Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Human fibrinogen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
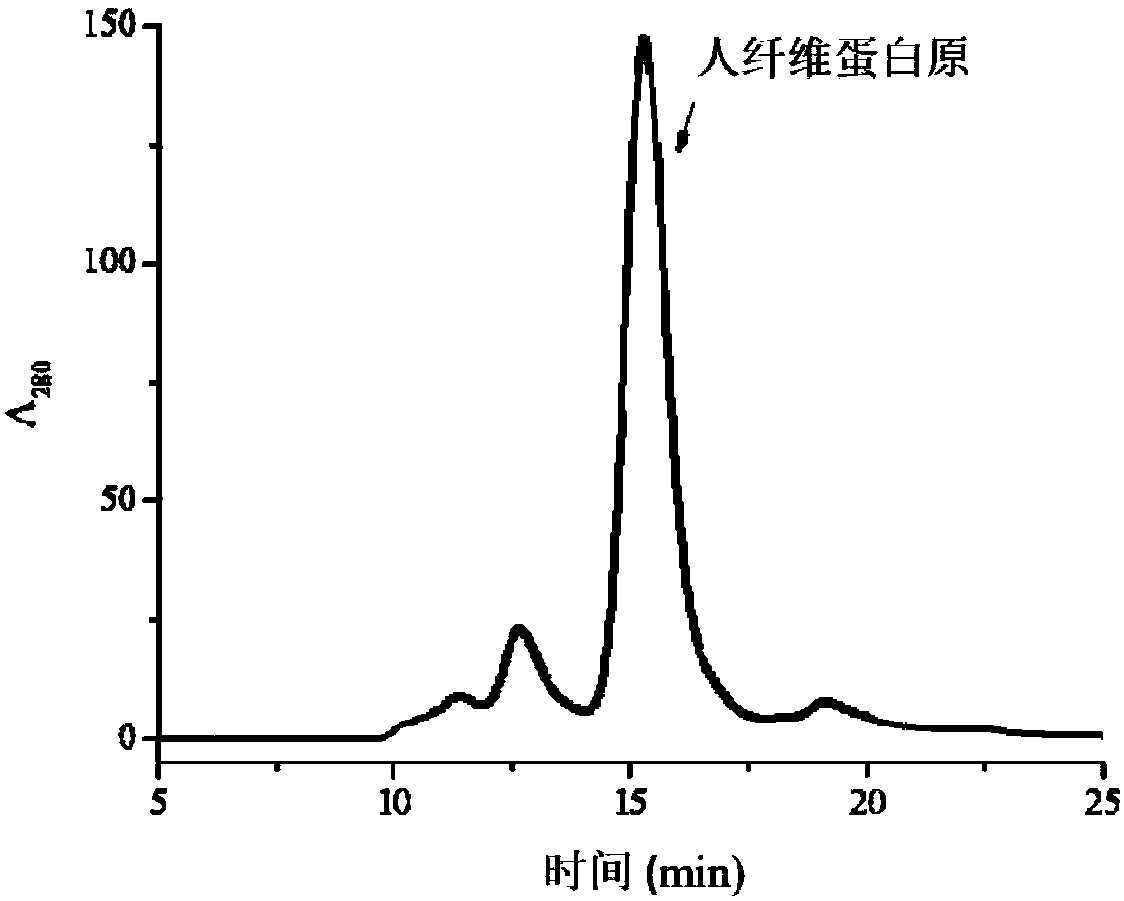
Human fibrinogen contains the soluble constituent of human plasma that is transformed to fibrin on the addition of thrombin. The fibrinogen molecule consists of three peptide chains, α (A), β (B), gamma (C), crosslinked by several disulfide bonds.
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm<3>, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsilon-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Carrier with solid fibrinogen and solid thrombin
InactiveUS7052713B2Safety and efficacyShorten hemostasis timePowder deliverySurgical adhesivesNatural sourceFiber
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibers. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
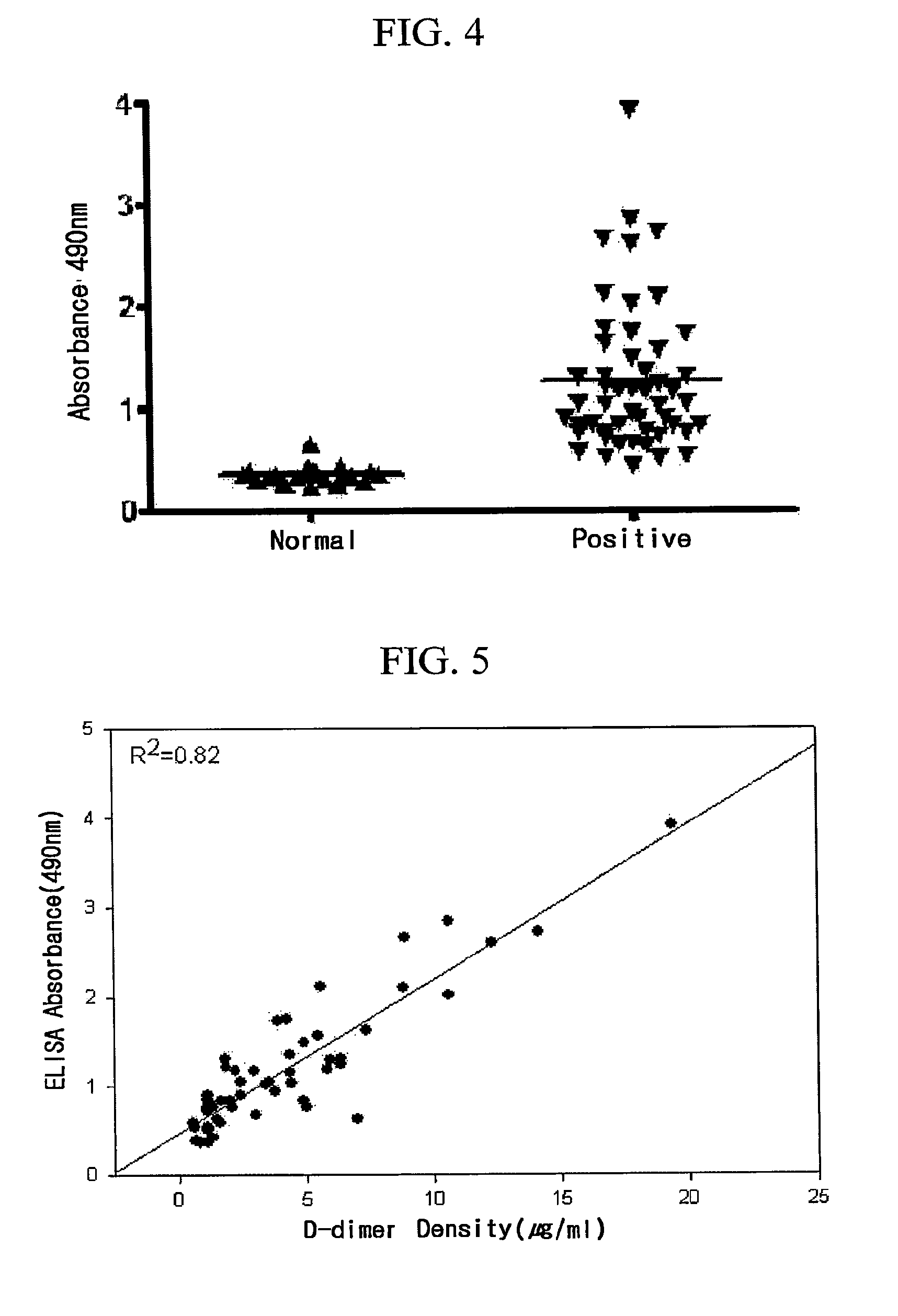
Monoclonal antibody and method of immunological analysis of e-D-dimer and e-DD/E complex
InactiveUS6132719AImmunoglobulins against animals/humansBiological material analysisFragment XMonoclonal antibody
PCT No. PCT / JP97 / 01639 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed May 15, 1997 PCT Pub. No. WO97 / 43315 PCT Pub. Date Nov. 20, 1997A monoclonal antibody which specifically reacts with D-monomer produced by digesting human fibrinogen with granulocyte elastase and D-domain containing digestion products produced by digesting human stabilized fibrin with granulocyte elastase, but does not react with fibrinogen, or fragment X, Y or E produced by digesting fibrinogen with granulocyte elastase is disclosed. The D-dimer or DD / E complex produced by digestion with granulocyte elastase in a sample from a living body can be analyzed without being interferred with fibrinogen, digestion products of fibrinogen with plasmin, or digestion products of stabilized fibrin with plasmin, using the monoclonal antibody.
