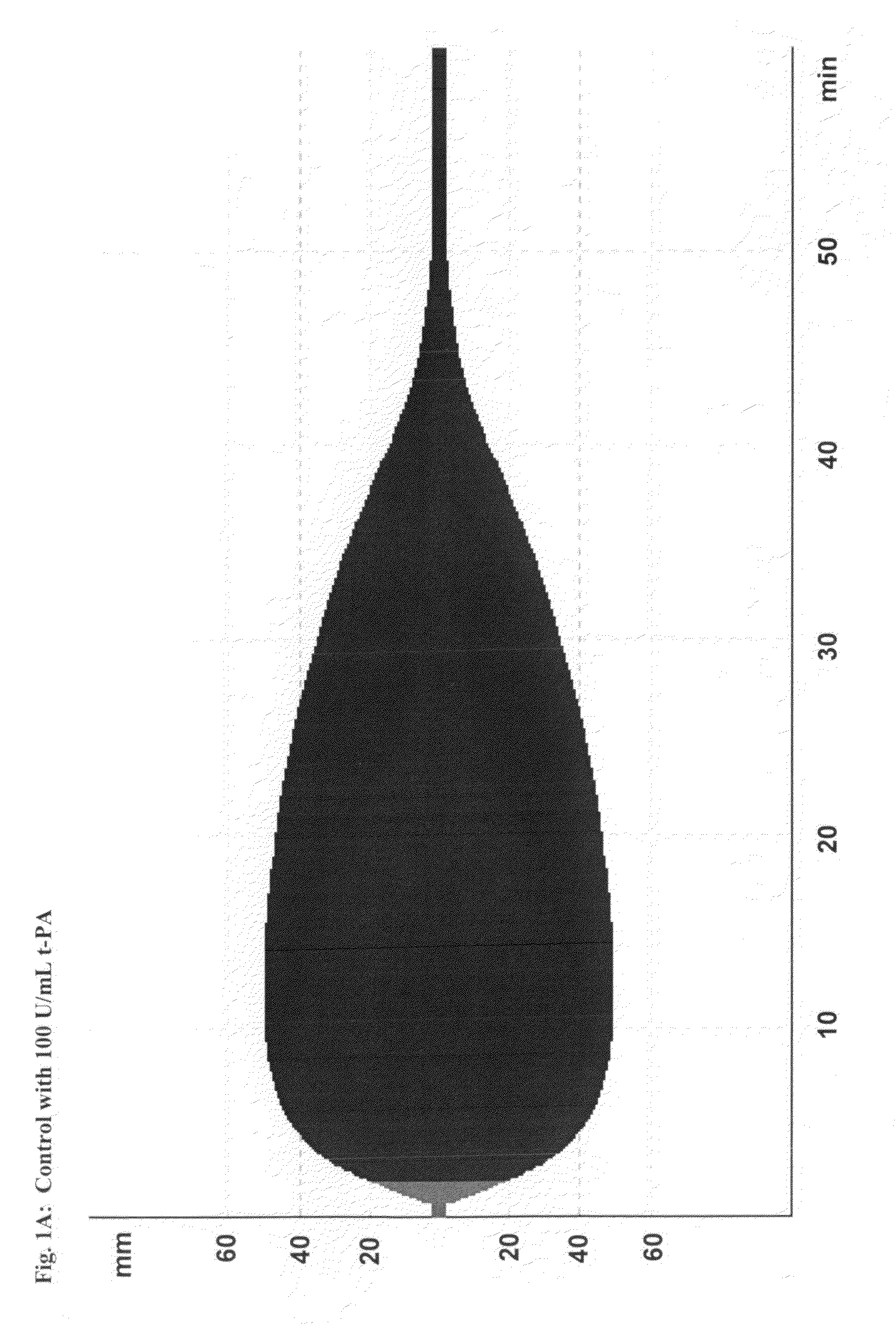
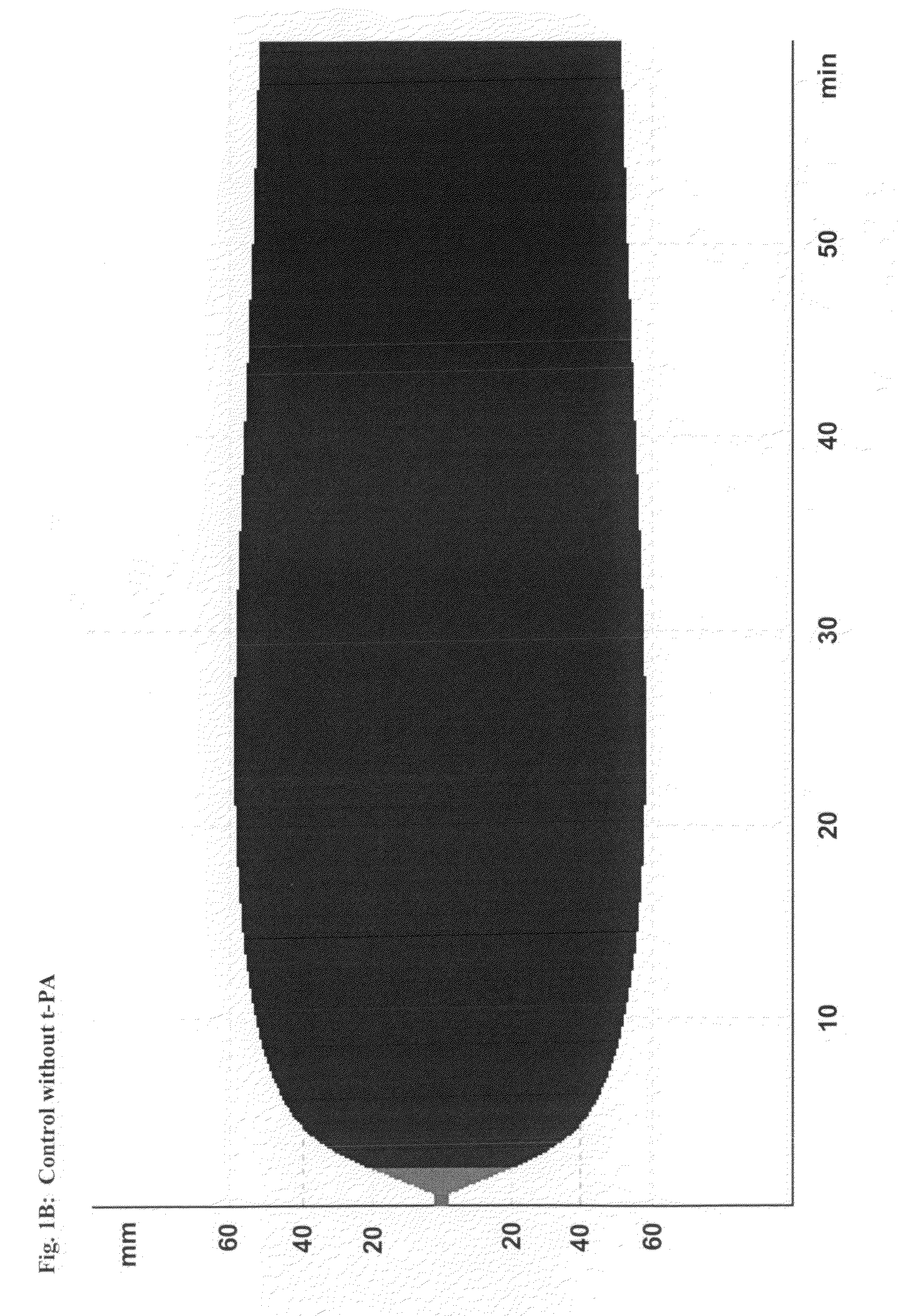
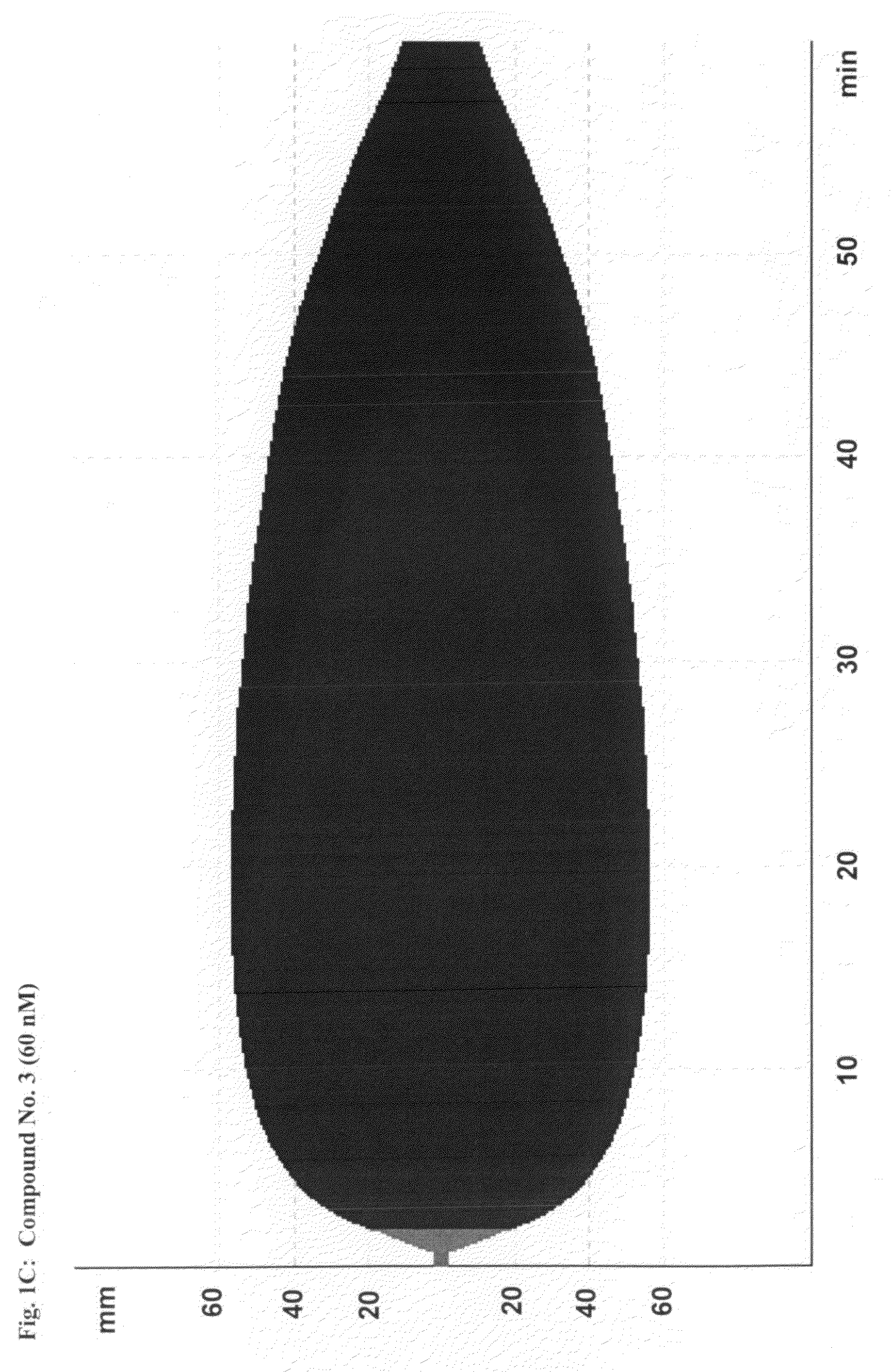
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
150 results about "Plasmin activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasmin is an important enzyme (EC 3.4.21.7) present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.
Compounds useful in the complement, coagulat and kallikrein pathways and method for their preparation
The present invention is concerned with new compounds, and particularly those having a fused bicyclic ring substituted with an amidine moiety. These compounds are each potent inhibitors of Factor D of the alternate pathway of complement, C1s of the classical pathway of complement, Factors Xa, XIIa, VIIa and thrombin of the coagulation pathway, plasmin in the fibrinolytic pathway, and kallikrein and high molecular weight kininogen in the inflammatory pathways. These proteases, which have serine in their active site, are called serine proteases and they are pivotal to most of the processes of inflammation and coagulation. In fact, these various systems are interactive with one another and it is difficult to activate one pathway without it influencing the others.
Owner:BIOCRYST PHARM INC
Removal of plasmin(ogen) from protein solutions
A method for specifically removing or isolating plasmin(ogen) or plasmin in presence of fibrinogen from a mixture containing plasmin(ogen) or plasmin by contacting the mixture with a rigid amino acid wherein the amino group of the amino acid and the carboxylic group of the amino acid are about 6–8 Angstroms, preferably about 7 Angstroms apart and the rigid amino acid is covalently bound to the support via the amino group of the amino acid.
Owner:OMRIX BIOPHARM
Method for purification of a blood component
InactiveUS6207066B1Efficient removalHydrolasesPeptide/protein ingredientsBlood componentCentrifugation
Owner:THROMBOGENICS NV
Prodrugs activated by plasmin and their use in cancer chemotherapy
InactiveUS7402556B2Low toxicityHigh specificity of actionSugar derivativesTetrapeptide ingredientsPeptide substrateOligopeptide
The product of the invention is a modified form of a therapeutic agent and comprises a therapeutic agent, an oligopeptide having a plasmin peptide substrate of 2-4 amino acids and mono- or di-peptide linkage, a stabilizing group and, optionally, a linker group. The prodrug is cleavable by plasmin. Also disclosed are methods of making and using the prodrug compounds.
