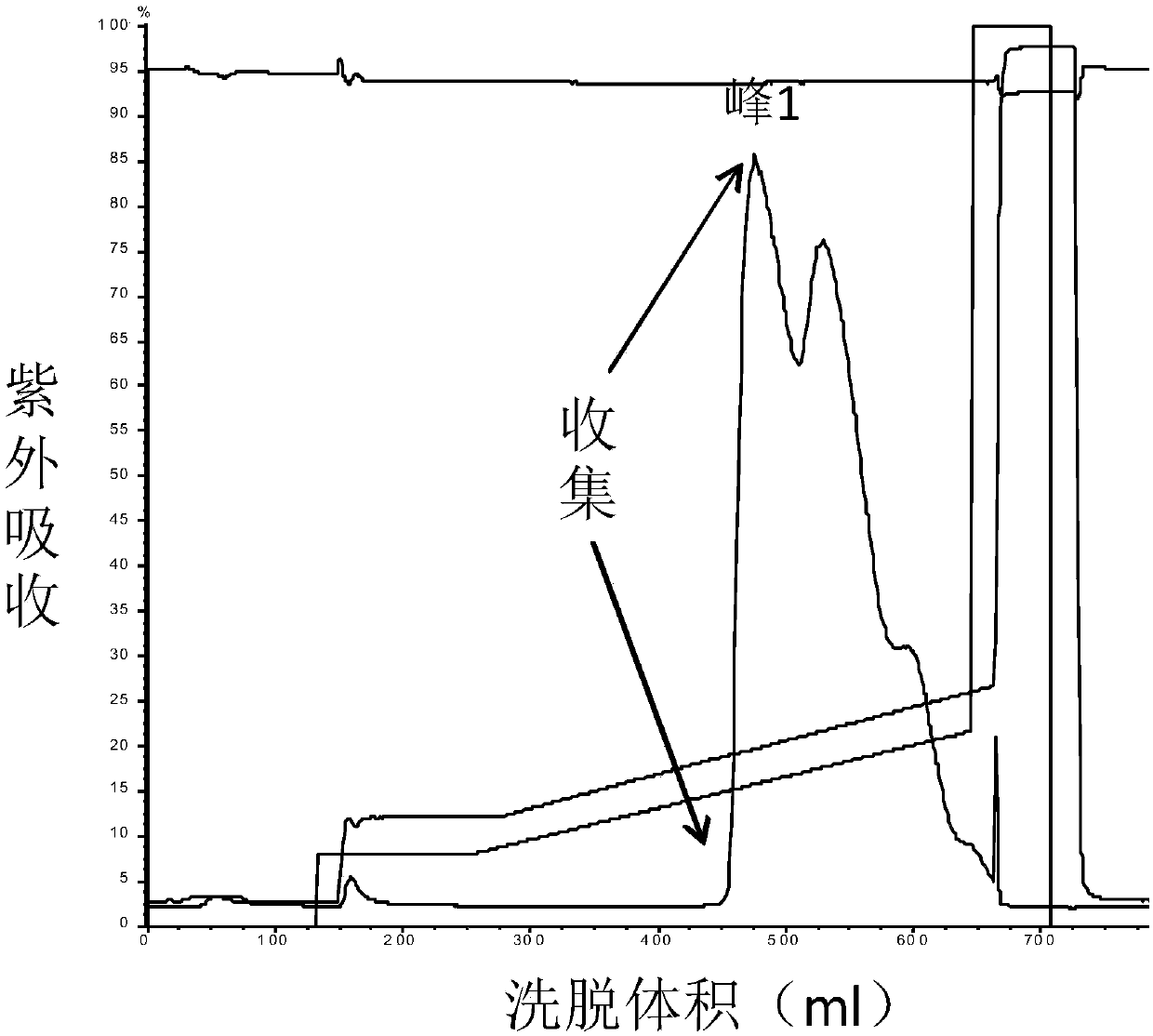
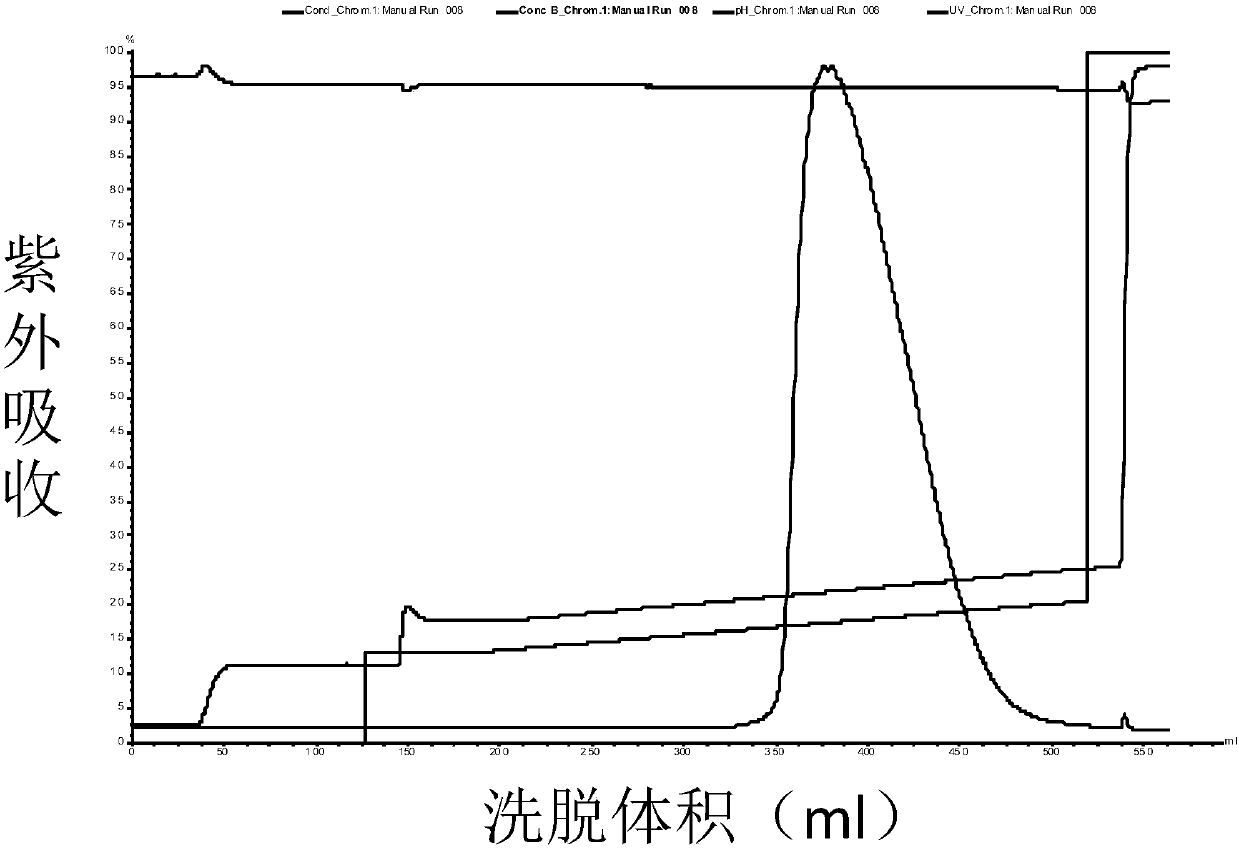
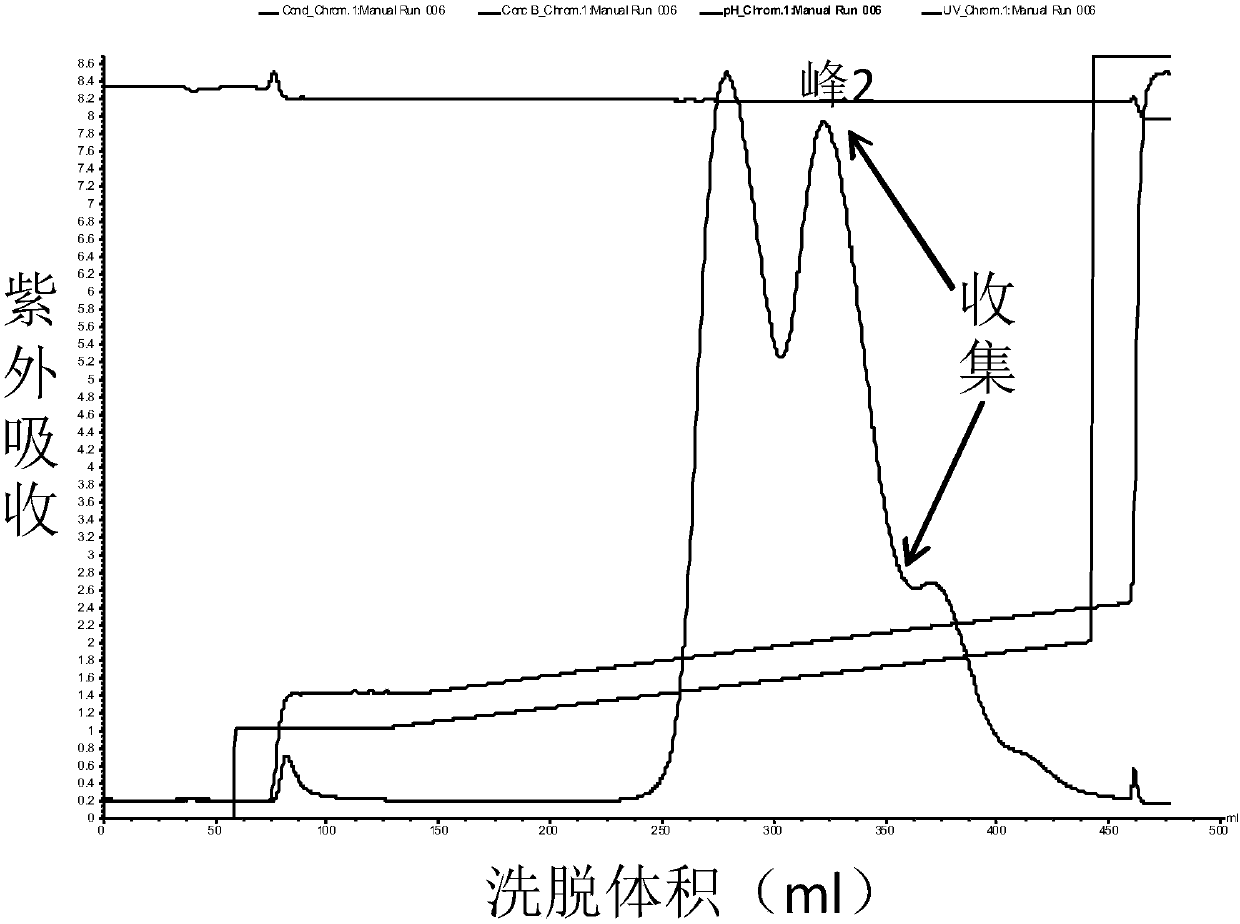
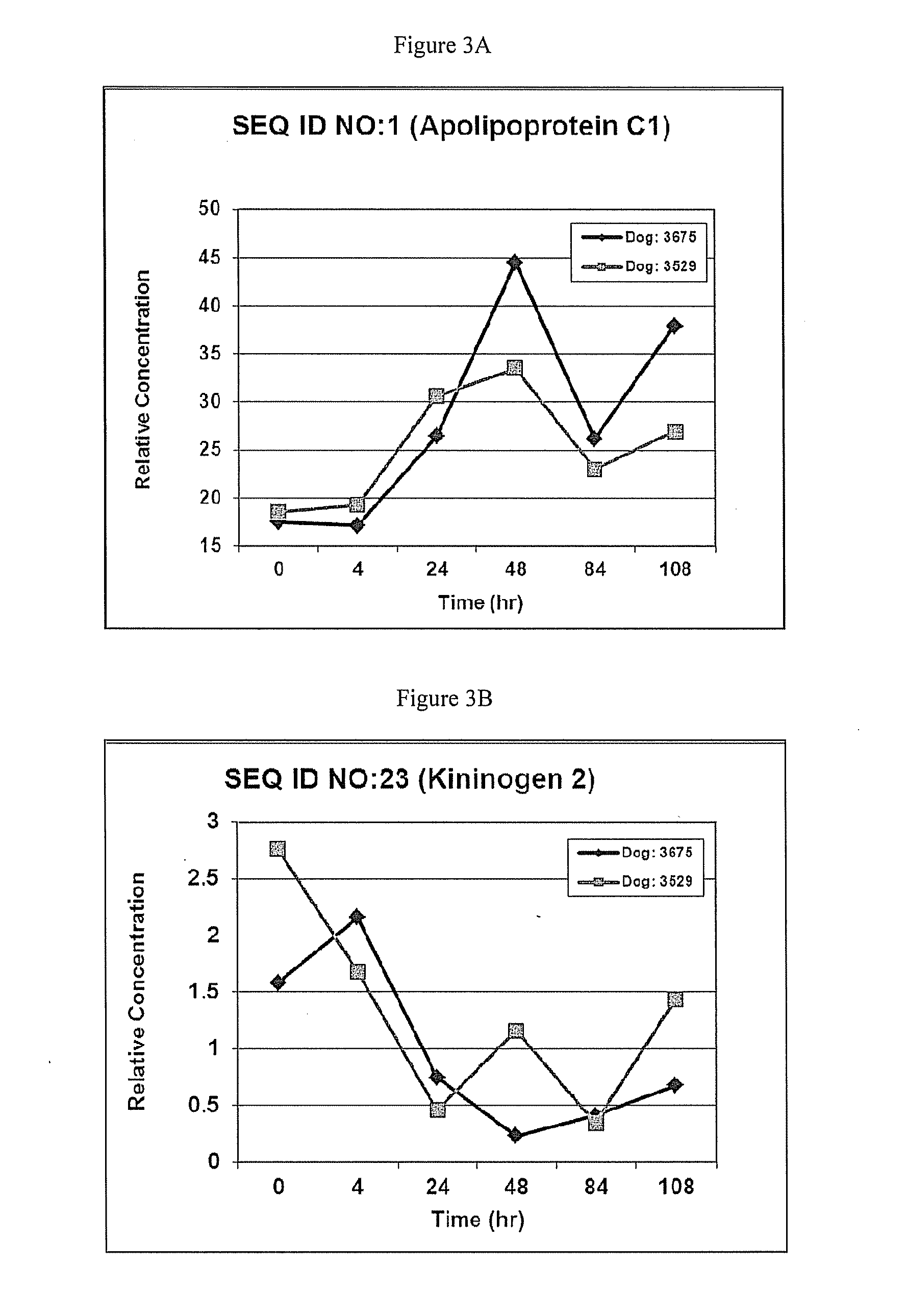
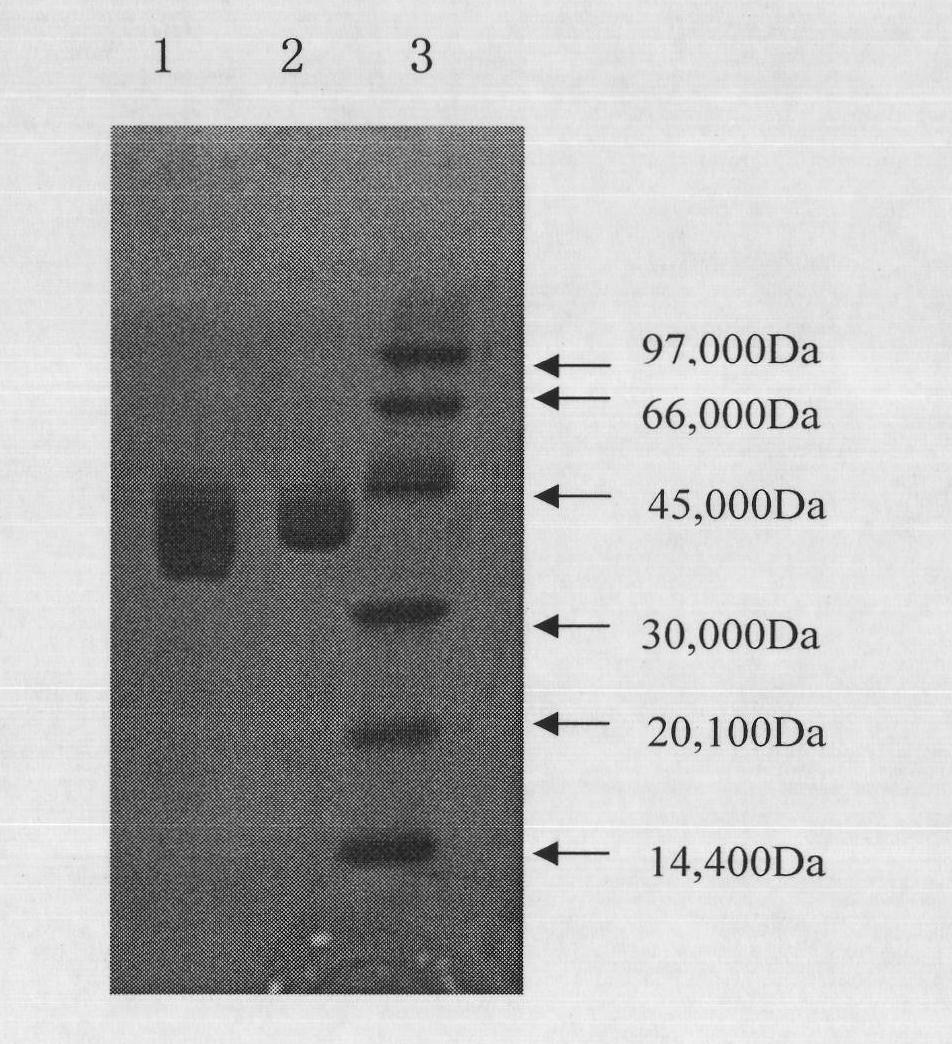
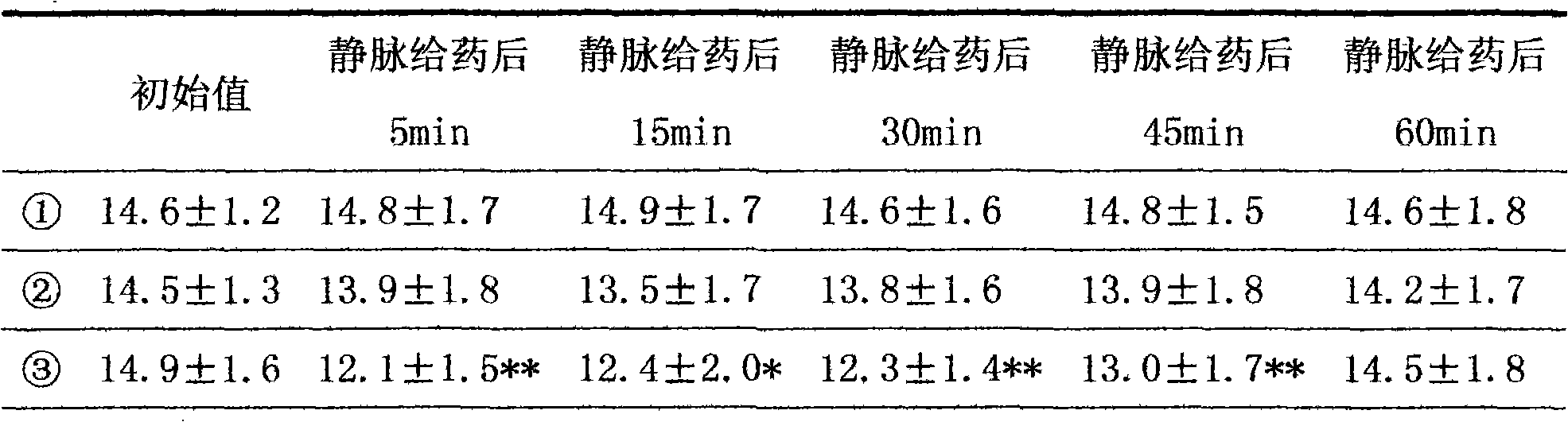
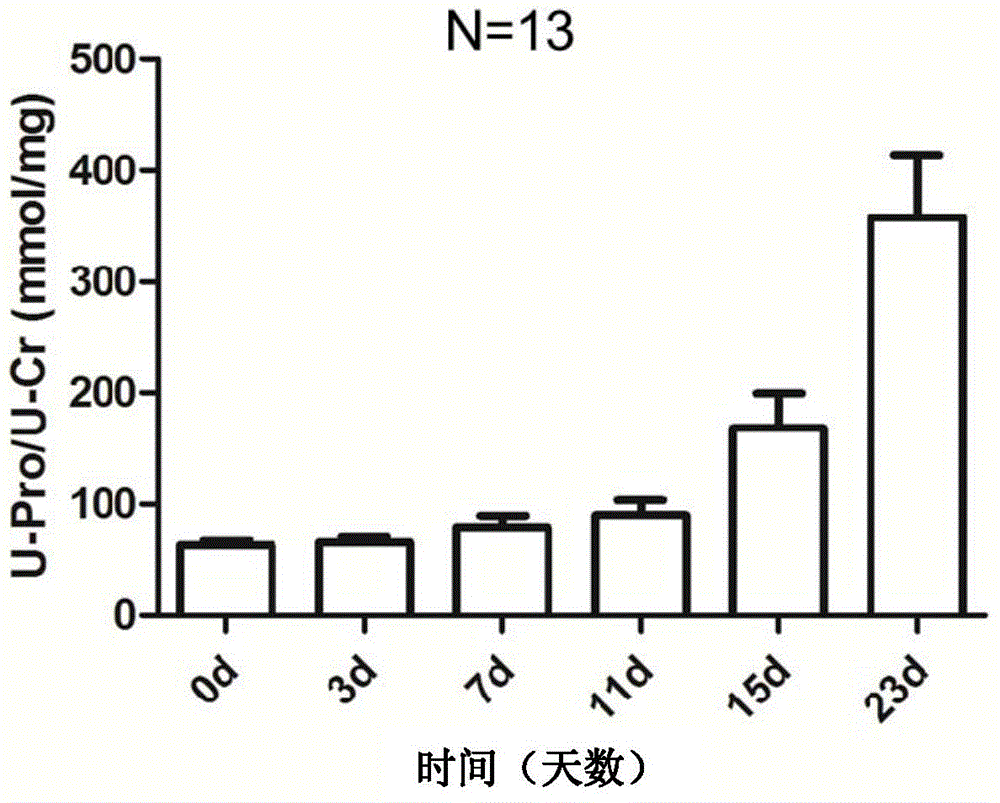
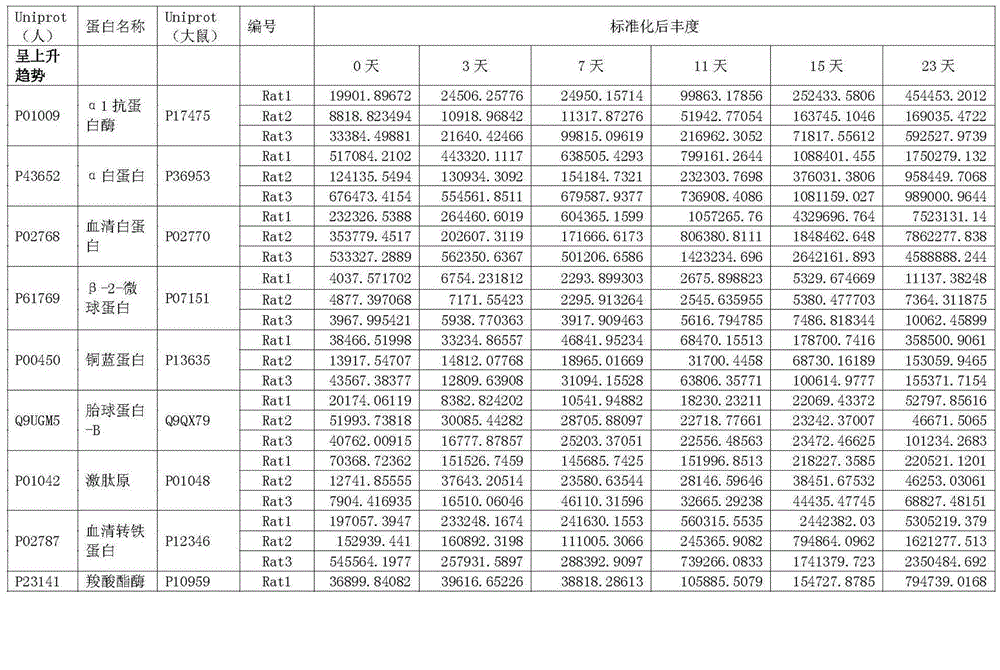
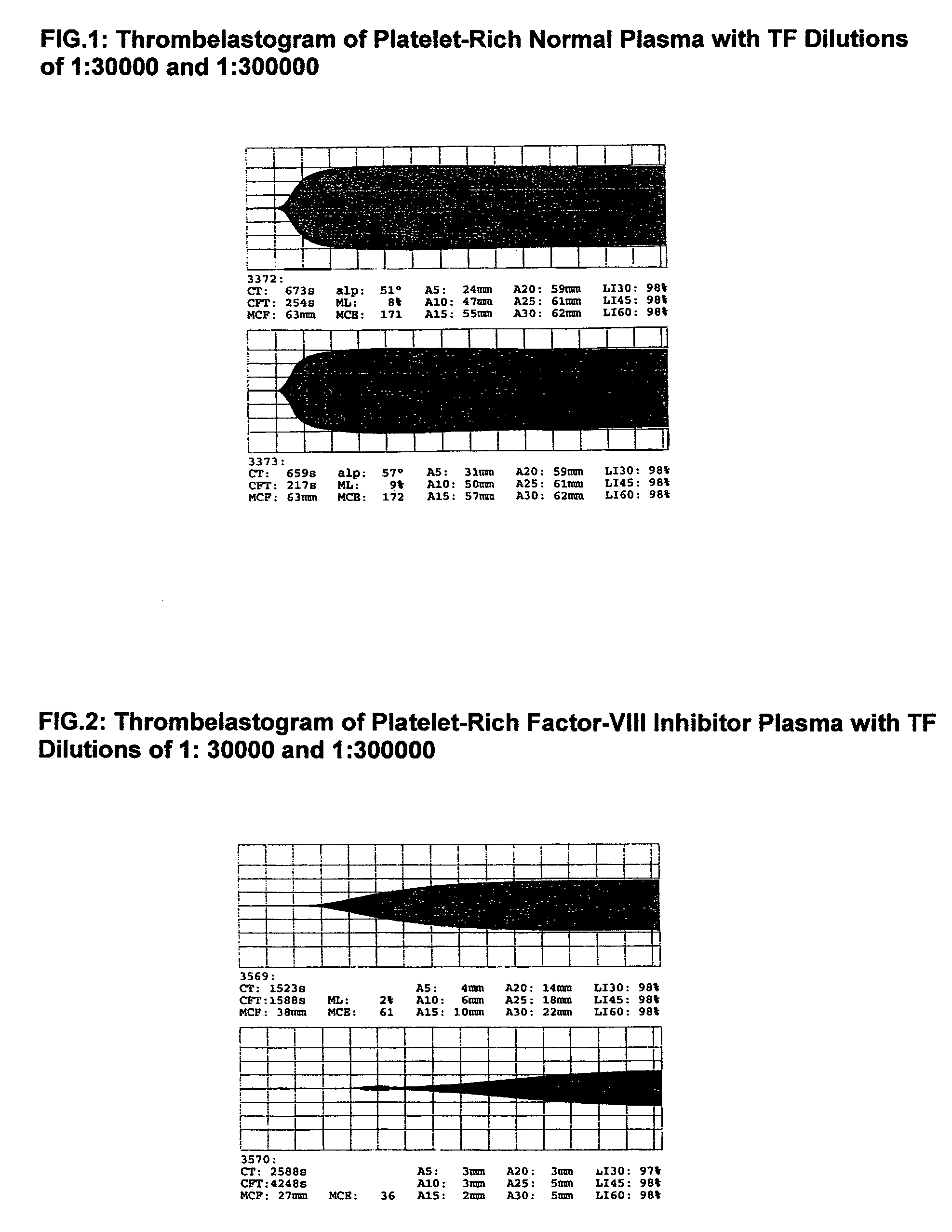
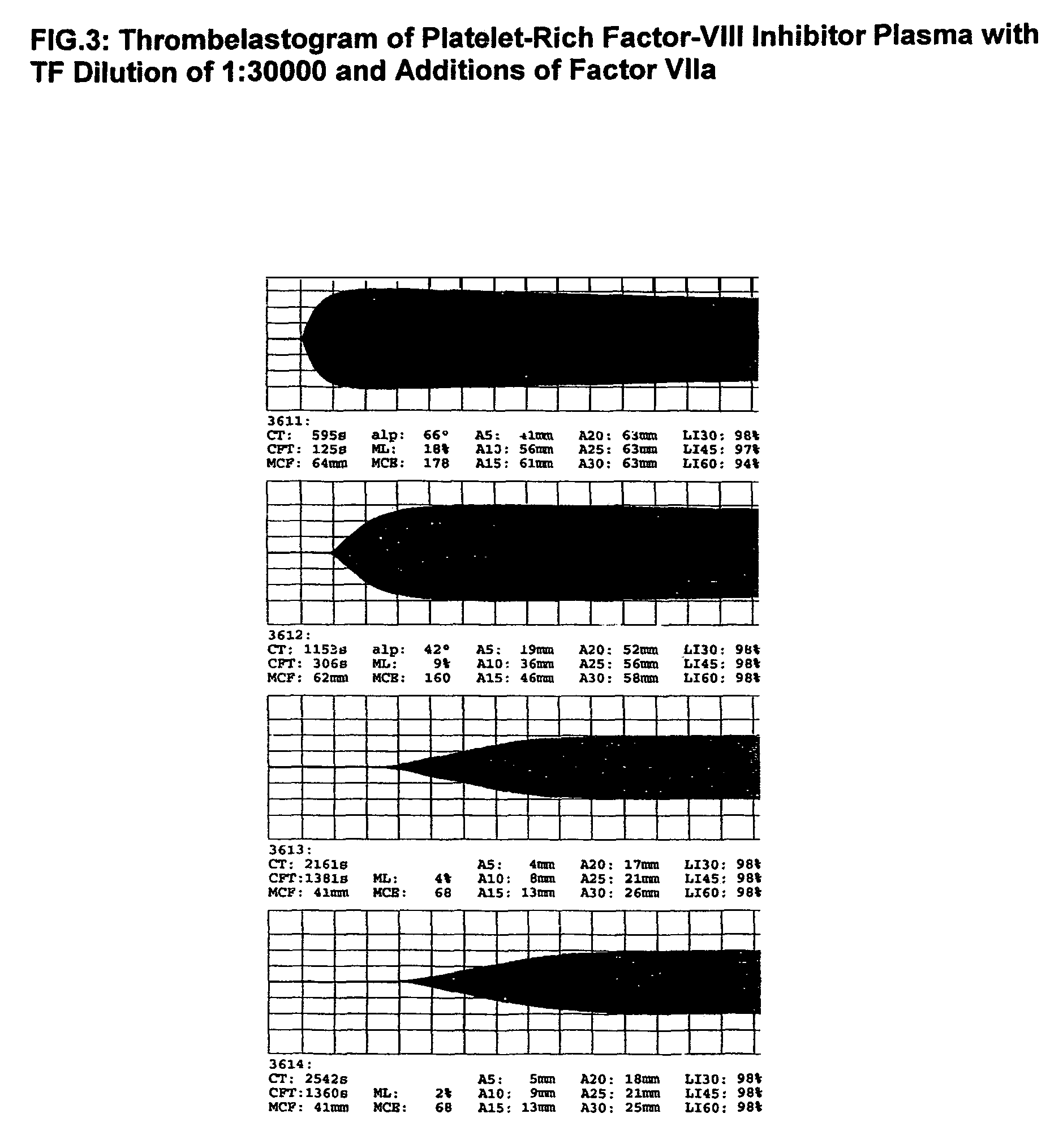
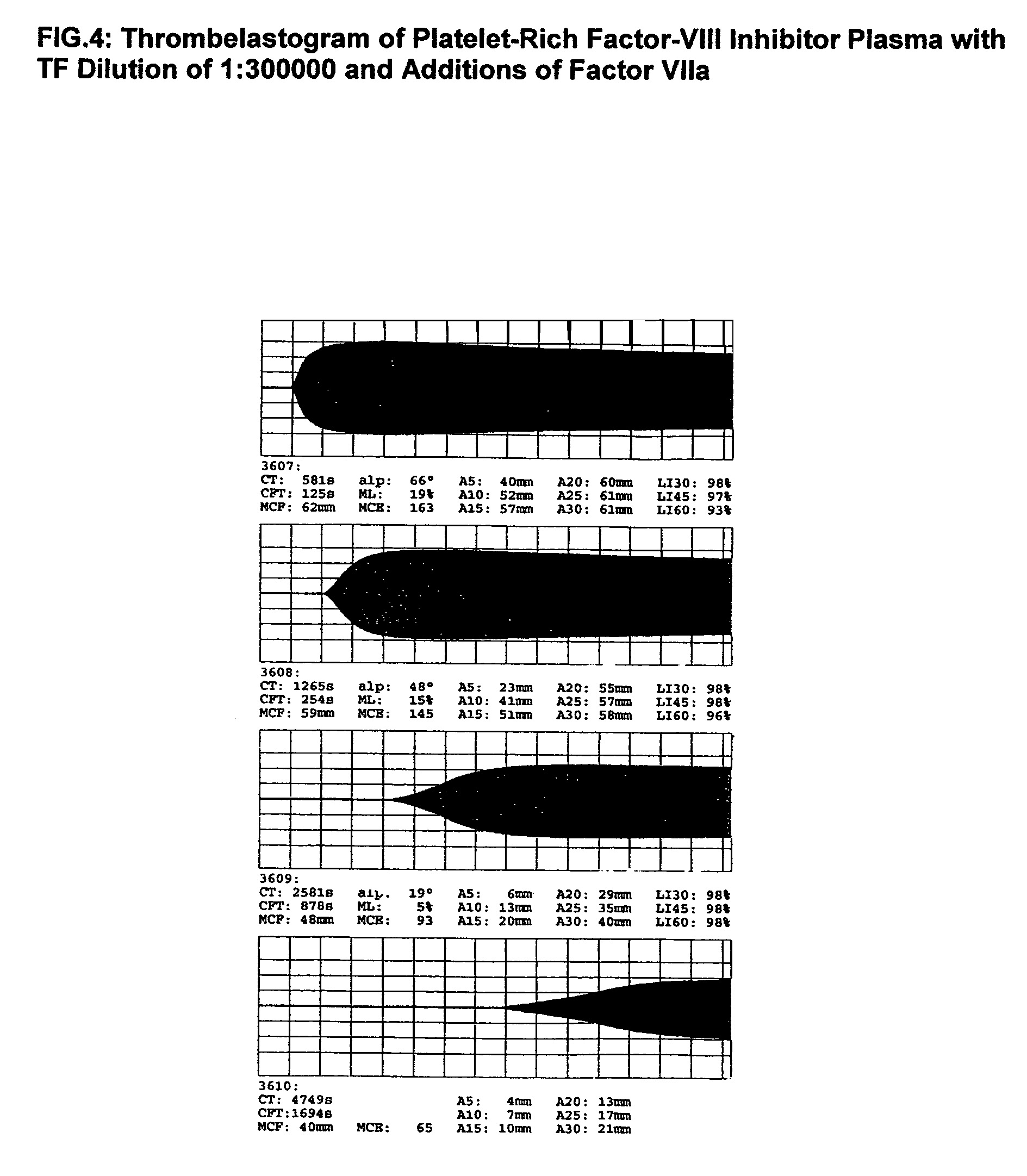
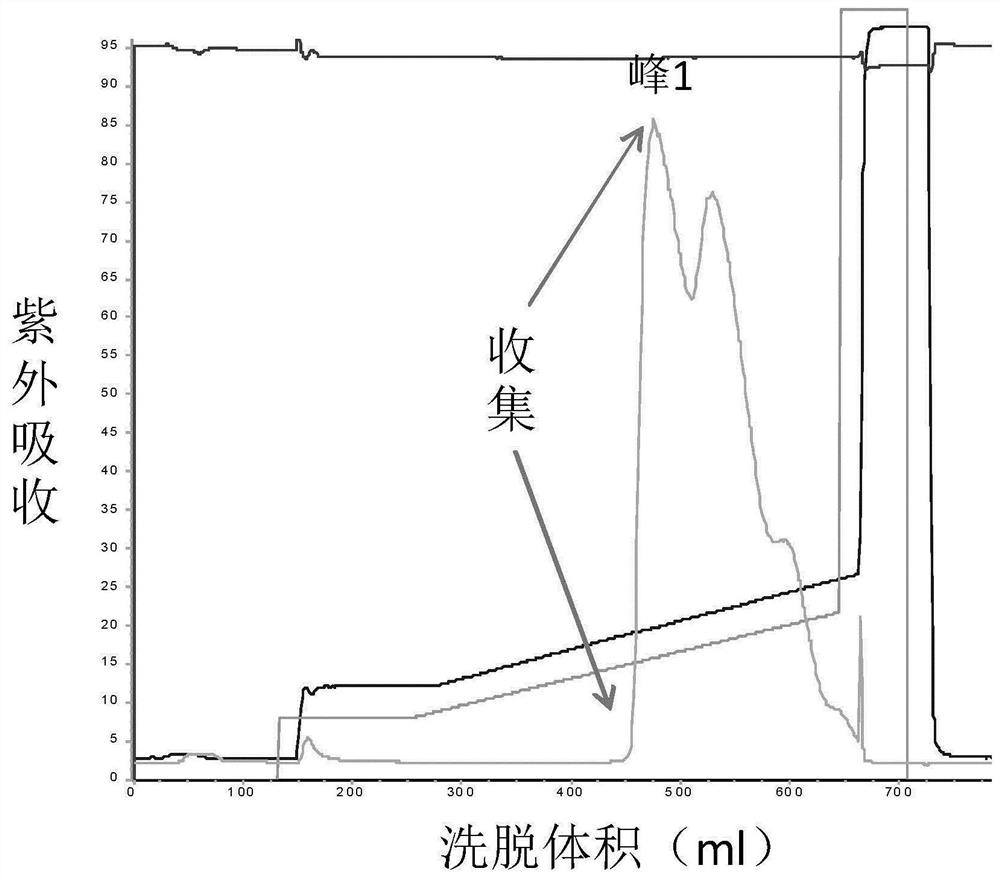
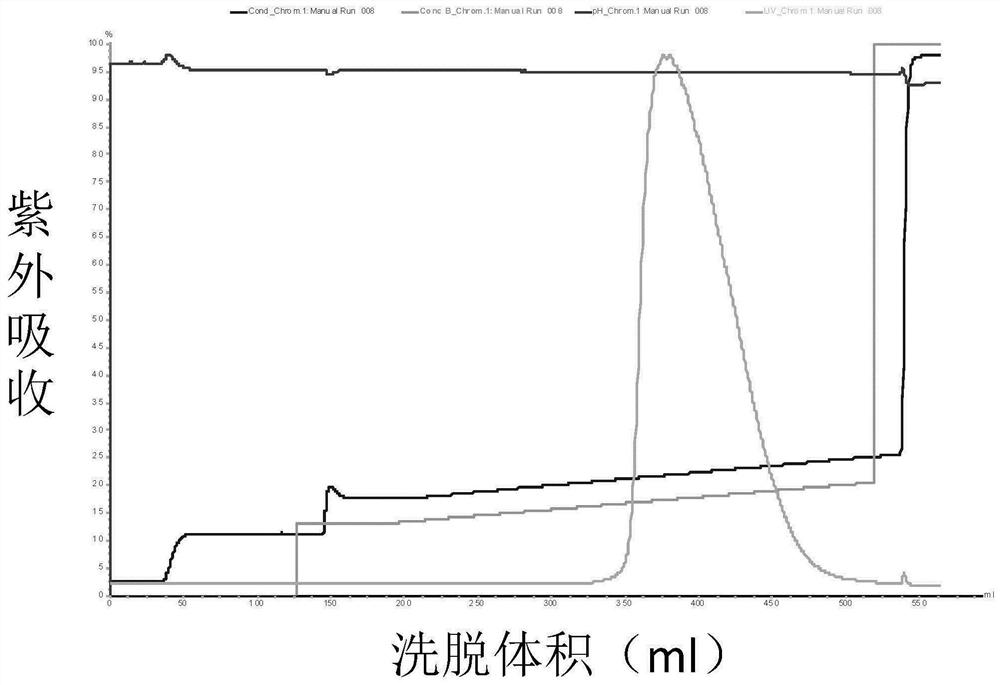
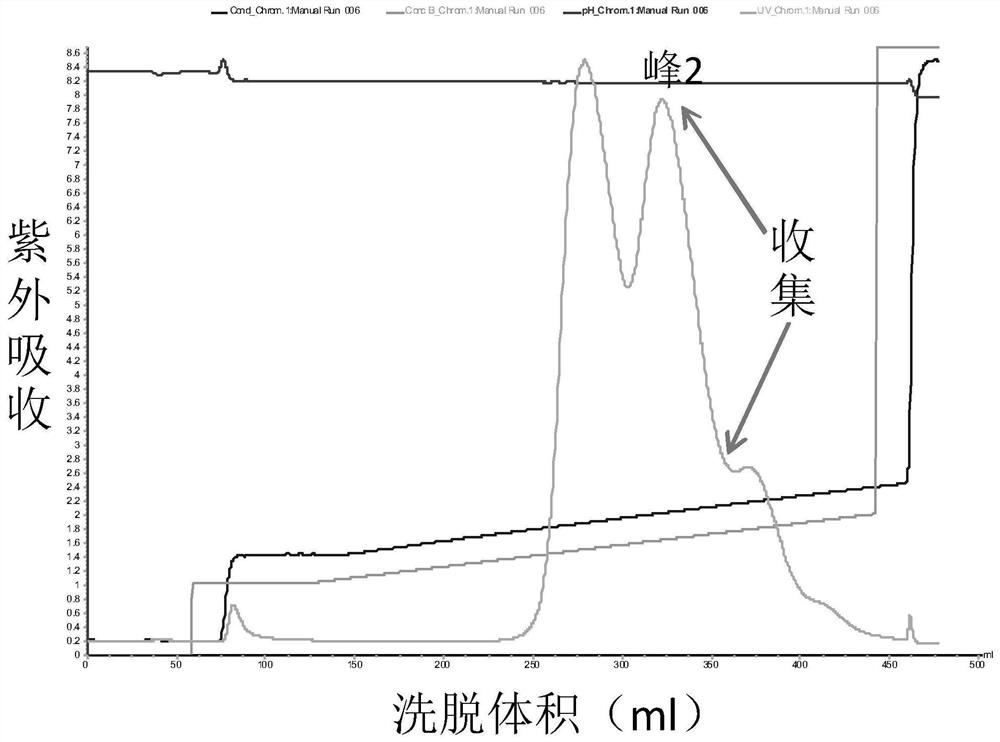
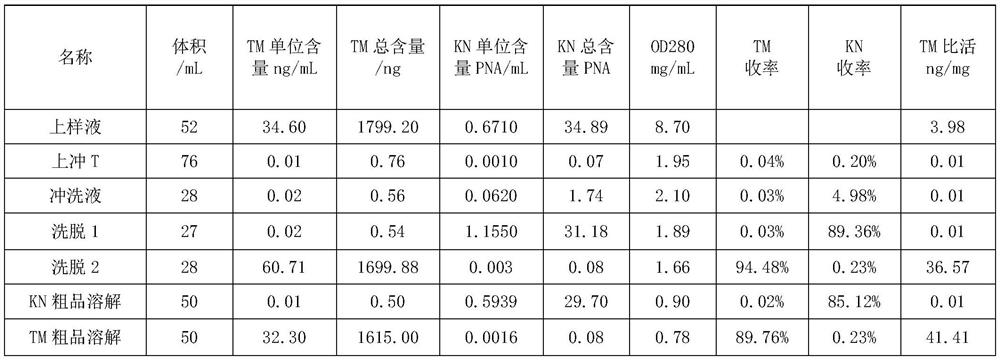
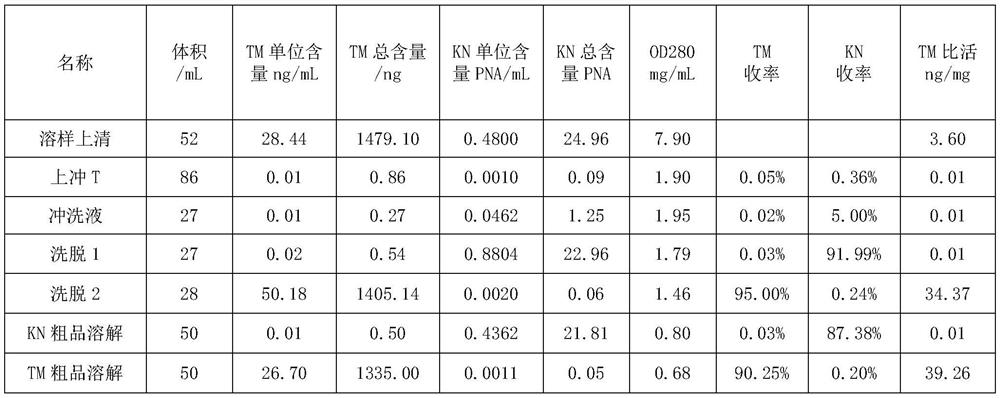
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Kininogen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
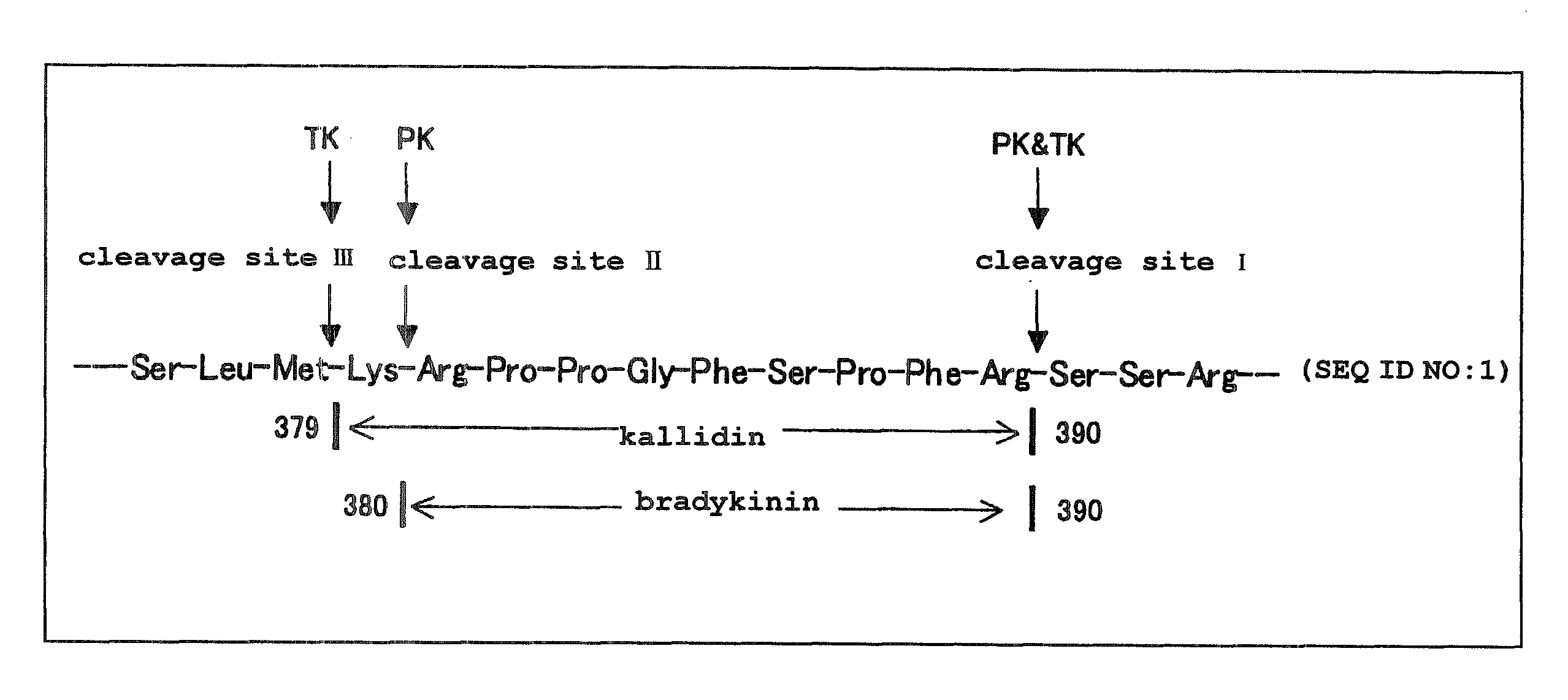
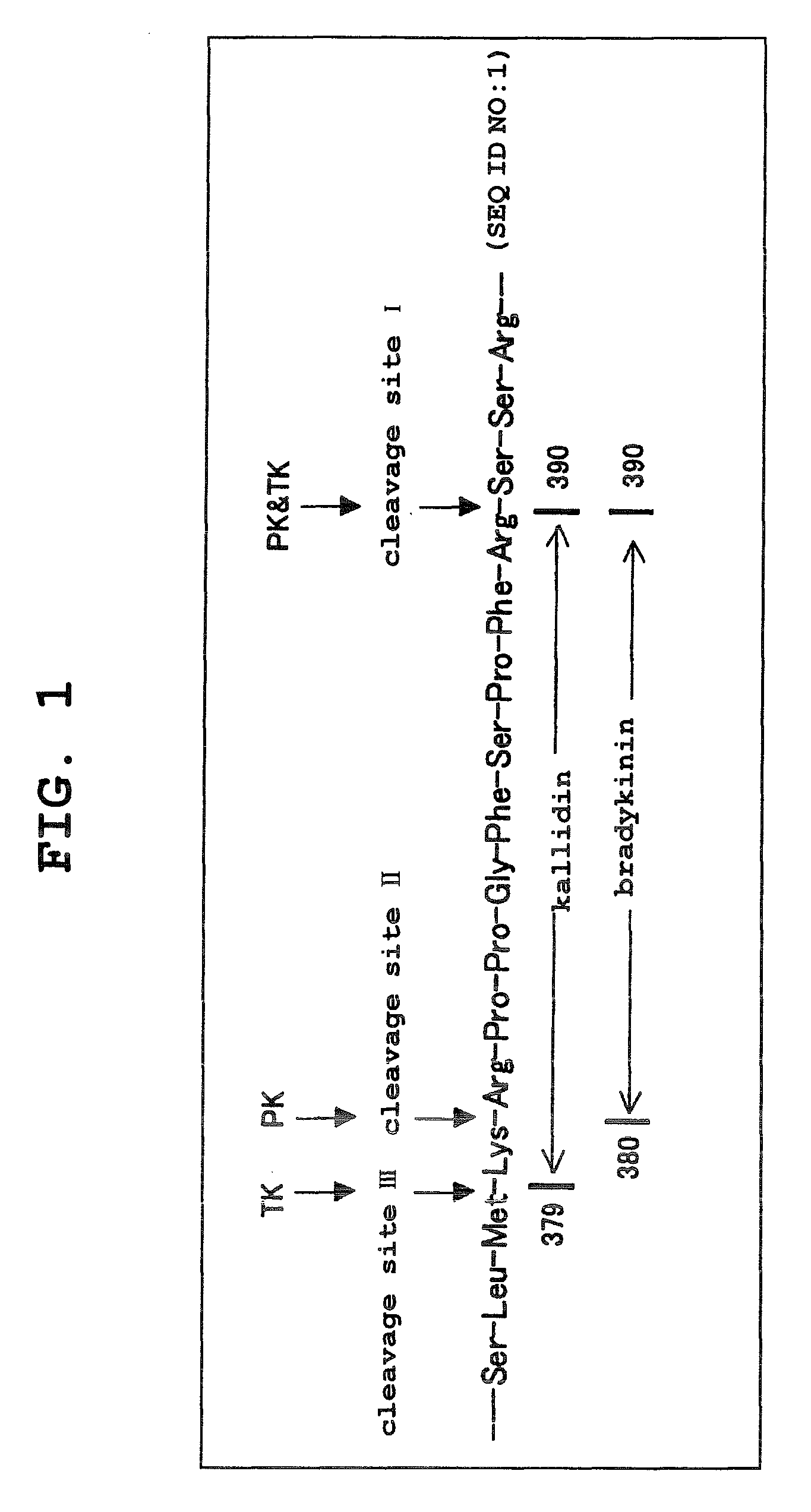
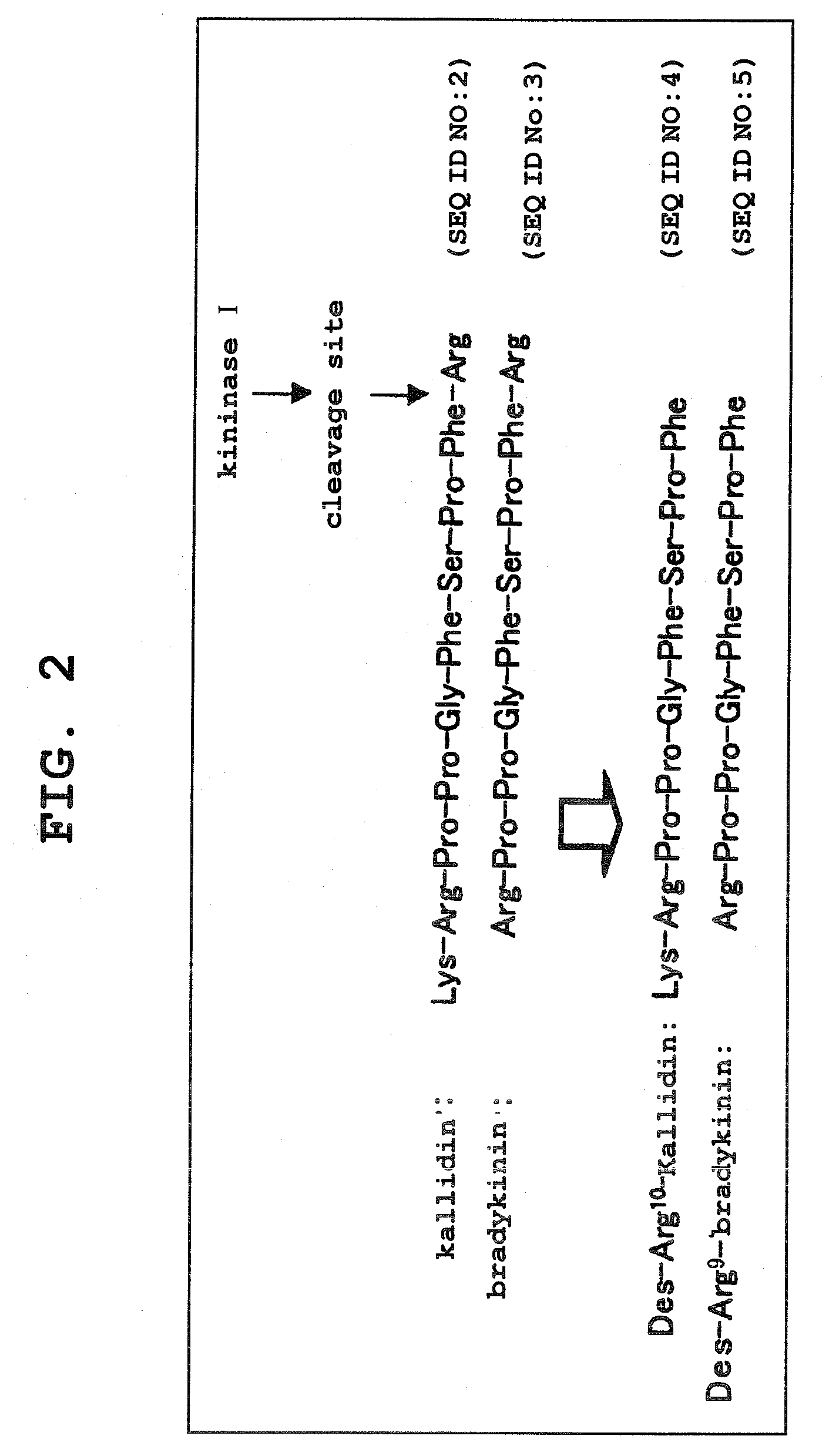
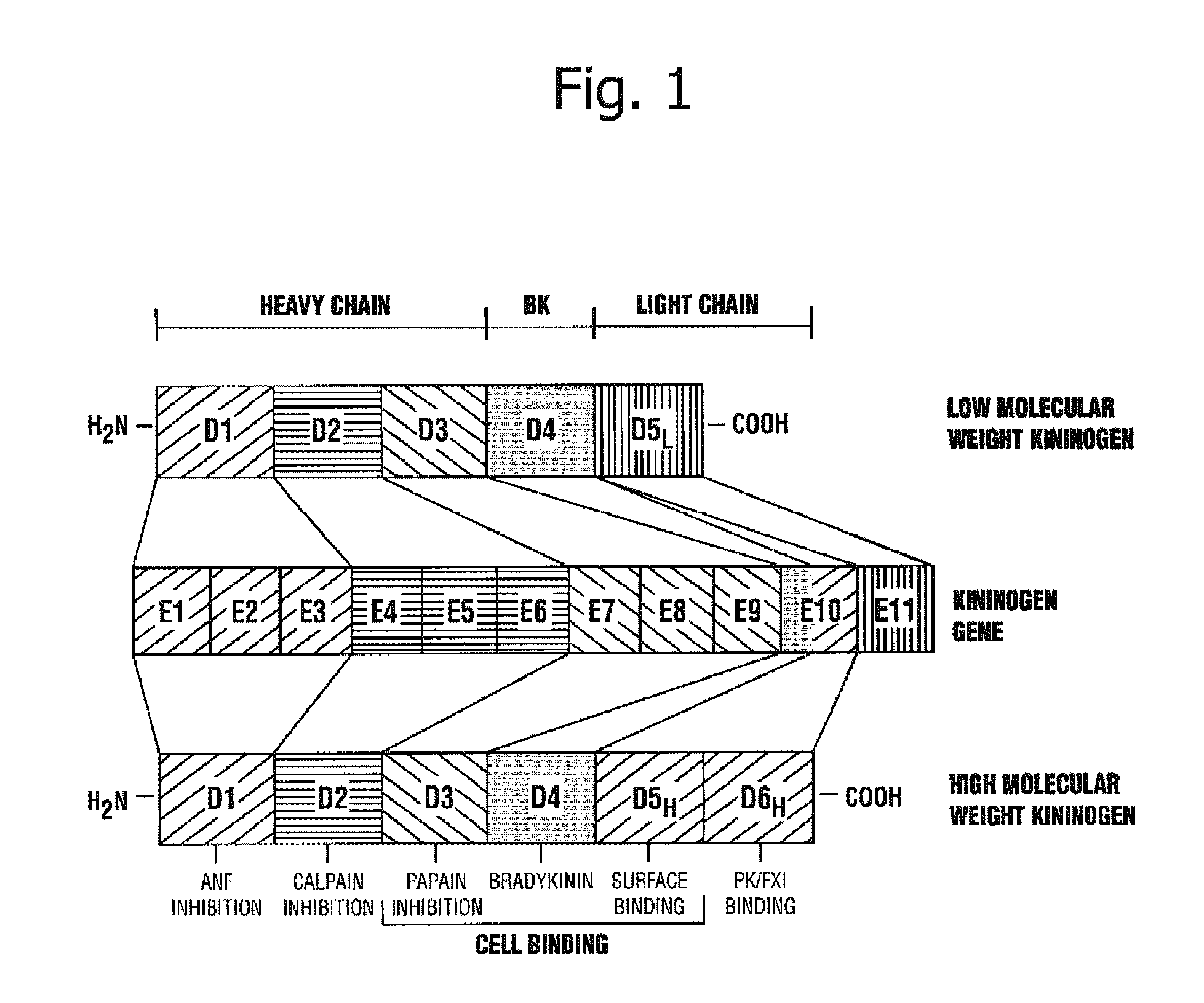
Kininogens are proteins that are defined by their role as precursors for kinins, but that also can have additional roles. Kinins are biologically active peptides, the parent form is bradykinin.