Owner:MITSUBISHI CHEM MEDIENCE
Technology for extracting human blood coagulation factor VIII and human fibrinogen from plasma constituent precipitation
The invention provides a technology for extracting human blood coagulation factor VIII and human fibrinogen from plasma constituent precipitation. The preparation technology comprises the following steps: fresh plasma is subjected to primary sedimentation, so that blood coagulation factor VIII and fibrinogen precipitation can be jointly precipitated from the plasma; the primary precipitation is subjected to suspension; the suspension liquid is subjected to centrifugal separation to obtain supernatant; the centrifugally separated supernatant is subjected to virus inactivation and chromatography refining to respectively obtain human blood coagulation factor VIII refined liquid used for preparing human blood coagulation factor VIII products and chromatography effluent used for preparing human fibrinogen products. According to the invention, the human blood coagulation factor VIII and the human fibrinogen are precipitated through the one-step plasma constituent precipitation, and an ion-exchange column chromatography technology is adopted to perform purification preparation of the human blood coagulation factor VIII and the human fibrinogen, so that the deficiency that the human fibrinogen cannot be normally produced as the human blood coagulation factor VIII is prepared through cryoprecipitation is overcome.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsi-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
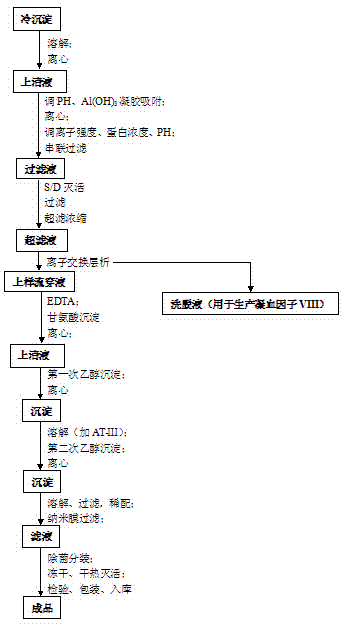
Preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII
ActiveCN104231072AHigh purityShort reconstitution timeFactor VIIFibrinogenBlood coagulation factor VIIIEthanol precipitation
The invention discloses a preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII. The preparation process is characterized by comprising the steps of cryoprecipitation dissolution, 2% aluminium hydroxide gel absorption, ion strength adjustment, series connection filtering, S / D viral inactivation, ion-exchange chromatography, EDTA Ca2+ removal, glycine precipitation, primary low-temperature ethanol precipitation, AT-III thrombin inhibition, secondary low-temperature ethanol precipitation, nanofilm filtering and dry-heat inactivation. For ensuring the safety, a nanofilm ia added to filter virus except S / D and dry-heat inactivation. By means of an added AT-III inactivated thrombin and EDTA Ca2+ removal process, fibrinogen in the production process is effectively prevented from being activated into fibrous protein. The glycine precipitation is utilized to remove fibrous protein monomers and polymers in products so as obtain high-purity human fibrinogen. The preparation products are safe and reliable, redissolution time is short, the clinic first-aid demand is met, and meanwhile the preparation process has important significance on indirect saving of scarce plasma resources.
Owner:华润博雅生物制药集团股份有限公司
Recombinant human fibrinogen for treatment of bleeding in trauma and platelet disorders
InactiveUS20100279939A1Save livesFast and efficient arrestSaccharide peptide ingredientsBlood disorderPlatelet disorderDisease
The present invention provides methods of using recombinant human fibrinogen to prevent or treat excessive bleeding in pre-hospital and hospital settings. In particular, the present invention relates to methods for treating bleeding using recombinant human fibrinogen in individuals suffering from traumatic hemorrhages in pre-hospital settings and in individuals having thrombocytopenia or qualitative platelet disorders.
Owner:FRIES DIETMAR RUDOLF +2
Method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen
InactiveCN105315360AHigh yieldIncrease productivityFactor VIIFibrinogenDEAE SephadexVirus inactivation
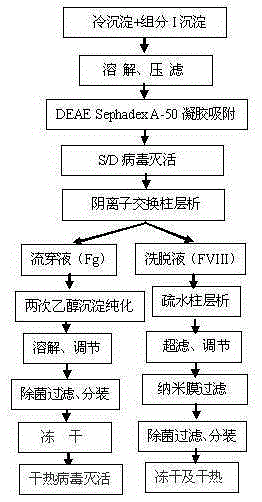
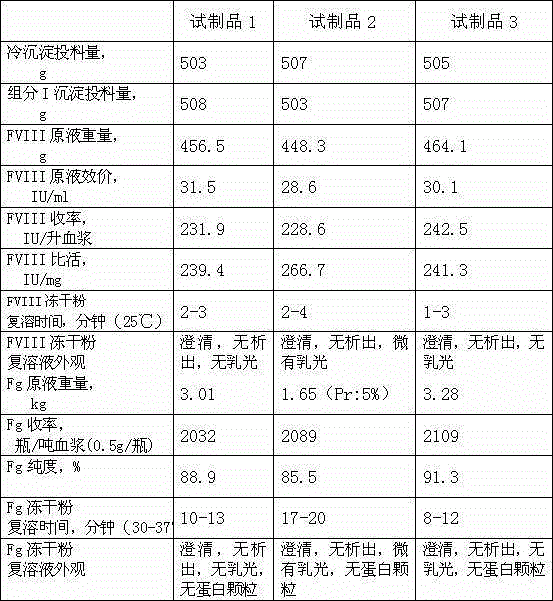
The invention discloses a method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen by cryoprecipitate and component I precipitation, mixing and feeding. The method comprises the following steps: (1) simultaneous feeding and dissolution of a cryoprecipitate and a component I; (2) DEAE Sephadex A-50 gel adsorption; (3) S / D virus inactivation; (4) anion exchange column chromatography; (5) two-step low-temperature ethanol precipitation and purification, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a chromatographic penetration liquid to obtain a human fibrinogen; (6) further hydrophobic column chromatography of a chromatographic eluant; (7) ultrafiltration, nanofilm filtration, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a hydrophobic eluant to obtain a high-purity human coagulation factor VIII. By the adoption of the process, FVIII and Fg in the two raw materials are extracted simultaneously, so that the yields of the two products are greatly improved, the yield of the human coagulation factor VIII can reach 200,000 IU / ton plasmas, the yield of the human fibrinogen exceeds 2,000 bottles / ton plasmas, and the yields are both far higher than those of a traditional process.
Owner:上海洲跃生物科技有限公司
Adhesion agent for fixing articular cartilage repair implant and use of human fibrinogen in preparing the adhesion agent
InactiveCN101352581AEasy to fixSimple operation techniqueSurgical adhesivesAdhesiveThrombin activity
The invention discloses an adhesive used for fixing articular cartilage repairing graft, which essentially comprises human fibrinogen and thrombin used as a coagulator. The adhesive has the advantages of simple fixing technique in repairing operation, good biological compatibility and easy absorption.