Owner:MEDAREX LLC
Means and methods for the recruitment and identification of stem cells
InactiveUS20100178297A1Inhibit recruitmentIncreased engraftment and isolationSalicyclic acid active ingredientsHydroxy compound active ingredientsProgenitorHematopoietic cell
Described are methods of modulating stem / progenitor cell recruitment involving molecules that agonize the formation of plasmin stimulating the recruitment of stem / progenitor cells, including hematopoietic and endothelial precursor cells. Conversely, antagonists of plasmin can inhibit recruitment of the stem cells. In addition, the identification of the uPA receptor (uPAR) as a retention signal for stem cells in their niche suggests a novel method for increased engraftment and isolation of multipotent stem cells.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
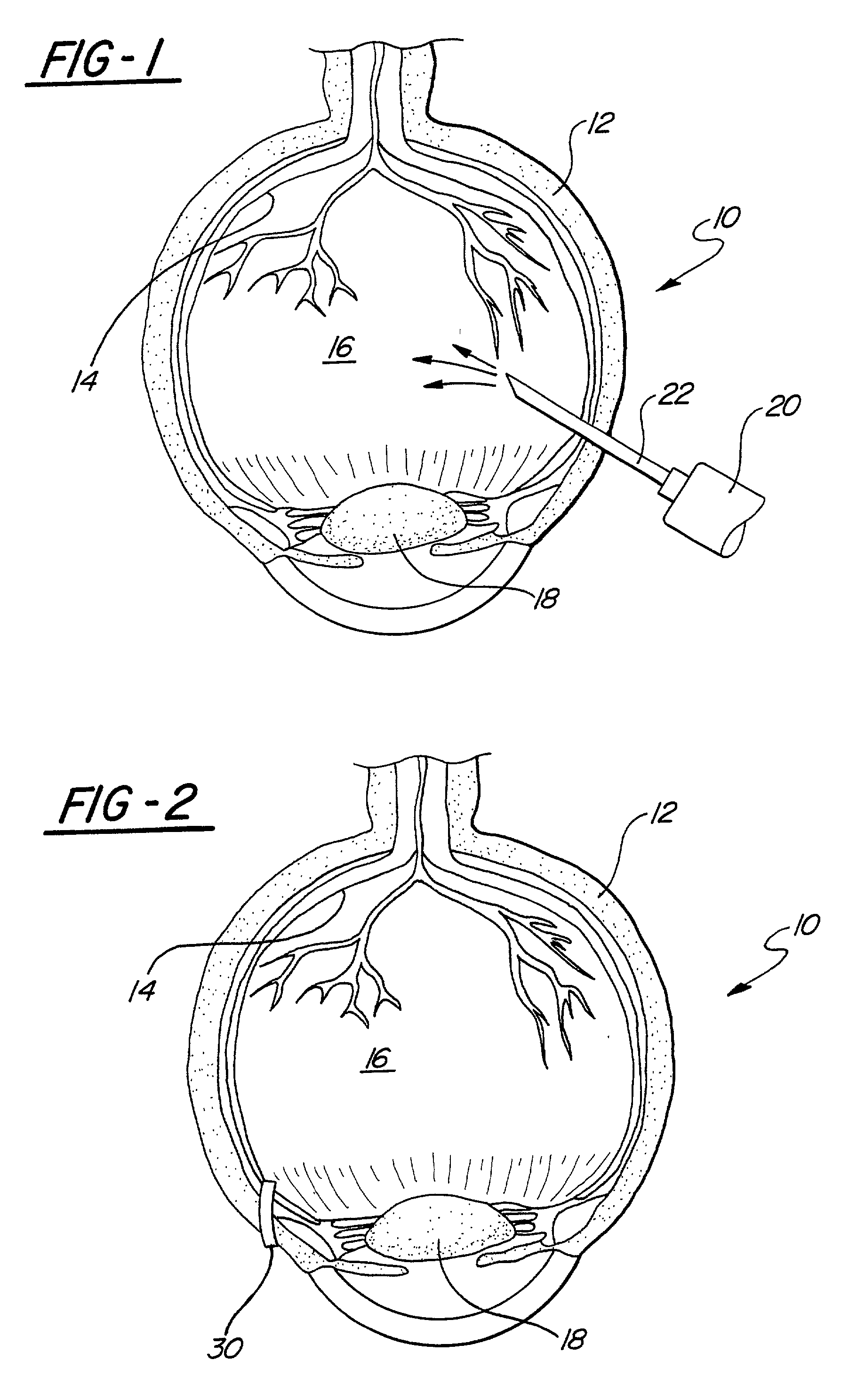
Method for creating a separation of posterior cortical vitreous from the retina of the eye
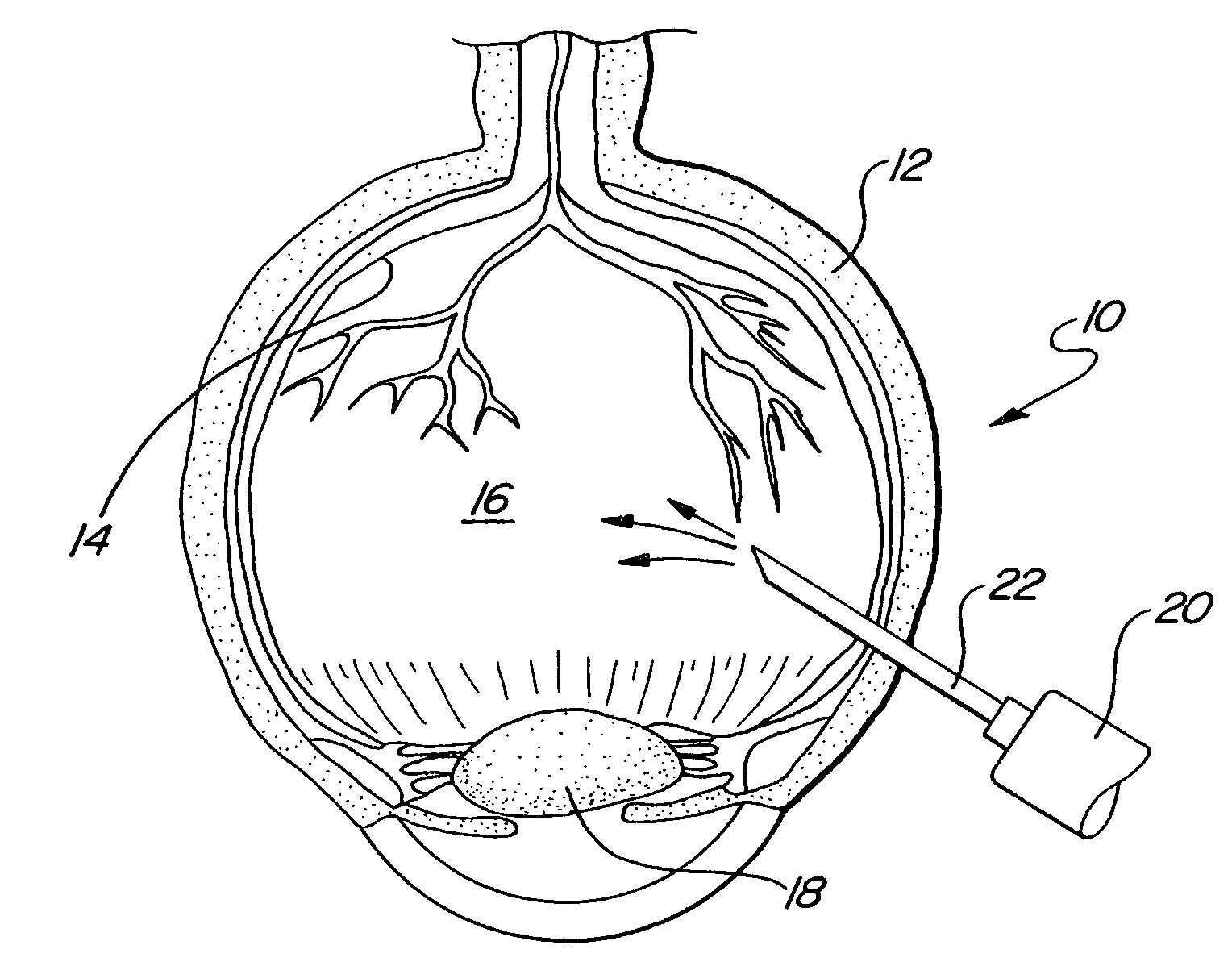
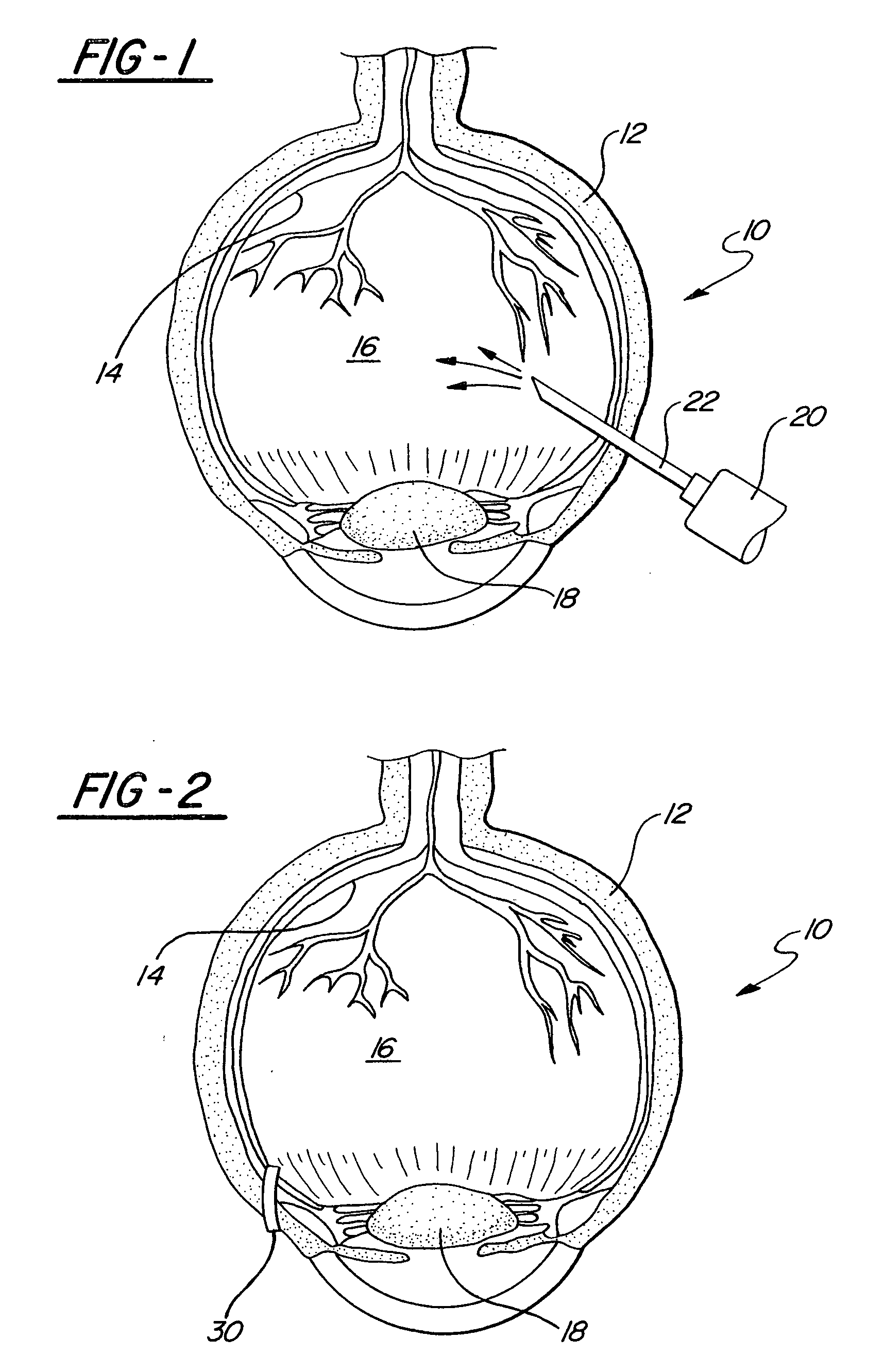
InactiveUS20020139378A1Minimize and altogether eliminateMinimize or altogether eliminate the vitreoretinal tractionSenses disorderDiagnosticsVitreous HumorsRetina
A method is disclosed for creating a separation of posterior cortical vitreous from the retina of the eye. The method includes the step of introducing plasmin into the vitreous humor of the eye. The plasmin may be introduced either by injection or through a sustained release device. Optionally, other enzymes, polysaccharides, and / or glycoproteins are intermixed with the plasmin.
Owner:NUVUE TECH
Enzyme-mediated modification of fibrin for tissue engineering: fibrin formulations with peptides
InactiveUS7241730B2Efficacious platformEnhanced andPeptide/protein ingredientsTransferasesCell Surface ProteinsADAMTS Proteins
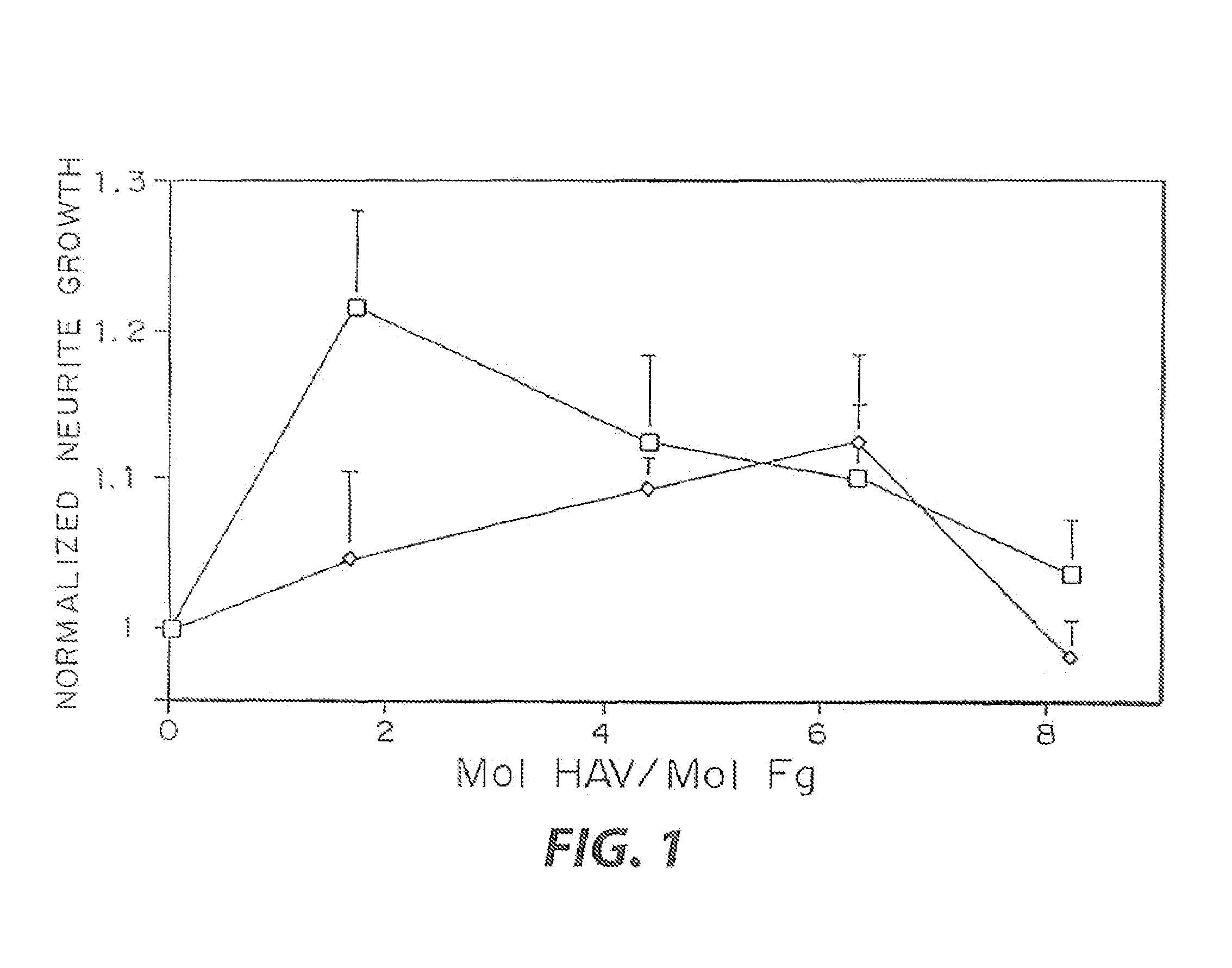
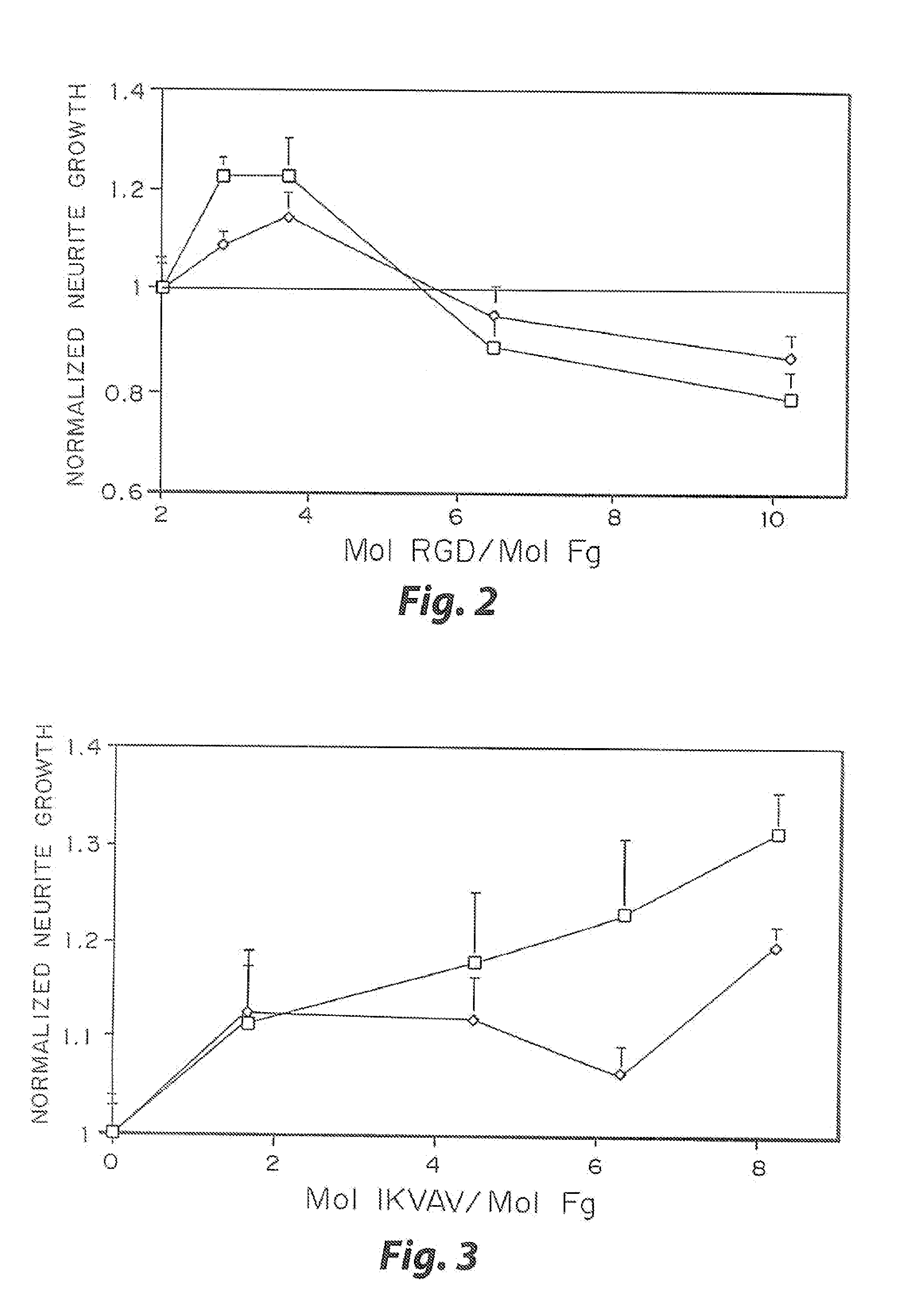
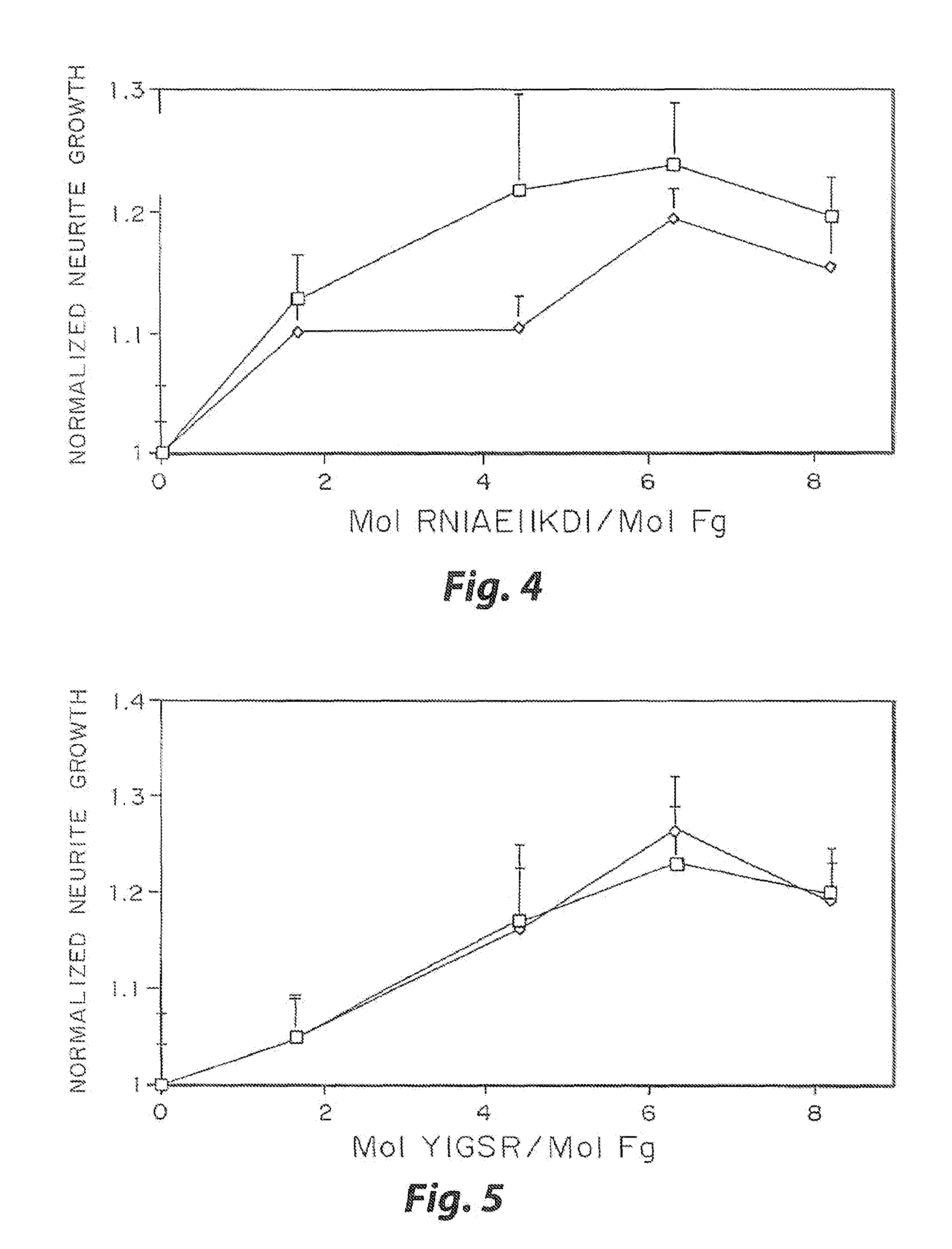
Heparin-binding regions of several proteins, such as neural cell adhesion molecule, fibronectin, laminin, midkine, and anti-thrombin III have been shown to promote neurite extension on two-dimensional surfaces. The effect of heparin-binding peptides on neurite extension through three-dimensional matrices was investigated by culturing embryonic chick dorsal root ganglia (DRG) within fibrin gels containing chemically attached heparin-binding peptide (HBP). The length of neurites within fibrin gels containing cross-linked HBP was increased by more than 70% over extension through fibrin gels containing no peptide. The HBP sequence of antithrombin III was incorporated into the fibrin gel as the C-terminal domain of a bidomian, chimeric peptide; the N-terminal second domain of this peptide contained the ∀2-plasmin inhibitor substrate for Factor XIIIa. Factor XIIIa, a transglutaminase, was used to chemically attach