cDNA sequence and amino acid sequence of lugworm fibrinolytic enzyme with thrombolysis activity
A sea earthworm and fibrinolytic enzyme technology, applied in the field of medical bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Cloning and sequencing of full-length cDNA of sea earthworm fibrinolytic enzyme.
[0050] Experimental Materials:
[0051] 1. Sea earthworms, collected from the offshore of Yantai City;
[0052] 2. Trizol reagent, 3′RACE kit, 5′RACE kit (Beijing Gibbett), reverse transcription kit (fermentas company), T-A Easy kit, Wizard purified DNA kit, DNase, RNase H (Promega company), high-fidelity Taq enzyme (Dalian Baobio).
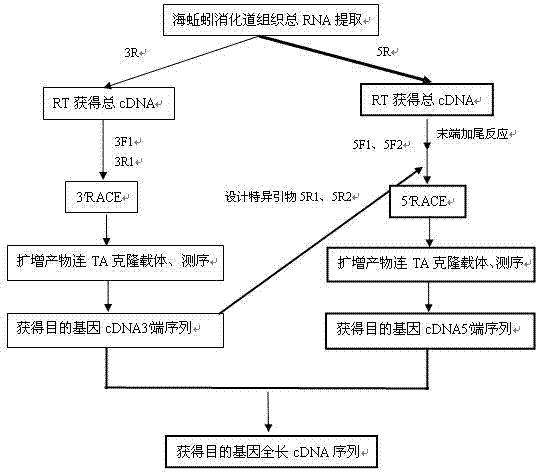
[0053] Experimental method: such as figure 1 shown, including the following steps:
[0054] 1. Design primer sequences:
[0055] Primer name sequence 3R 5′-GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT-3′ 3F1 5′-GGATCTTGCAATGGTGACAGCGGTG-3′ 3R1 5′-GGCCACGCGTCGACTAGTAC-3′ 5R 5′-TTTTTTTTTTTTTTTTTT-3′ 5F1 5′-GGCCACGCGTCGACTAGTACGGGGGGGGGGGGG-3′ 5R1 5′-CACCCTCTCTGATCCAGTCACGGAAG-3′ 5F2 5′-CCACGCGTCGACTAGTACG-3′ 5R2 5′-GGAGTAGGCAGATGGGTAGGAGGTC-3′
[0056] 3R is a reverse transcription primer containi...
Embodiment 2
[0110] Construction of recombinant expression vector and expression in Escherichia coli.
[0111]Experimental materials: Endonucleases BamHI and XhoI (Dalian Bao Biology), expression vectors pET-21a, E.coliBL21 are preserved in our laboratory, IPTG (Beijing Dongsheng Taibo), ultrasonic cracker (Shanghai Bilang).
[0112] Experimental method: such as figure 2 shown, including the following steps:
[0113] 1. According to the obtained cDNA sequence, design a pair of primers:
[0114] F 5′-TTAT GGATCC ATTGTTGGTGGCGATGAG-3′ R 5′-AATA CTCGAG GATGTCAGACACCTCTCTGATC-3′
[0115] upstream primer F contains BamHI Restriction site, contained in the downstream primer R wxya Restriction sites.
[0116] Using the cDNA of sea earthworm fibrinolytic enzyme as a template, pfu DNA polymerase was used to amplify the cDNA fragment without the coding region of the signal peptide, that is, the active peptide.
[0117] 2. The PCR product was connected with the pro...
Embodiment 3
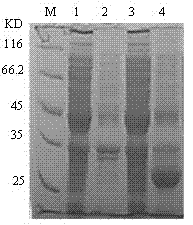
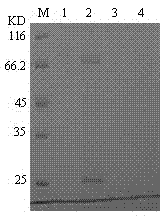
[0121] Isolation and purification of recombinant sea worm fibrinolytic enzyme.
[0122] Experimental materials: nickel column packing, ultrafiltration tube (Beijing Dongsheng Taibo).
[0123] Experimental method: such as figure 2 shown, including the following steps:
[0124] 1. Pick a single colony of engineered bacteria, inoculate it in 5ml LB medium containing 100μg / ml ampicillin, and cultivate overnight at 37°C with shaking. Transfer to 500ml of the same medium and continue to culture at 37°C for 4h. Add IPTG (isopropyl-β-D-thiogalactoside) to 1.0 mM, continue to culture at 30°C for 4 hours, and collect the bacteria by centrifugation at 8000×g for 5 minutes.
[0125] 2. Resuspend the bacteria in 25ml Tris-CL buffer (20mM, pH7.4, 10mM imidazole, 0.5M NaCl), sonicate the cells, centrifuge at 12000×g for 30min at 4°C, and take the supernatant.
[0126] 3. Flow the supernatant over Ni 2+ Resin column, washed with 50ml washing solution (20mM Tris-CL, pH7.4, 10mM imidazole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com