Owner:ARMY MEDICAL UNIV
Storage-stable human fibrinogen solutions
InactiveUS20070014780A1Good storage stabilityImprove efficiencyFibrinogenPeptide/protein ingredientsFiberPresent method
Methods are provided for the stable storage of ready-to-use, biocompatible human fibrinogen, which despite its concentration, remains available in fluid form, and which will permit long-term rapid and easy processing into a tissue adhesive preparation. Also provided is the sterile, storage-stable aqueous fibrinogen product resulting from the use of the present methods, wherein the fibrinogen remains long term in ready-to-use in liquid form, it has not spontaneously clotted (i.e., formed a clot even in the absence of an activator, such as thrombin / Ca++), and it retains its biological activity (i.e., the ability to rapidly form a fibrin clot upon exposure and vigorous mixing with thrombin and Ca++).
Owner:STATSEAL
Human fibrinogen preparation method
ActiveCN107827974AEfficient removalHigh purityFibrinogenPeptide preparation methodsFiberChromatography liquid
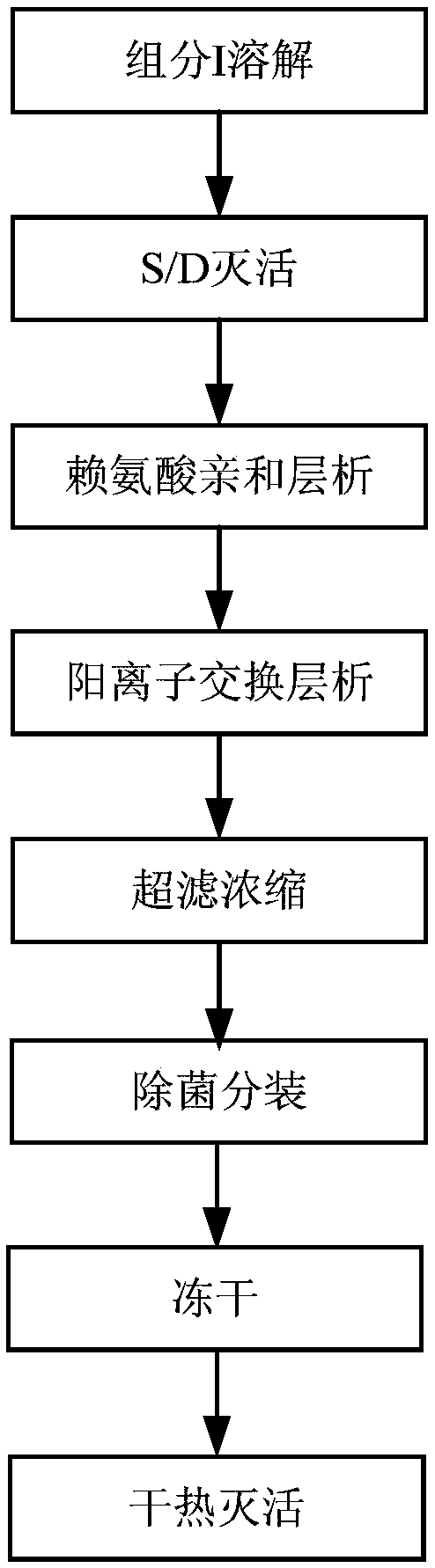
The invention provides a human fibrinogen preparation method which comprises the following steps: (1) dissolving a human plasma low-temperature ethanol precipitation component I into an extracting solution to obtain a component I solution; (2) performing S / D inactivation on the component I solution obtained in the step (1); (3) balancing a lysine affinity chromatography medium by equilibrium liquid, then performing column chromatography on the solution obtained in the step (2) and collecting a penetration peak; (4) balancing a cation exchange chromatography medium by the equilibrium liquid, then performing column chromatography on the penetration peak obtained in the step (3), leaching a chromatography column by leachate after chromatography finishes, finally eluting by an eluant and collecting an elution peak to obtain human fibrinogen. The method disclosed by the invention has simpleness in operation and can effectively remove plasminogen; a prepared human fibrinogen product has theadvantages of higher purity and stability.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +2