the HBP-containing chimeric peptide to the fibrin gels during polymerization. The amount of HBP cross-linked into the fibrin gels was determined, after degradation by plasmin using gel permeation chromatography, to be approximately 8 moles of peptide per mole fibrinogen. A peptide (HBP), where the cross-linking glutamine was replaced with glycine, showed no increase in extension in comparison with fibrin gels. The additional of heparin to the gel percursors resulted in no increase in neurite extension in comparison with fibrin gels. HBPs promote neurite extension by binding to cell surface proteoglycans on the DRG.
Owner:UNIV ZURICH +1
Kit for purification of plasmin
InactiveUS6183692B1Efficient removalIon-exchange process apparatusPeptide/protein ingredientsBlood componentCentrifugation
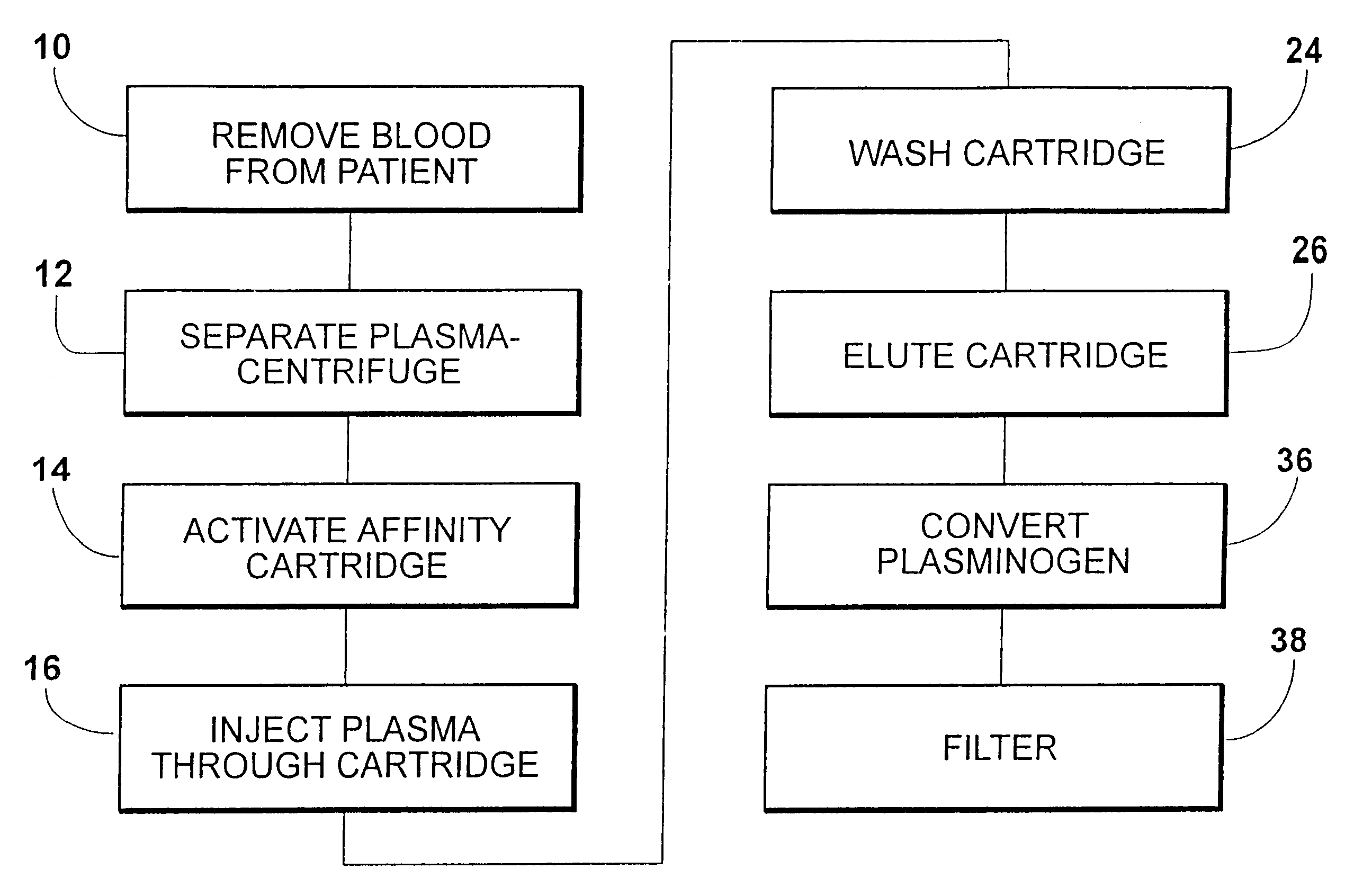
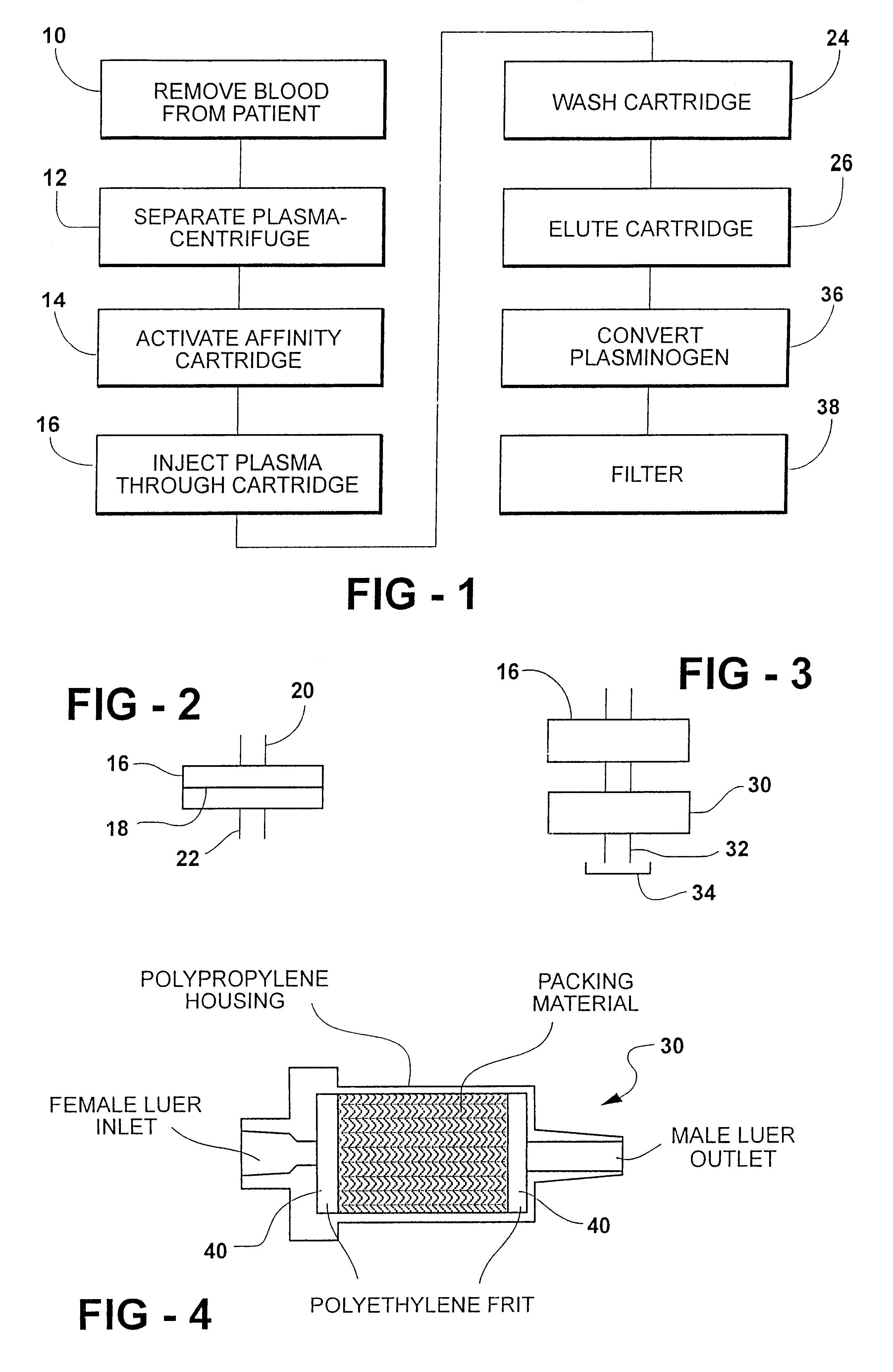
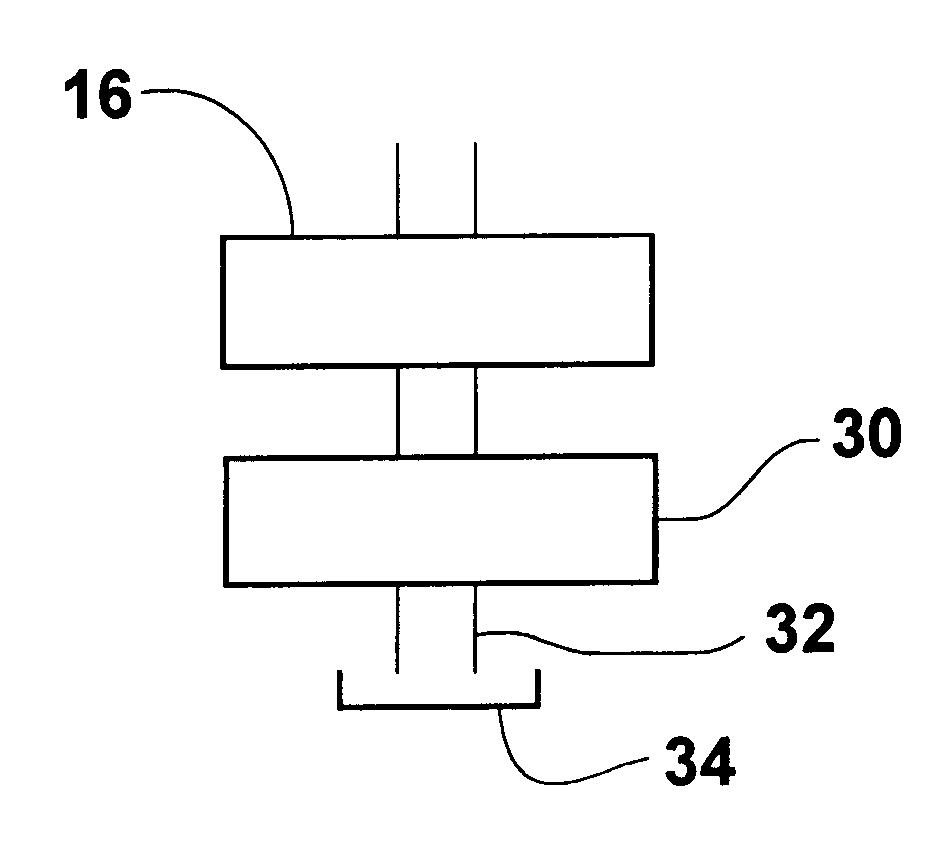
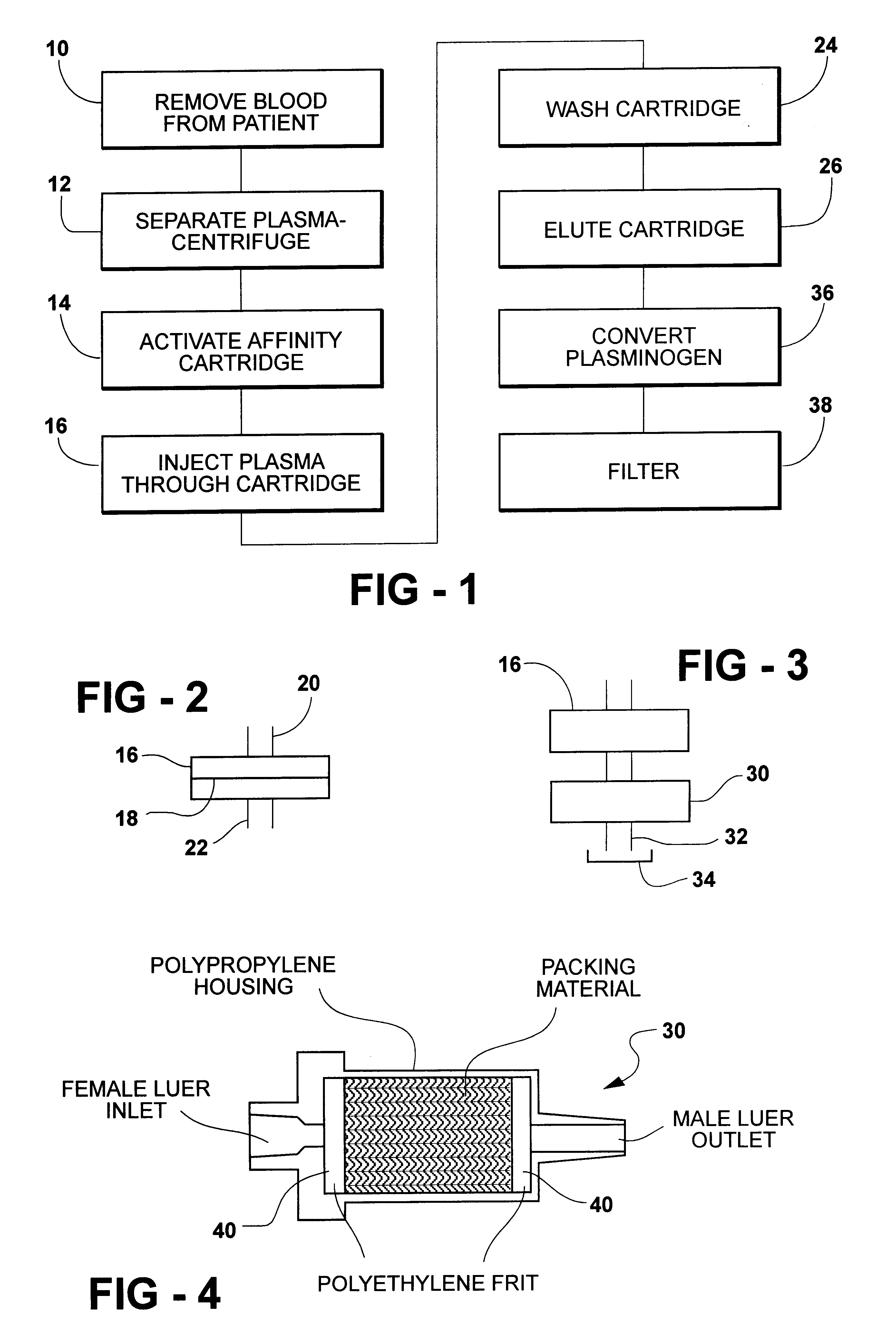
A method for rapid purification of a blood component from blood is described in which the blood plasma is first separated from the cellular blood elements by any conventional means, such as centrifugation. An affinity cartridge is then activated with a molecule, such as an amino acid, which binds with a blood component such as plasminogen. The separated blood plasma is then passed through the affinity cartridge such that the blood component is retained by the affinity cartridge. Thereafter, the blood component is eluted from the affinity cartridge by passing a buffer solution containing a releasing agent through the affinity cartridge. This releasing agent disengages the blood component from the affinity cartridge. The releasing agent is then separated from the eluted solution by passing the eluted solution through a device, such as an ion exchange or size exclusion device. The separated blood component, e.g. plasminogen, is then converted to plasmin by adding a known amount of an enzyme to the solution from which the releasing agent has been removed.
Owner:THROMBOGENICS NV
Application of earthworm fibrinolytic enzyme in resistance to liver fibrosis
InactiveCN101628113AAnthropod material medical ingredientsPeptide/protein ingredientsEarthworm fibrinolytic enzymeCollagenan
The invention relates to an application of an earthworm fibrinolytic enzyme in preparation of liver fibrosis resistant medicine or liver protection health-care food, which belongs to the technical field of biology. The effective dose of an oral liver fibrosis resistant earthworm fibrinolytic enzyme is 1,000-3,000 urokinase unit / person / day. Proved by experiments, earthworm collagenase can degrade collagen but cannot degrade (ox blood) fibrin, and the earthworm fibrinolytic enzyme can degrade the (ox blood) fibrin and can also degrade the collagen, thus the activity of the earthworm fibrinolytic enzyme is higher than the activity of the earthworm collagenase and the activity of a collagenase standard product (Sigma I type). Proved by a liver fibrosis resistant experiment by using a rat, the oral liver fibrosis resistant earthworm fibrinolytic enzyme has obvious effect and no obvious poisonous and side effect, but the oral liver fibrosis resistant earthworm collagenase does not have obvious effect.