Aniline derivatives
InactiveUS7879863B2Inhibition releaseInhibit growthBiocideNervous disorderCombinatorial chemistryPharmaceutical medicine
Owner:AJINOMOTO CO INC
High purity ulinastatin and its prepn process and medicine composition
ActiveCN1931875AHigh purityImprove stabilityPeptide/protein ingredientsAntipyreticMetalloproteinPhosphate
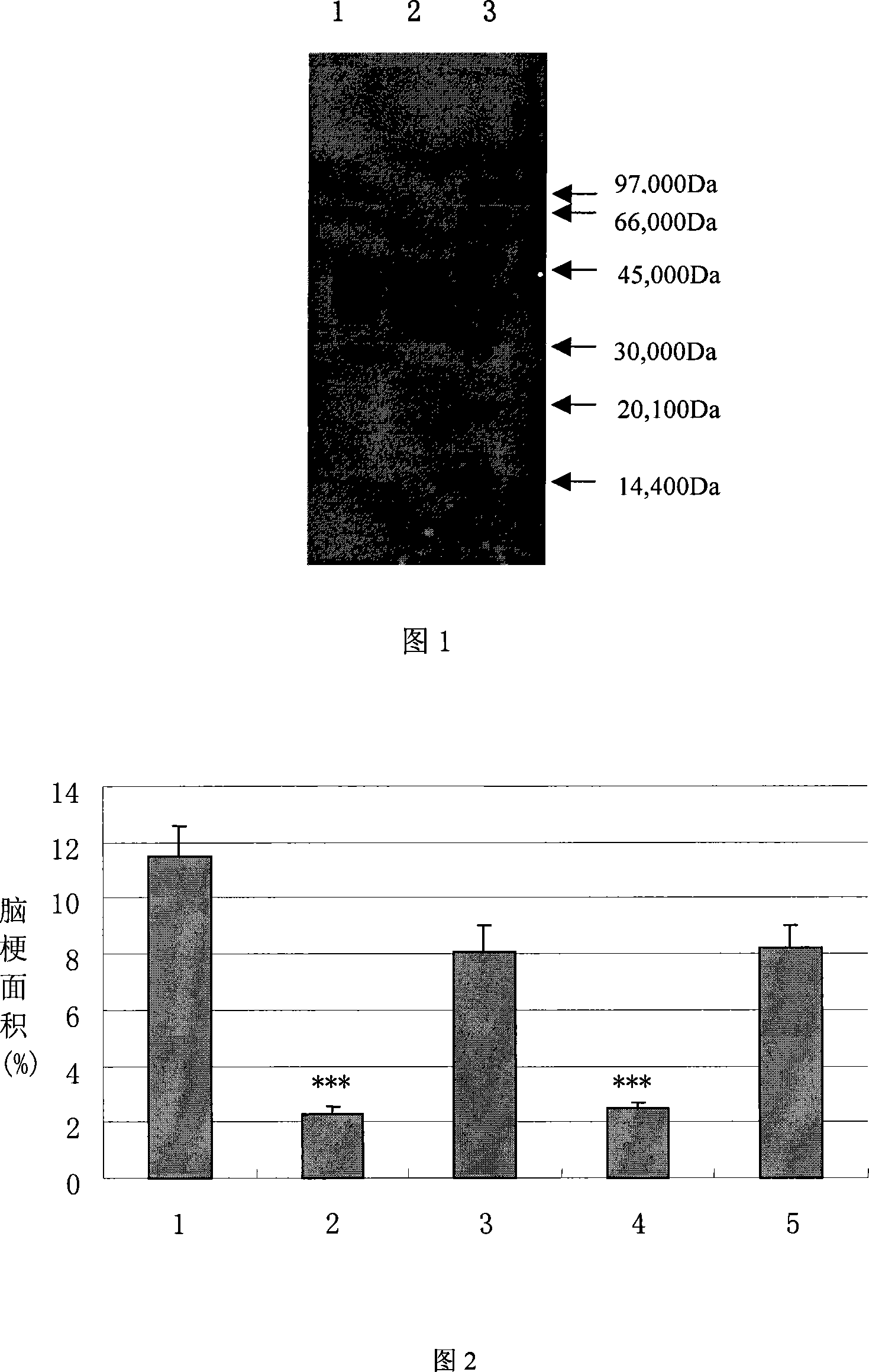
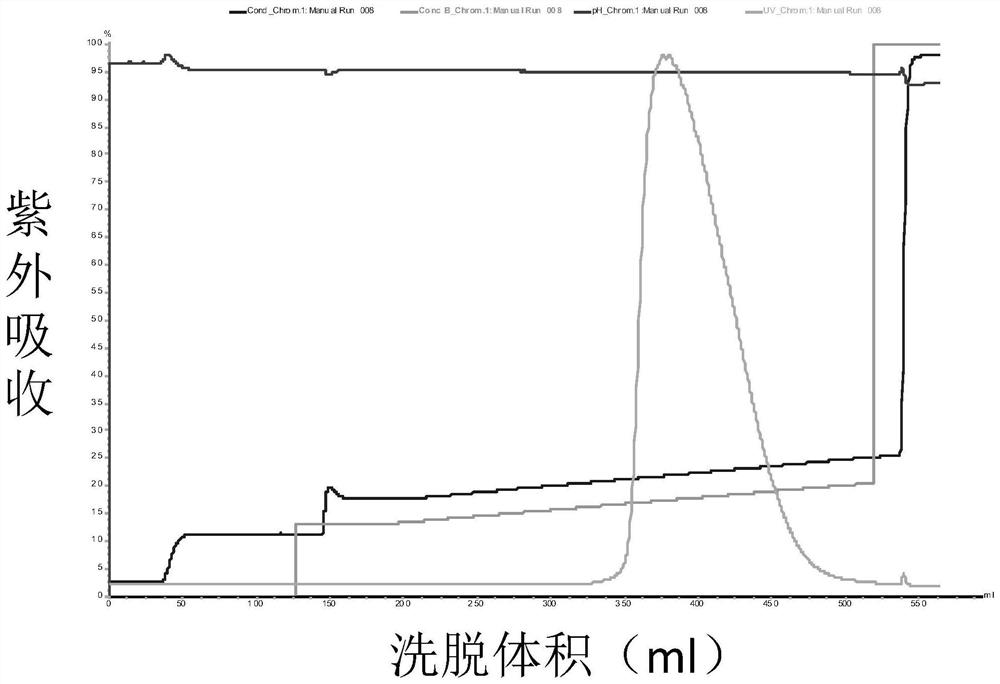
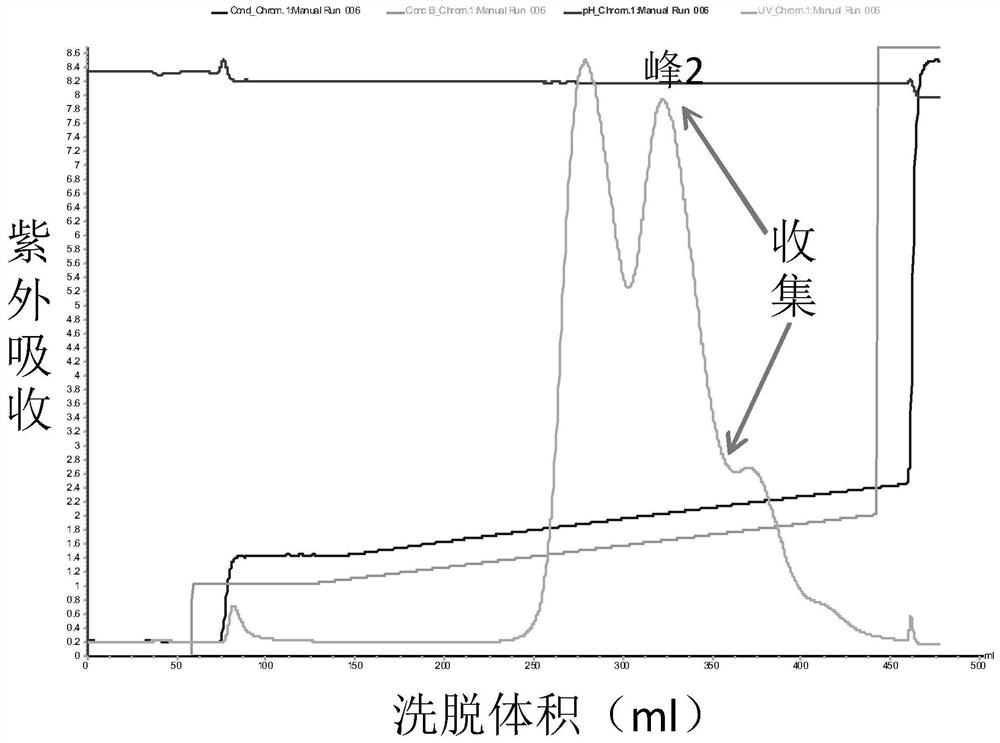
The present invention relates to high purity ulinastatin and its medicine composition and their preparation process. Specially, the high purity ulinastatin in 50,000 U / ml concentration has optical absorption value at 405 nm not exceeding 0.05 and human urea kininogenase content not exceeding 0.0003 PNAU. The present invention purifies ulinastatin product through adsorption with hydrophobic column, purification in hydrophilic column, combination with metalloprotein in metal chelating column and elution with buffering phosphate solution.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Pharmaceutical composition containing recombination human pancreatic kininogenase for treating and/or preventing cerebral infarction