Method for preparing human fibrinogen preparation and preparation prepared by method
InactiveCN104436171AAvoid destructionLow impurity contentFibrinogenPeptide/protein ingredientsLipid formationFreeze-drying
The invention discloses a method for preparing a human fibrinogen preparation. The method comprises the following step: with healthy human plasma as raw material, separating and purifying by a low-temperature ethanol protein separation method, wherein a centrifugal method is replaced with a mild filter press method; top washing is carried out on sediments by using a low-temperature ethanol solution; a multi-stage deep filtration is added; a freeze-dry process is improved; the freeze-dry cycle is shortened to 2-3 days from 4-8 days; and inactivation treatment is carried out on viruses by combining an S / D method, a UVC radiation method and a dry heat process, so that the removal effect on lipid envelop viruses and non-lipid envelope viruses, especially heat-resistant parvovirus is ensured. The invention also discloses a fibrinogen preparation prepared by the method. The fibrinogen preparation produced by the method has the advantages of relatively stable quality, relatively low impurity content, and relatively high virus safety; the safety risk which can be brought for a patient by the infusion of the preparation can be reduced to the maximal extent; side reaction during clinical application is reduced; and medication is relatively safe.
Owner:HUALAN BIOLOGICAL ENG INC +1
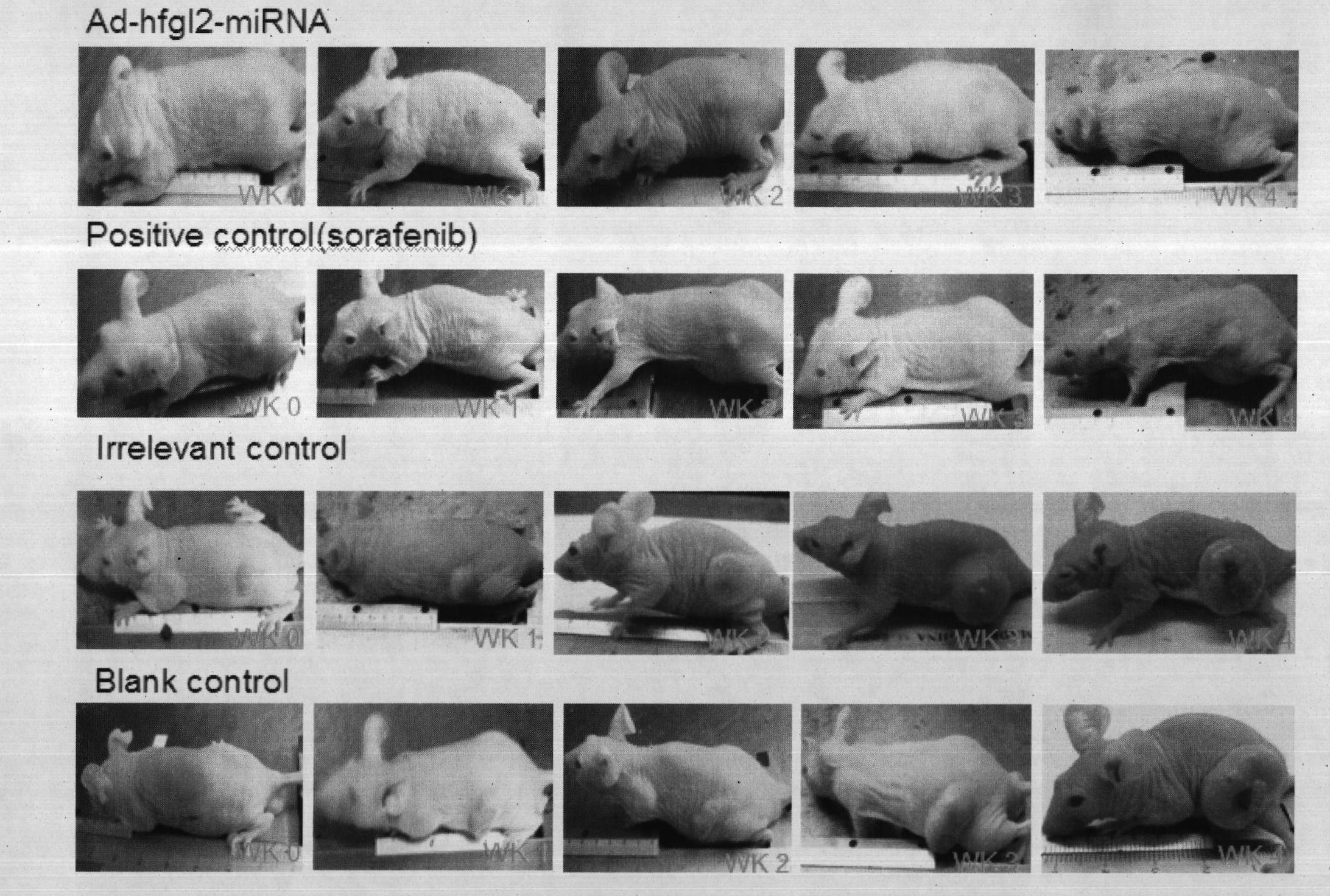
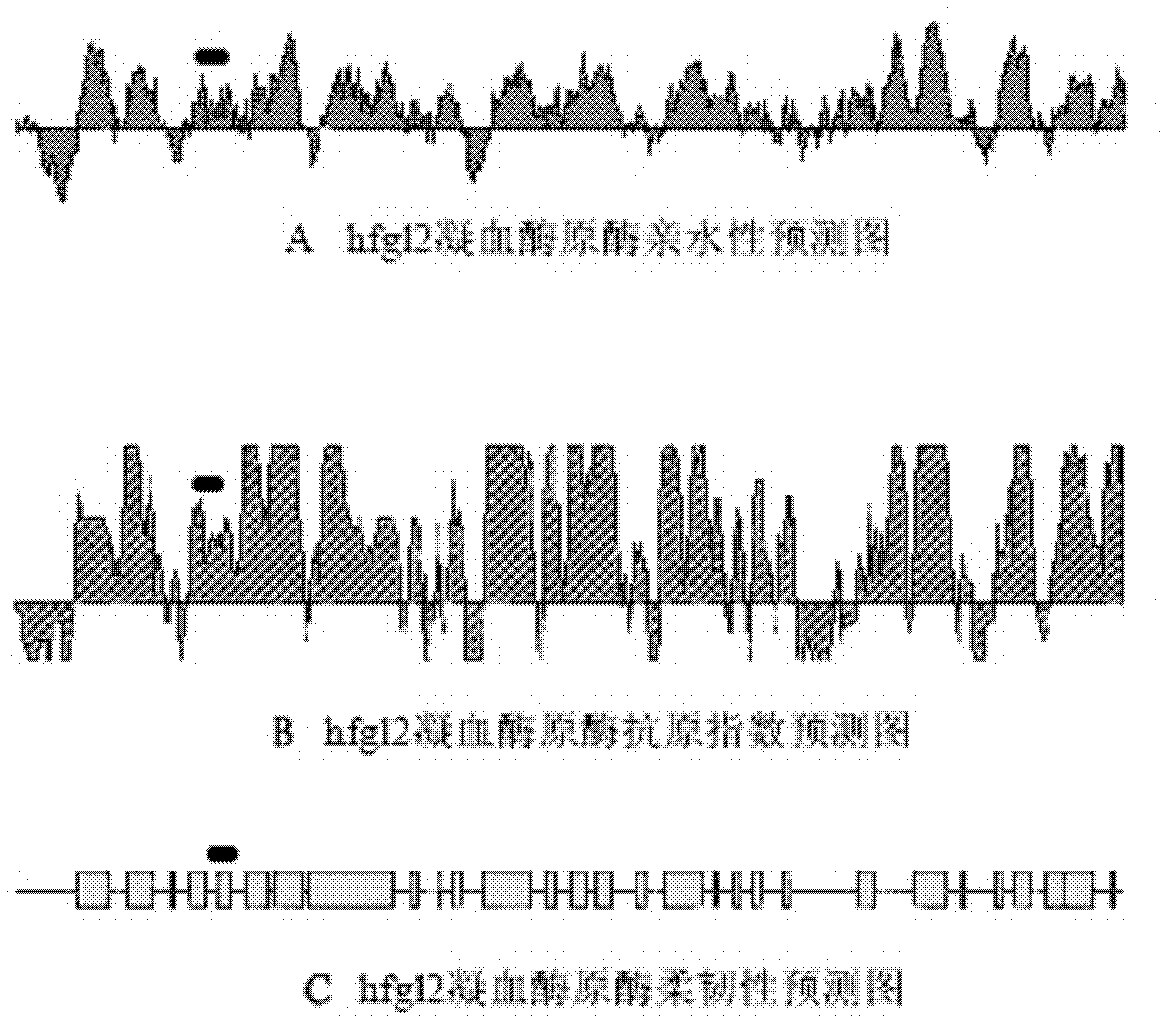
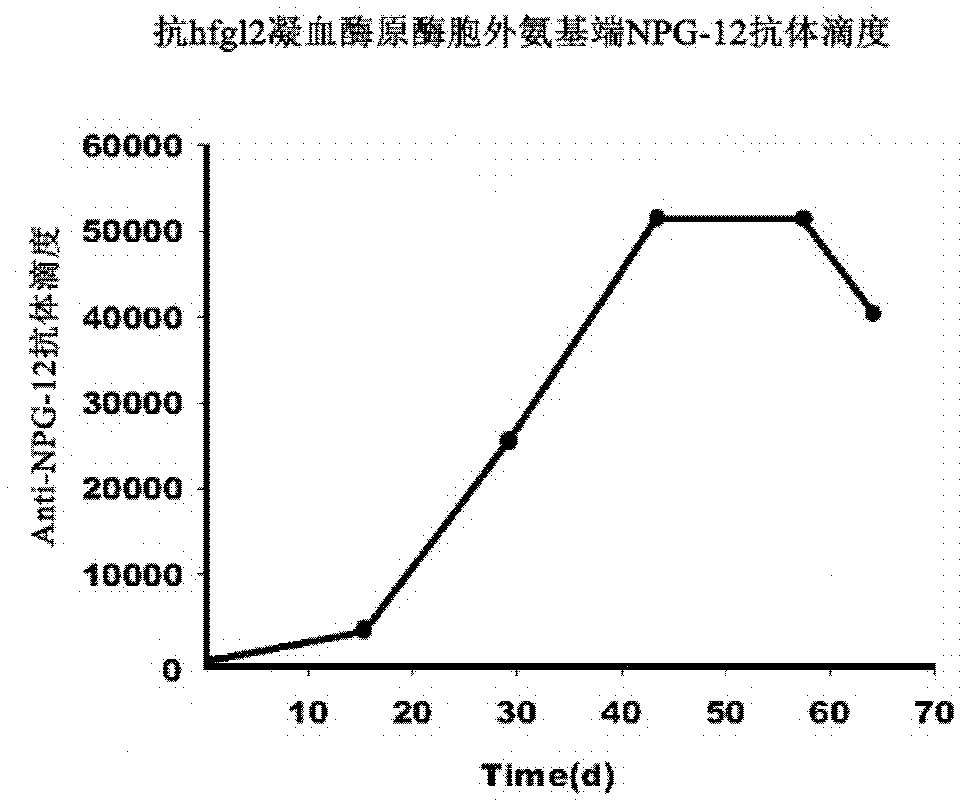
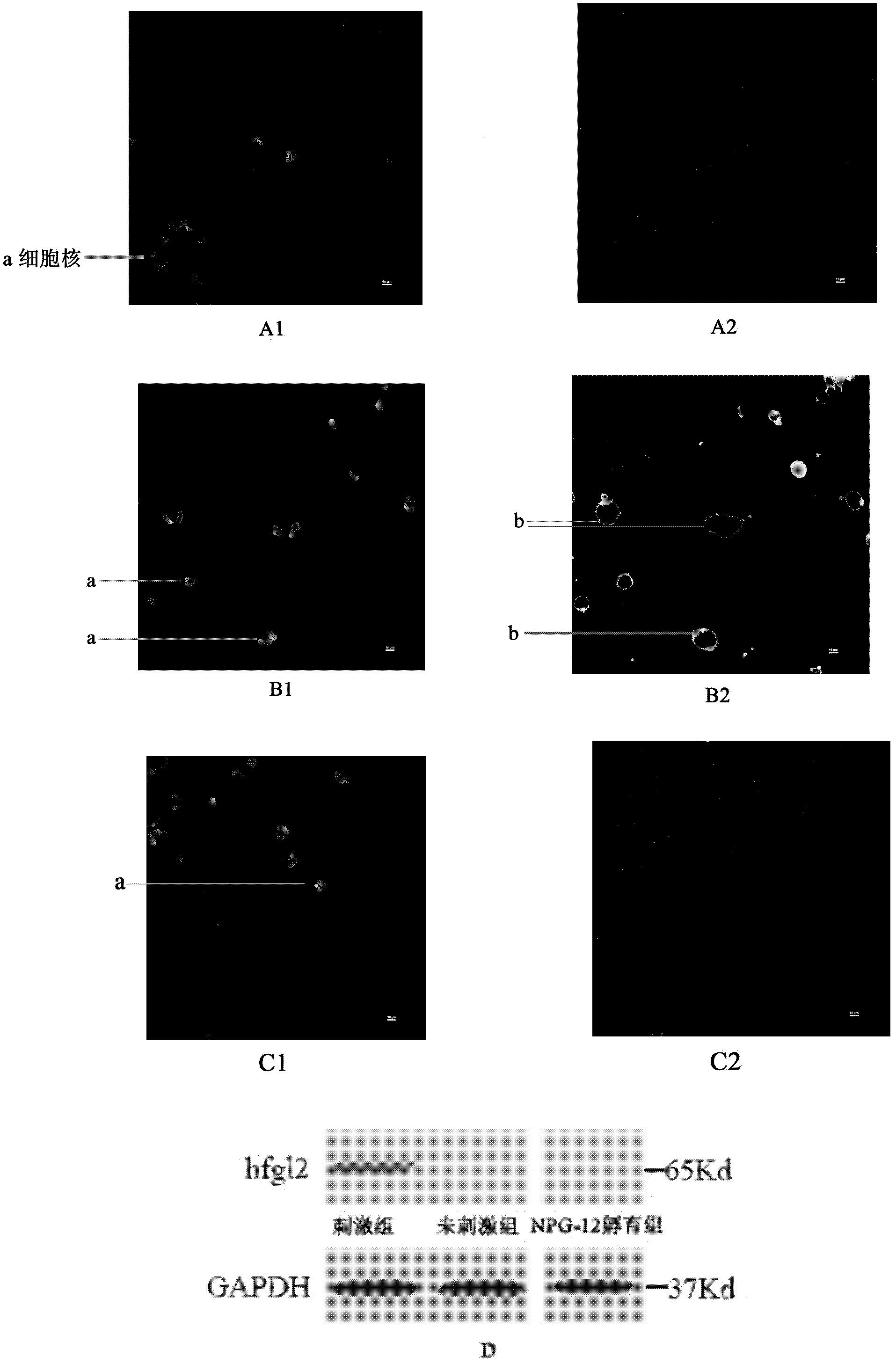
Application of hfgl2 (Human Fibrinogen-like protein 2) inhibitor in preparation of medicaments for treating liver cancer
ActiveCN102085378AGrowth inhibitionGood treatment effectOrganic active ingredientsGenetic material ingredientsTherapeutic effectWilms' tumor
