Pharmaceutical composition containing recombination human pancreatic kininogenase for treating and/or preventing cerebral infarction
A technology of pancreatic kininogenase and composition, applied in the field of recombinant human pancreatic kininogenase pharmaceutical composition, which can solve the problems of unknown influence on enzyme activity, difficulty in collecting human urine, limited human urine protein resources, etc., and achieve side effects Small, less side effects, excellent efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
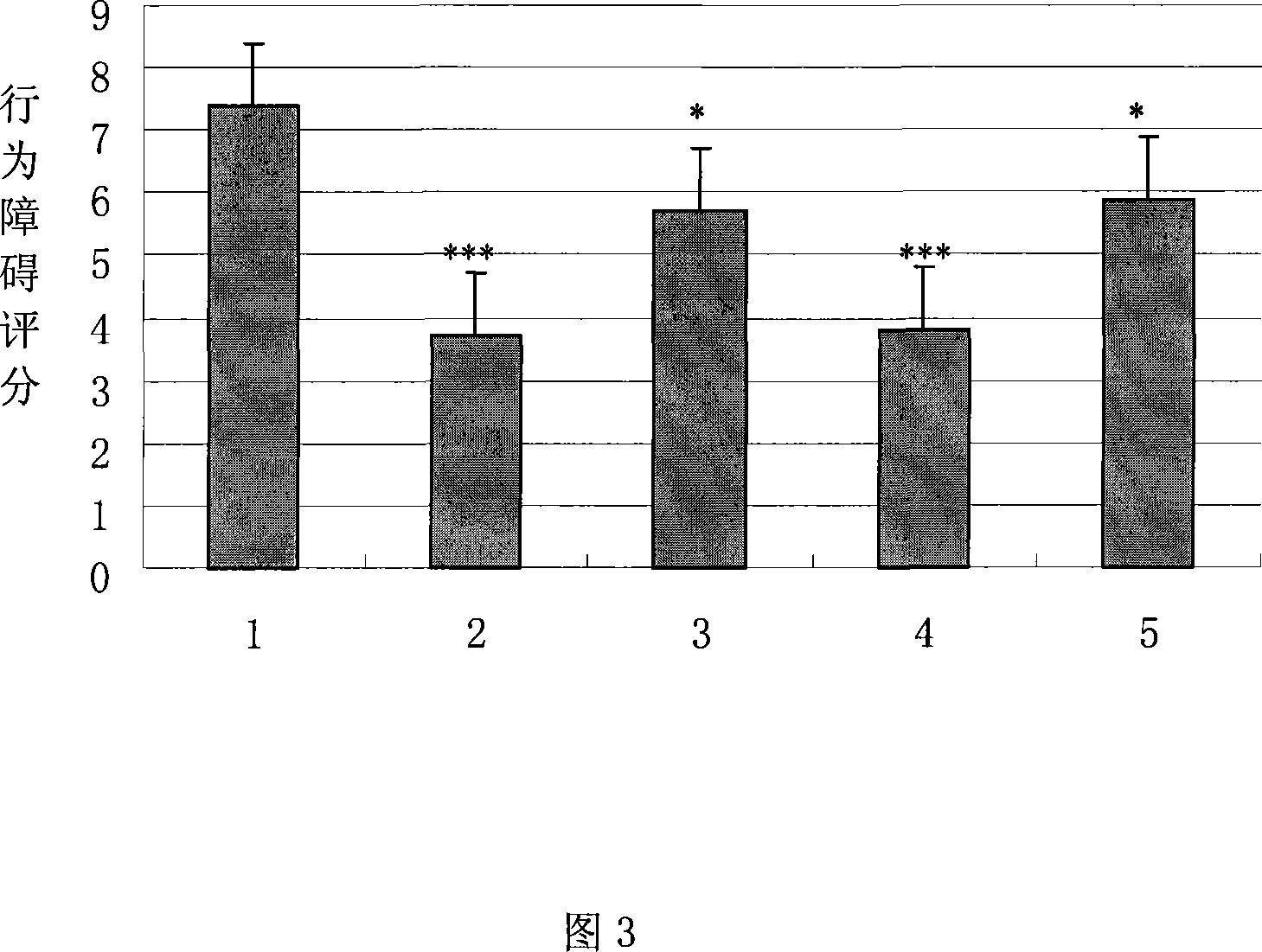
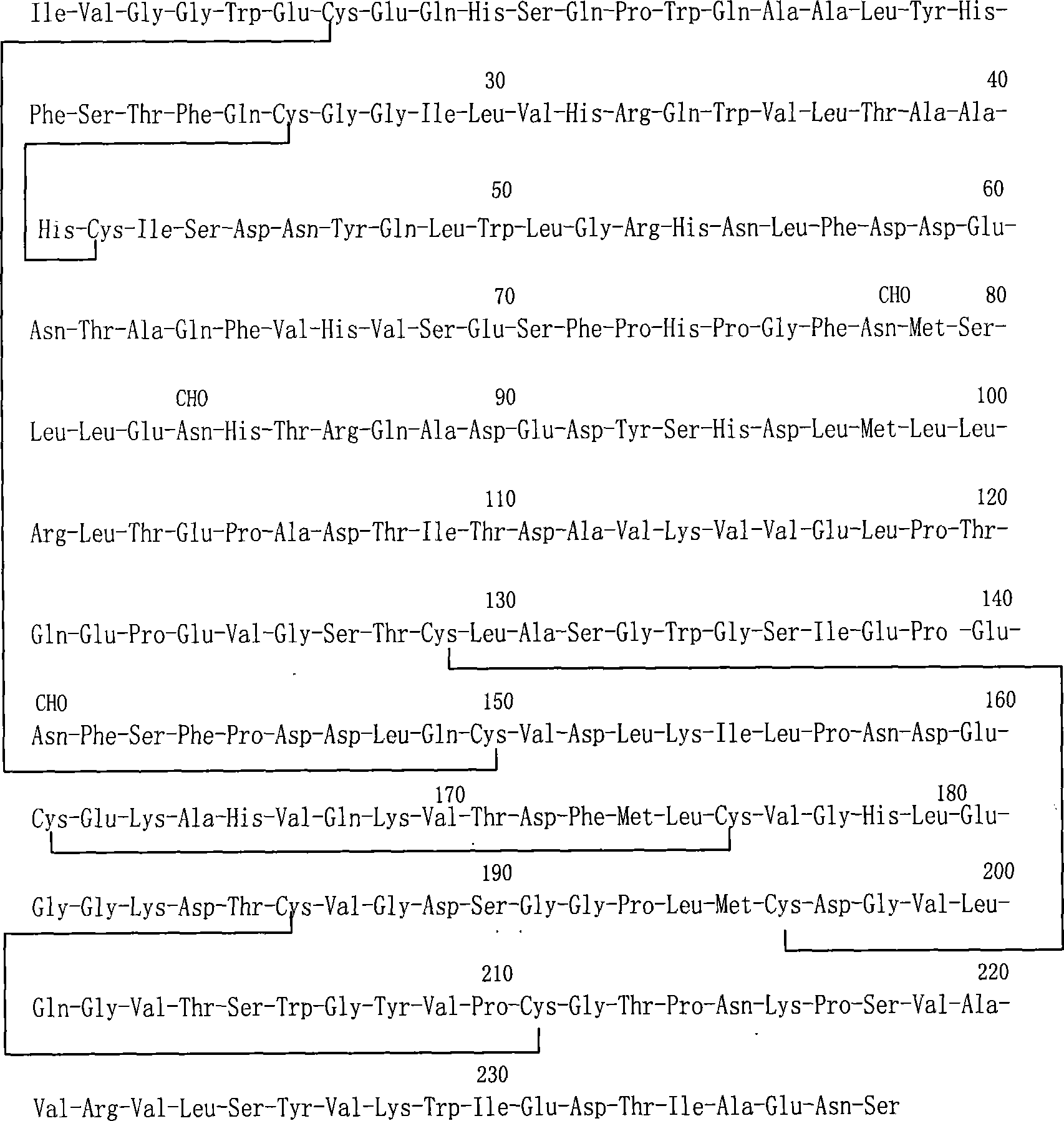
Examples
Embodiment 1
[0048] Example 1: Cloning of human pancreatic kininogenase gene (KLK1 gene)
[0049] Materials and methods: Using human kidney total RNA as a template, the full-length cDNA of KLK was first obtained through a reverse