ActiveCN101134105ALittle side effectsBlood pressure dropPowder deliveryPeptide/protein ingredientsFreeze-dryingAcute cerebral infarction
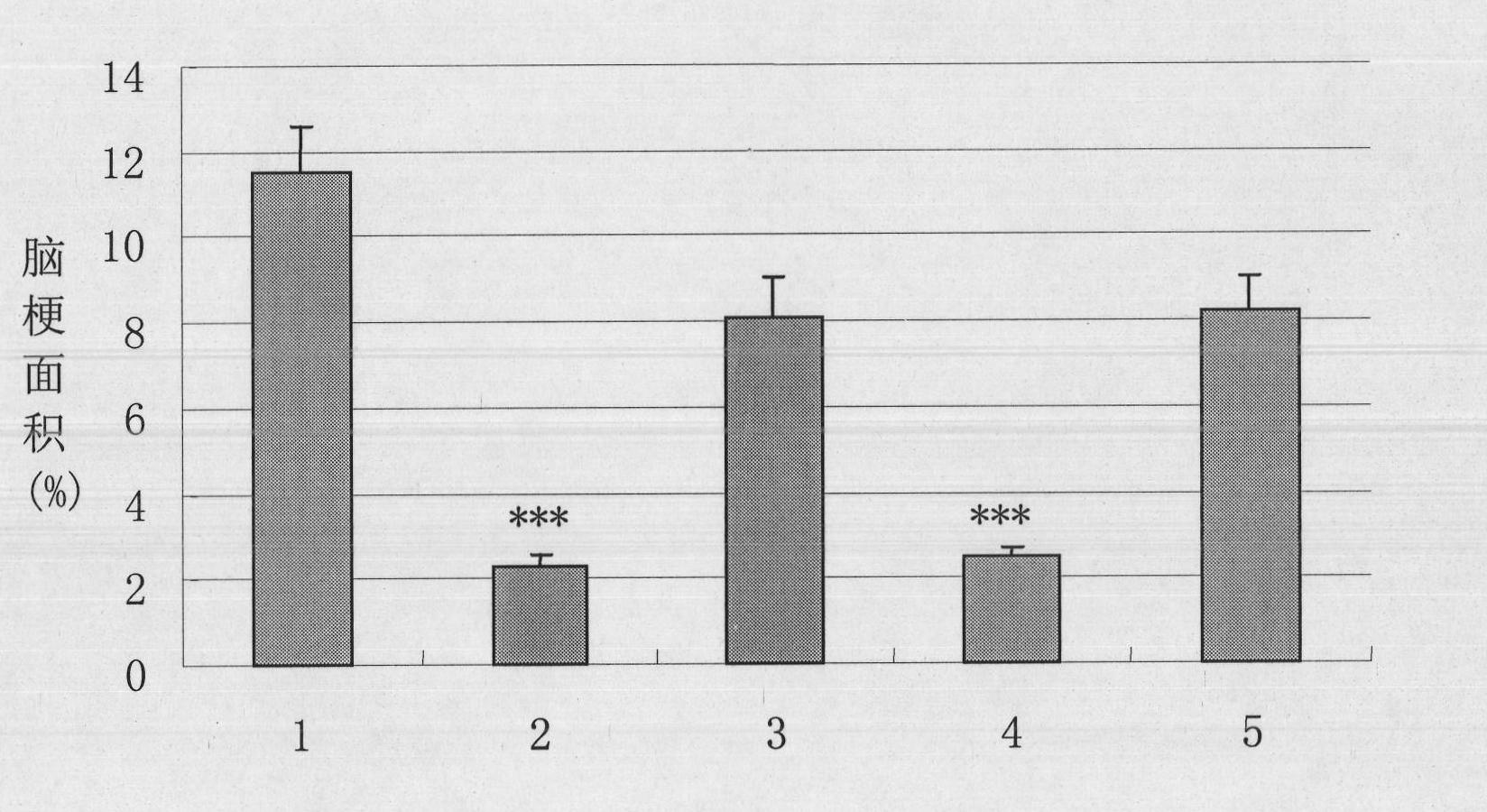
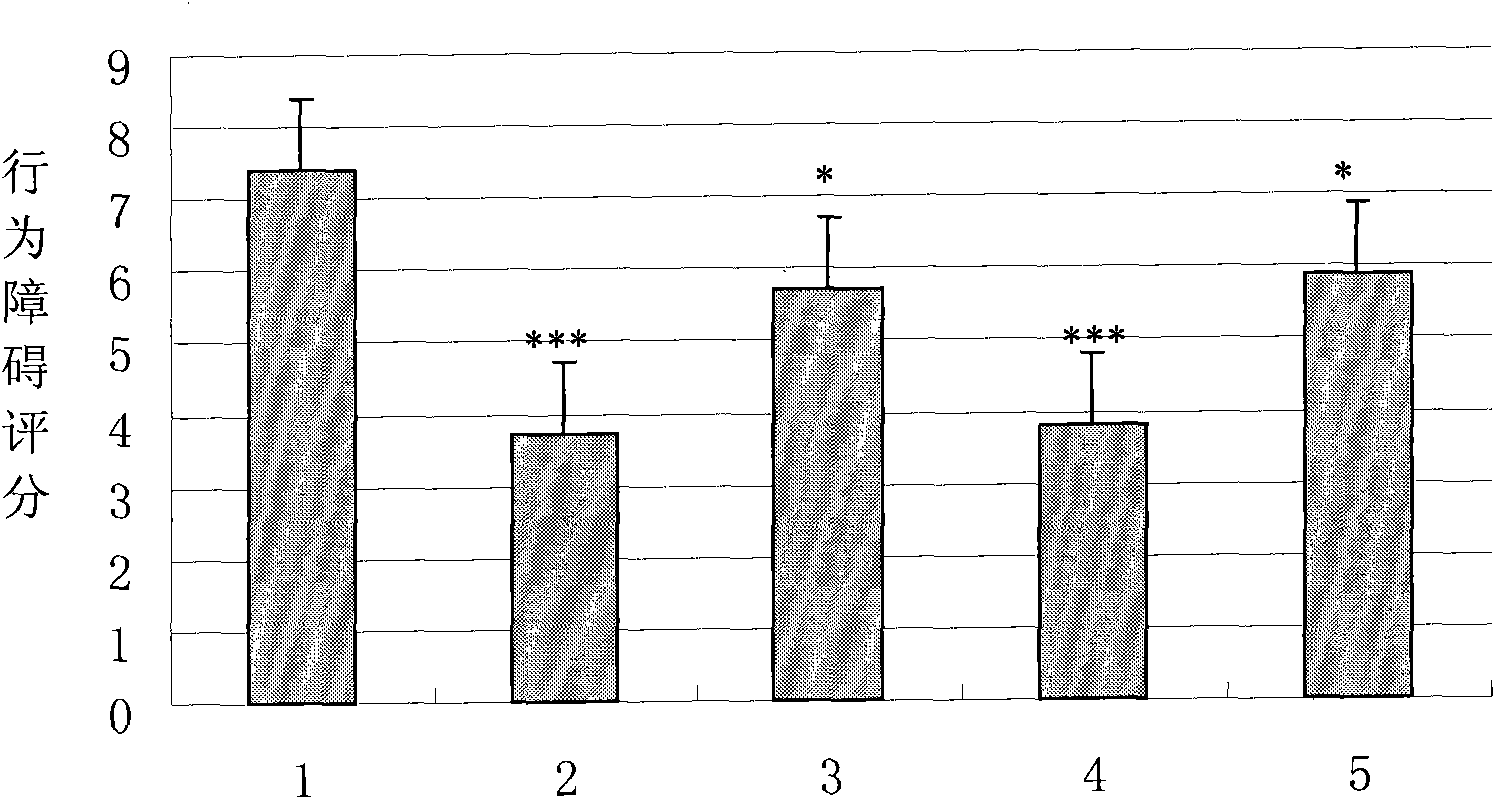
The present invention relates to the use of recombinant human kallidinogenase composition in preparing medicine for preventing and treating cerebral infarction. The recombinant human kallidinogenase composition is prepared with recombinant human kallidinogenase through combination with the host cell expressing the recombinant protein by means of molecular biology technology. It has obvious effect of preventing and treating cerebral infarction. The recombinant human kallidinogenase composition of the present invention is normally in the form of medicine composition, such as freeze dried powder for injection or liquid injection.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Biomarker for diagnosis of liver disease
InactiveUS20110129859A1Conveniently and correctly diagnosedHeavy burdenPeptide/protein ingredientsImmunoglobulins against animals/humansKininChronic hepatitis