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method of thrombolysis by local delivery of reversibly inactivated acidified plasmin
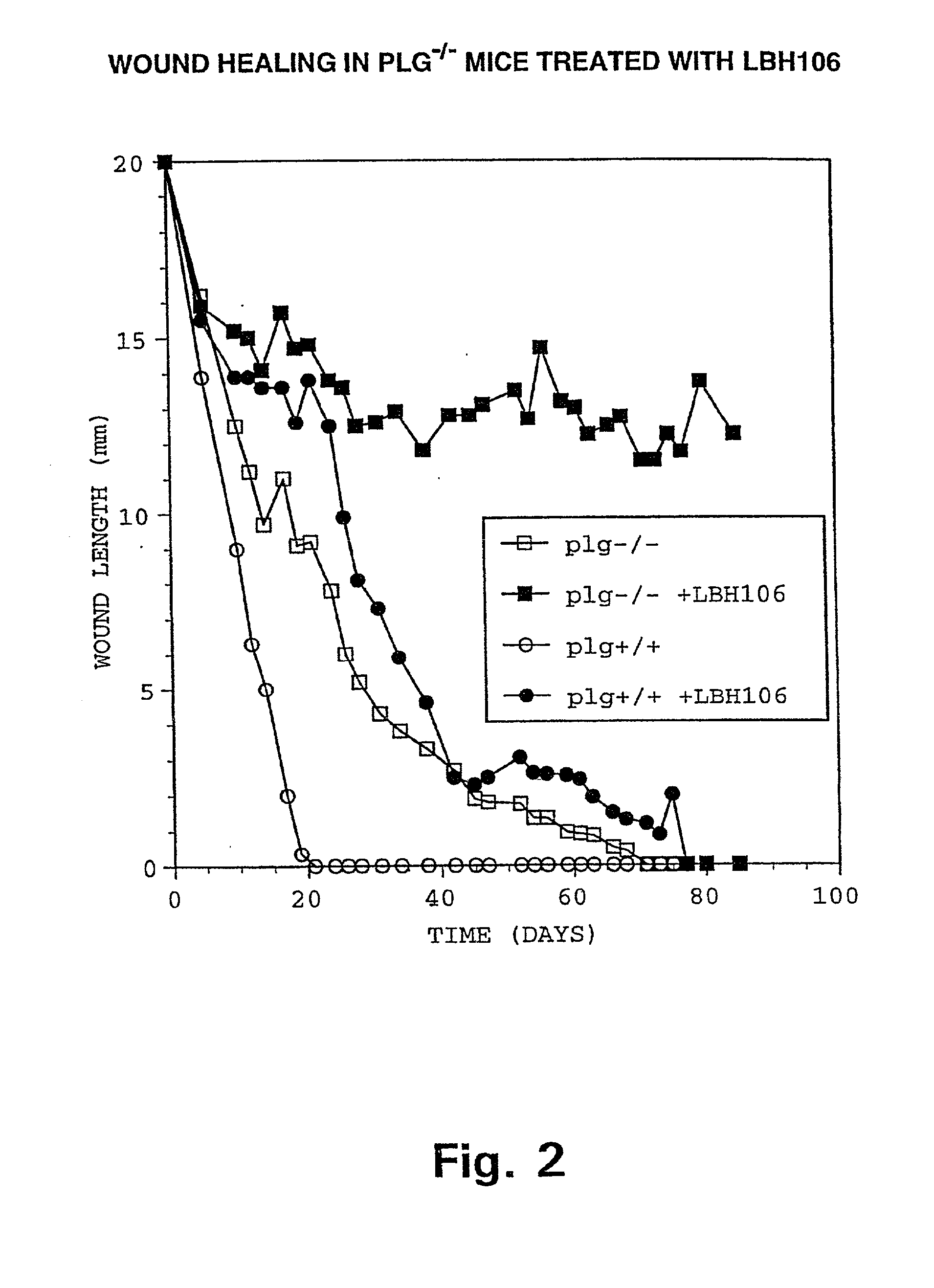
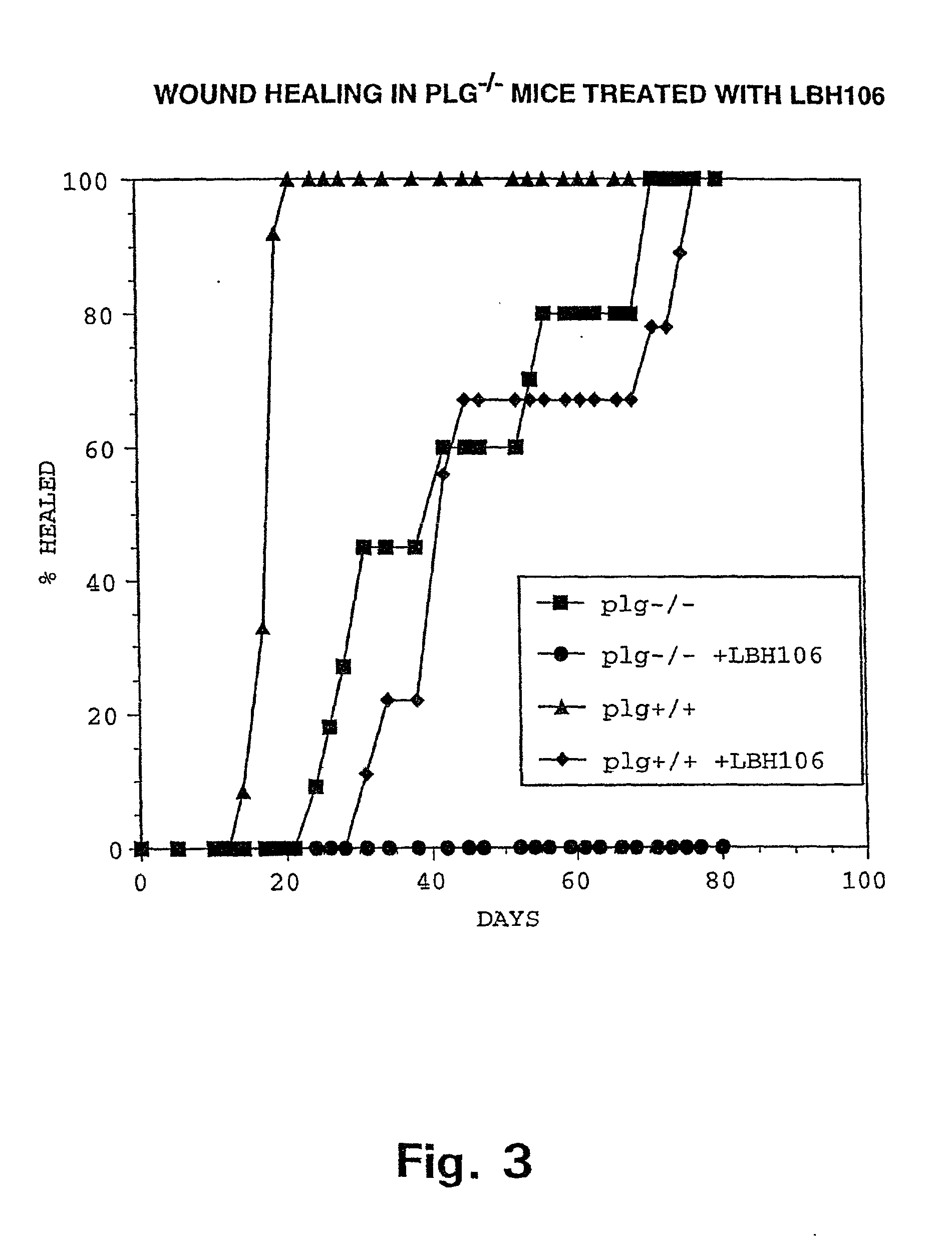
InactiveUS6964764B2Formula stableReduce capacityPeptide/protein ingredientsInorganic non-active ingredientsWhole bodyThrombus
Methods of thrombolysis that allow the use of a fibrinolytic composition comprising reversibly inactivated acidified plasmin and the localized delivery of the plasmin to a vascular thrombotic occlusion are disclosed. Further disclosed is a method for administering a therapeutic dose of a fibrinolytic composition substantially free of plasminogen activator to a human or animal having a vascular thrombotic occlusion. The fibrinolytic composition includes a reversibly inactivated acidified plasmin substantially free of plasminogen activator. Intravascular catheter delivery of the fibrinolytic composition directly into or in the immediate vicinity of the thrombus is disclosed to minimize the systemic degradation of fibrin while retaining the maximum plasmin activity against the thrombus.
Owner:GRIFOLS THERAPEUTICS LLC
Non-surgical method for preventing or reducing the rate of the progression of non-proliferative diabetic retinopathy and the treatment of other ocular conditions
InactiveUS20060257391A1Prevent and reduce rateReduce inflammationSenses disorderPeptide/protein ingredientsSerine proteinasesDisease
A non-surgical method for preventing or reducing the rate of the progression of non-proliferative diabetic retinopathy to the proliferative form of diabetic retinopathy comprising intravitreally administering to a patient suffering from non-proliferative diabetic retinopathy an effective amount of serine proteinase enzyme sufficient to create, without surgery, a posterior vitreal detachment to prevent or reduce the progression of proliferative diabetic retinopathy in said patient. Also disclosed is a non-surgical method of treating ocular conditions such as retinal ischemia, retinal inflammation, retinal edema tractional retinal detachment, tractional retinopathy, vitreous hemorrhage and tractional maculopathy by intravitreally administering to a patient suffering from one or more of these conditions with an effective amount of a serine proteinase enzyme to reduce or treat that particular ocular condition. Plasmin, microplasmin and miniplasmin are preferred serine proteinase enzymes and plasmin is the most preferred.