transcription kit (Invitrogen, USA). Then using the cDNA as a template, first amplify the two fragments of 1-496bp (taking ATG as 1) and 476-789bp of KLK respectively, and then use the 5' and 3' end primers to splice the two fragments into Complete KLK gene. PCR reaction conditions for amplifying the KLK fragment: denaturation at 94°C for 3 minutes; 30s at 94°C; 30s at 62°C; 30s at 72°C; 34 cycles of amplification;
[0050] The primers used to amplify KLK 1-496 are:
[0051] Upstream primer: 5'GCCTCGCCCTGTCCCTGGGGGGGACTGGTGCTGCGCCCCCGATTCAGT
[0052] CCCGGATTGTGG3'
[0053] Downstream primer: 5'AATTCTCTGGTTCGATGCTGC3'
[0054] The primers used for PCR amplification of 476-789bp fragments are:
[0055] Upstream primer: 5'GCAGCATCGAACCAGAGAATTTCTC...
Embodiment 2
[0064] Embodiment 2: Contain the construction of recombinant KLK1 gene expression plasmid
[0065] The full-length KLK1 gene was inserted into the Xho I and EcoR I sites of the vector pcDNA3.1 / myc-His(-)A containing a strong CMV promoter, and the pcDNA3.1-KLK1 eukaryotic expression plasmid was constructed.
[0066] Primers used to construct pcDNA3.1-KLK1:
[0067] Upstream primer: 5'GTGA CTCGAG ACCATGGGGTTCCTGGTTCTGTGC3' (Xho I restriction site is underlined)
[0068] Downstream primer: 5'ATCT GAATTC TCAGGAGTTCTCCGCTATGGTGTC3' (the underline is the EcoR I restriction site).
[0069] In addition, in order to construct a fusion protein containing human pancreatic kininogenase and human IgG1 Fc fragment in order to express a more stable fusion protein in vivo, human IgG1 Fc fragment was inserted at the EcoR I and BamH I sites of pcDNA3.1-KLK1 to construct The pcDNA3.1-KLK1-Fc eukaryotic expression plasmid was obtained. The Fc fragment is located at the C-terminus of the KLK...