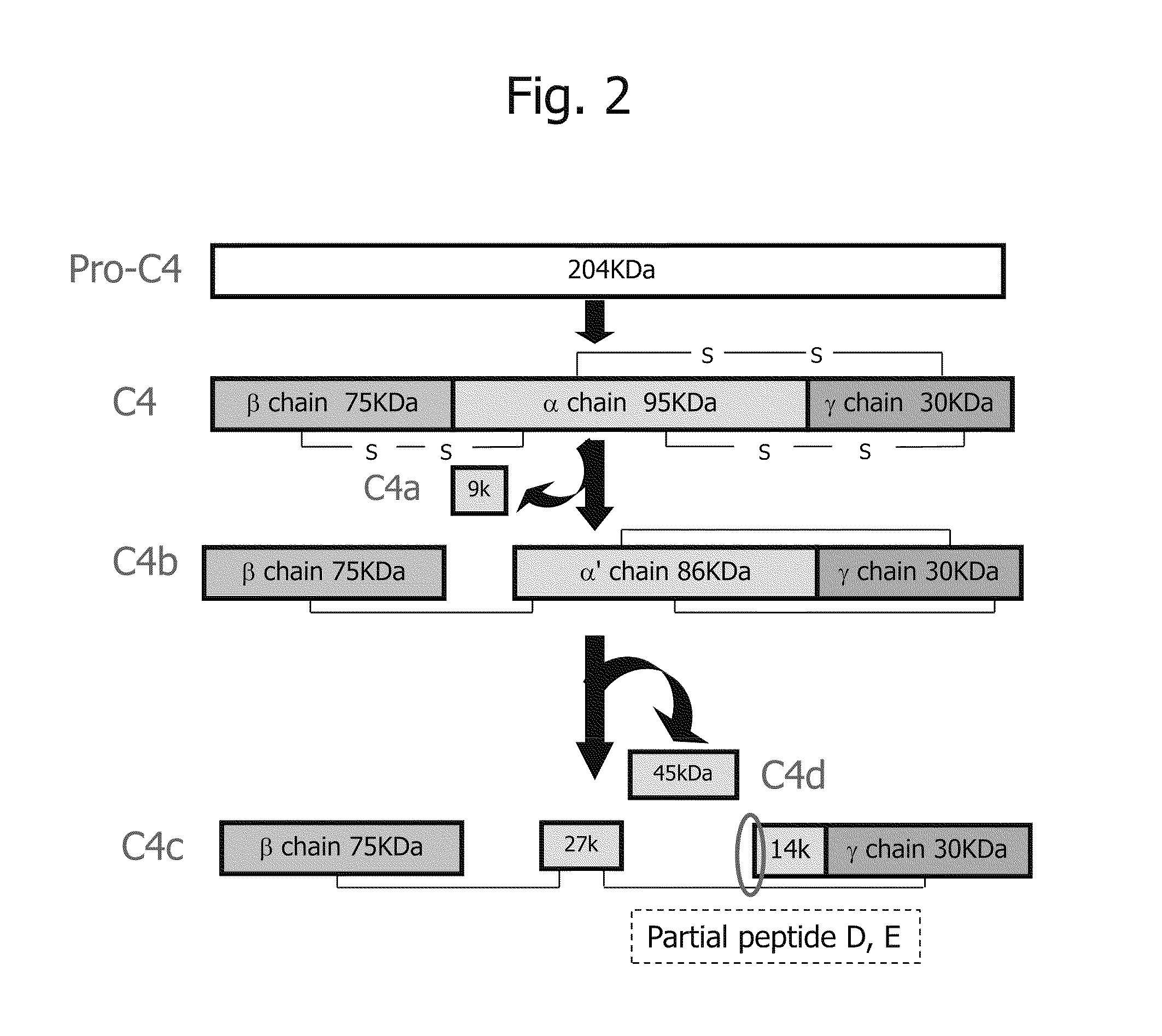
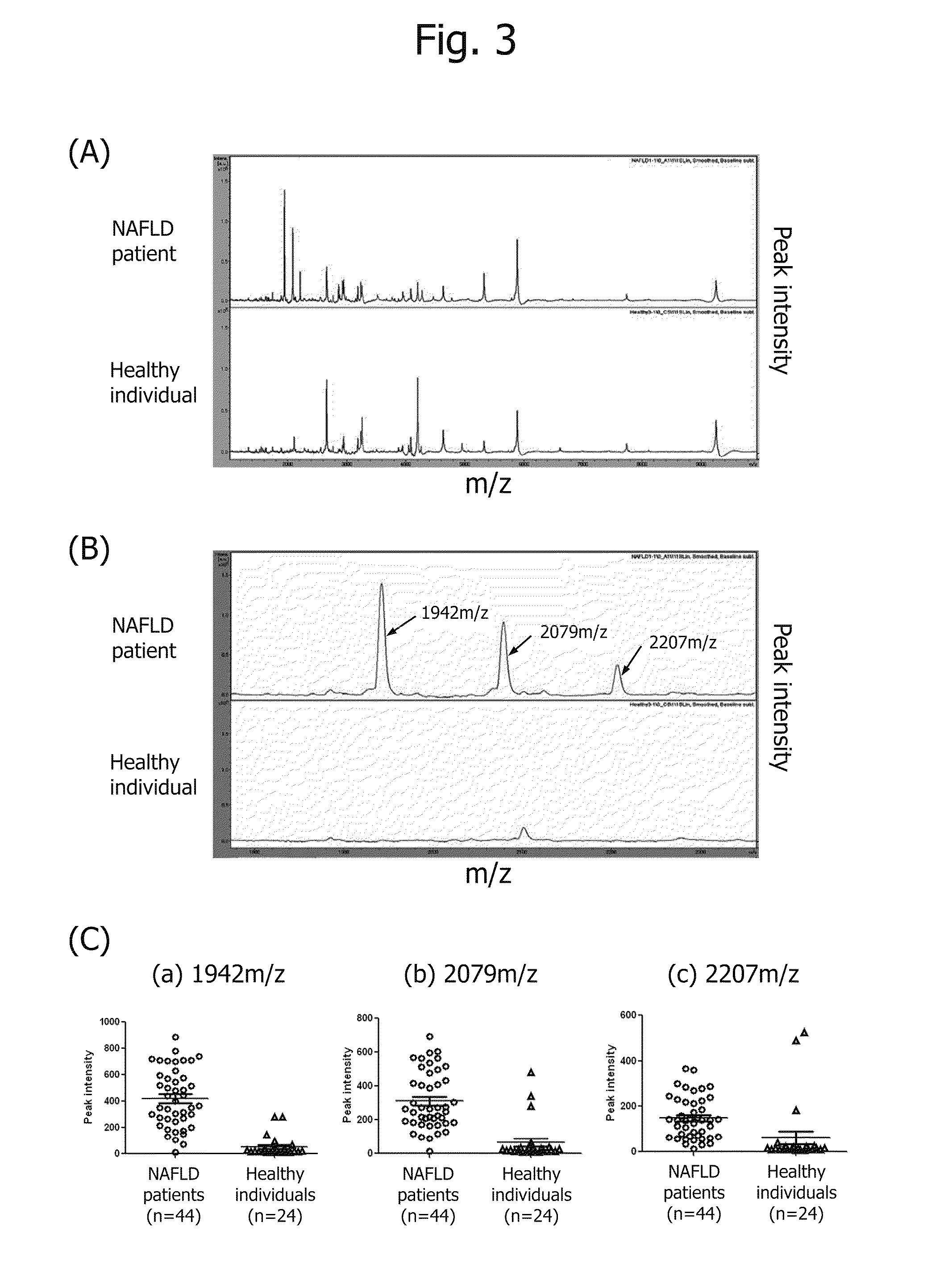
Disclosed are: a marker for the diagnosis of a liver disease, which can determine the disease in a simple manner; an antibody directed against the marker; a diagnostic agent; a diagnosis method; and a method for marker detection in blood or serum. Proteome analysis revealed that quantities of the full-length kininogen and three partial peptides thereof (sequence A: position-440 to position-456, sequence B: position-439 to position-456, and sequence C: position-438 to position-456) in sera of patients with non-alcoholic fatty liver disease are significantly different from those in sera of healthy individuals; and a diagnostic agent and a detecting method for the non-alcoholic fatty liver disease that can be conveniently used for medical examination are established. The use of a combination of a kininogen-based marker and a C4-based marker (the full length sequence or partial peptides thereof) enables identification of chronic hepatitis and an asymptomatic virus carrier, as well as non-alcoholic fatty liver disease.