The invention relates to application of recombinant hfgl2 (Human Fibrinogen-like protein 2) micro RNA (ribonucleic acid) in liver cancer, in particular to application of micro RNA adenovirus expression particles for constructing hfgl2 prothrombinase genes related to liver cancer in the treatment of liver cancer as well as verification of pharmaceutical application of the micro RNA adenovirus expression particles. The adenovirus-mediated micro RNA interference technology, namely the recombinant hfgl2 micro RNA (hfgl2-miRNA adenovirus injection), is used for targeted treatment of liver cancer; a constructed highly-aggressive human liver cancer cell nude mouse subcutaneous transplantation tumor model is adopted; the recombinant hfgl2-miRNA adenovirus injection is injected into tumors throughmultiple points; and thus, the optimized therapeutic dose of the hfgl2-miRNA adenovirus injection is found by comparing the sizes of the tumors, implementing immunohistochemistry and using other methods. Besides, the result shows that a remarkable therapeutic effect is achieved and the silence of the hfgl2 has a potential clinical application value for the treatment of liver cancer.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Monoclonal Antibody Against D-Dimer and Diagnosis Agent for Detecting D-Dimer, Crosslinked Fibrin and its Derivatives Containing D-Dimer by Using the Antibody
ActiveUS20090298100A1High activityEasy to useImmunoglobulins against blood coagulation factorsDisease diagnosisEpitopeMonoclonal antibody
Disclosed are a monoclonal antibody against human D-dimer produced in a mouse and high molecular weight crosslinked fibrin including a corresponding epitope, a cell line secreting the monoclonal antibody, and a method for manufacturing the same. The anti-D-dimer monoclonal antibody of the present invention may be effectively used as a diagnosis agent for screening and detecting in vivo D-dimer, and high molecular weight crosslinked fibrin and its derivatives containing the D-dimer since the monoclonal antibody specifically reacts with D-dimer, and crosslinked fibrin and its derivatives containing the D-dimer, which do not bind to human fibrinogen or fibrin.