Owner:BAUSCH & LOMB INC
Process for producing well-preserving low-lactose or lactose-free milk product
InactiveUS20100215828A1Extension of timeWeakening of nutritional valueMilk preparationMilk preservationLactose free milkEnzyme system
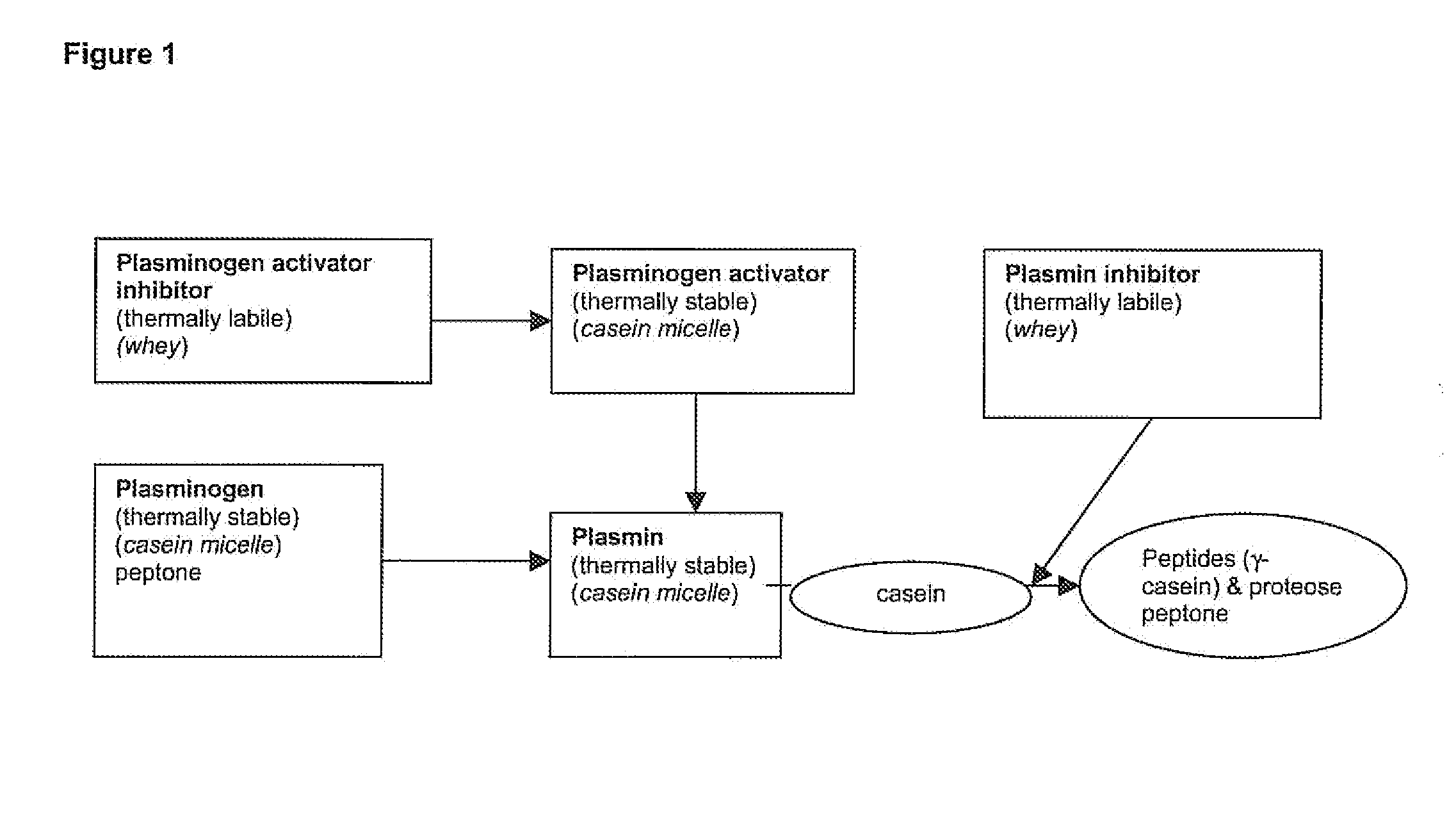
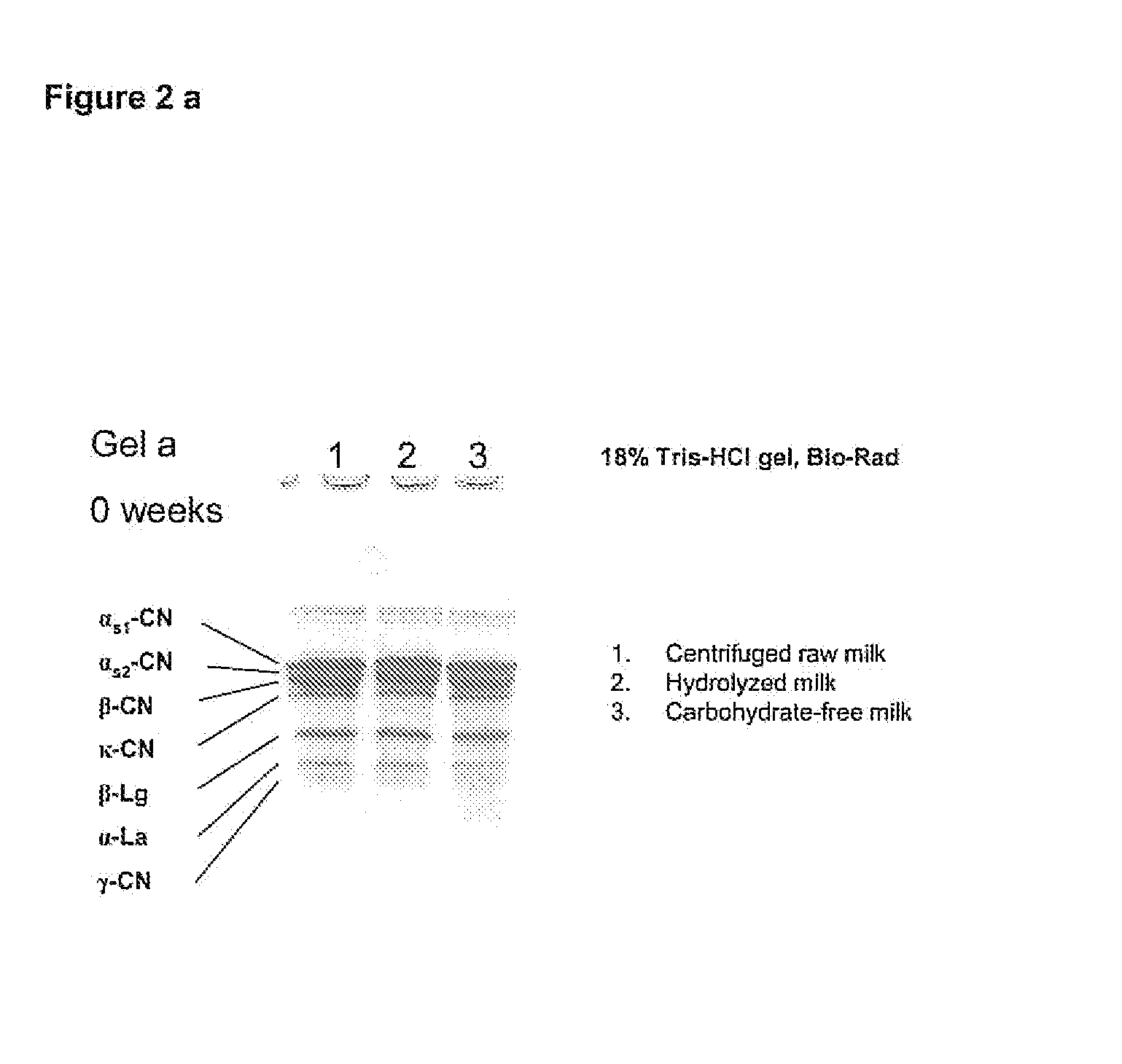
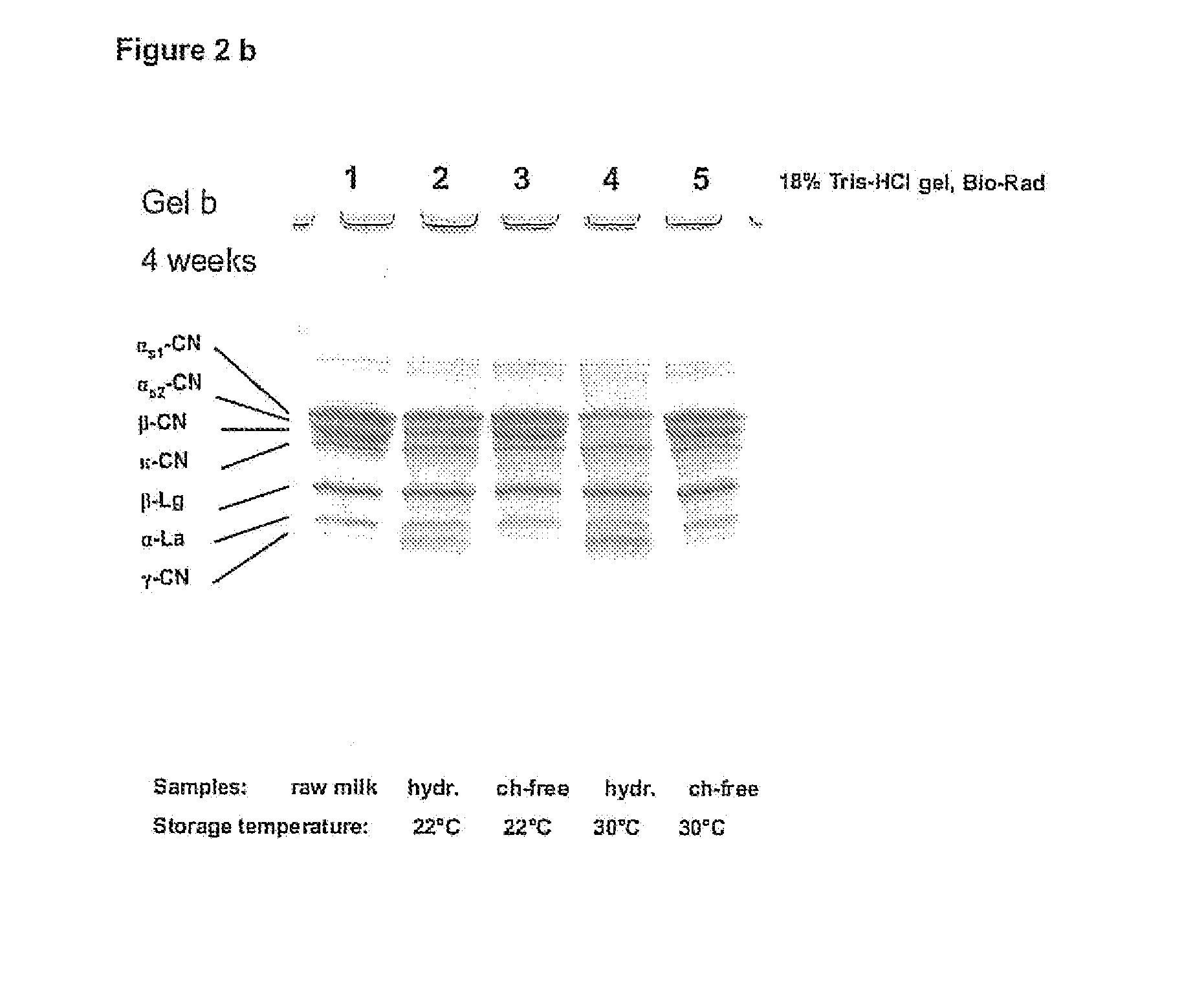
The invention relates to a process for the production of well-preserving lactose-free and low-lactose milk products. The process of the invention is characterized by separating the sugars and proteins in a raw material, thermally treating them in such a manner that the plasmin enzyme system and other proteolytic enzymes are inactivated, and combining the fractions and other preparation agents into a drink with a required composition and properties.