Owner:KAGOSHIMA UNIV
Recombinant human pancreas kininogenase
The present invention relates to one kind of recombinant kallidinogenase and its preparation process. The recombinant kallidinogenase is produced by means of molecular biological technology and with host cell for expressing recombinant protein, and is purified through an affinity chromatographic process. It has several clinical uses and less side effects.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Novel antimicrobial peptides with heparin binding activity
InactiveUS20090074864A1Facilitate efficient prevention and reduction and eliminationProbability be reduced and evenAntibacterial agentsAntimycoticsMicroorganismLaminin
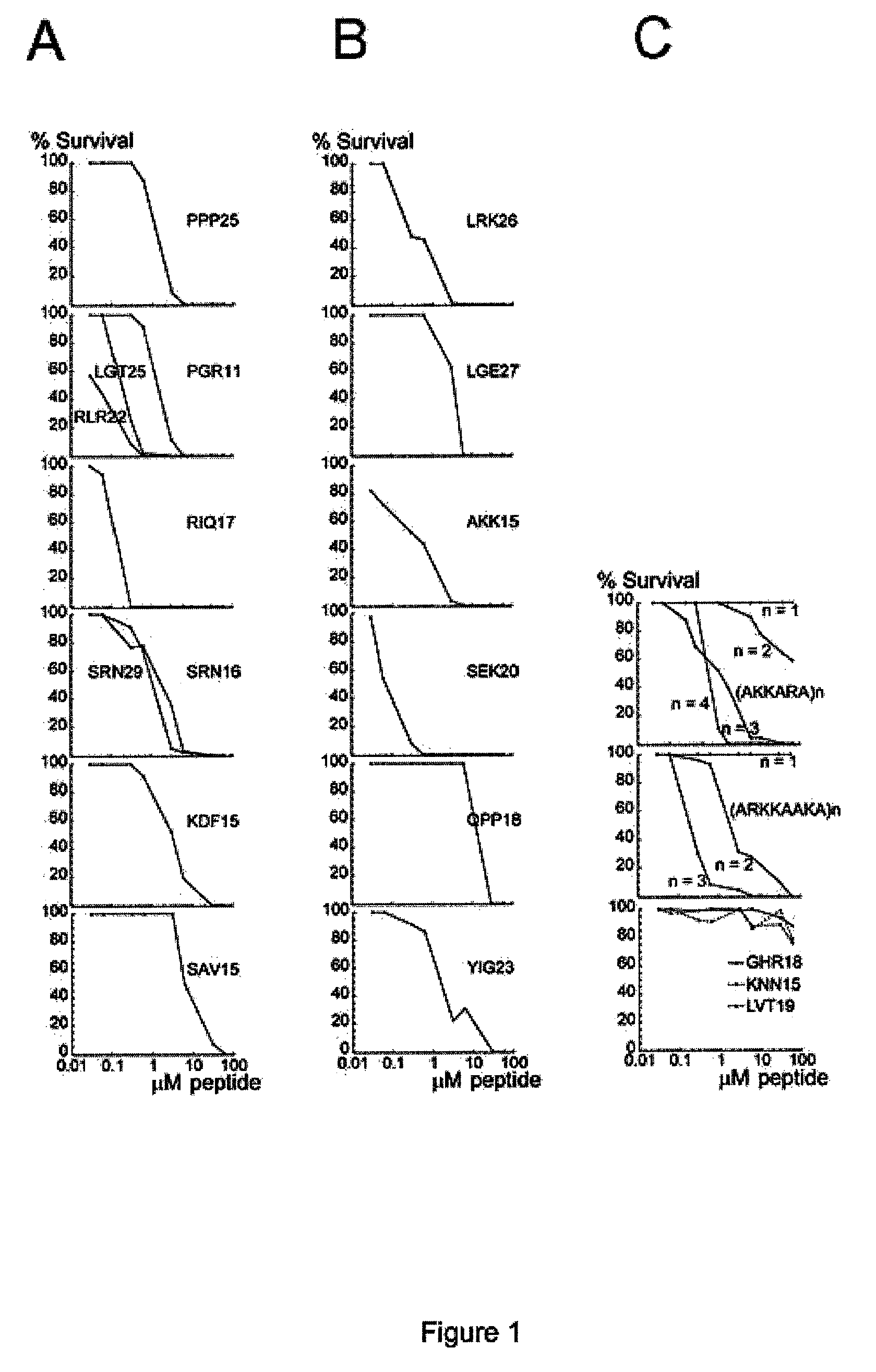
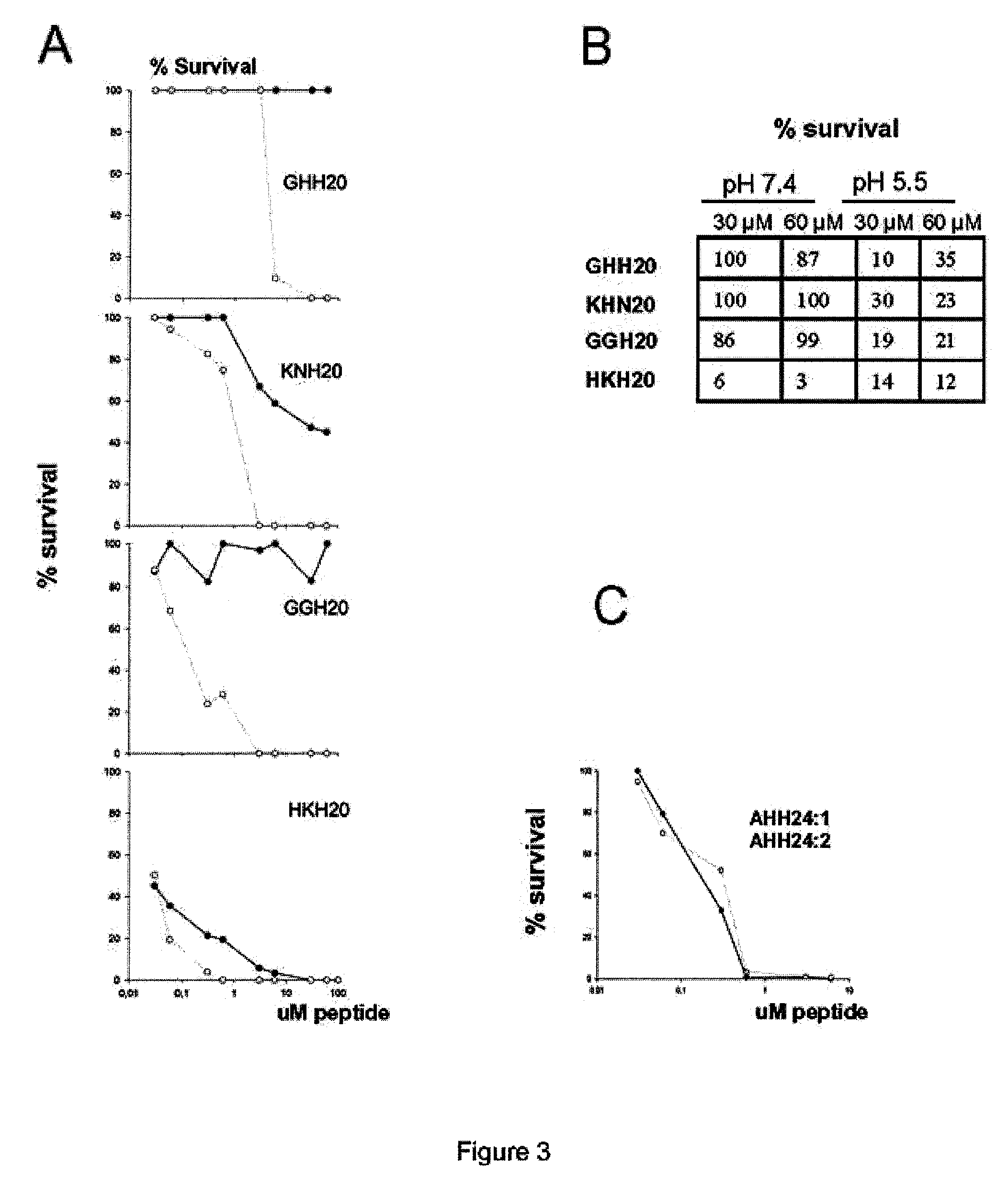
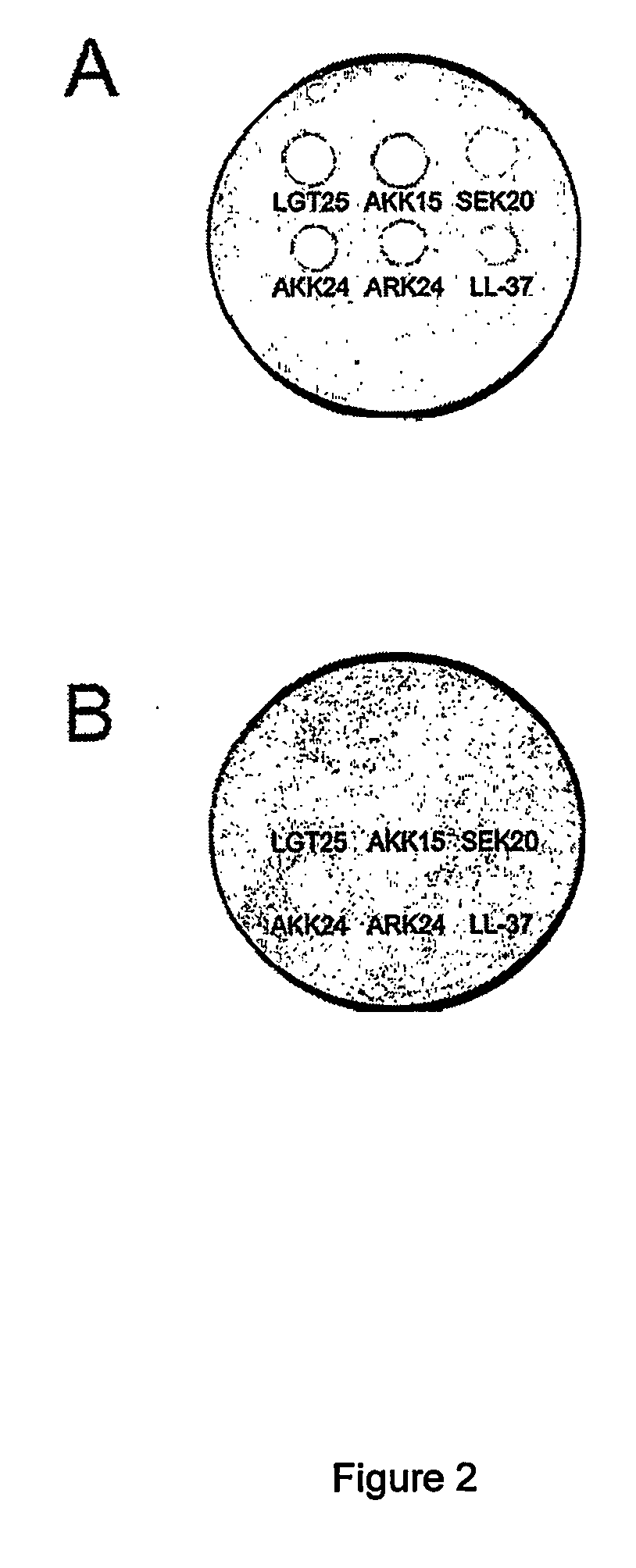
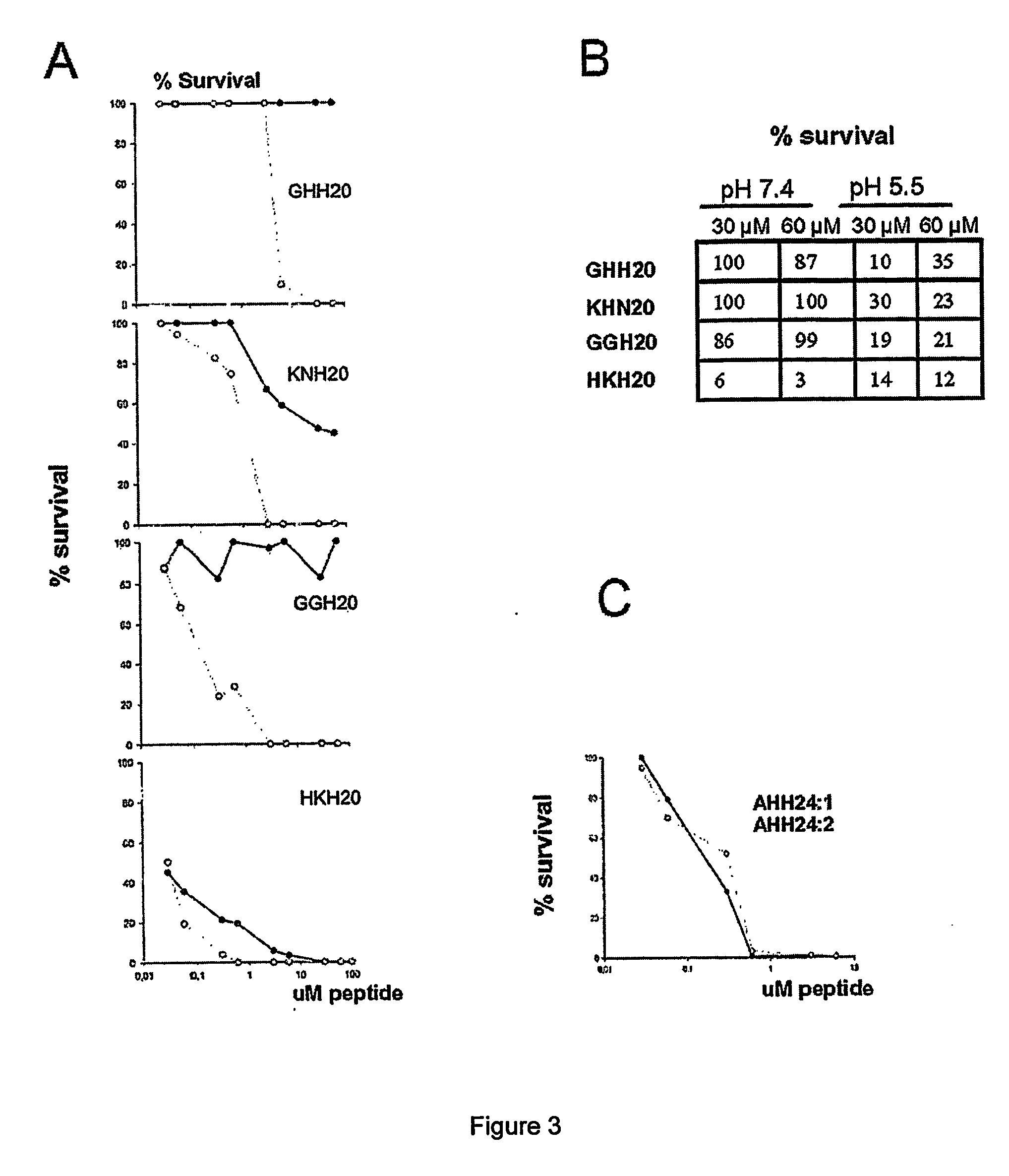
The invention relates to an antimicrobial peptide with heparin binding activity, being derived from endogenous mammalian proteins being substantially free from antimicrobial activity selected from the group consisting of laminin isoforms, complement factor C3, histidin rich glycoprotein and kininogen and having from 10 to 36 amino acid residues, wherein the antimicrobial peptide consists of at least four amino acid residues selected from the group consisting of K, R and H. The invention also relates to pharmaceutical compositions comprising said antimicrobial peptide and use of the antimicrobial peptide and / or antimicrobial / pharmaceutical composition.
Owner:PERGAMUM AB
Method for preparing urokinase
ActiveCN105087531AImprove adsorption capacitySimple structurePeptidasesURINARY TRYPSIN INHIBITORSilica gel
The invention relates to a urinary protein enriching method which comprises the steps of directly adsorbing urinary proteins in urine in urinals or urinating buckets with filter cloth bags loaded with modified silica gel, and transporting the urinary protein adsorbed filter cloth bags to working points for subsequent treatment. According to the method, the isoelectric point property of specific urinary proteins is utilized, and urinary proteins such as urinary trypsin inhibitors, human urinary kininogenase, urokinase and the like are effectively absorbed directly by using the modified silica gel, macroporous resin, chitin, ionic resin and the like, so that a urine collecting step is avoided. The method is free of obvious influence on sanitary conditions of toilets, and the cost for urine transportation and a series of environmental problems resulting from urine transportation are greatly reduced.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
PEG-modified medicinal kininogenase and preparation method and application thereof
ActiveCN107760661AHigh purityImprove stabilityPeptide/protein ingredientsHydrolasesSide effectHalf-life
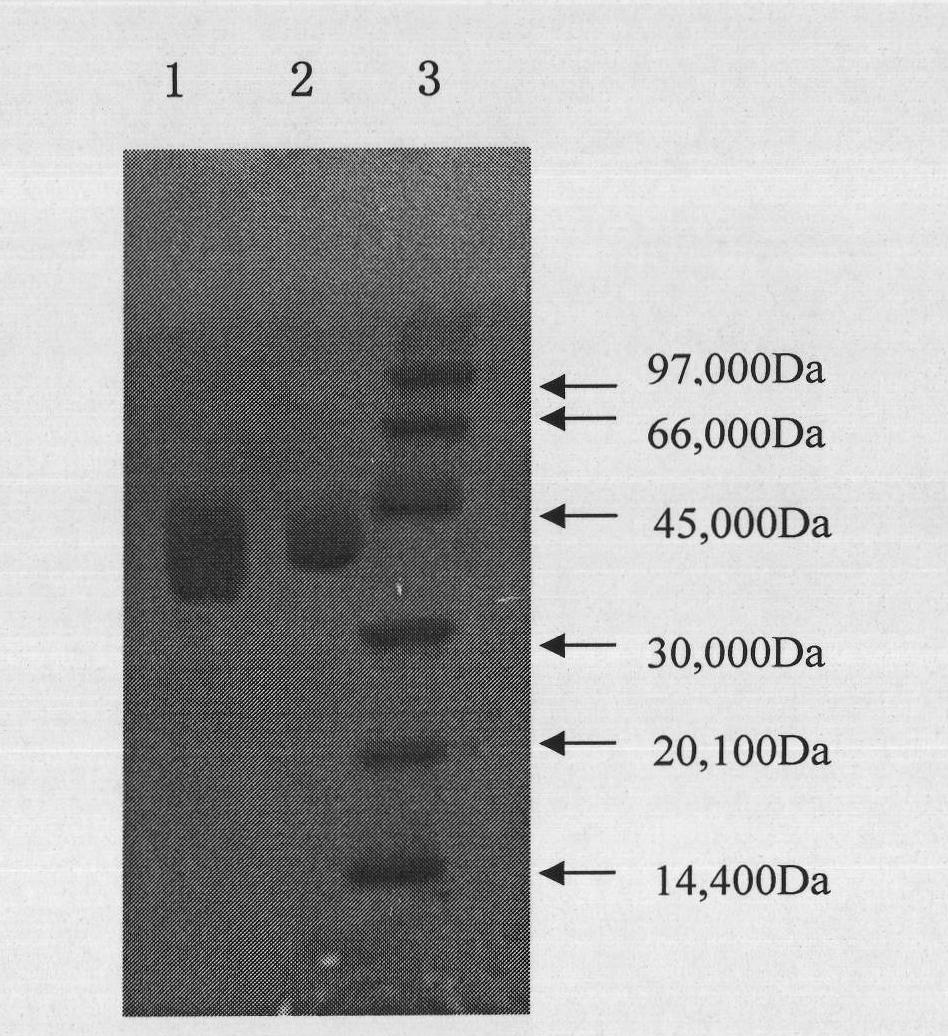
The invention relates to PEG-modified medicinal kininogenase and a preparation method and application thereof. The kininogenase does not contain lowly-glycosylated KLK1, and the lowly-glycosylated KLK1 is a stripe with the lowest molecular weight in three stripes during SDS-PAGE protein electrophoresis of porcine pancreas-derived KLK1; PEG adopts a structural general formula shown as a formula (1)or a formula (2). According to the pegylated kininogenase, on one hand, component nonuniformity caused by different glycosylation modifications of the kininogenase is eliminated, so that the purity,the stability, the bioactivity and the medicinal efficacy are improved; on the other hand, after PEG modification of the kininogenase, the half-life period is significantly prolonged, the immunogenicity is significantly reduced, and side effects of a raw medicine are effectively reduced.
Owner:ZONHON BIOPHARMA INST +1
Markers for Renal Disease
ActiveUS20130130285A1Less discomfortThe detection method is simplePeptide/protein ingredientsComponent separationDiseaseMetabolite
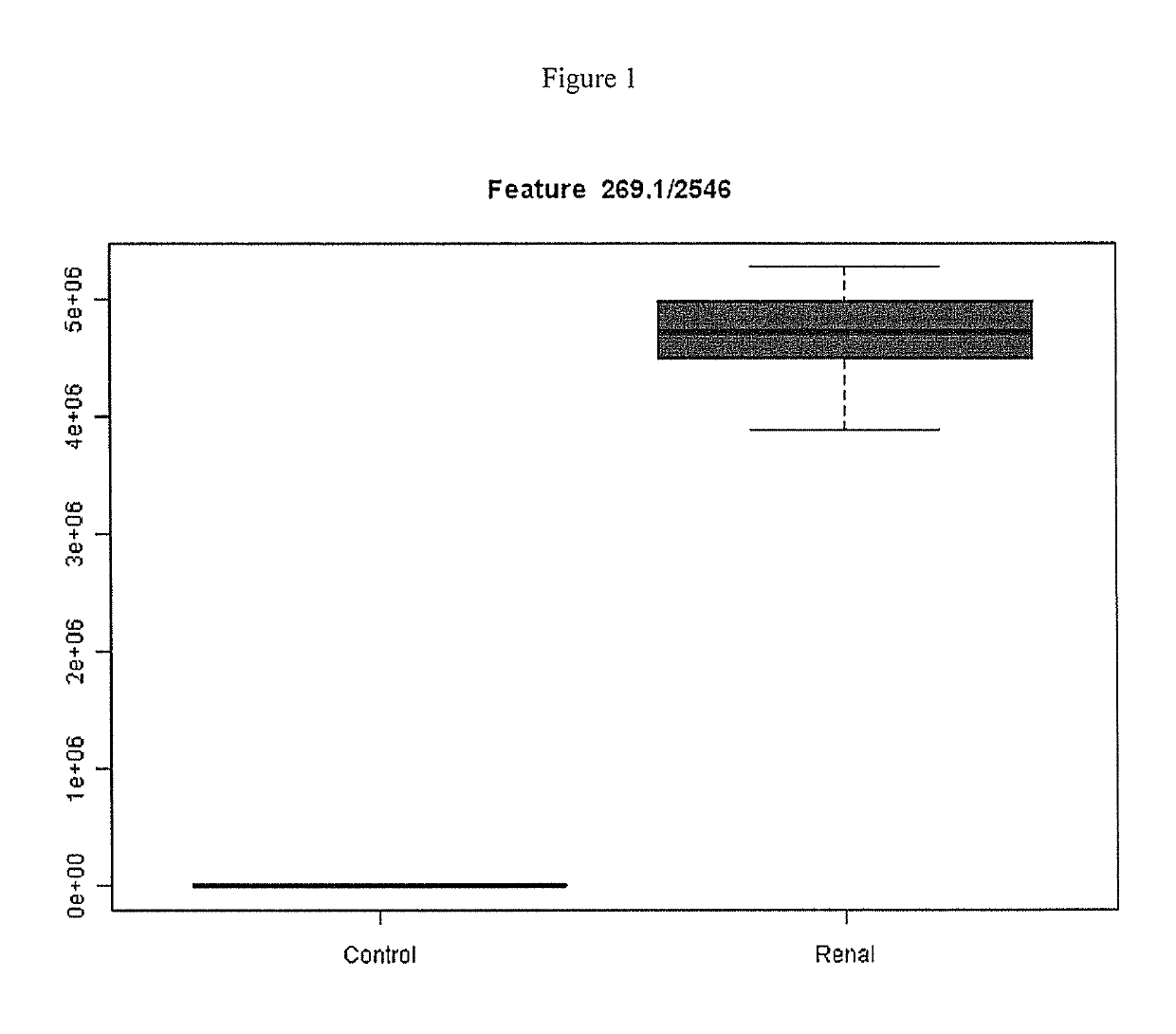
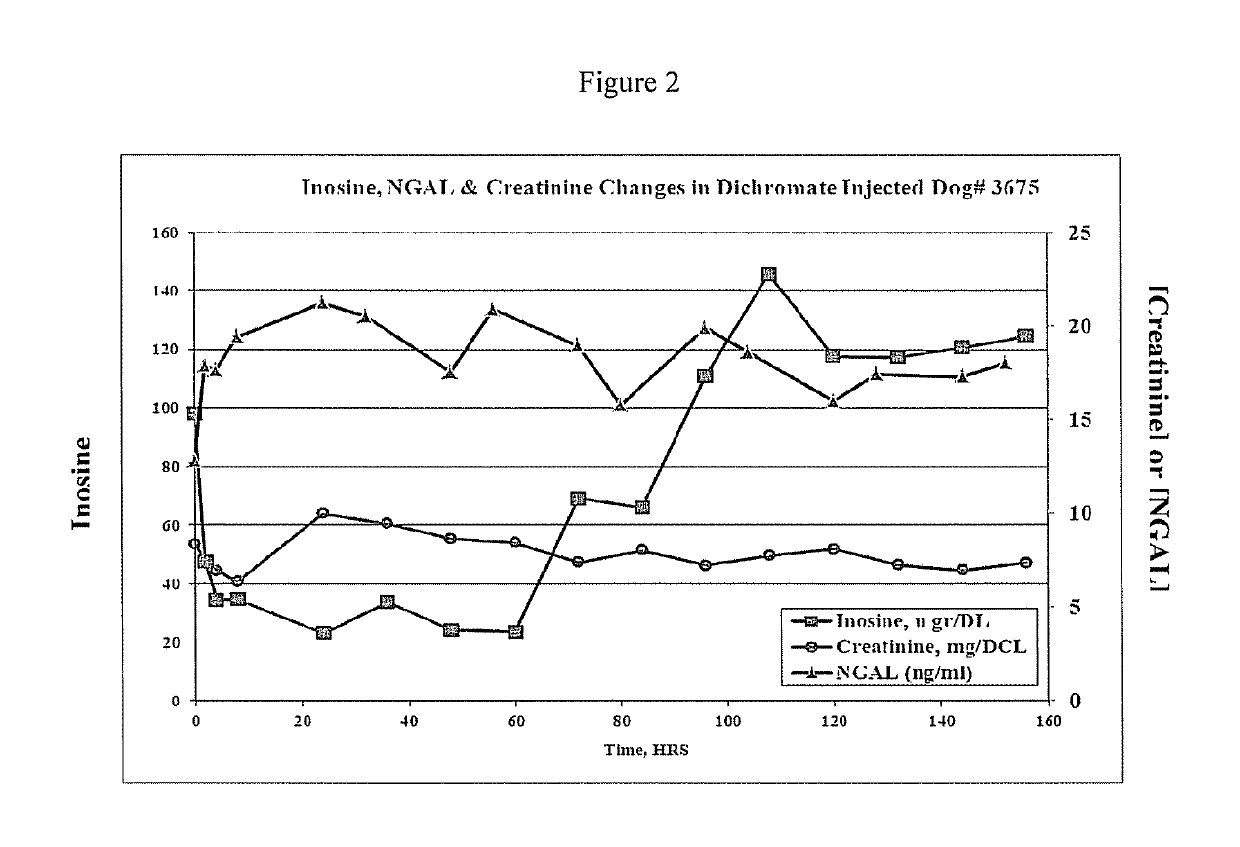
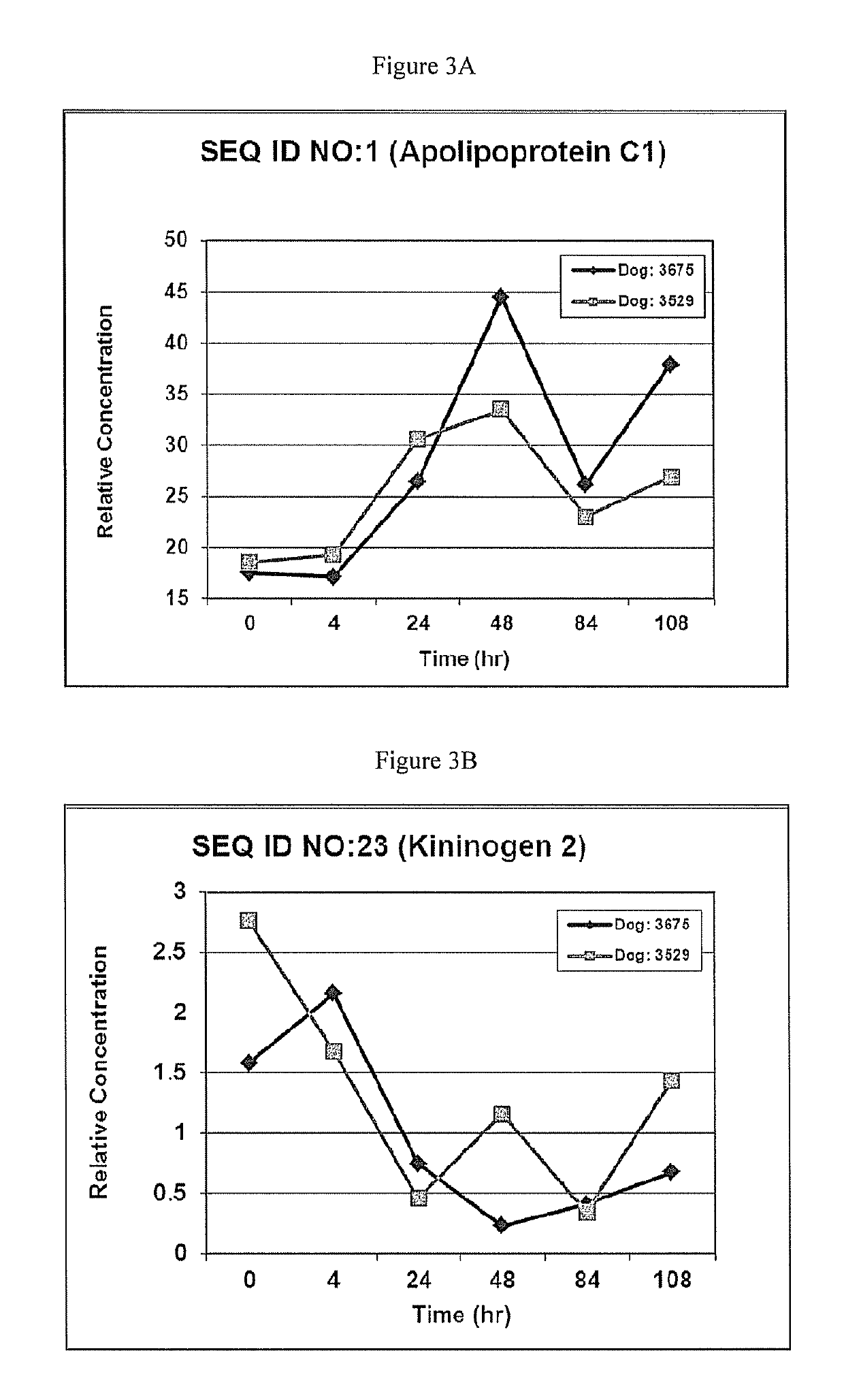
This invention provides reagents and methods for diagnosing renal disease. Differential levels of inosine metabolite, and proteins: apolipoprotein C-I, apolipoprotein C-II, fibrinogen alpha chain, or fibrinogen A-alpha chain, kininogen, Inter-Alpha Inhibitor H4 (ITIH4), keratin Type I cytoskeletol 10 cystatin A, cystatin B and other polypeptides and fragments thereof provide biomarkers of renal disease and are described herein.