Owner:DOH HYUN JU +2
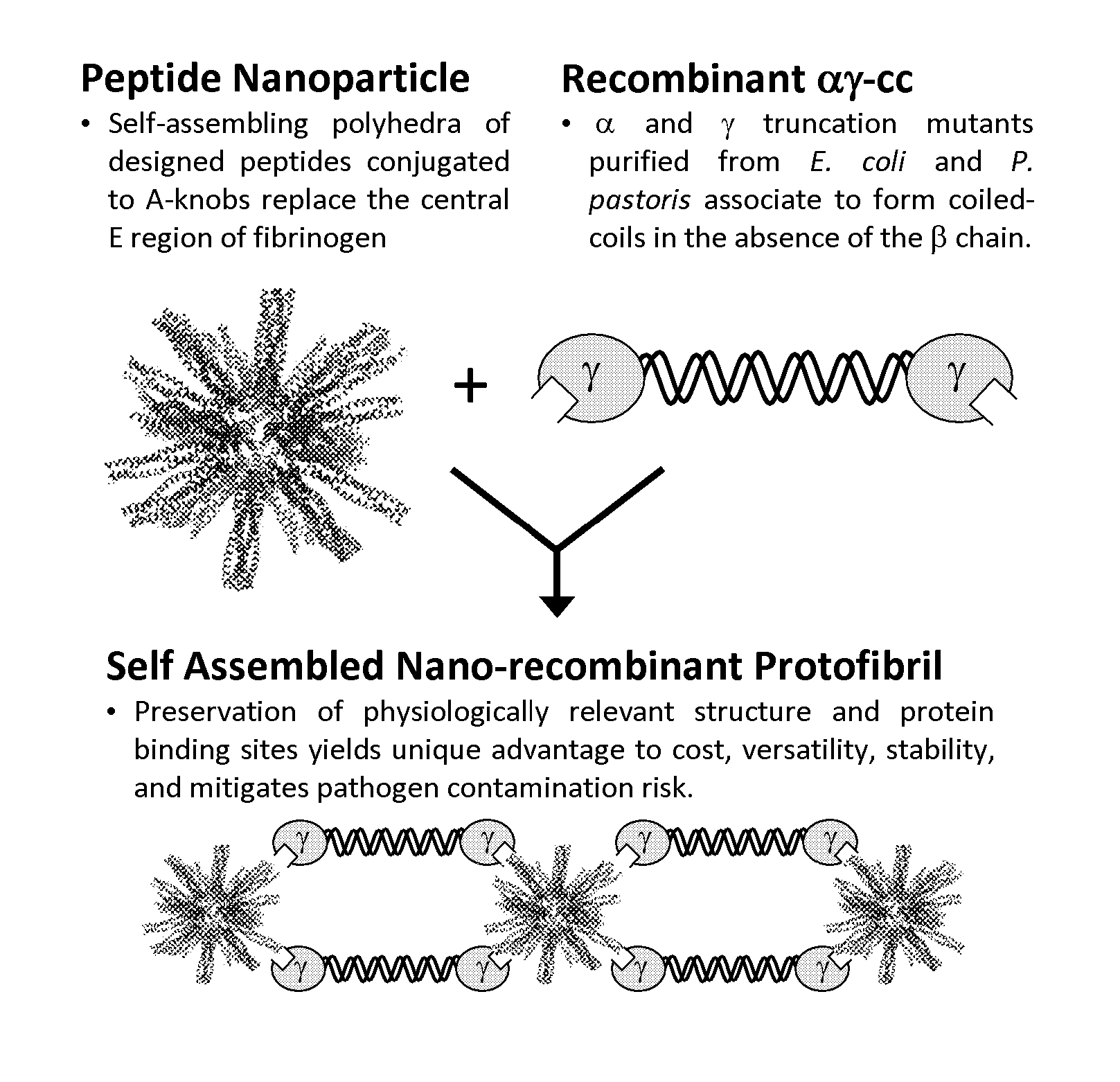
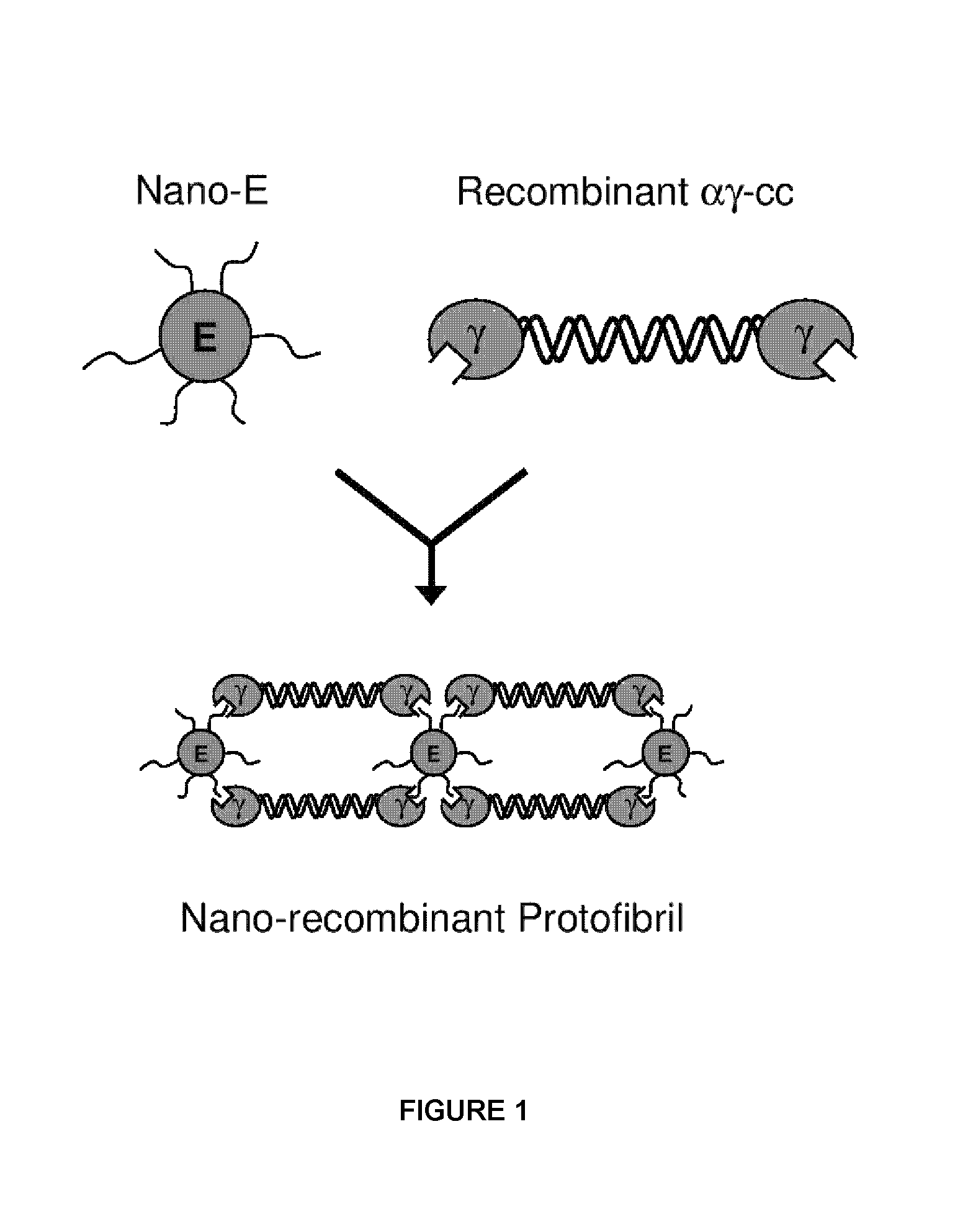
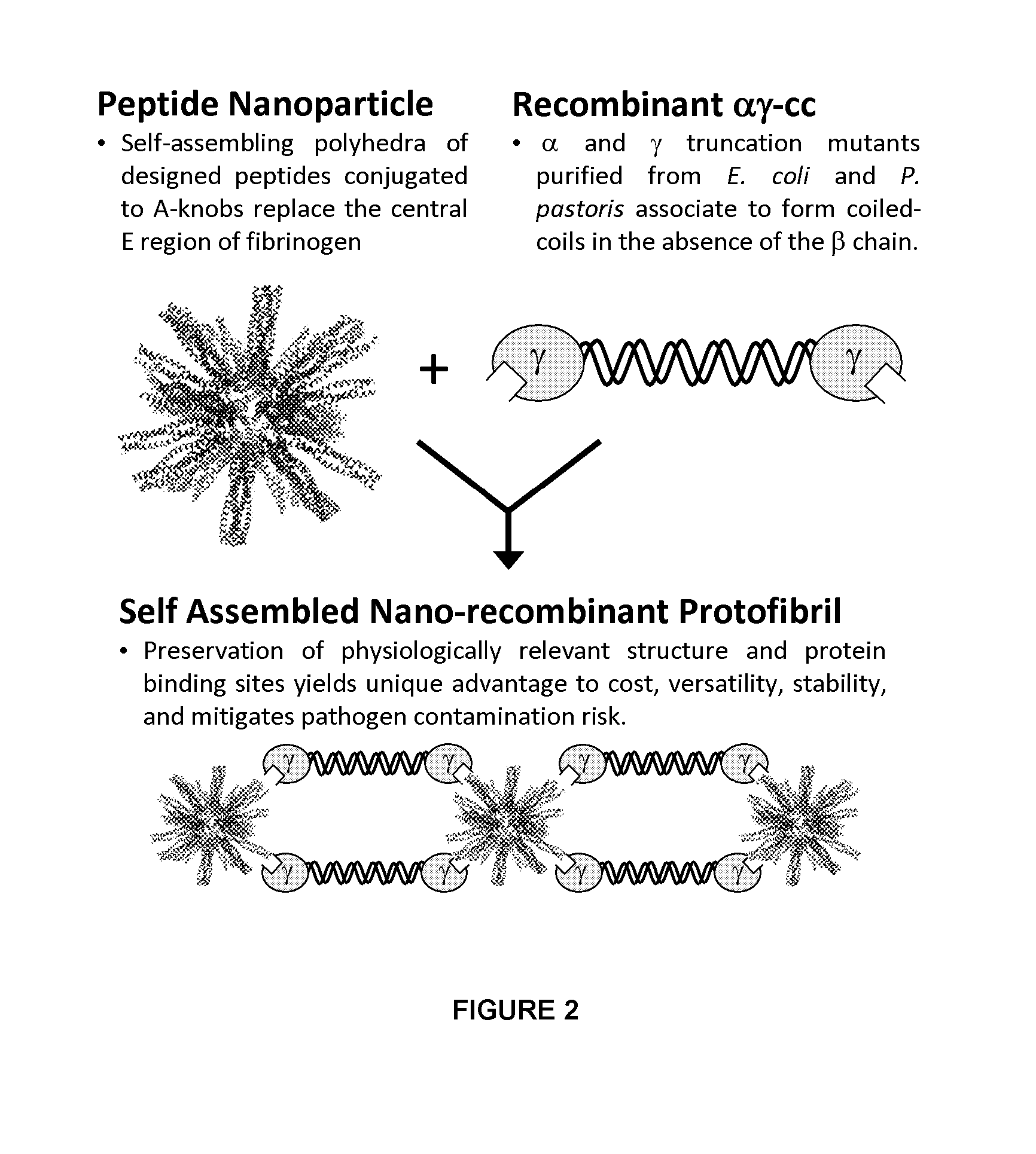
Nano-recombinant fibrinogen for fibrin sealants
A fibrin-based hemostatic agent suitable for both civilian and military use is disclosed. The hemostatic agent comprises (i) nanoparticles to which a plurality of Knob-A recognition sequences are attached, and (ii) coiled-coils of recombinantly-produced human fibrinogen α and chains and the γ chain globular domain. A delivery system for the hemostatic agent also is disclosed, which additionally comprises means for delivering (i) and (ii) to a wound site. The delivery means may be a CO2 canister or a shaker jet.
Owner:BAE SYST INFORMATION & ELECTRONICS SYST INTERGRATION INC
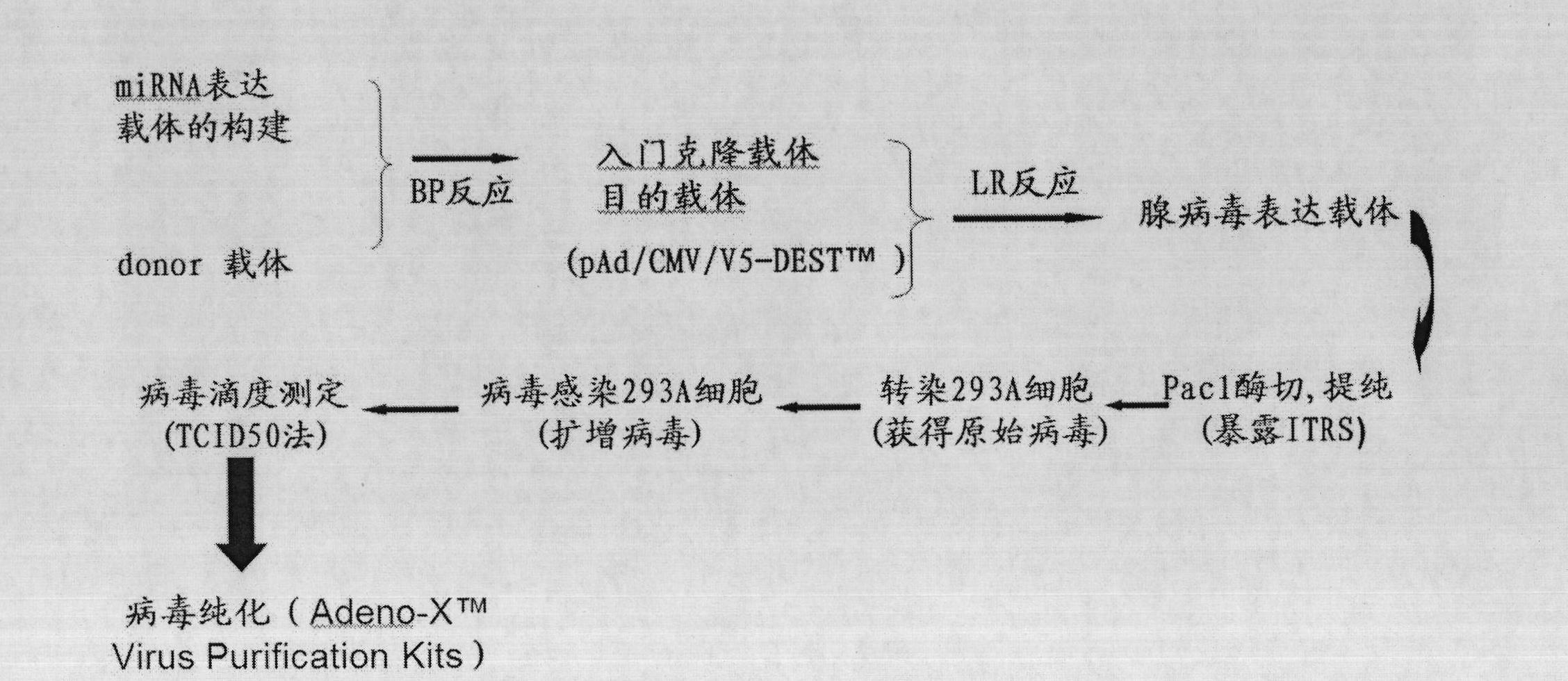
Construction method and pharmaceutical use of microRNA adenovirus expression plasmid for severe hepatitis related gene hfg12, hFas and hTNFR1
The invention relates to a group of genetic biological agent, namely microRNA adenovirus expression plasmids which construct severe hepatitis related human fgl2 (Human Fibrinogen-like protein 2, hfgl2) prothrombinase, Fas and tumor necrosis factor recipient I (tumor necrosis factor, TNFR1) genes. The effectiveness and the specificity of microRNA interference plasmids are detected in the cellular level and three kinds of the microRNA adenovirus expression plasmids are constructed and applied together to treat severe hepatitis. The group of the genetic biological agent adopts pAd / CMV / V5-DEST vectors and pcDNA expression plasmids of the hfgl2, the hFas and the hTNFR1 to construct pAd-hfgl2, pAd-hFas and pAd-hTNFR1 through the Gateway technology.