Owner:VALIO LTD
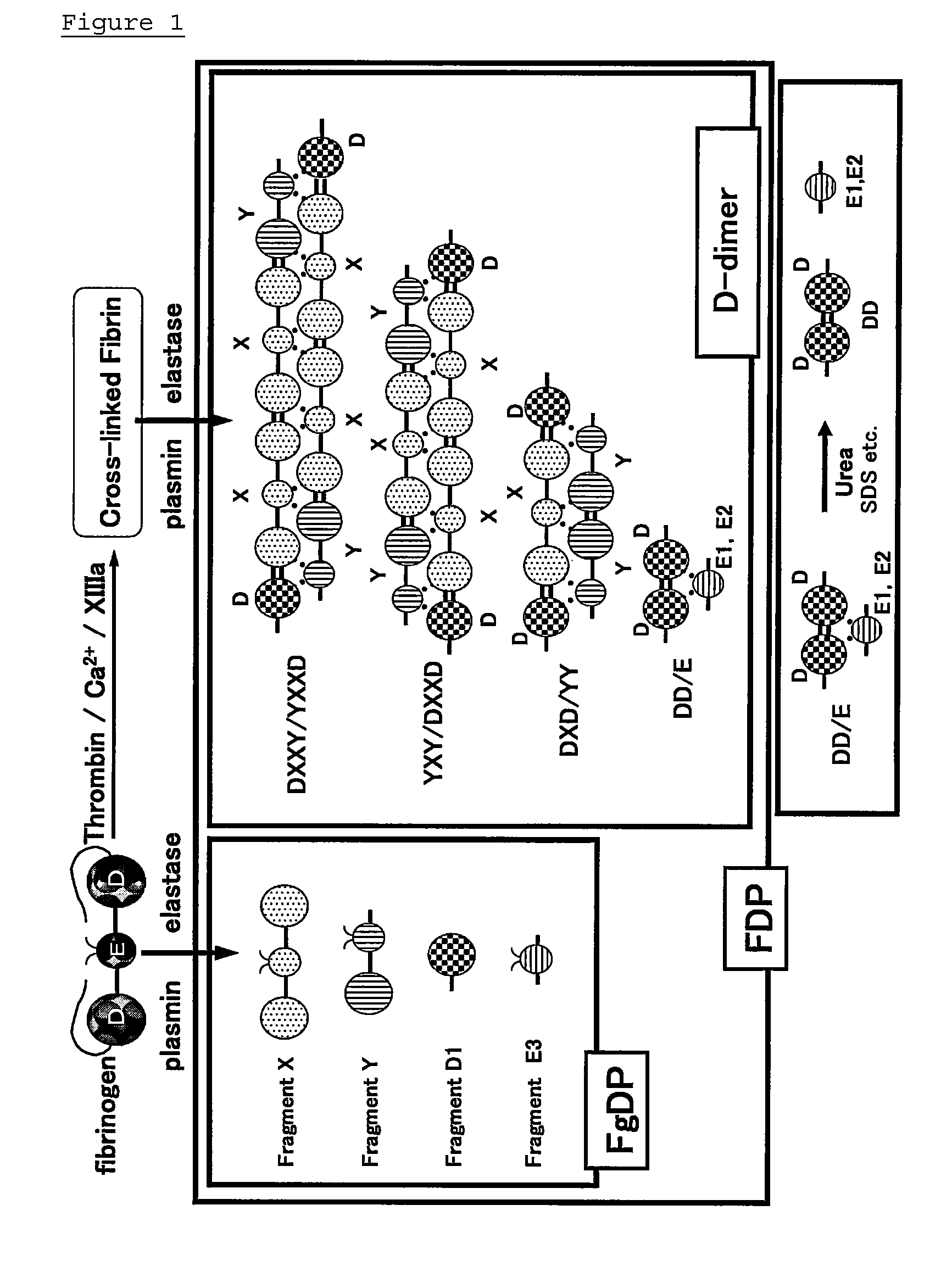
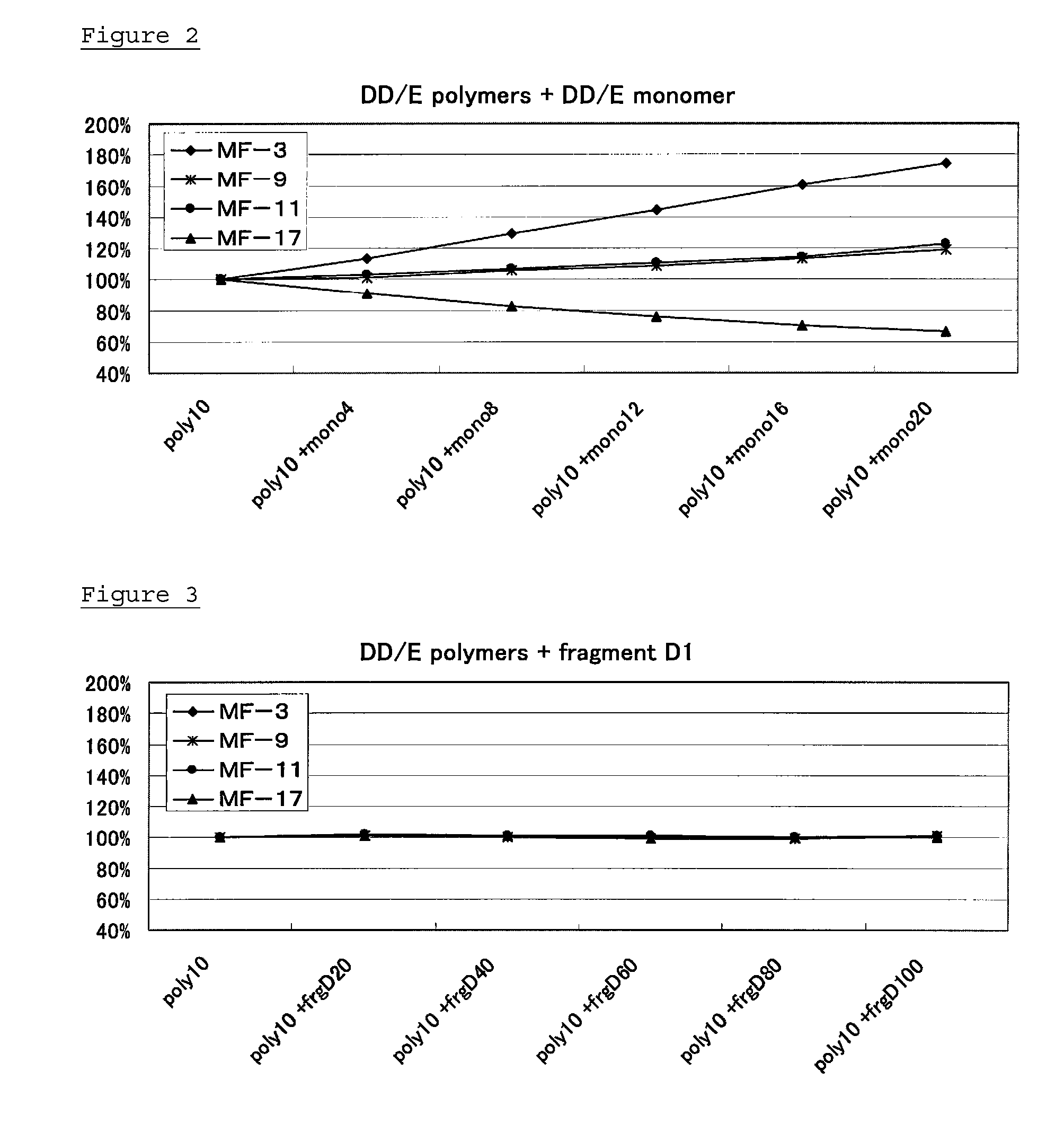
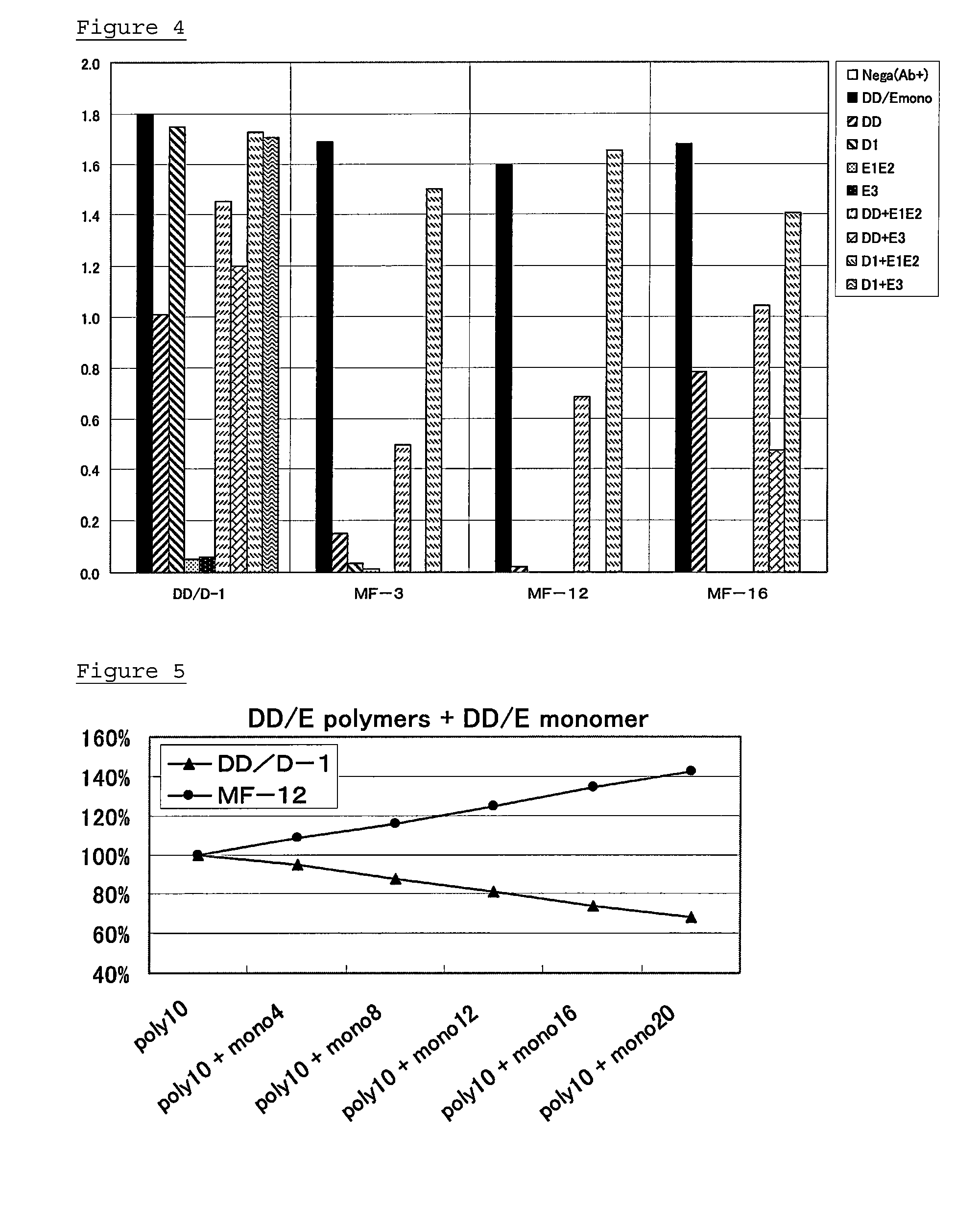
Monoclonal antibody and method of immunological analysis of e-D-dimer and e-DD/E complex
InactiveUS6132719AImmunoglobulins against animals/humansBiological material analysisFragment XMonoclonal antibody
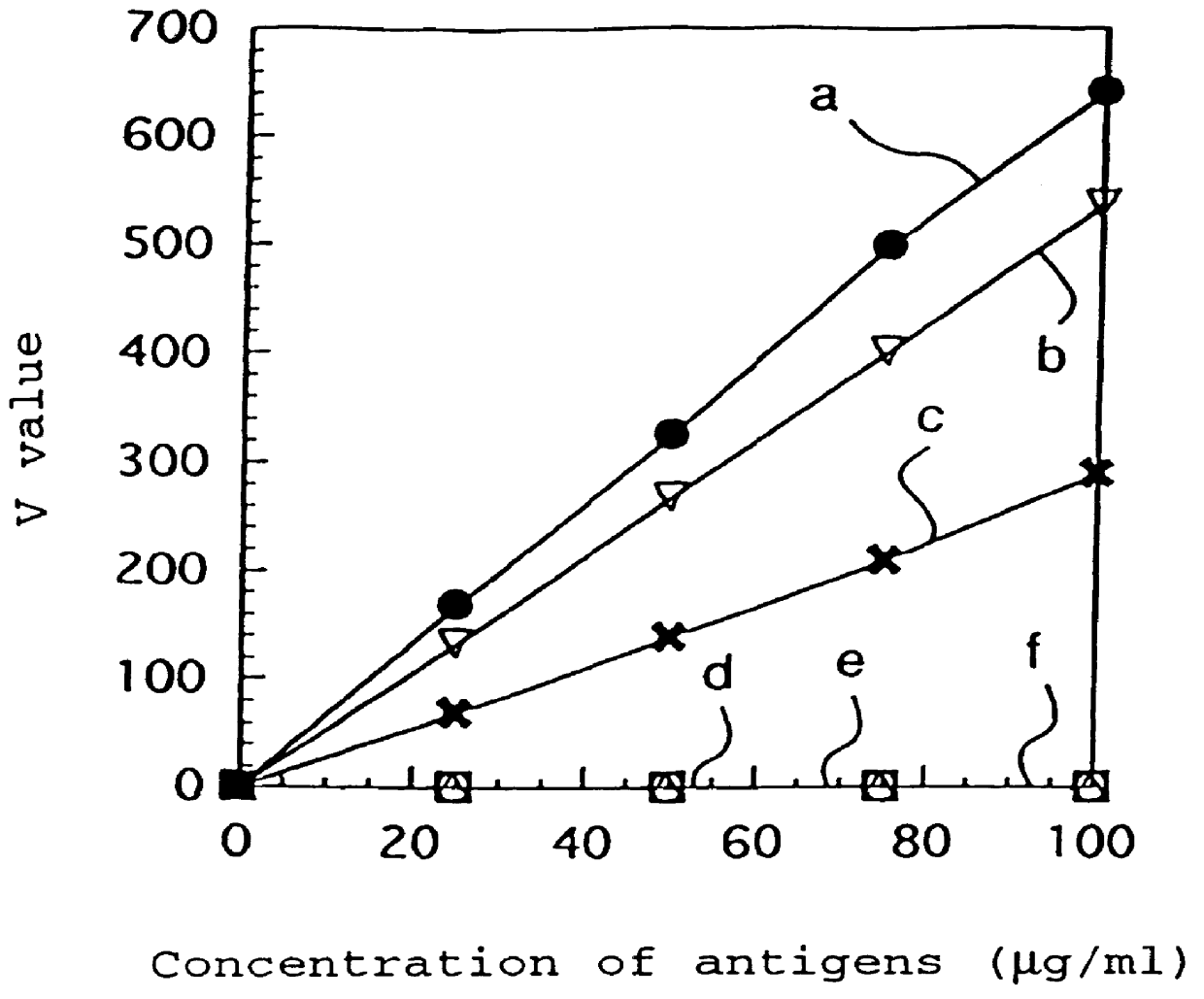
PCT No. PCT / JP97 / 01639 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed May 15, 1997 PCT Pub. No. WO97 / 43315 PCT Pub. Date Nov. 20, 1997A monoclonal antibody which specifically reacts with D-monomer produced by digesting human fibrinogen with granulocyte elastase and D-domain containing digestion products produced by digesting human stabilized fibrin with granulocyte elastase, but does not react with fibrinogen, or fragment X, Y or E produced by digesting fibrinogen with granulocyte elastase is disclosed. The D-dimer or DD / E complex produced by digestion with granulocyte elastase in a sample from a living body can be analyzed without being interferred with fibrinogen, digestion products of fibrinogen with plasmin, or digestion products of stabilized fibrin with plasmin, using the monoclonal antibody.