Owner:IDEXX LABORATORIES
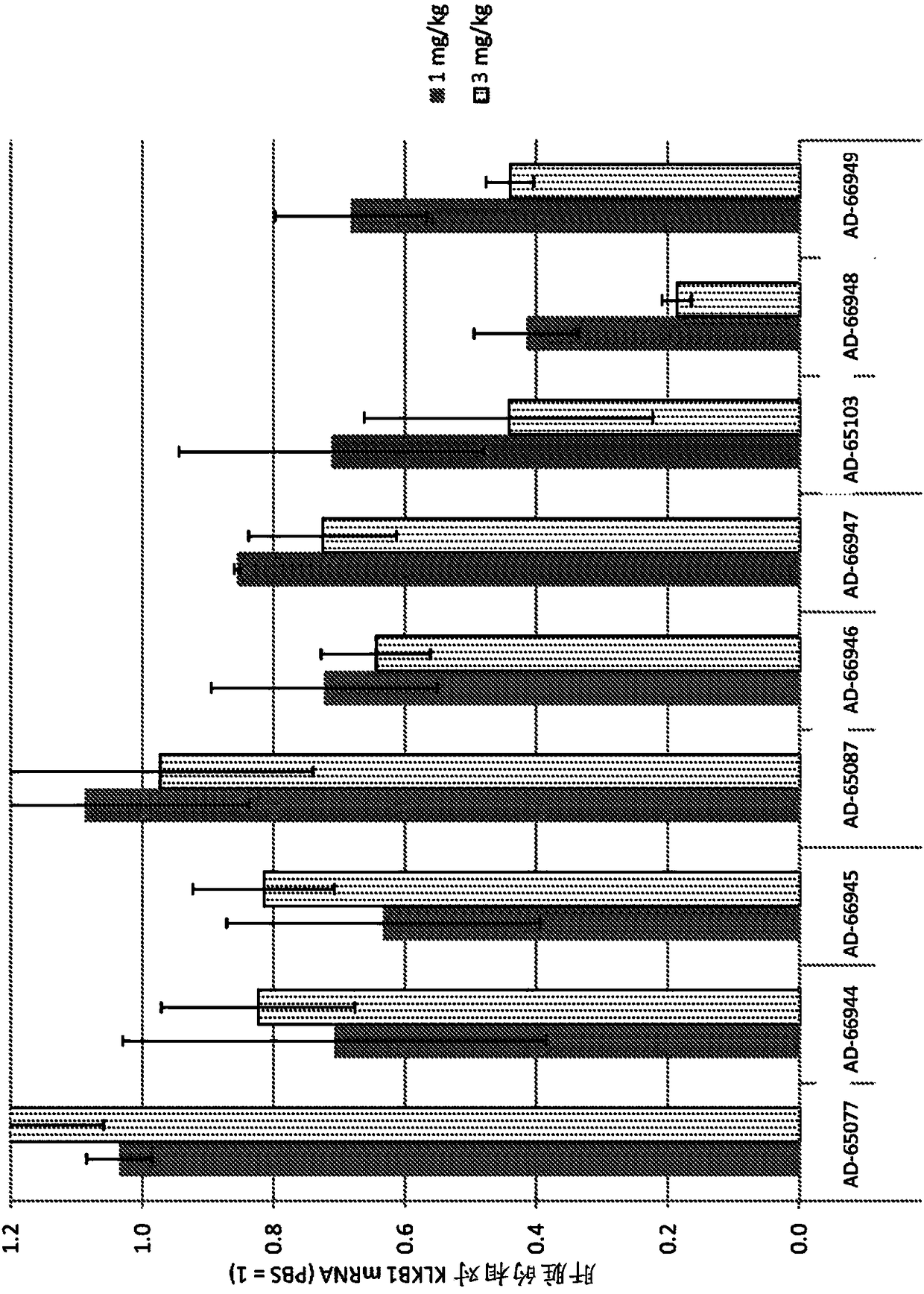
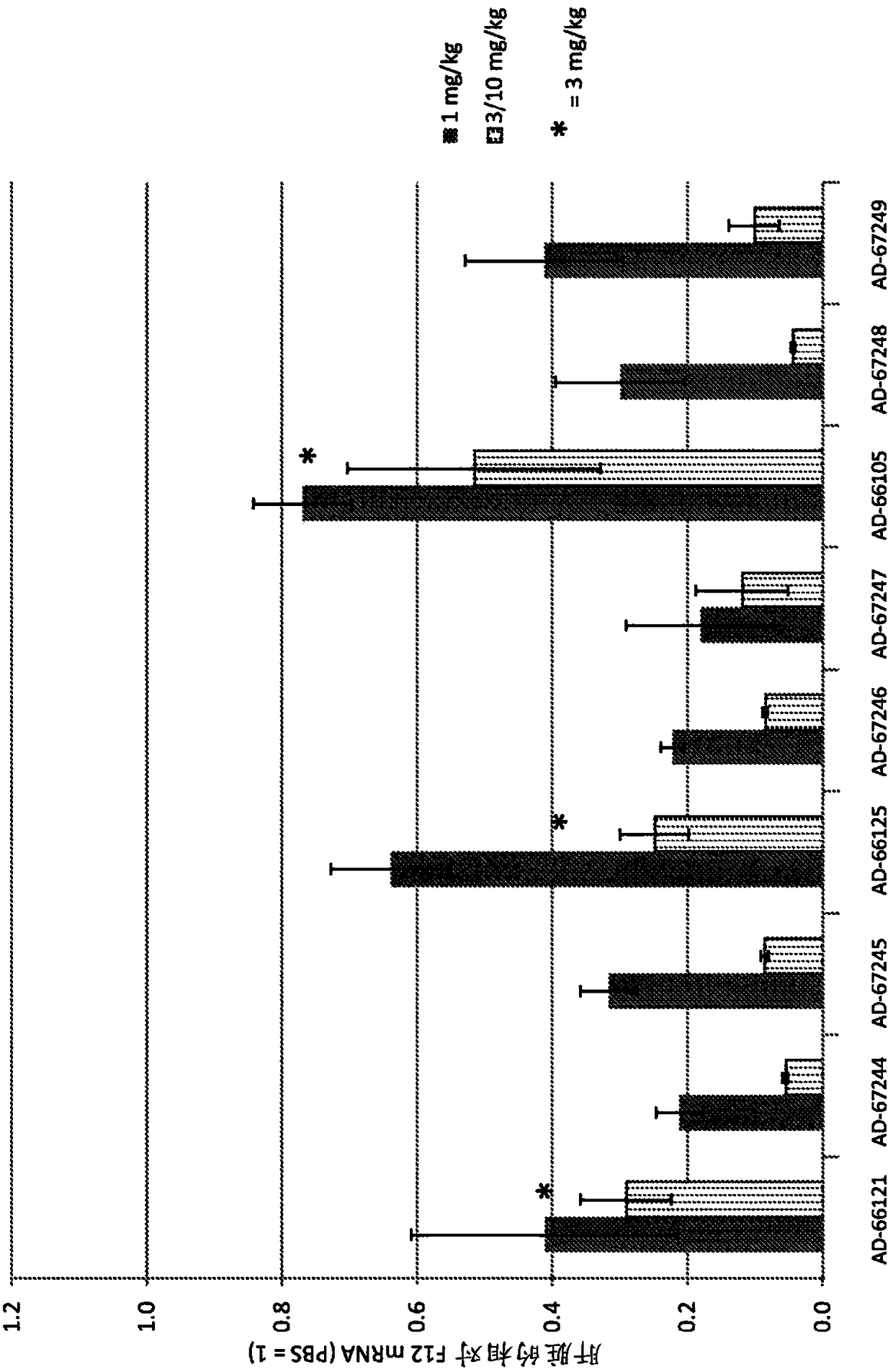
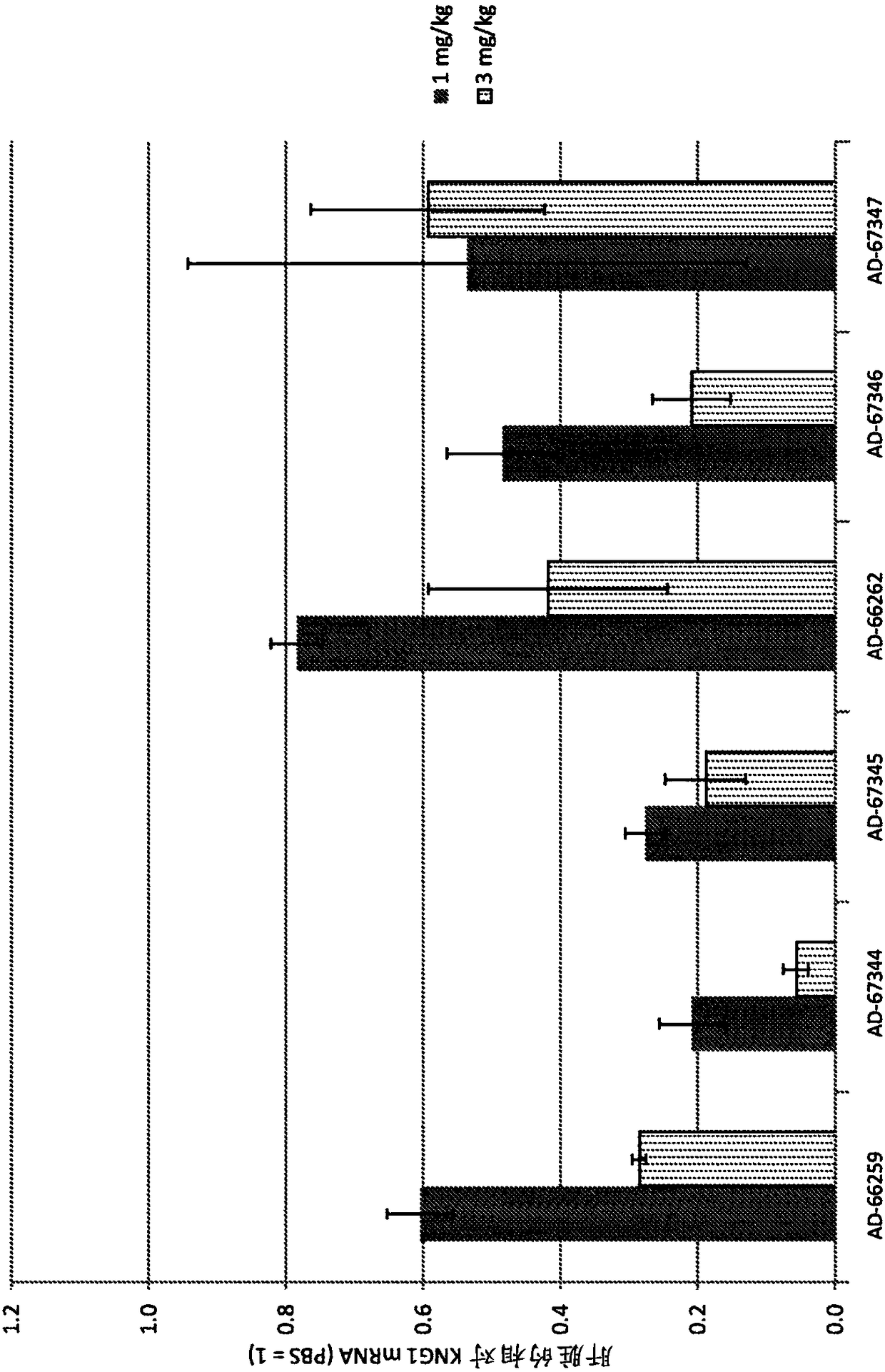
Factor xii (hageman factor) (F12), kallikrein b, plasma (fletcher factor) 1 (KLKB1), and kininogen 1 (KNG1) irna compositions and methods of use thereof
ActiveCN108271386AOrganic active ingredientsMicrobiological testing/measurementContact activationKinin
The present invention relates to RNAi agents, e.g., double stranded RNAi agents, targeting the Kallikrein B, Plasma (Fletcher Factor) 1 (KLKB1) gene, the Factor XII (Hageman Factor (F12) gene, or theKininogen 1 (KNG1) gene, and methods of using such RNAi agents to inhibit expression of a KLKB1 gene, an F12 gene, and / or a KNG1 gene, and methods of treating subjects having an hereditary angioedema(HAE) and / or a contact activation pathway-associated disorder.
Owner:ALNYLAM PHARM INC
Recombinant human kallidinogenase
InactiveCN101967468ALittle side effectsBlood pressure dropHydrolasesPeptide/protein ingredientsSide effectBiochemistry
The invention relates to human kallidinogenase and a production method thereof. The recombinant human kallidinogenase is produced by using molecular biological technology and host cells for expressing recombinant proteins and by purifying the recombinant proteins by using affinity chromatography. The recombinant human kallidinogenase has various applications in clinic, and the side effect of the recombinant human kallidinogenase is reduced obviously.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Protein marker of focal segmental glomerulosclerosis
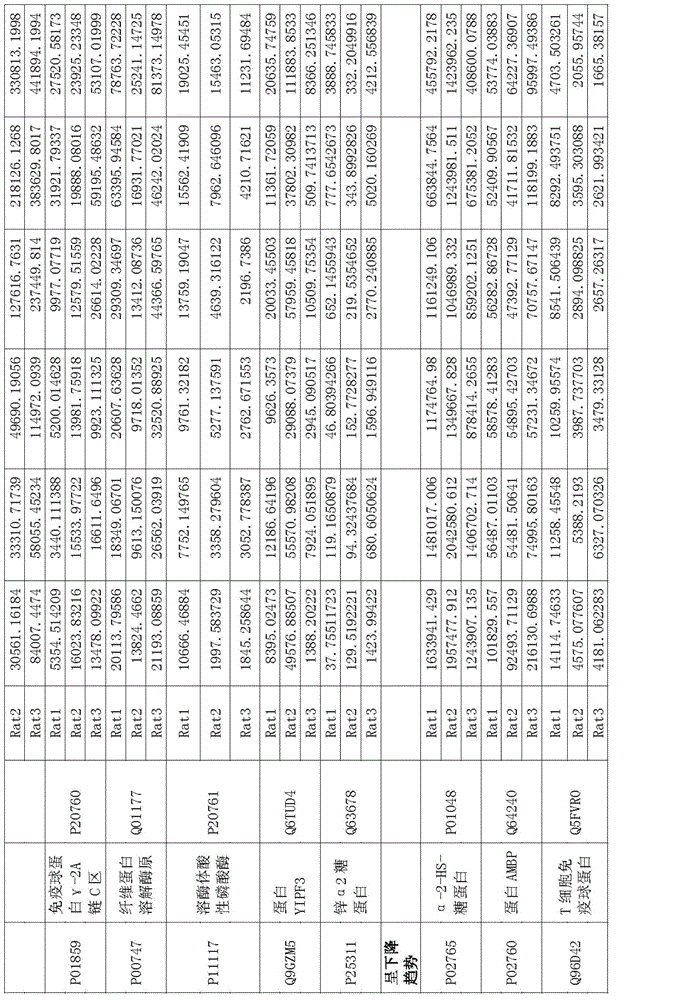
The invention relates to a protein marker of focal segmental glomerulosclerosis, and more specifically relates to an application of a connecting part selectively connected to one or more proteins in preparing a diagnostic reagent in a development process of human focal segmental glomerulosclerosis. The one or more proteins are selected from [alpha]1 antiprotease, alpha albumin, serum albumin, beta-2-microglobulin, ceruloplasmin, fetuin-B, kininogen, serum transferrin, carboxylesterase, immune globulin gamma-2A chain C region, plasminogen, lysosomal acid phosphatase, protein YIPF3, zinc [alpha]2 glycoprotein, alpha-2-HS-glycoprotein, protein AMBP or T cell immune globulin. Specifically, the invention relates to the application of a urine protein potential marker of human focal segmental glomerulosclerosis obtained by a rat model and mass spectrometry.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
New application of human urine kininogenase and medicine composition comprising same
ActiveCN104940914ADelay disease progressionRestore memoryNervous disorderPeptide/protein ingredientsDiseasePentoxyfylline
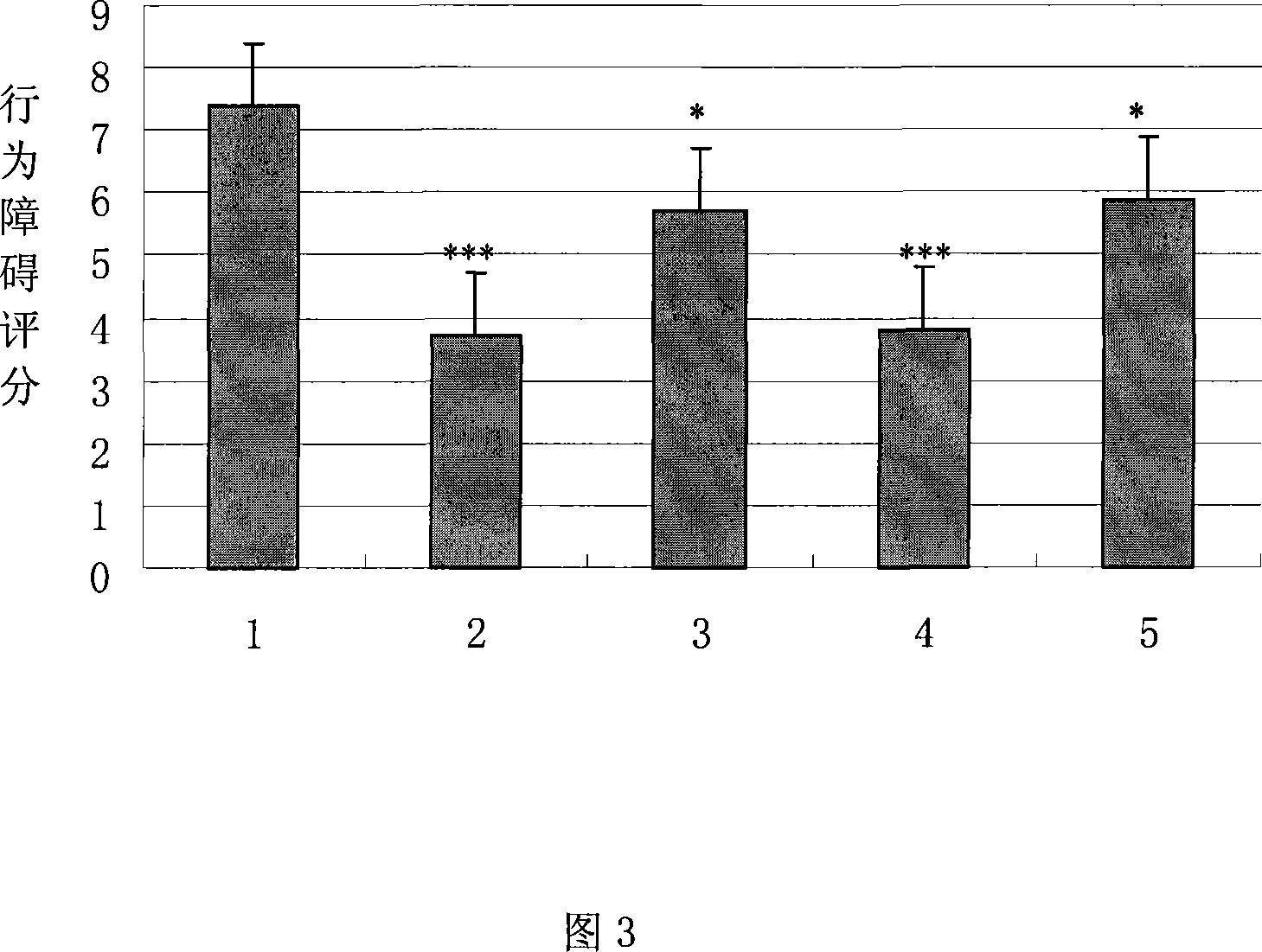
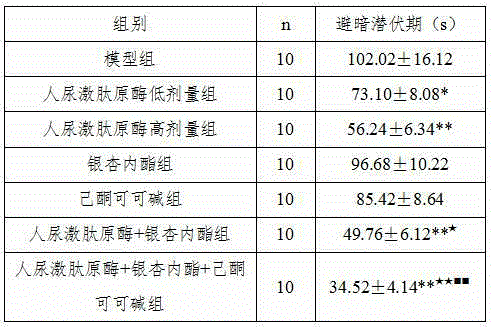
The invention belongs to the field of medicine, and particularly relates to a new application of human urine kininogenase and a medicine composition comprising the human urine kininogenase. The human urine kininogenase is applied to prepare medicine for treating the Alzheimer's disease. The invention further provides the medicine composition for treating the Alzheimer's disease. The medicine composition comprises the human urine kininogenase and bilobalide, and further comprises pentoxifyline. The medicine composition can remarkably recover the memory capacity of a patient suffering with the Alzheimer's disease, improve the cognition capacity of the patient suffering with the Alzheimer's disease, delay the disease progress of the Alzheimer's disease, greatly improve the life quality of the patient suffering with the Alzheimer's disease, improve the treatment compliance of the Alzheimer's disease, improve the treatment effect, provide the effective treatment medicine for the patient suffering with the Alzheimer's disease, and greatly relieve pain of the patient of the Alzheimer's disease.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
New application of human urinary kallidinogenase and pharmaceutical composition with the human urinary kallidinogenase
InactiveCN104906564ARelieve painGood blood pressure effectPeptide/protein ingredientsPharmaceutical delivery mechanismSide effectEfficacy
The invention belongs to the field of medicine, in particular to a new application of human urinary kallidinogenase and a pharmaceutical composition with the human urinary kallidinogenase. According to the new application of the human urinary kallidinogenase, the human urinary kallidinogenase is used for preparing medicine for treating hypertensive nephropathy, and the test discovers that the human urinary kallidinogenase is capable of lowering the arterial pressure of a rat with hypertensive nephropathy and improving the glomerular filtration rate and renal plasma flow of the rat with hypertensive nephropathy through regulating the micro-circulation of glomerulus, and has a certain treatment effect for renal damage. The pharmaceutical composition for treating the hypertensive nephropathy comprises the human urinary kallidinogenase and betahistine hydrochloride and further comprises pentoxifylline, the pharmaceutical composition has an obvious blood pressure reducing efficiency and has an obvious treatment effect for the damaged kidney, and the pharmaceutical composition for treating the hypertensive nephropathy is definite in effect, low in side effect and better for the recovery of a patient with hypertensive nephropathy.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Antimicrobial peptides with heparin binding activity
InactiveUS8551954B2Facilitate efficient prevention and reduction and eliminationProbability be reduced and evenAntibacterial agentsAntimycoticsMicroorganismLaminin
An antimicrobial peptide with heparin binding activity is described. It can be derived from endogenous mammalian proteins being substantially free from antimicrobial activity selected from the group consisting of laminin isoforms, complement factor C3, histidin rich glycoprotein and kininogen and having from 10 to 36 amino acids residues, wherein the antimicrobial peptide consists of at least four amino acid residues selected from the group consisting of K,R, and H. Also described are pharmaceutical compositions comprising said antimicrobial peptide and use of the antimicrobial peptide and / or antimicrobial / pharmaceutical composition.