Embodiment 3
[0070] Example 3: Expression and preparation of recombinant human pancreatic kininogenase in eukaryotic cells
[0071] method:
[0072] 1. Expression of KLK1 gene in CHO cells:
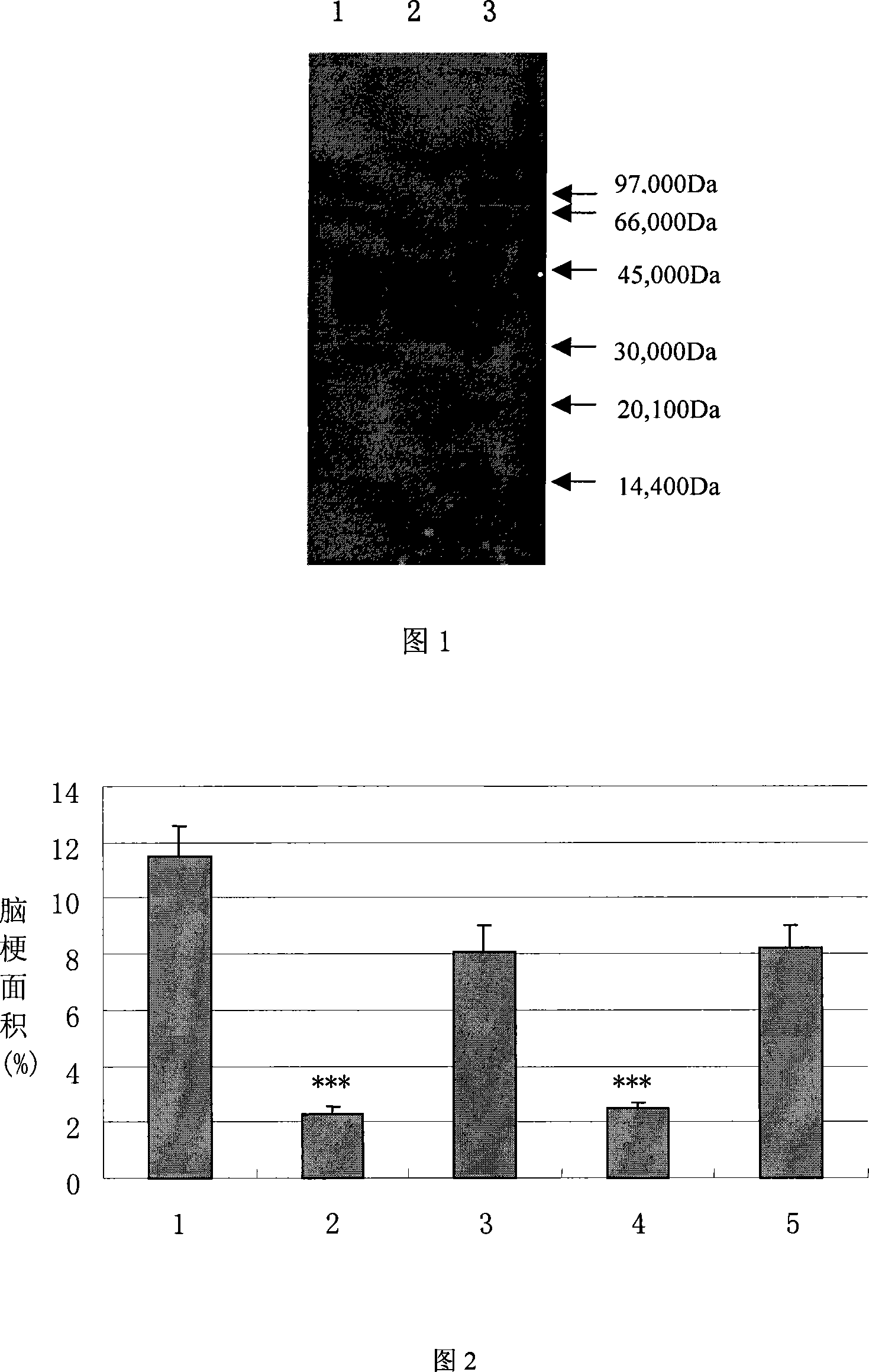
[0073]CHO cells were cultured in DMEM medium containing 10% fetal bovine serum and incubated at 37°C with 5% CO2. The pcDNA3.1-KLK eukaryotic expression plasmid prepared in the above-mentioned embodiment 2 was transfected into CHO cells with cationic liposomes (LipofectAMINE2000, Invitrogen Company), and the transfected cells were screened with G418; then the G418 screening was identified by enzyme activity assay and Western Blot The latter monoclonal stable cell line.
[0074] 2. Large-scale culture of host cells expressing recombinant human pancreatic kininogenase:
[0075] a) The cell line containing the expressed recombinant protein is cultured with DMEM medium (pH7.20) containing 5% fetal bovine serum, and subcultured in a serum-free medium using a conventional method, and then cultured in sma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com