Method for preparing recombinant human octoplasmin
A technology of plasmin and plasminogen, which is applied in the field of biomedicine, can solve the problems of staphylokinase rarity and high production cost, and achieve the effects of increasing expression, simple operation, and reducing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: The process of fermenting recombinant human Oak plasminogen
[0074] The processing steps of the present embodiment are as follows:
[0075] 1. Preparation of seed solution
[0076] The glycerin seeds of engineering bacteria were thawed, inoculated into BMGY medium shake flasks and amplified after shaking culture to obtain OD 600 Seed solution with a value between 5.0 and 15.0.
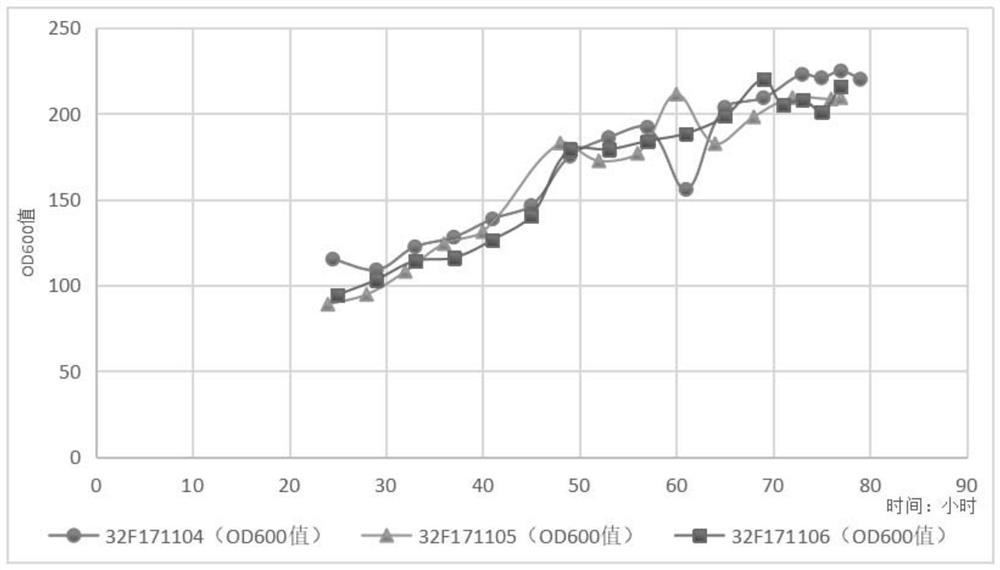
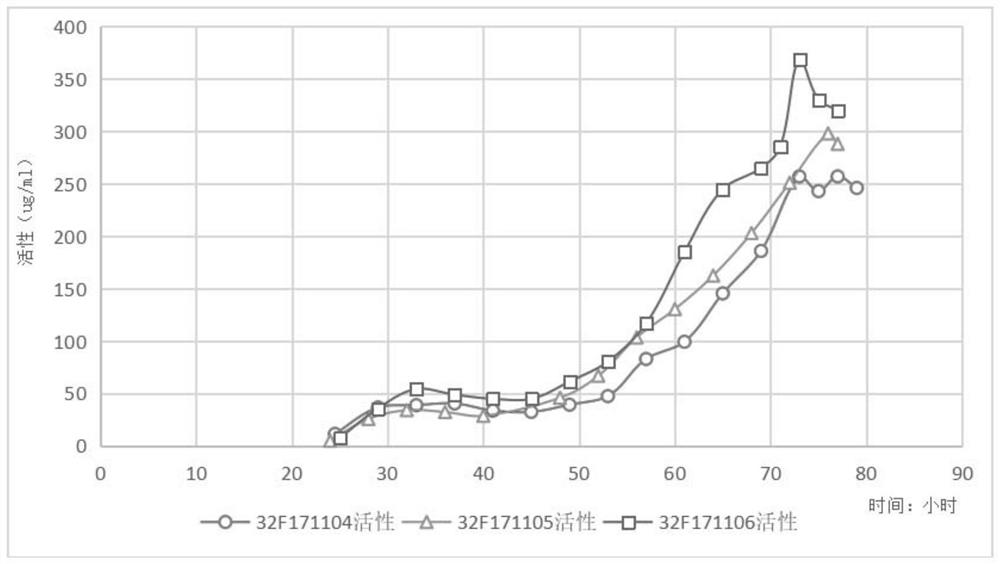
[0077] 2. Fermentation culture in fermenter (batch 32F171104)
[0078] Inoculate the seed liquid prepared above to BSM basal medium for fermentation (the volume of the inoculated seed liquid is 10% of the volume of the BSM basal medium), the culture temperature is 30.0±1.0°C, the pH of the fermented liquid is controlled at 5.0±0.5, and the dissolved oxygen is maintained. (D.O) is above 10%. Cultivate until the initial glycerol in the fermentation broth is exhausted (dissolved oxygen in the fermentation broth rises to 100% at this time), supplement 50% glycerol with 3% of the init...
