Recombinant human pancreatic kininogenase-containing medicine for treating and/or preventing cerebral infarction
A kallikrein and drug technology, applied in the field of recombinant human kallikrein drugs, can solve the problems of unknown influence of enzyme activity, limited human urine protein resources, difficulty in collecting human urine, etc. The effect of excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
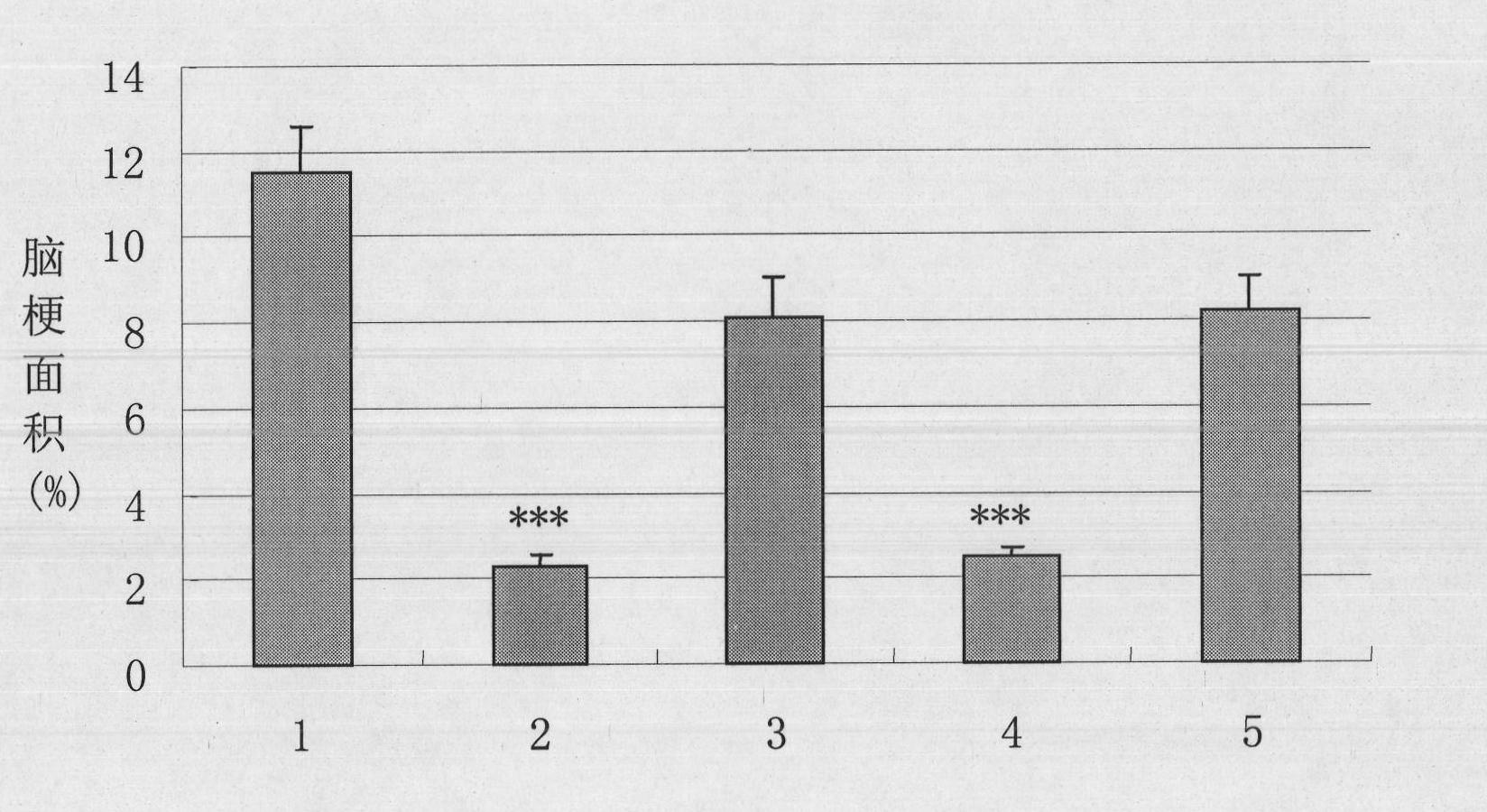
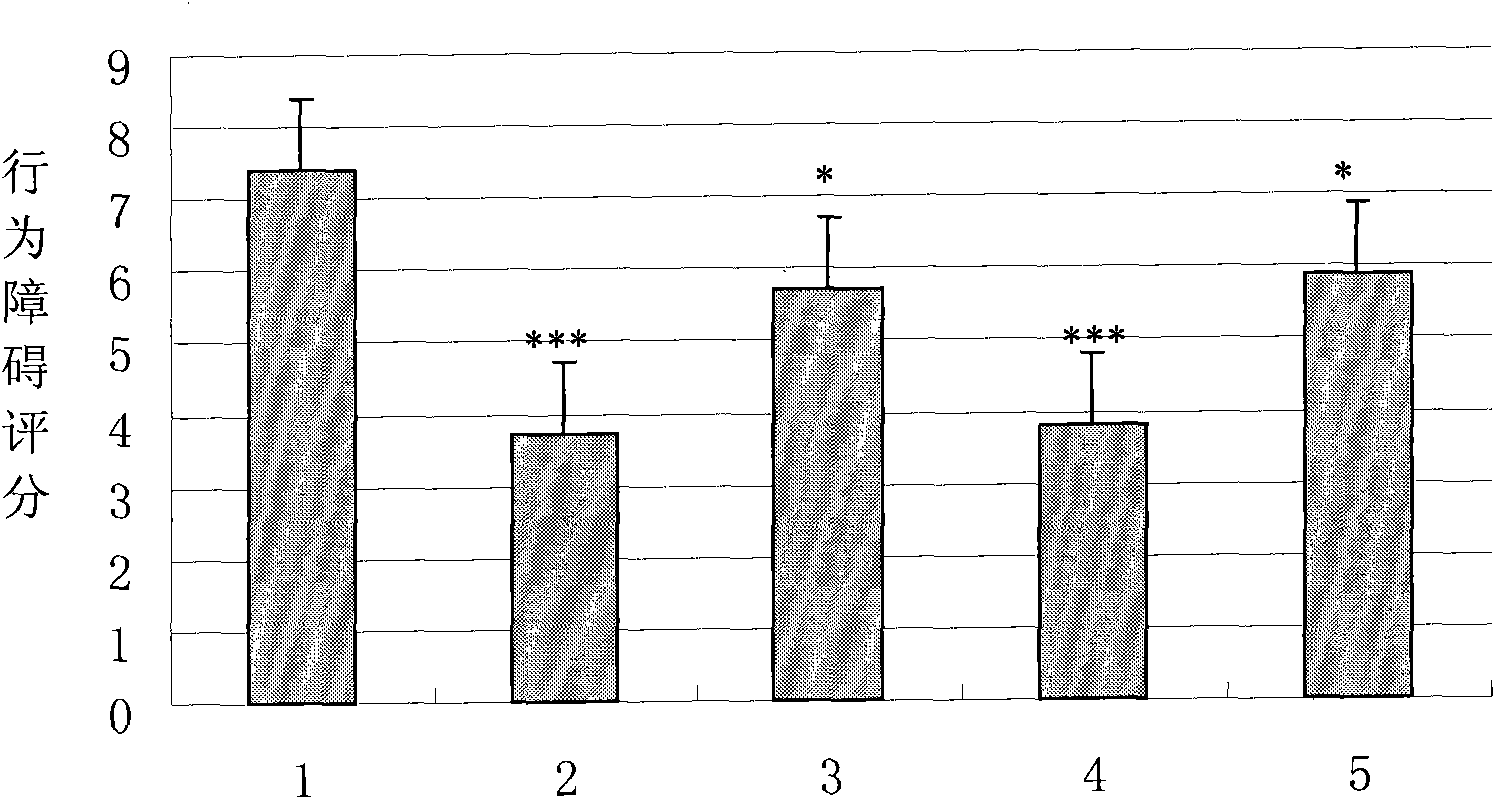
Examples
Embodiment 1
[0046] Example 1: Cloning of human pancreatic kininogenase gene (KLK1 gene)
[0047] Materials and methods: Using human kidney total RNA as a template, the full-length cDNA of KLK was firstly obtained through a reverse transcription kit (Invitrogen, USA). Then using the cDNA as a template, first amplify the two fragments of 1-496bp (taking ATG as 1) and 476-789bp of KLK respectively, and then use the 5' and 3' end primers to splice the two fragments into Complete KLK gene. PCR reaction conditions for amplifying the KLK fragment: denaturation at 94°C for 3 minutes; 30s at 94°C; 30s at 62°C; 30s at 72°C; 34 cycles of amplification;
[0048] The primers used to amplify KLK 1-496 are:
[0049] Upstream primer: 5'GCCTCGCCCTGTCCCTGGGGGGGACTGGTGCTGCGCCCCCGATTCAGTCCCGGATTGTGG3'
[0050] Downstream primer: 5'AATTCTCTGGTTCGATGCTGC 3'
[0051] The primers used for PCR amplification of 476-789bp fragments are:
[0052] Upstream primer: 5'GCAGCATCGAACCAGAGAATTTCTCATTTCCAGATGATCTC3'
...
Embodiment 2
[0060] Embodiment 2: Contain the construction of recombinant KLK1 gene expression plasmid
[0061] The full-length KLK1 gene was inserted into the Xho I and EcoR I sites of the vector pcDNA3.1 / myc-His(-)A containing a strong CMV promoter, and the pcDNA3.1-KLK1 eukaryotic expression plasmid was constructed.