Owner:PERGAMUM AB
Recombinant human pancreatic kininogenase-containing medicine for treating and/or preventing cerebral infarction
InactiveCN101897959ALittle side effectsBlood pressure dropPeptide/protein ingredientsEnzymesMedicineFreeze-drying
The invention relates to the use of recombinant human pancreatic kininogenase composite in the preparation of medicine for treating and / or preventing the cerebral infarction. Recombinant human pancreatic kininogenase is prepared by being combined with a host cell expressing the recombinant protein by means of molecular biology technology, thus having obvious treatment and / or prevention function(s) for the cerebral infarction. The recombinant human pancreatic kininogenase is generally used in a form of pharmaceutical composite such as freeze-dried powder injection or liquid injection.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for producing kallidin proenzyme raw-material medicine
The invention is concerned with the method for prepareing high purity organism pharmaceutical product, that is, the method of the high purity pancreatic kiniogenase pharmaceutical product. The method includes: isolates the pancreatic kinigenase midbody perfectly, passes it through the QAE-Sepharose Fast Flow that generates the pharamaceutical prodct of the kinigenase by hydronium column chromatographic purification. This pharamaceutical produc approaches high level of purity with 75%, increases the titer to 55 unit / mg and above, and boots up the specific activity to 350 unit / mg albumen and above.
Owner:上海丽珠制药有限公司
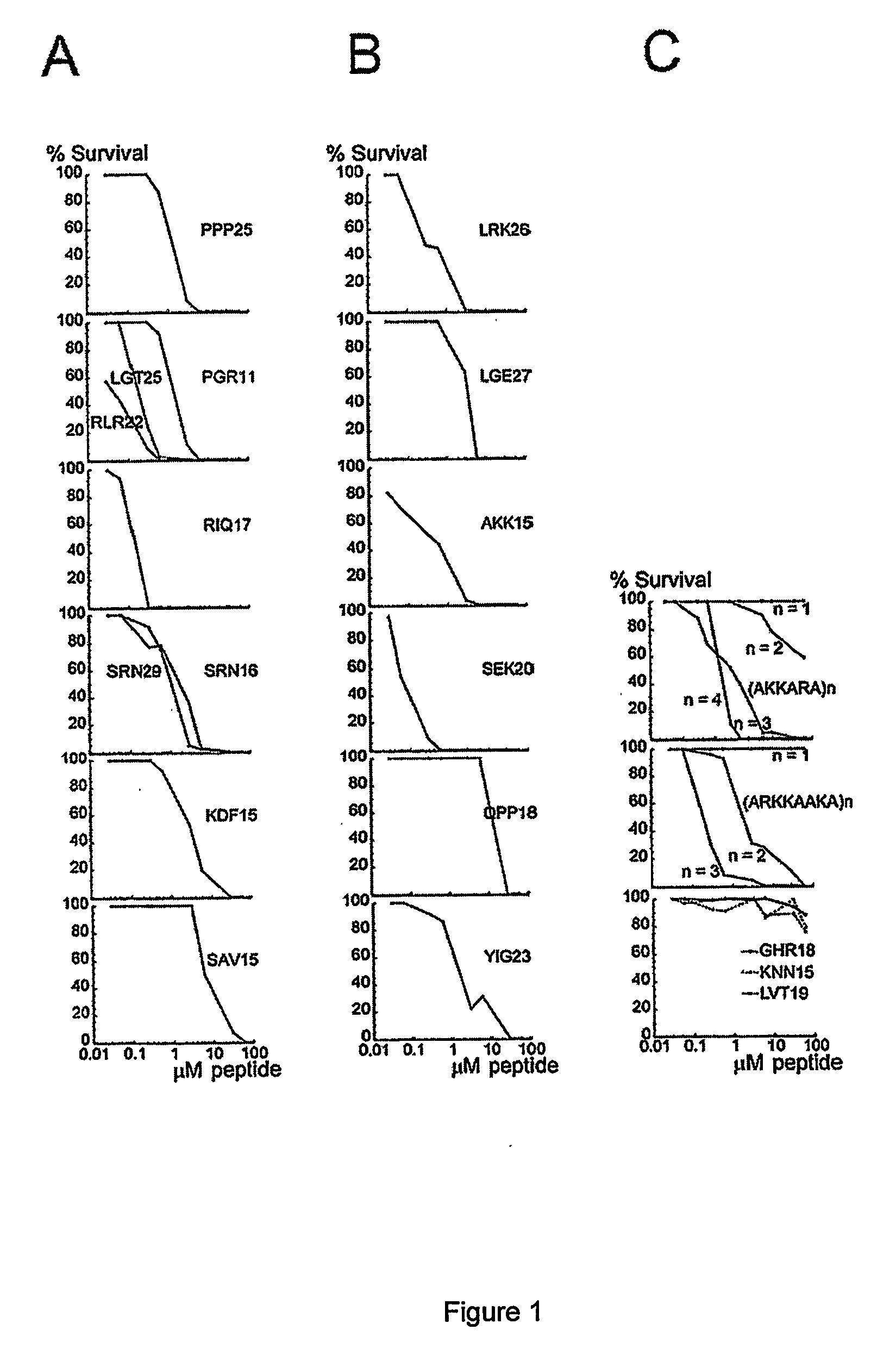
Inhibitors of plasma kallikrein and uses thereof
Provided herein are compounds that inhibit pKal, a serine protease whose activity is responsible for proteolytically cleaving kininogen and generating the potent vasodilator and pro-inflammatory peptide bradykinin, which can lead to painful and debilitating inflammatory attacks (e.g., edema). Also provided are pharmaceutical compositions and kits comprising the compounds, and methods of treating pKal-related diseases and disorders (e.g., edema) with the compounds in a subject, by administering the compounds and / or compositions described herein.
Owner:TAKEDA PHARMA CO LTD
Preparation method for pancreatic kininogenase enteric-coated tablets
ActiveCN105168168AAvoid degradationStable potencyPeptide/protein ingredientsUrinary disorderFormularyCoated tablets
The invention discloses a preparation method for pancreatic kininogenase enteric-coated tablets. The method comprises the steps of weighing, adhesive preparing, wet granule preparing, drying, total blending and tabletting. According to the preparation method for the pancreatic kininogenase enteric-coated tablets, compatibility is achieved by adopting a reasonable formula, degradation of pancreatic kininogenase in the preparing process is avoided by controlling technological parameters in the preparing process, and therefore it is guaranteed that the drug potency and quality are stable; a unique low-temperature drying technology is adopted when drying is performed, and it can be guaranteed that the product potency is not reduced; in the tabletting process, the temperature and the humidity are strictly controlled, and therefore it is guaranteed that in the tabletting process, the product potency cannot be lost due to the fact that the product is excessively heated; the preparation method is easy to operate, low in cost and suitable for industrialized mass production.
Owner:CHENGDU TONGDE PHARMA
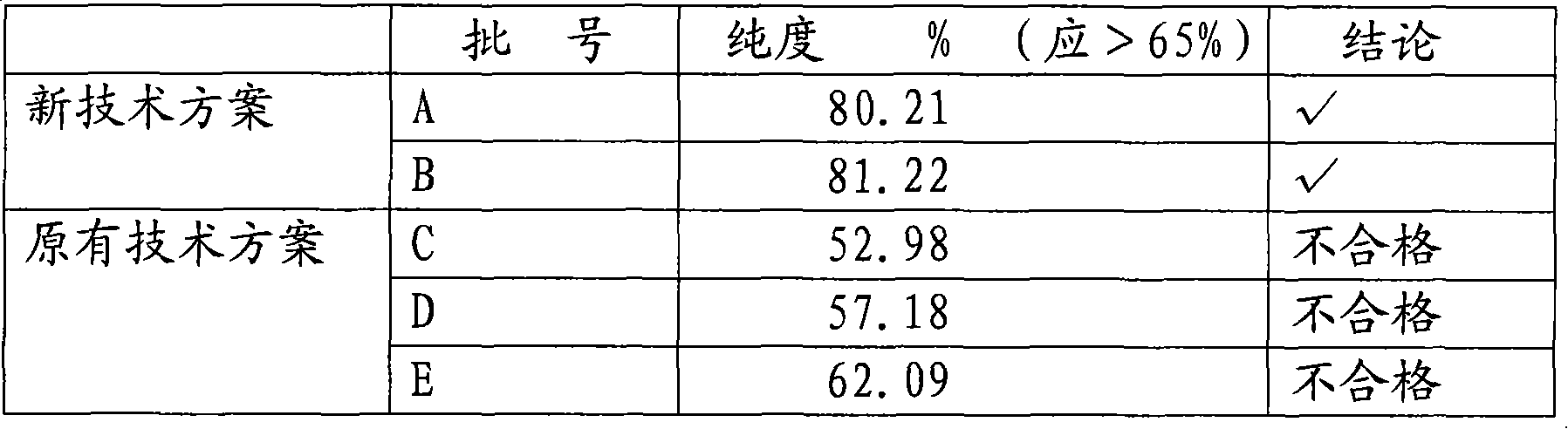
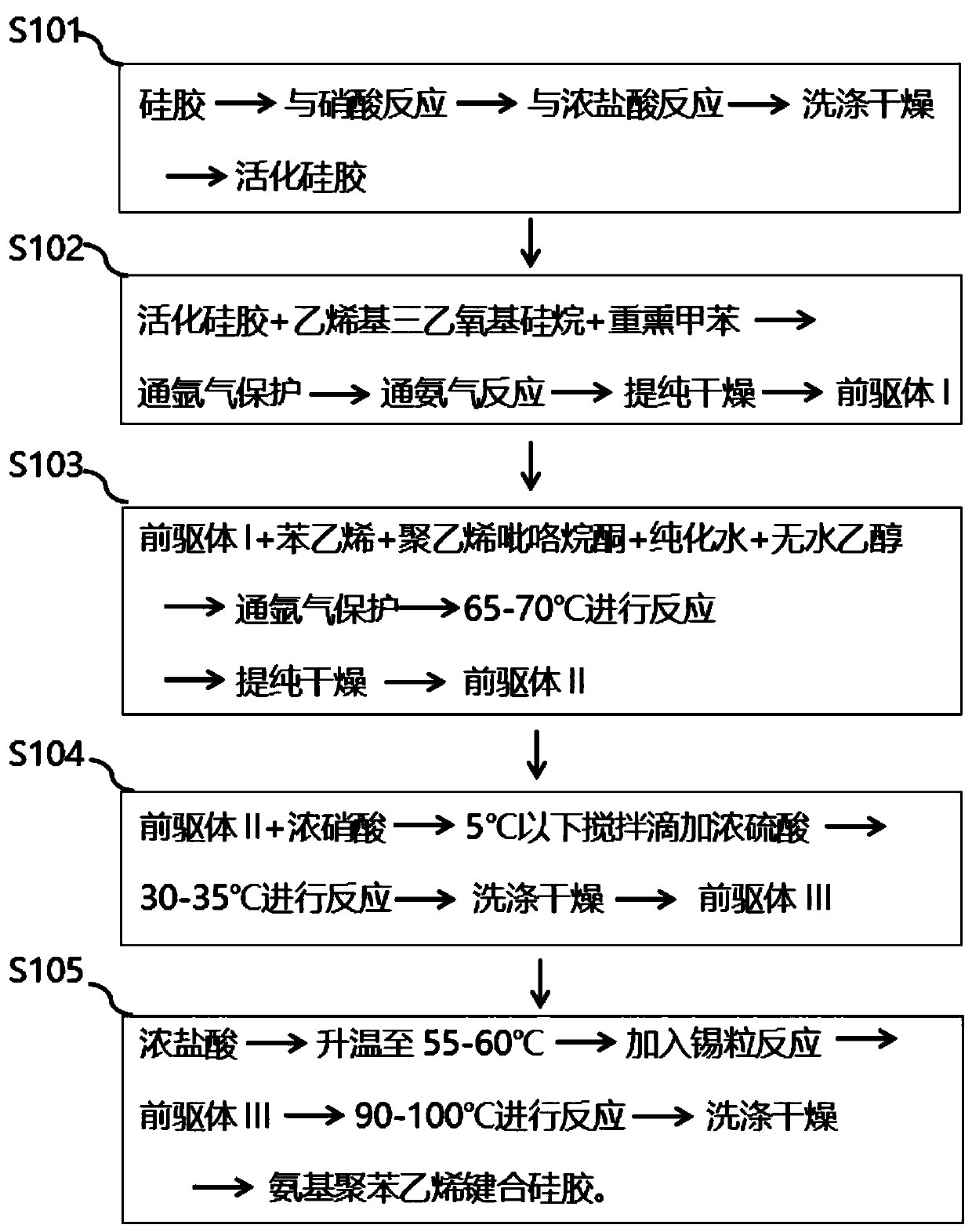
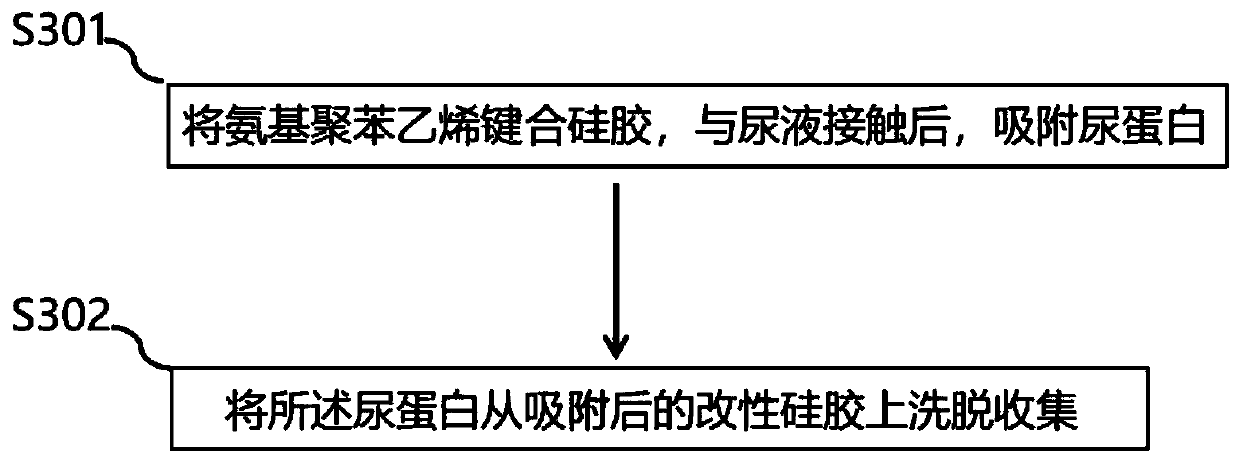
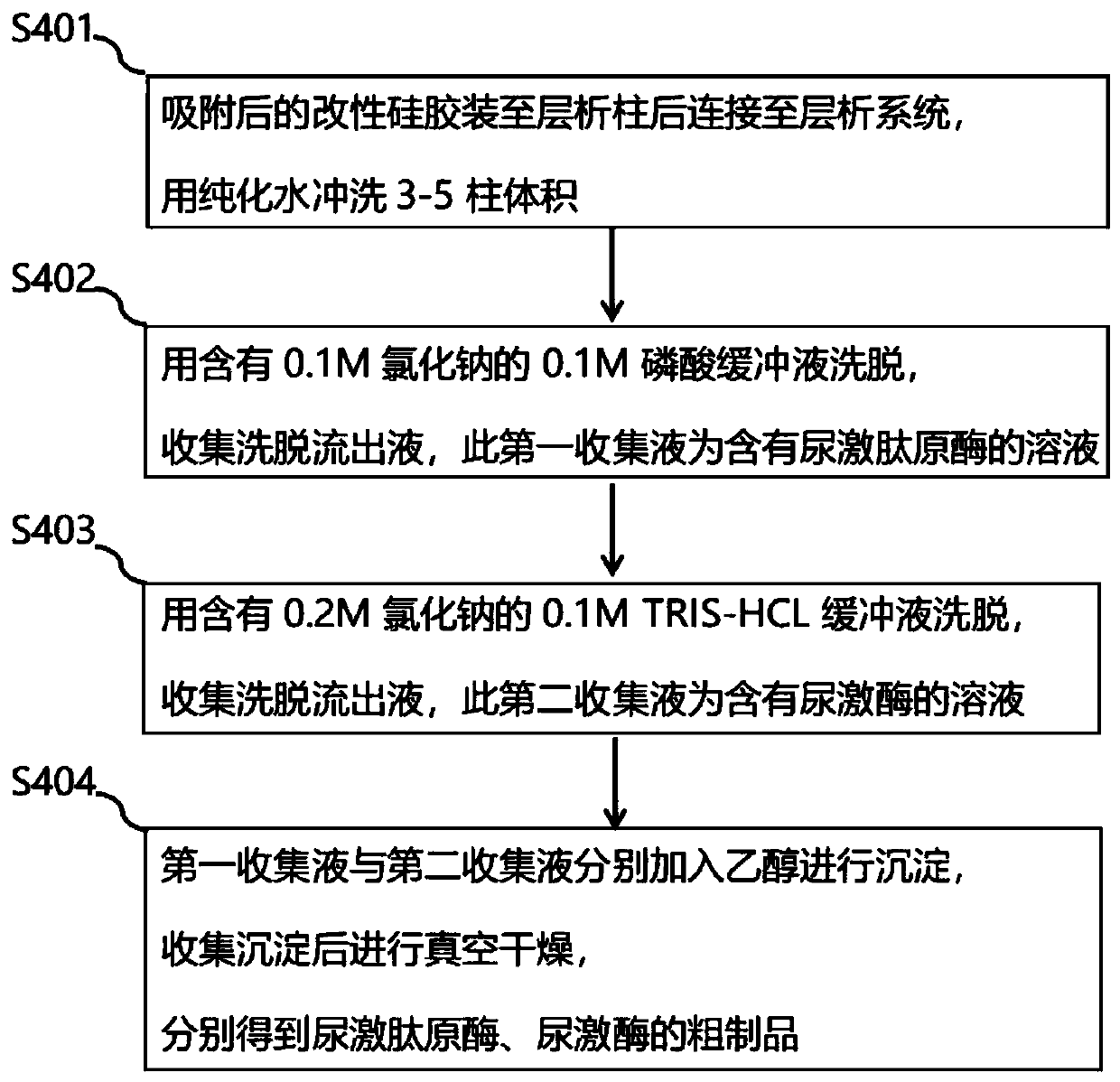
Preparation method and application of modified silica gel for extracting urine protein
ActiveCN111185141AIncrease profitImprove adsorption capacityOther chemical processesPeptidasesSilica gelIsoelectric point
The invention relates to a preparation method and application of modified silica gel for extracting urine protein. According to the invention, silica gel is modified into amino polystyrene bonded silica gel; urine proteins such as urokinase, human urokininogenase and the like in urine can be directly adsorbed by utilizing the modified silica gel, then the urine proteins are sequentially eluted byadopting different elution conditions according to the properties of different isoelectric points of the different urine proteins, and finally effective adsorption of the urine proteins and separationand enrichment of the different urine proteins can be realized; and the silica gel is high in utilization rate, good in adsorption separation effect, small in environmental pollution, convenient to use, low in cost and suitable for large-scale industrial production, and urine does not need to be collected and a urinal does not need to be transformed.
