolytic effectExcellent thrombolytic activityBacteriaHydrolasesDiseaseCerebrovascular disorder
The invention discloses a staphylokinase and an expression vector thereof. The staphylokinase is polypeptide with amino acid sequence shown in SEQ ID No: 1, and the gene coding sequence thereof is shown in SEQ ID No: 2. The invention also discloses an expression vector containing a staphylokinase gene. The thrombolysis of the staphylokinase has strong effect, so that the staphylokinase can be used for preparing medicine for remedying cardio-cerebrovascular diseases, in particular to medicine for remedying myocardial infarction and other thrombotic diseases; the thrombolysis activity of the staphylokinase is high, and the activity rate thereof is 4.0*10<4>AU / mg; in addition, the expression amount of the expression vector of the aphylokinase is high, and the expression amount of the aphylokinase accounts for more than 45 percent of total protein of thallus.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Reformed lyophylization preparation of recombinant staphylokinase (r-Sak), its preparing method and application
InactiveCN100580082CReduce manufacturing costBacteriaPeptide/protein ingredientsMANNITOL/SORBITOLIntramuscular injection
A freeze-dried preparation of recombinant glucokinase in the form free dried powder, injection, lipoplasm, or microcapsule for thrombolytic purpose contains the glucokinase with mutation of amino acids at positions 7, 3 and 43, and one or more of mannitol, phosphate, EDTA and sodium chloride. Its preparing process is also disclosed.
Owner:BEIJING YILING BIOENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com