Embodiment 2
[0084] Example 2: The process of fermenting recombinant human Oak plasminogen
[0085] The processing steps of the present embodiment are as follows:
[0086] 1. Preparation of seed solution
[0087] The glycerin seeds of engineering bacteria were thawed, inoculated into BMGY medium shake flasks and amplified after shaking culture to obtain OD 600 Seed solution with a value between 5.0 and 15.0.
[0088] 2. Fermentation tank culture (32F171105)
[0089] Inoculate the seed solution prepared above into BSM medium for fermentation (the volume of the inoculated seed solution is 10% of the volume of the BSM basal medium), the culture temperature is 30.0±1.0°C, the pH of the fermentation solution is controlled at 5.0±0.5, and the D.O is maintained at 10 %above. Cultivate until the initial glycerol in the fermentation broth is exhausted (dissolved oxygen in the fermentation broth rises to 100% at this time), replenish 50% glycerol with an initial fermentation broth volume of 6.25...
Embodiment 3
[0093] Example 3: Process for fermenting recombinant human Oak plasminogen
[0094] 1. Preparation of seed solution
[0095] The glycerin seeds of engineering bacteria were thawed, inoculated into BMGY medium shake flasks and amplified after shaking culture to obtain OD 600 Seed solution with a value between 5.0 and 15.0.
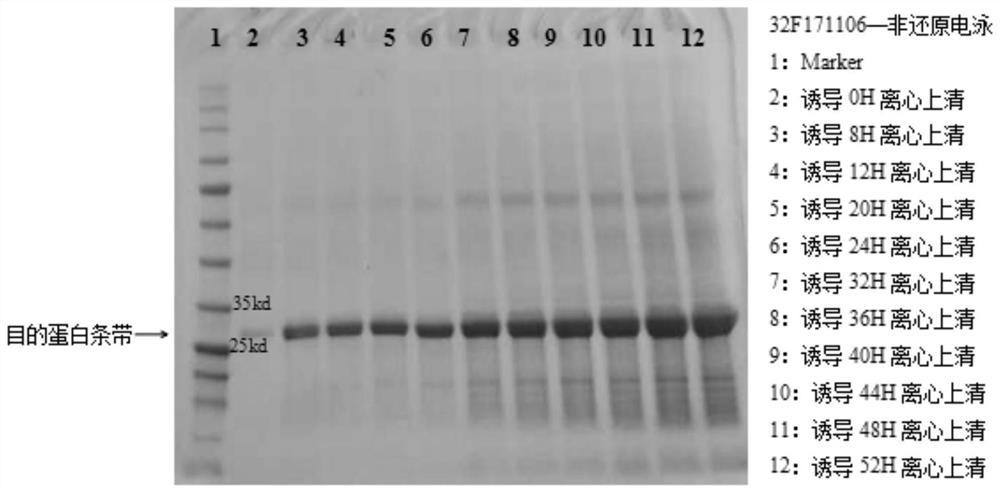
[0096] 2. Fermentation tank culture (32F171106)
[0097]The fermentation base medium is BSM medium, the culture temperature is 30.0±1.0°C, the pH of the fermentation broth is controlled at 5.0±0.5, and the D.O is maintained above 10%. Cultivate until the initial glycerol in the fermentation broth is exhausted (dissolved oxygen in the fermentation broth rises to 100% at this time), supplement 50% glycerol with an initial fermentation broth volume of 8.3%, and stop when the cell wet weight in the fermentation broth reaches 185g / L Add glycerin. When the added glycerin in the fermentation broth is exhausted (the dissolved oxygen in the fermentation broth ri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com