[0062] Primers used to construct pcDNA3.1-KLK1:
[0063] Upstream primer: 5'GTGA CTCGAG ACCATGGGGTTCCTGGTTCTGTGC 3' (Xho I restriction site is underlined)
[0064] Downstream primer: 5'ATCT GAATTC TCAGGAGTTCTCCGCTATGGTGTC 3' (the underline is the EcoR I restriction site).
[0065] In addition, in order to construct a fusion protein containing human pancreatic kininogenase and human IgG1 Fc fragment to express more stable in vivo, the human IgG1 Fc fragment was inserted at the EcoR I and BamH I sites of pcDNA3.1-KLK1, and the pcDNA3.1-KLK1-Fc eukaryotic expression plasmid. The Fc fragment is located at the C-terminus of the KLK1 gene, connected by an EcoR I restricti...
Embodiment 3
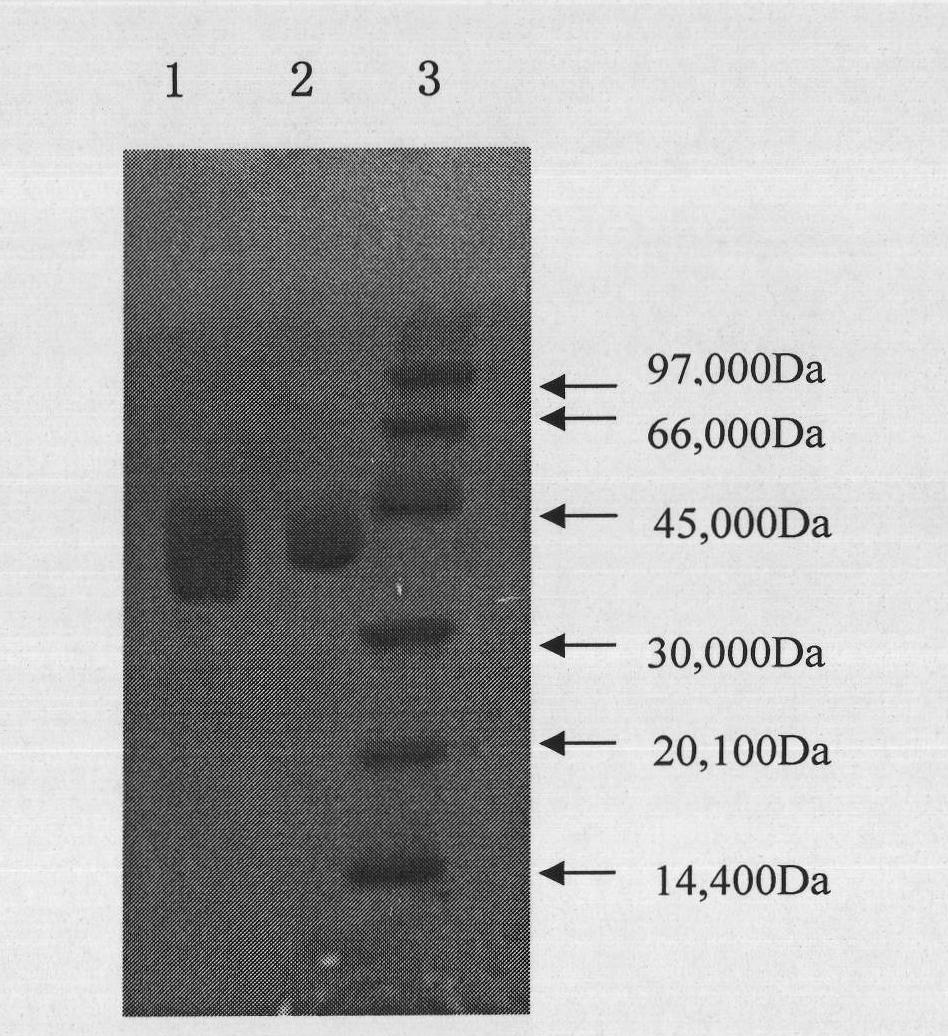
[0066] Example 3: Expression and preparation of recombinant human pancreatic kininogenase in eukaryotic cells
[0067] method:
[0068] 1. Expression of KLK1 gene in CHO cells:
[0069] CHO cells were cultured in DMEM medium containing 10% fetal bovine serum in 5% CO 2, Incubated at 37°C. Cationic liposomes (LipofectAMINE 2000, Invitrogen Corporation) were used to transfect CHO cells with the pcDNA3.1-KLK eukaryotic expression plasmid prepared in Example 2, and to screen transfected cells with G418; and then to identify G418 by enzyme activity assay and Western Blot Screened monoclonal stable cell lines.
[0070] 2. Large-scale culture of host cells expressing recombinant human pancreatic kininogenase:
[0071] a) The cell line containing the expressed recombinant protein is cultured with DMEM medium (pH7.20) containing 5% fetal bovine serum, and subcultured in a serum-free medium using a conventional method, and then cultured in small-scale suspension batches , placed i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com