Owner:江西浩然生物制药有限公司
Anti-human kininogenase antibody and application thereof
ActiveCN105985436AMeet the needs of large-scale clinical applicationsEase of mass productionFermentationVector-based foreign material introductionPlasma samplesImmunofluorescence
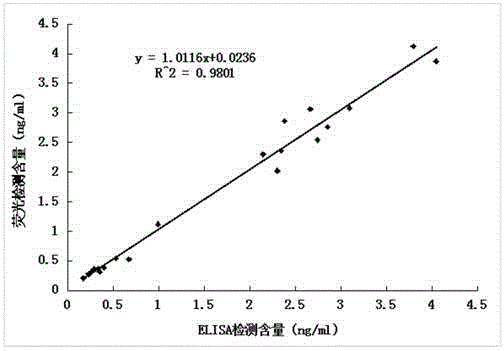
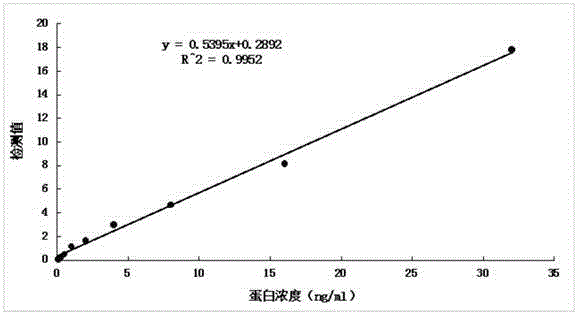
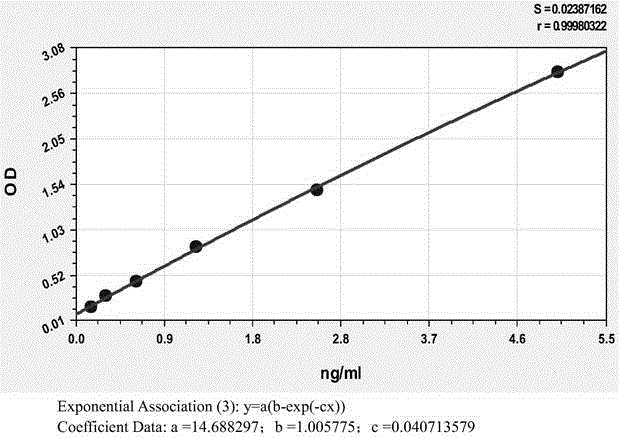
The invention relates to an anti-human kininogenase antibody and application thereof. According to the invention, a plurality of monoclonal antibodies are prepared and are paired and screened, so an antibody combination (A24 and A32) with sensitivity and specificity meeting demands is obtained; and the antibody combination can be conveniently produced in a large scale and can meet demands of large-scale clinical application in the future. The antibody combination is subjected to adjustment and optimization of a detection system so as to obtain an ELISA quantitative detection kit for human kininogenase, a colloidal gold immunochromatographic quantitative detection card for human kininogenase and a time-resolved immunofluorescent chromatographic quantitative detection card for human kininogenase, wherein the kit and the cards are simple operation and have sensitivity, specificity and related detection performance meeting requirements on detection of human blood plasma samples.
Owner:ZONHON BIOPHARMA INST
Novel antimicrobial peptides with heparin binding activity
InactiveUS20070185019A1Reduce riskImprove stabilityAntibacterial agentsBiocideAnti microbial peptideMicroorganism
The invention relates to an antimicrobial peptide with heparin binding activity, being derived from endogenous mammalian proteins being substantially free from antimicrobial activity selected from the group consisting of laminin isoforms, complement factor C3, histidin rich glycoprotein and kininogen and having from 10 to 36 amino acid residues, wherein the antimicrobial peptide consists of at least four amino acid residues selected from the group consisting of K, R and H. The invention also relates to pharmaceutical compositions comprising said antimicrobial peptide and use of the antimicrobial peptide and / or antimicrobial / pharmaceutical composition.
Owner:DERMAGEN AB
Markers for renal disease
ActiveUS10436797B2Less discomfortMore timely assessmentsDisease diagnosisBiological testingDiseaseMetabolite
This invention provides reagents and methods for diagnosing renal disease. Differential levels of inosine metabolite, and proteins: apolipoprotein C-I, apolipoprotein C-II, fibrinogen alpha chain, or fibrinogen A-alpha chain, kininogen, Inter-Alpha Inhibitor H4 (ITIH4), keratin Type I cytoskeletol 10 cystatin A, cystatin B and other polypeptides and fragments thereof provide biomarkers of renal disease and are described herein.
Owner:IDEXX LABORATORIES
PEG (pegylation) kininogenase preparation and application thereof
ActiveCN107753953AImprove biological activityImprove efficacyPowder deliveryPeptide/protein ingredientsMiddle molecular weightDrug product
The invention relates to an injection containing PEG (pegylation) kininogenase and application of injection. The kininogenase does not contain a component: low glycosylated KLK1, and the low glycosylated KLK1 is a band with the lowest molecular weight in three bands when in SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) on the component KLK1 from a pig pancreas; the general molecular formula of PEG is shown as the formula (1) or the formula (2). The prescription of a PEG kininogenase preparation highly maintains the biological activity of the PEG kininogenase and has comparatively good protection for PEG kininogenase, and the stability of a drug is effectively improved.
Owner:ZONHON BIOPHARMA INST +1
Thrombin-free composition containing freeze-dried, virally inactivated FXIa and serpins and/or kininogen
InactiveUS8889624B2EfficaciousQuick controlPeptide/protein ingredientsHydrolasesFreeze-dryingFractionation
A virally safe, thrombin-free factor-XIa concentrate or a coagulation factor concentrate which contains factor XIa as an active pharmaceutical ingredient and which is obtained by fractionation of plasma or serum or by genetic engineering and is suitable for the treatment of coagulation disorders attributable to diminished and / or delayed thrombin formation.
Owner:BIO PRODS & BIO ENG AKTIENGES
Preparation and application of pegylated kininogenase
ActiveCN107753953BImprove biological activityImprove efficacyPowder deliveryPeptide/protein ingredientsElectrophoresesMedicine
The present invention relates to an injection containing pegylated kininogenase and its application. The kininogenase does not contain lowly glycosylated KLK1, and the lowly glycosylated KLK1 is KLK1 derived from porcine pancreas The band with the lowest molecular weight among the three bands during SDS-PAGE protein electrophoresis; the general structural formula of the PEG is as shown in formula (1) or formula (2). The pegylated kininogenase formulation of the present invention highly maintains the biological activity of the pegylated kininogenase, has a better protective effect on it, and effectively improves the stability of the drug.
Owner:ZONHON BIOPHARMA INST +1
Method for separating human urinary kallidinogenase and thrombin regulatory protein
PendingCN113913415AEasy to operateLow costPeptide preparation methodsAnimals/human peptidesCellulosePhenyl group
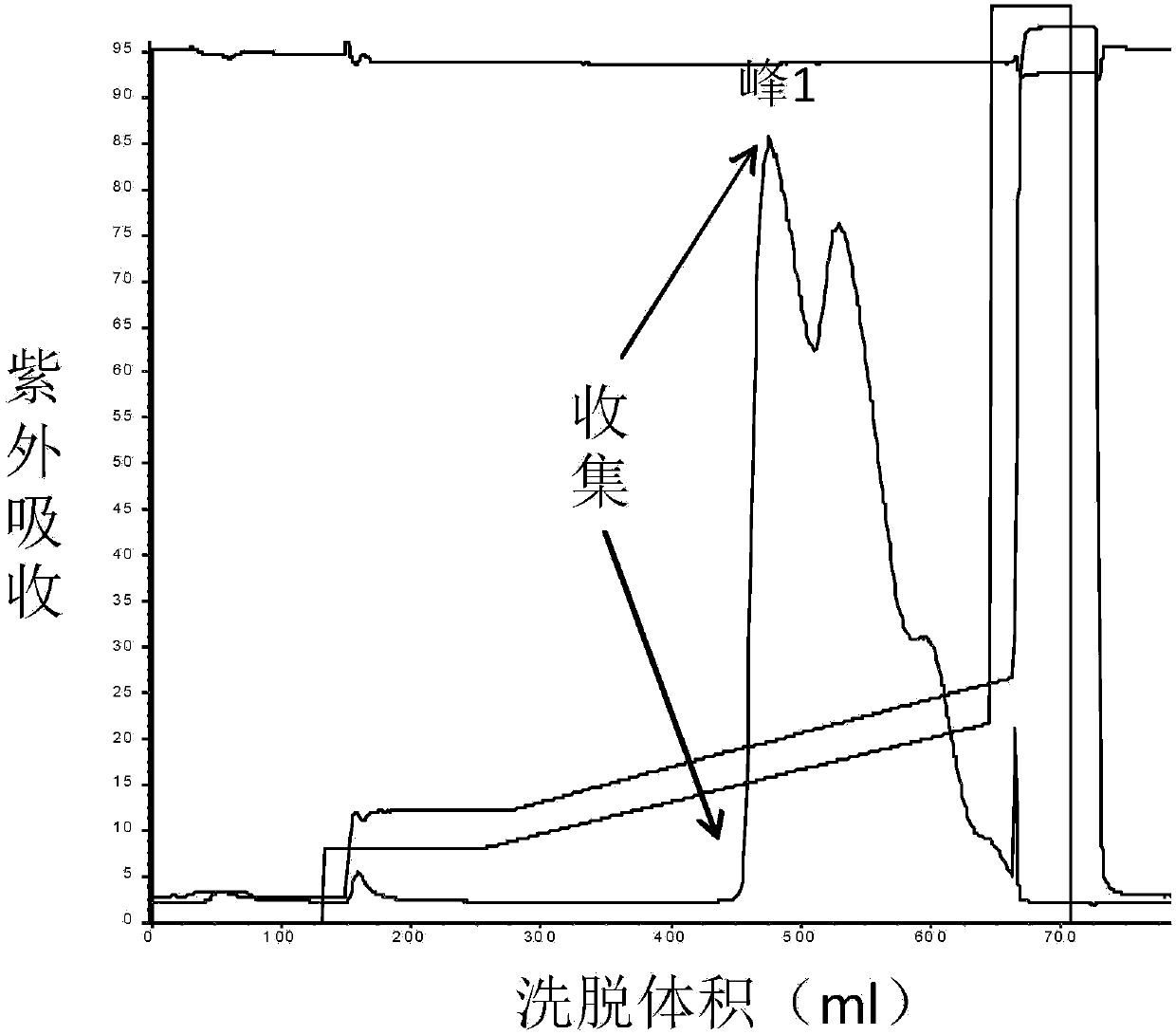
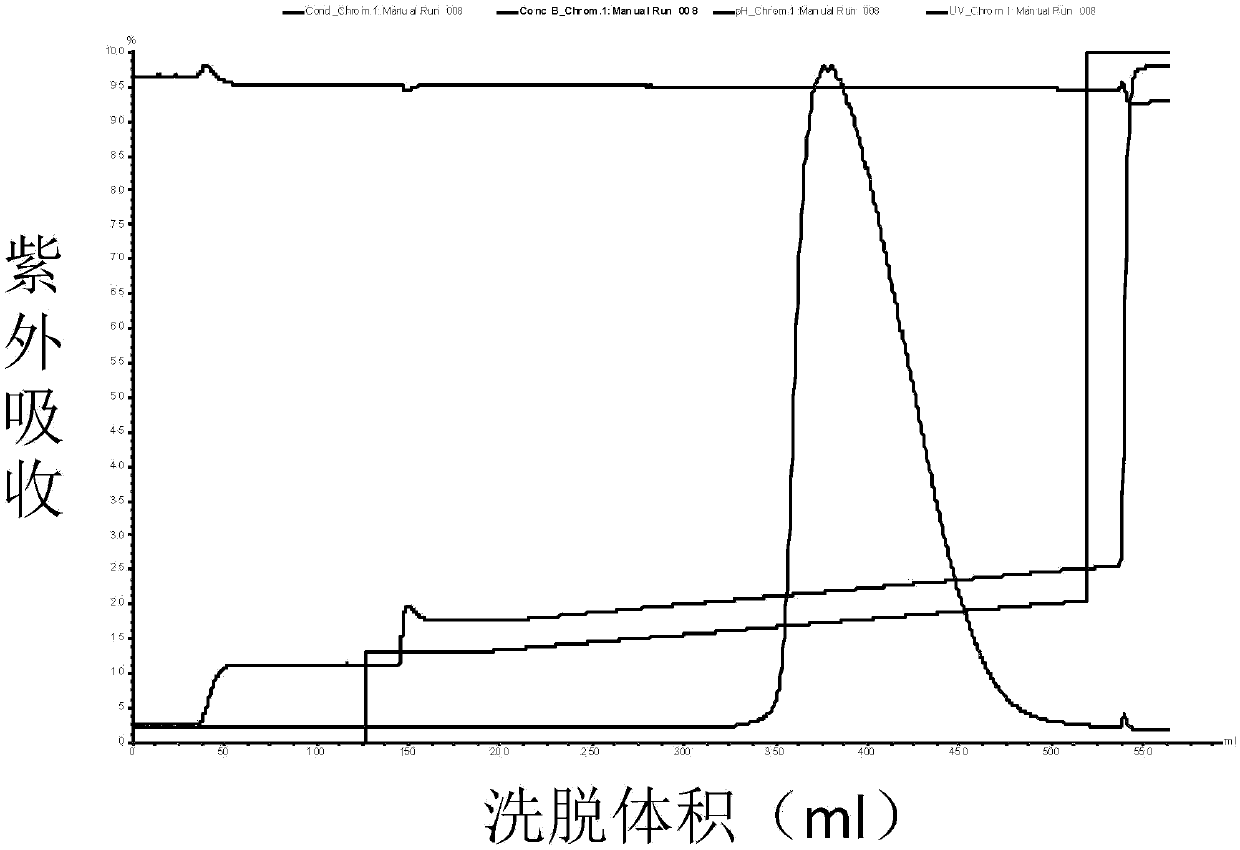
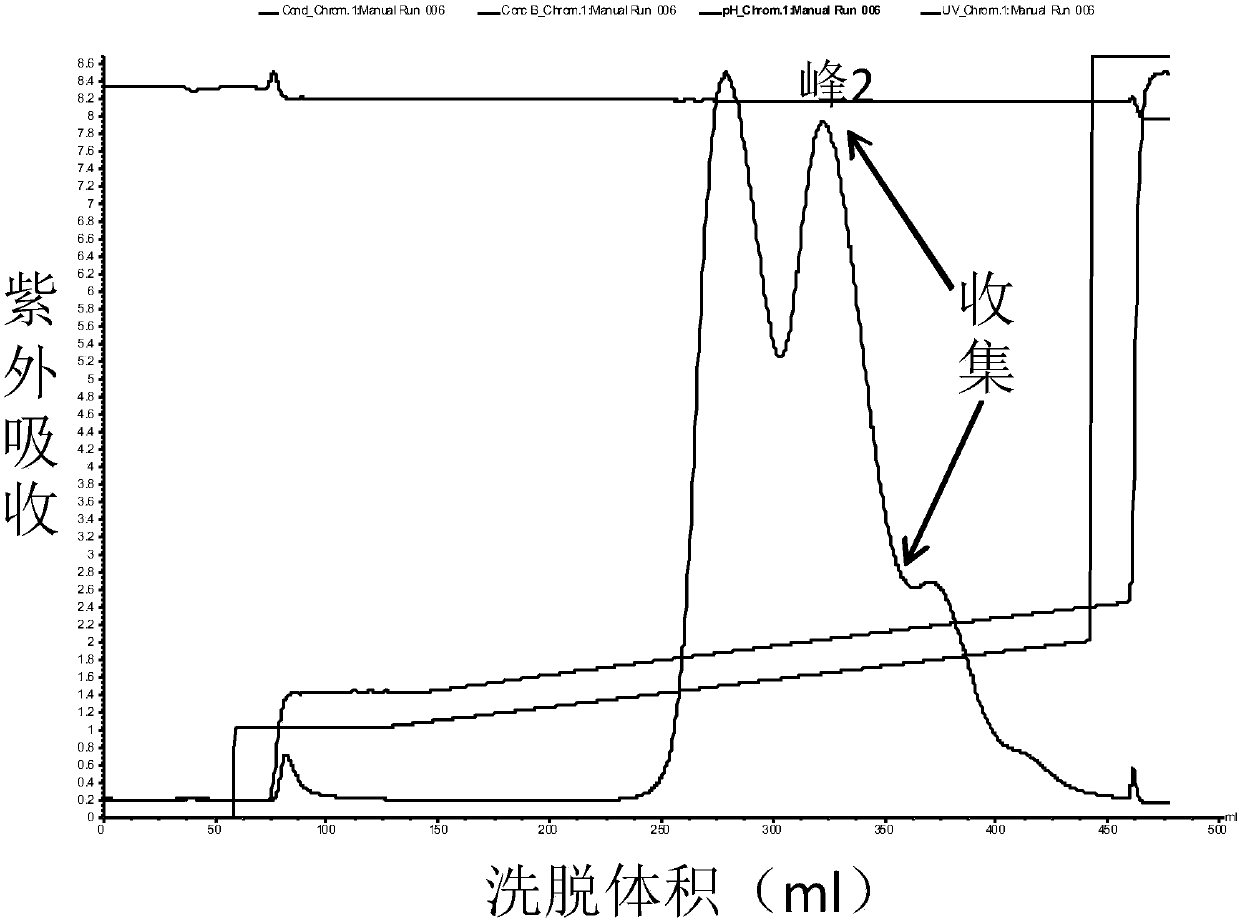
The invention discloses a method for separating human urinary kallidinogenase and thrombin regulatory protein in the technical field of biology. The method adopts hydrophobic chromatography to separate and obtain a human urinary kallidinogenase crude product and a thrombin regulatory protein crude product in one step for the first time, wherein a hydrophobic ligand of a hydrophobic chromatography column is one of phenyl, butyl sulfur and octyl; and a filler main body framework of the hydrophobic chromatography column is one of agarose, cellulose or polymer. The method has the advantages of simple operation, low cost, easy industrialized amplification, high yield of the two separated products and obvious impurity removal effect.
Owner:YANGZHOU AIDEA BIOTECH
A kind of pancreatic kininogenase and its preparation process
ActiveCN107043760BFully activatedHigh pancreatic elastase activityPeptidasesPancreatic elastasePancreas
The invention relates to a pancreatic kininogenase and a preparation process thereof. The preparation steps of the pancreatic kininogenase include: thawing frozen porcine pancreas, mincing, grinding, pretreatment, fiber separation, activation, activated pancreatic pulp filtration, 19 steps including alcohol precipitation, heavy phase filtration, granulation, etc., finally obtain pancreatic kininogenase. The present invention realizes the full activation of pancreatic kininogenase and elastase in the pancreas, creating the possibility for the further joint production of pancreatic kininogenase and pancreatic elastase; the activity of pancreatic elastase in the pancreatic kininogenase obtained in the present invention is very high High; the invention greatly improves the filtration speed and realizes industrialized production; the invention adopts the boiling drying method, which has small investment, short time consumption and low energy loss.
Owner:临沂新程金锣肉制品集团有限公司
PEG modification of medicinal kininogenase and its preparation method and application
ActiveCN107760661BHigh purityImprove stabilityPeptide/protein ingredientsHydrolasesSide effectEfficacy
The invention relates to PEG-modified medicinal kininogenase and a preparation method and application thereof. The kininogenase does not contain lowly-glycosylated KLK1, and the lowly-glycosylated KLK1 is a stripe with the lowest molecular weight in three stripes during SDS-PAGE protein electrophoresis of porcine pancreas-derived KLK1; PEG adopts a structural general formula shown as a formula (1)or a formula (2). According to the pegylated kininogenase, on one hand, component nonuniformity caused by different glycosylation modifications of the kininogenase is eliminated, so that the purity,the stability, the bioactivity and the medicinal efficacy are improved; on the other hand, after PEG modification of the kininogenase, the half-life period is significantly prolonged, the immunogenicity is significantly reduced, and side effects of a raw medicine are effectively reduced.
Owner:ZONHON BIOPHARMA INST +1
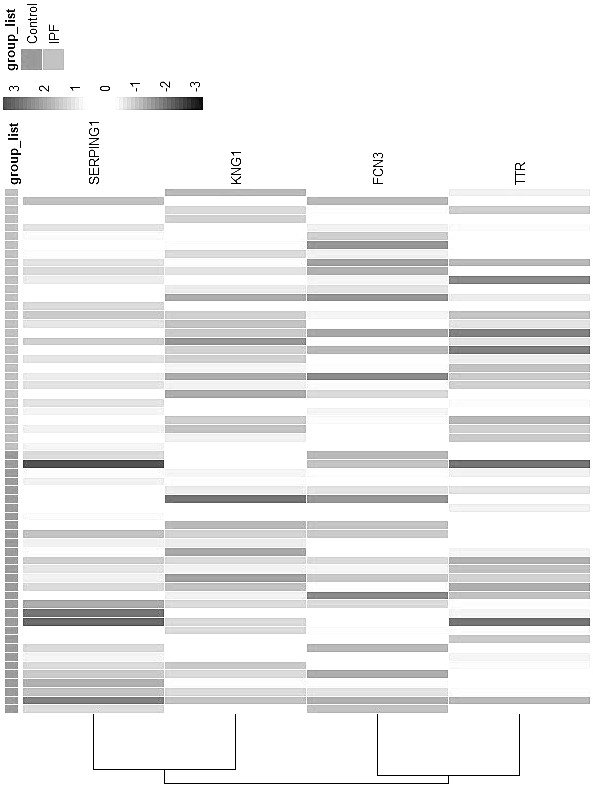
Idiopathic pulmonary fibrosis plasma protein marker and application thereof in preparation of detection reagent or diagnostic tool
PendingCN114878832ABiological material analysisBiological testingSerine Protease InhibitorsIdiopathic pulmonary fibrosis
The invention discloses an idiopathic pulmonary fibrosis plasma protein marker and application thereof in preparation of a detection reagent or a diagnostic tool, and the plasma protein marker is a serine protease inhibitor family G1, kininogen 1, fibrogelling protein 3 and transthyretin. The invention also discloses application of the idiopathic pulmonary fibrosis plasma protein marker in preparation of a detection reagent or a diagnostic tool. Results show that the four proteins are combined to predict area (AUC) values under a working characteristic curve (ROC) of a subject suffering from pulmonary fibrosis to be 0.799 and 0.848 (a verification data set), expression quantities of the four proteins in normal people and patients suffering from pulmonary fibrosis have significant differences, and variable coefficients are all less than 0.5; the robustness and the accuracy of predicting idiopathic pulmonary fibrosis through the combination of the four proteins are further determined.
Owner:HENAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com