Owner:MITSUBISHI CHEM MEDIENCE
Composition and method for enhancing fibrinolysis
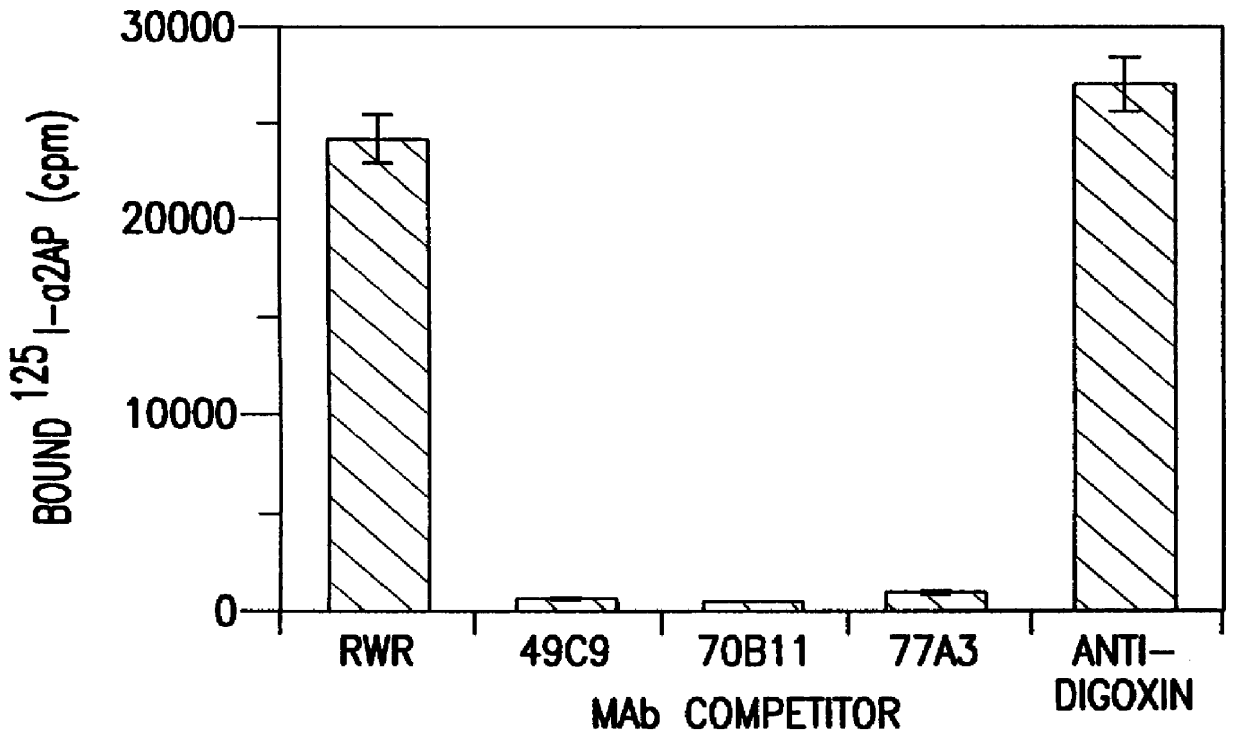
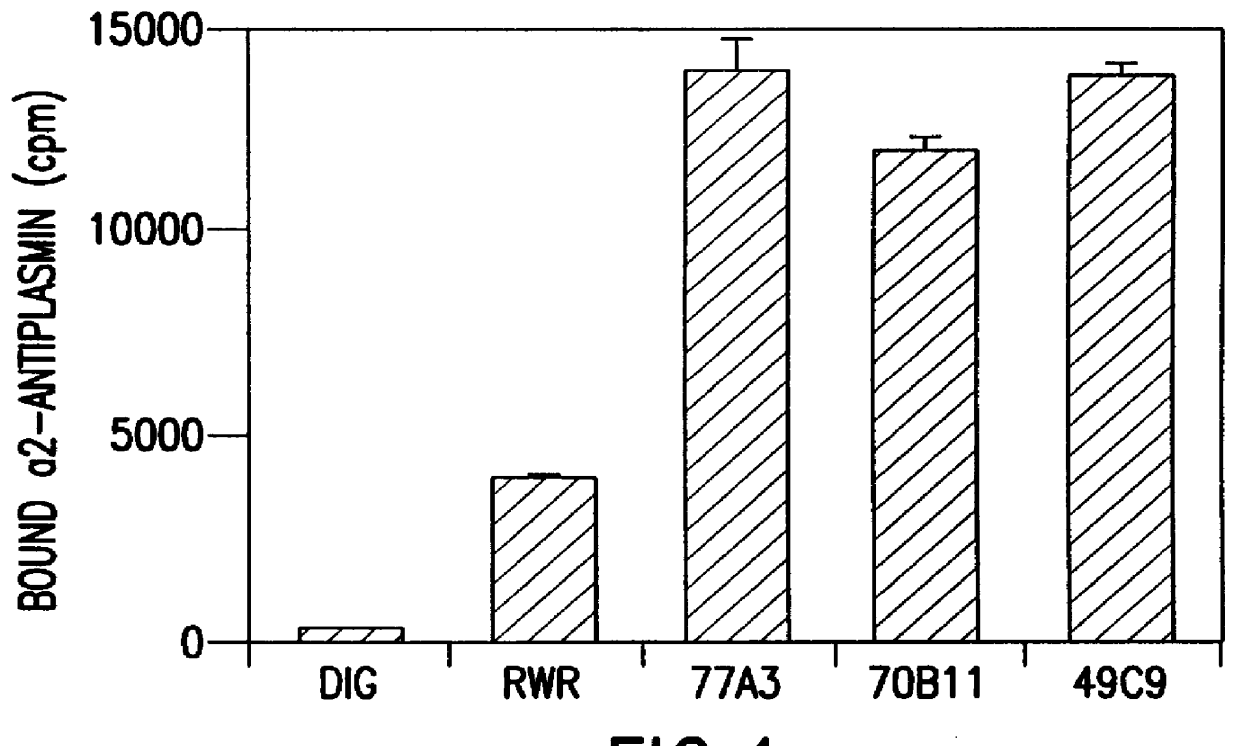
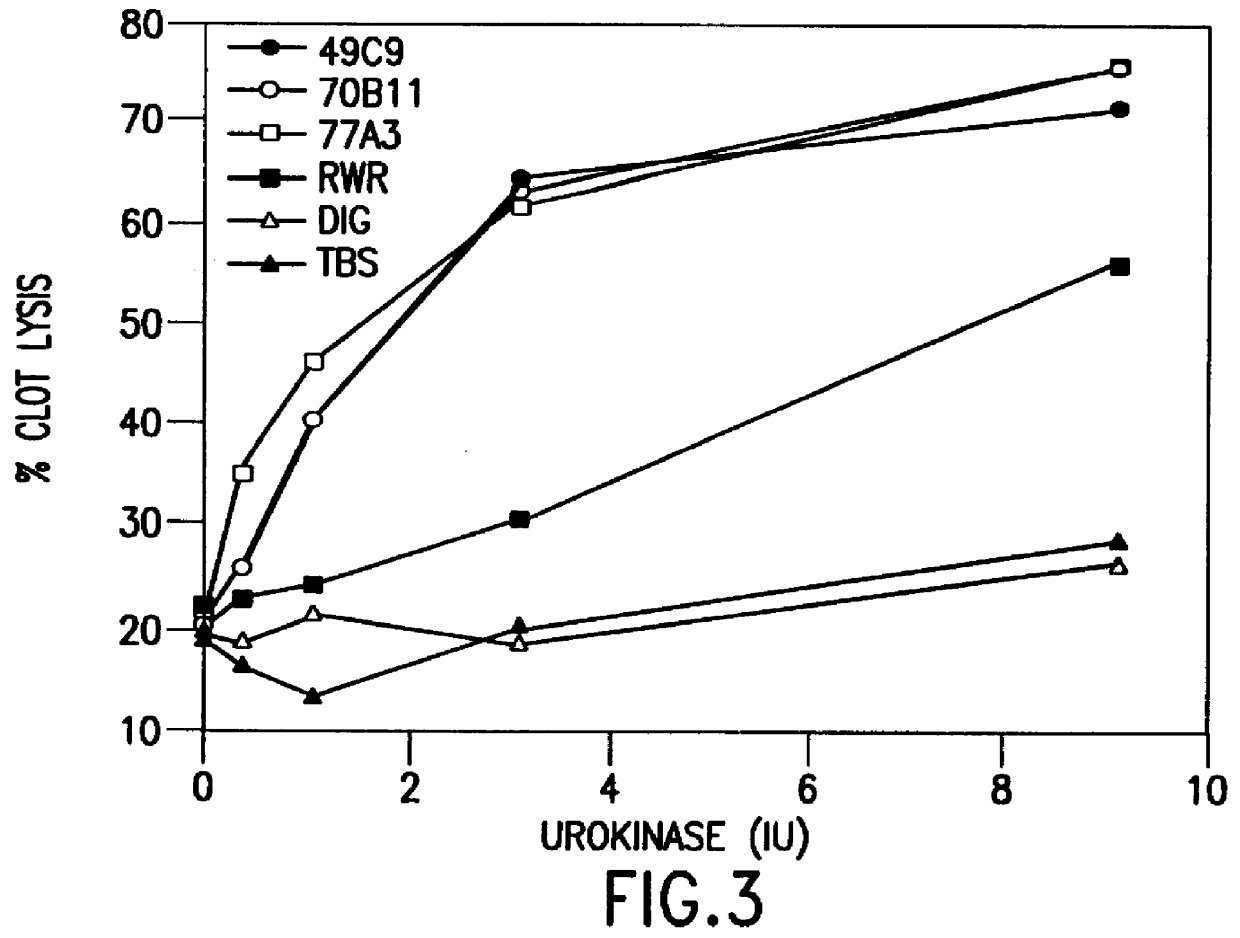
The present invention relates to a novel alpha-2-antiplasmin-binding molecules and treatment for pulmonary embolism, myocardial infarction, thrombosis or stroke in a patient which comprises administering an alpha-2-antiplasmin-binding molecule capable of preventing inhibition of plasmin by endogenous alpha-2-antiplasmin. The invention also relates to a treatment for pulmonary embolism, myocardial infarction, thrombosis or stroke in a patient comprising coadministrating an alpha-2-antiplasmin-binding molecule of the invention together with a thrombolytic agent.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Non-Surgical Method for Preventing or Reducing the Rate of the Progression of Non-Proliferative Diabetic Retinopathy and the Treatment of Other Ocular Conditions
InactiveUS20070128182A1Reduce inflammationSenses disorderPeptide/protein ingredientsDiseaseSerine proteinases
A non-surgical method for preventing or reducing the rate of the progression of non-proliferative diabetic retinopathy to the proliferative form of diabetic retinopathy comprising intravitreally administering to a patient suffering from non-proliferative diabetic retinopathy an effective amount of serine proteinase enzyme sufficient to create, without surgery, a posterior vitreal detachment to prevent or reduce the progression of proliferative diabetic retinopathy in said patient. Also disclosed is a non-surgical method of treating ocular conditions such as retinal ischemia, retinal inflammation, retinal edema tractional retinal detachment, tractional retinopathy, vitreous hemorrhage and tractional maculopathy by intravitreally administering to a patient suffering from one or more of these conditions with an effective amount of a serine proteinase enzyme to reduce or treat that particular ocular condition. Plasmin, microplasmin and miniplasmin are preferred serine proteinase enzymes and plasmin is the most preferred.
Owner:BARTELS STEPHEN P +3
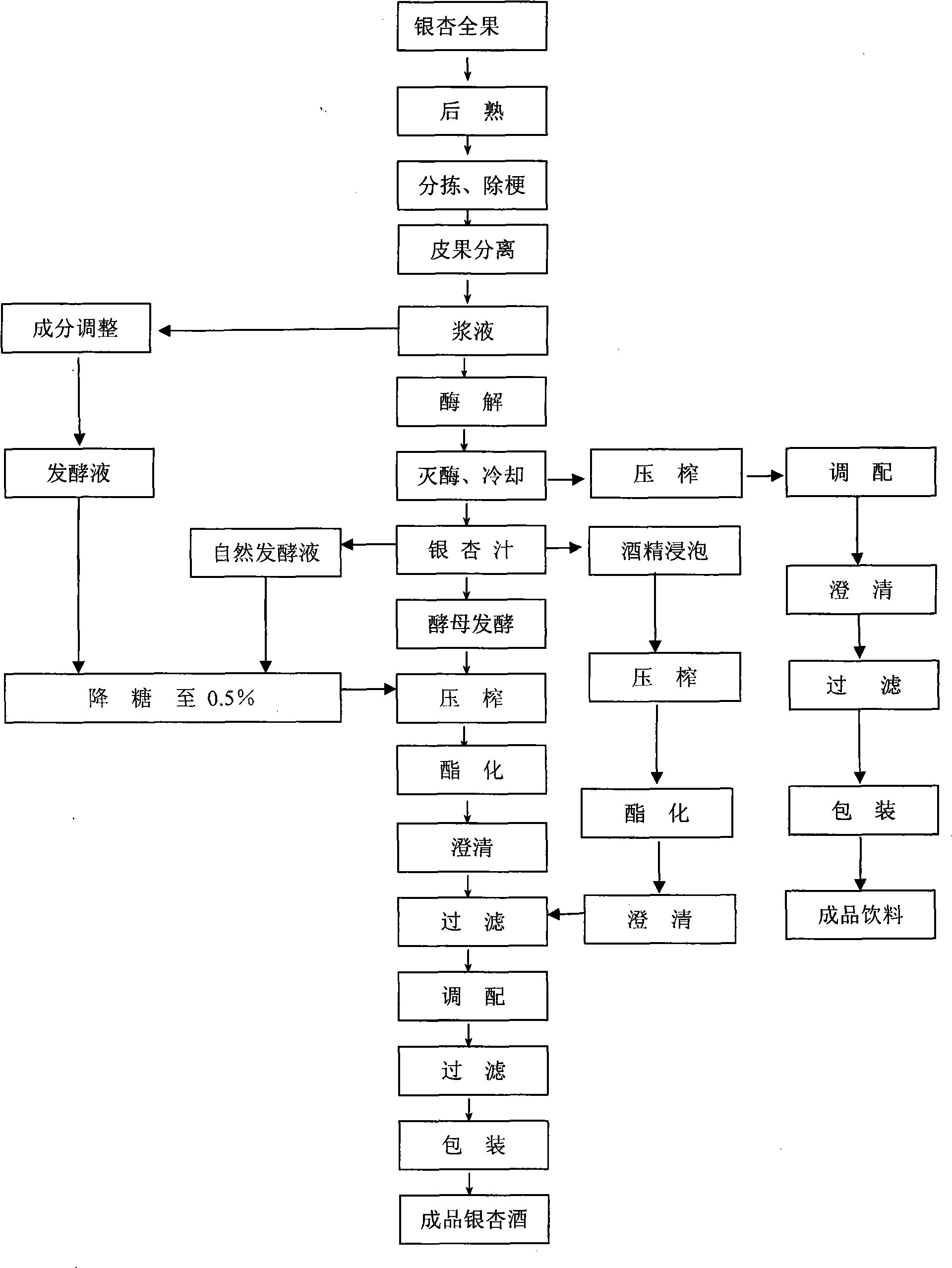
Gingko wine production process
InactiveCN101519629AShort production cycleHealth care functionAlcoholic beverage preparationCerebral vasospasmYeast
The invention relates to the preparation field of alcoholic beverage, in particular to gingko wine preparation with high health care component content, which is produced by taking deserted ginkgo biloba sarcotesta as the raw material, and adopting biological enzyme degrading pectic substance, cellulose, amylum, and other substances, and adopting the processes of wine yeast and natural grape fermentation or off-flavor alcohol infusing after enzyme destruction and defecation. The problems of foreign flavor and toxic component are simultaneously solved. The health care function of the gingko wine is more powerful than that of grape wine, the wine body is unique, the fruit aroma is stronger, the gingko wine is golden-colored and full of nutriments, with pure taste; and the gingko wine has the effects of dilating coronary arteries, enhancing blood flow, inhibiting blood coagulation, activating plasmin system, improving cardiotrophin, promoting cholesterin conversion, maintaining blood vessel elasticity, preventing and treating thromboembolia, coronary heart disease, angina pectoris, cerebral vasospasm and preventing cancer and restraining cancer, and the like. The gingko wine has remarkable economic benefit and social benefit.
Owner:徐琛
Trypsin-like serine protease inhibitors, and their preparation and use
InactiveUS20100022781A1Good effectAvoid constantOrganic compound preparationPeptidesMedicineOrgan transplanting
The invention relates to inhibitors of trypsin-like serine proteases of the general formula (I) which, as well as plasmin, also inhibit plasma kallikrien, and to their preparation and use as medicaments, preferably for treatment of blood loss, especially in the case of hyperfibrinolytic states, in organ transplants or heart surgery interventions, in particular with a cardiopulmonary bypass, or as a constituent of a fibrin adhesive.
Owner:THE MEDICINES CO (LEIPZIG) GMBH
Inhibition of invasive remodelling
InactiveUS20020099004A1Blocks invasive tissue remodellingImprove survivalFibrinogenPeptide/protein ingredientsProteolytic enzymesCell biology
Owner:LUND LEIF ROGE +6
Variants of plasminogen and plasmin
InactiveUS20120114630A1Excellent long-term storage stabilityReducing circulating fibrinogenSenses disorderFungiProteinase activityPlasmin
The invention relates to variants of plasminogen and plasmin comprising one or more point mutations in the catalytic domain which reduce or prevent autocatylic destruction of the protease activity of plasmin. Compositions, uses and methods of using said variants of plasminogen and plasmin are also disclosed.
Owner:THROMBOGENICS NV
Method for creating a separation of posterior cortical vitreous from the retina of the eye
InactiveUS20060024349A1Minimize or altogether eliminate the vitreoretinal tractionMinimize tractionSenses disorderSaccharide peptide ingredientsVitreous HumorsBody fluid
A method is disclosed for creating a separation of posterior cortical vitreous from the retina of the eye. The method includes the step of introducing plasmin into the vitreous humor of the eye. The plasmin may be introduced either by injection or through a sustained release device. Optionally, other enzymes, polysaccharides, and / or glycoproteins are intermixed with the plasmin.
Owner:NUVUE TECH
TGF-beta activation and use
InactiveUS20050065089A1Improve biological activityPeptide/protein ingredientsMammal material medical ingredientsPlasminChemistry
Proteases are added to Platelet Rich Plasma (PRP) along with calcium and thrombin to activate latent TGF-β. The proteases include plasmin, calpin, MMP-9, thrombospondin, transglutaminase, the mannose 6-phosphate receptor (M6PR), furin, substilisin-like endoproteases, and integrins. Dermatopontin can also be added to the PRP to enhance its biologic activity.
Owner:FERREE BRET A
Compositions and Methods for Effecting Controlled Posterior Vitreous Detachment
InactiveUS20070212358A1Eliminate side effectsLower potentialBiocidePeptide/protein ingredientsPosterior vitreous detachmentVitreous Bodies
A composition comprises plasmin or an enzymatically equivalent derivative thereof and an inhibitor of at least an enzyme that is activatable, directly or indirectly, by plasmin or one of its enzymatically equivalent derivatives. The composition can be used to effect or induce a controlled posterior vitreous detachment (“PVD”) to prevent, treat, or ameliorate a potential complication of a pathological ocular condition. Such a composition can be administered intravitreally.
Owner:BAUSCH & LOMB INC
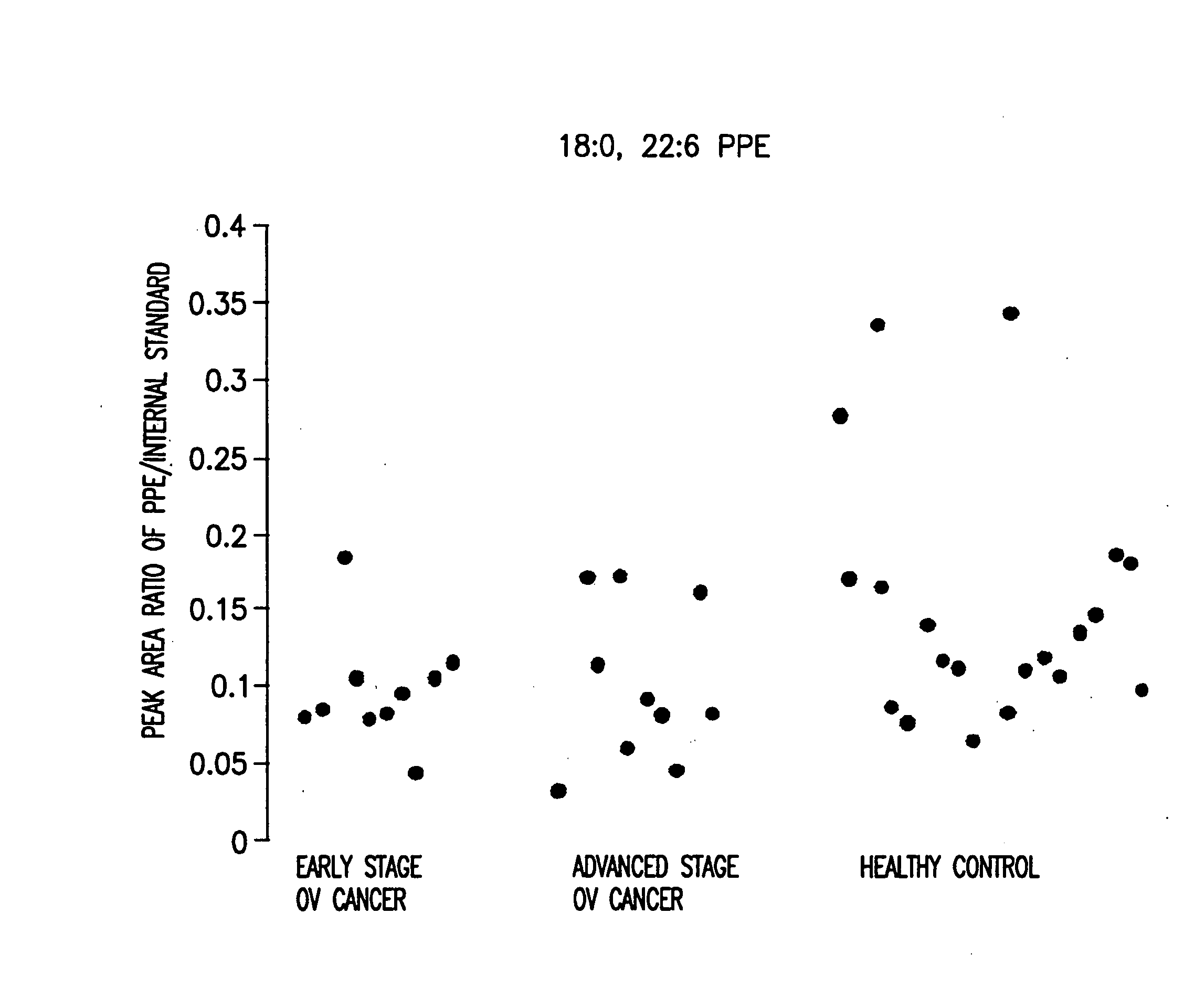
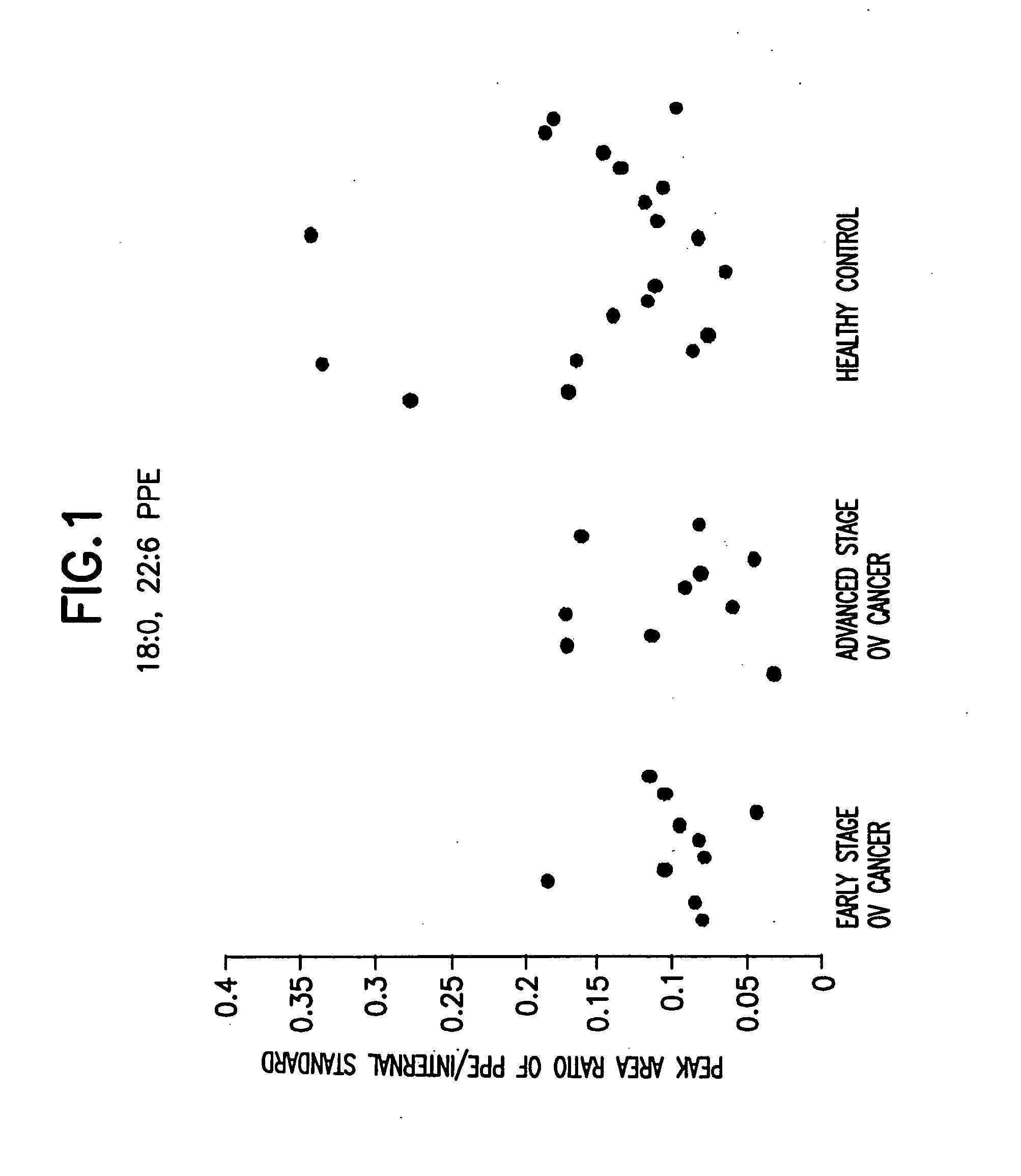
Method for detecting an inflammatory disease or cancer
A method of detecting an inflammatory disease or a cancer in a test subject comprising determining the amount of plasmenyl-PE or a biomarker having a mass charge ratio of approximately 698.2, 722.2, 726.2 or 750.2 in a sample of bodily fluid taken from the test subject and comparing the amount of plasmenyl-PE (or the biomarker) in the sample of the bodily fluid from the test subject to a range of amounts of plasmenyl-PE (or the biomarker) found in samples of the bodily fluid from a group of normal subjects of the same species as the test subject and lacking the inflammatory disease or the cancer, whereby a change in the amount of the plasmenyl-PE (or the biomarker) (such as a lower amount) in the sample of the bodily fluid from the test subject indicates the presence of the inflammatory disease or the cancer.
Owner:FRANTZ BIOMARKERS
Polypeptide and use thereof in medicament preparation
ActiveCN101914150AGood hypoglycemicPeptide/protein ingredientsMetabolism disorderBlood sugarPharmacology
The present invention belongs to the field of bio-pharmaceuticals and diabetes, and discloses a polypetide and use of the polypetide in medicament preparation. The polypetide is (a) or (b): (a) polypeptide which is composed of amino acid sequence represented by SEQ ID NO:22; (b) polypetide which is composed of the amino acid sequence in (a) through replacement, deletion or adding one or several amino acids, has blood sugar reduction and / or blood fat reduction function and is derived from (a). The polypeptide and a plasmin inhibitor thereof have the following functions: reducing blood sugar, reducing triglyceride, total cholesterol or LDL in blood. The polypeptide can be used for preparing the blood sugar reduction medicament or blood fat reduction medicament, and particularly for the diabetes treatment medicament.
Owner:刘建宁
Plasmin inhibitors from the australian brown snake Pseudonaja textilis textilis
InactiveUS7070969B1High enzymeReduced inhibition efficiencyPeptide/protein ingredientsAntibody ingredientsSingle stageNucleotide
The invention provides novel single stage competitive inhibitors of plasmin from the Australian brown snake Pseudonaja textilis textilis. The invention also features polynucleotides and polynucleotide homologues encoding these inhibitors. Pharmaceutical compositions containing the plasmin inhibitors of the invention are also disclosed as well as methods useful for treatment of blood loss.
Owner:THE UNIV OF QUEENSLAND +1
Novel monoclonal antibodies and method of immunological analysis of d-dimer
ActiveUS20130011869A1Accurate measurementAvoid inhibitionImmunoglobulins against blood coagulation factorsAnimal cellsDimerFibrinogen
Provided are an antibody capable of specifically and accurately measuring digested products of stabilized fibrin (D-dimer), and a method and a reagent for measuring D-dimer using the antibody. The antibody specifically reacts with D-dimer, which is plasmin-digested products of stabilized fibrin, but does not react with fibrinogen or plasmin-digested products of fibrinogen, which include fragment X, fragment Y, fragment D1, and fragment E3, and does not react with dissociation products of DD / E monomer, which include fragment DD, fragment E1, and fragment E2.
Owner:MITSUBISHI CHEM MEDIENCE
Method for purifying plasmin in animal medicine and preparing traditional Chinese medicine composition
ActiveCN103656630ASimple extraction processGood effectPowder deliveryAnthropod material medical ingredientsBiotechnologyMedicinal herbs
The invention discloses a method for separating and purifying the plasmin component in an animal medicine by a membrane separation technology and preparing a traditional Chinese medicine composition by use of the component. The method comprises the following steps: (1) weighing a proper quantity of ants or ground beeltles, and crushing into coarse powder; (2) soaking the coarse powder in water of which the amount is 6 times that of the coarse powder, and stirring to obtain homogenate; and fetching the homogenate and placing in a constant-temperature water bath, and stirring; (3) centrifuging and discarding the precipitate; (4) filtering the centrifugate with a pore membrane; (5) performing ultrafiltration concentration of the filtrate with an ultrafilter (molecular weight cut-off is 3,000-20,000) until the volume ratio of the medicine to the medicine liquid is about 1:1; and freeze-drying the concentrated liquid into powder for later use; and (6) independently using the freeze-dried powder or matching the freeze-dried powder with other components to obtain a composition for use. The invention provides a new method for separating and purifying the plasmin component in the animal medicine; and the extraction process is simple, and the effect is remarkable.
Owner:JIANGSU TIANZHAO PHARMA
Method for culturing cordyceps militaris mycelium and plasmin
ActiveCN104911169AExtensive growth conditionsEnzyme production cycle is shortFungiMicroorganism based processesBiotechnologyNutrient solution
The invention discloses a method for culturing cordyceps militaris mycelium and plasmin. The used cordyceps militaris bacterial strain has a preservation number of CGMCC No.2577 in the General Microbiological Center of China Committee of Culture Collection for Microorganisms on 4th, July, 2008. The taxology name is cordyceps militaris. The culture method comprises bacterial classification activation, seed nutrient solution preparation and solid fermentation, a solid fermentation matrix contains cordyceps militaris and plasmi, its plasmin vitality can reach 927.77U / g. The method uses rice and wheat bran as culture matrix for culturing cordyceps militaris and producing cordyceps militaris mycelium and plasmin, bacterial classification and production raw material are safe and reliable, the raw material has the advantages of low cost and easy acquisition, compared with a traditional cultivation technology, the production period is shortened by 5 / 6, operation is simple, and realization is easy. The method provides a novel thinking and research base for research and development of health food for preventing and treating pulmonary thromboembolism, and provides a novel approach for producing cordyceps militaris mycelium.
Owner:QIQIHAR UNIVERSITY
Traditional Chinese medicine preparation for purifying blood
InactiveCN101804110AGood hypolipidemic effectImprove solubilityAnthropod material medical ingredientsMetabolism disorderDiseasePropolis
The invention discloses a traditional Chinese medicine preparation for purifying blood, which comprises the following components: hawthorn, Astragalus root, leech, cortex moutan, safflower, hemlock parsley, suberect spatholobus stem, vinegar-radix paeoniae alba, angelica, trogopterus dung, salviae miltiorrhizae, propolis, calendula officinalis root, ginkgo leaf and gynostemma pentaphylla. The traditional Chinese medicine composite has the obvious effect of reducing blood lipid, can promote the decomposition of blood lipid, reduces blood viscosity, and resists atherosclerosis; the traditional Chinese medicine composite has the obvious effects of anticoagulation and anti-thrombosis, can inhibit the aggregation of thrombocyte, improves the activity of plasmin, and promotes fibrinolysis; the traditional Chinese medicine composite has favorable effects of heart strengthening, decompression and arrhythmia resistance, can slow heart rate, dilates coronary artery and blood vessel of brain, increases blood flow, lightens myocardial ischemic injury degree, lowers myocardial oxygen consumption, has obvious prevention effect on ischemic cerebrovascular diseases, can dilate peripheral vessel, reduces vascular resistance and promotes blood circulation; and the traditional Chinese medicine composite can promote the generation of haemoglobin and erythrocyte.
Owner:杨勤峰
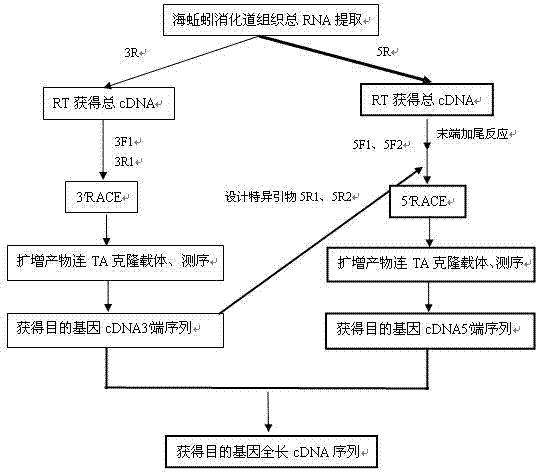
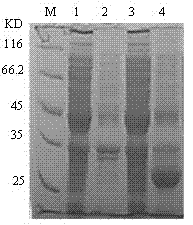
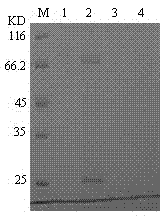
cDNA sequence and amino acid sequence of lugworm fibrinolytic enzyme with thrombolysis activity
The invention discloses a cDNA sequence and an amino acid sequence of coding lugworm fibrinolytic enzyme which are respectively shown in SEQIDNo.1 and SEQIDNo.2 in a sequence table, and belongs to the field of medical biological engineering. The cDNA sequence has the beneficial effects that according to the cDNA sequence of the lugworm fibrinolytic enzyme, the coding gene of the fibrinolytic enzyme can be cloned from digestive tract tissue of lugworm, and can be expressed in a prokaryotic expression system or a eukaryotic expression system through a gene recombination technology. The recombinant lugworm fibrinolytic enzyme has plasminogen activation activity, and can be used as a new thrombolytic medicament in genetic engineering.
Owner:WEIFANG MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com