Owner:武汉大风生物科技有限公司
Solubilization technology for producing human fibrinogen
ActiveCN103405754AImprove utilizationEffective inactivationPowder deliveryFibrinogenSolubilityLipid formation
The invention relates to a solubilization technology for producing human fibrinogen. The solubilization technology comprises following main steps: (1) source plasma treatment; (2) dissolving and centrifuging of cryoprecipitate; (3) inactivation of viruses; (4) chromatography purification; (5) centrifugal separation; (6) dissolving of sediments; (7) degerming and filtering; (8) split charging and freeze drying. The solubilization technology has the advantages that the purity of the extracted product can be up to over 95%; the content of foreign protein is low; a lipid envelope virus and a non-lipid envelope virus can be effectively activated; the safety of the product is ensured; arginine hydrochloride and glycine are adopted as stabilizers; the product can be fully protected in a freeze-drying process; the temperature change of the product in the freeze-drying process is stable by prolonging the freeze-drying time (about 4-8 days, and about 3 days for general factories); a freeze-dried product with a uniform structure can be obtained; the solubility of the human fibrinogen is increased; the product is quick to dissolve after being re-dissolved; no highly visible denatured protein is generated.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
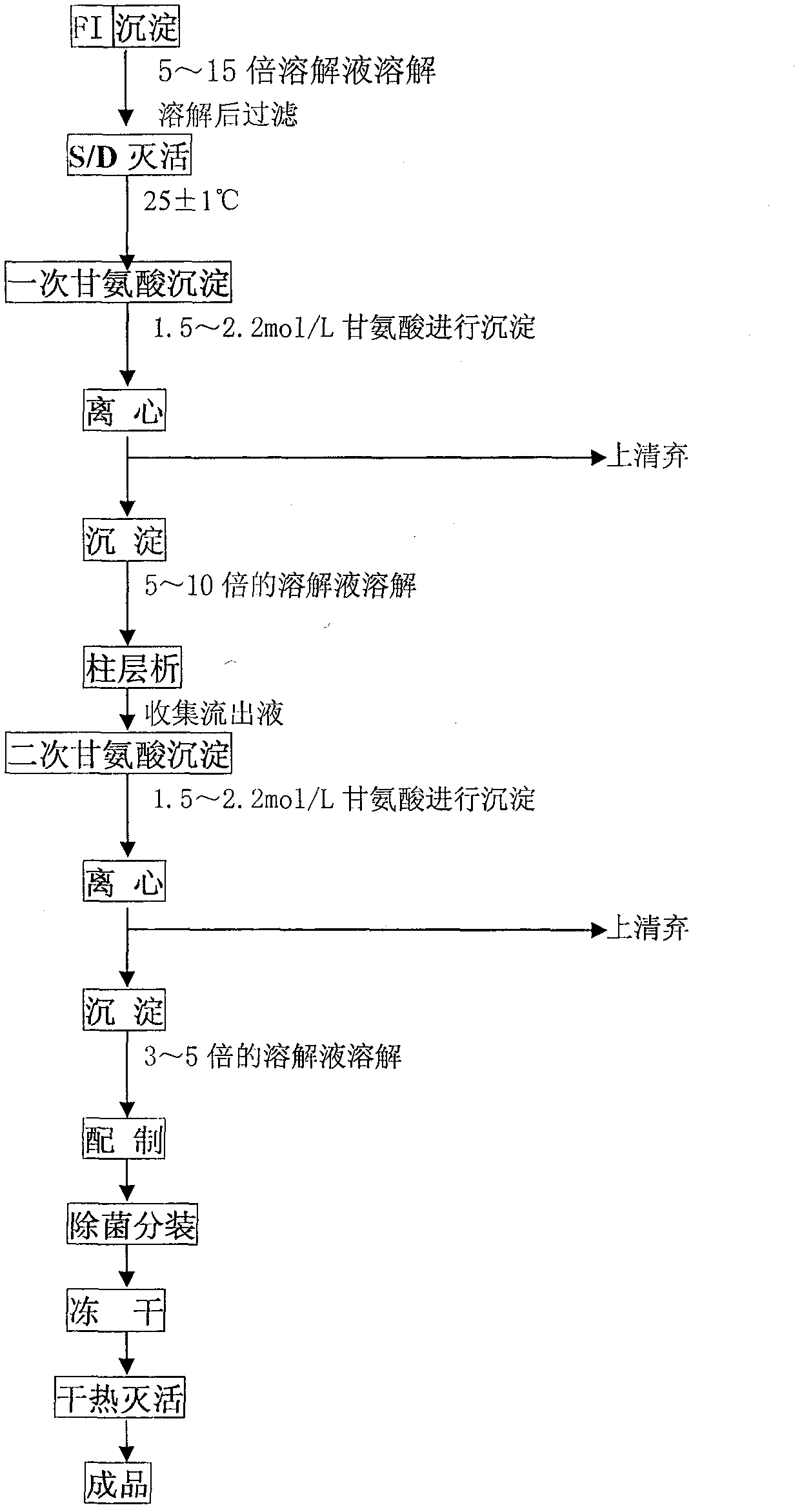
Method for extracting human fibrinogen from component I through column chromatography
The invention relates to a method for extracting human fibrinogen from a component I through column chromatography. The problem of low purity, high stability and high yield of the human fibrinogen can be effectively solved. The method comprises the following steps of: (1) dissolving and filtering FI precipitate; (2) inactivating S / D ; (3) precipitating glycine for the first time; (4) dissolving and filtering the precipitate obtained in the precipitation for the first time; (5) performing column chromatography; (6) precipitating glycine for the second time; (7) dissolving and filtering the precipitate obtained in the precipitation for the second time; (8) precipitating; (9) packing the product in bags and freeze-drying the product; and (10) inactivating the product through hot air. In the method, glycine serves as a precipitator, so the finally obtained product contains about 0.5 percent of glycine; and the glycine and arginine hydrochloride are combined to serve as a stabilizer of the product, so the stability of freeze-dried product can be improved and the quality, the purity and the yield of the final product are improved. Therefore, the method is an innovation for preparing the human fibrinogen.
Owner:BANGHE PHARMA CO LTD
Method for constructing interference plasmid of human fibrinogen hf12 shRNA, and pharmic usage
InactiveCN1958801AImprove survival rateProlong survival timeBacteriaGenetic material ingredientsU6 promoterHuman fibrinogen
This invention discloses a method for constructing shRNA interference plasmid targeting human fibrinogen-like protein 2 (hfgl2) and its medical application. The method comprises: screening the target sequence according to hfgl2 cDNA sequence, designing a template of hfgl2 cDNA according to the target sequence, designing a reverse primer containing the template, performing PCR to amplift U6 promoter, inserting BamH I and Hind III sites at both ends respectively, cutting pMSCVneo plasmid with BamH I and Hind III, and inserting U6 promoter sequence with shRNA template sequence at the downstream into pMSCVneo. It is proved by cell level experiments that hfgl2 shRNA interference plasmid can effectively inhibit the expression of hfgl2 gene.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Human fibrinogen-like protein 2 prothrombinase immunogenicity peptide and use thereof
The invention provides a human fibrinogen-like protein 2 prothrombinase immunogenicity peptide and a use thereof. The human fibrinogen-like protein 2 prothrombinase immunogenicity peptide comprises 12 continuous glutamic acid-rich amino acids near by a serine residue located at an exocellular amino terminal locus 91 of human fibrinogen-like protein 2 prothrombinase. An amino acid sequence of the human fibrinogen-like protein 2 prothrombinase immunogenicity peptide is Glu-Glu-Val-Phe-Lys-Glu-Val-Gln-Asn-Leu-Lys-Glu. A monoclonal antibody of the human fibrinogen-like protein 2 prothrombinase immunogenicity peptide can be prepared by an immunological method and can be used as a novel and specific inhibitor of human fibrinogen-like protein 2 prothrombinase. The monoclonal antibody can be usedfor research of adjustment on blood coagulation promotion functions of human fibrinogen-like protein 2 prothrombinase. The monoclonal antibody prepared according to the human fibrinogen-like protein 2 prothrombinase immunogenicity peptide is beneficial for clinic intervention of disease associated with microcirculatory disturbance.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Preparation method of human fibrinogen
InactiveCN105504046AEasy extractionImprove utilizationFibrinogenPeptide preparation methodsFiberFiltration
The invention discloses a preparation method of human fibrinogen. The method comprises the following steps that 1, cryoprecipitate serves as a staring material and is dissolved; 2, clarification and filtration are conducted; 3, solvent / detergent (S / D) inactivation is conducted; 4, Q Sepharose fastflow gel adsorption chromatographic processing is conducted; 5, centrifugation is conducted, precipitation is collected, sterilizing, subpackaging, freeze-drying, capping and dry-heating inactivating are conducted, and the product is obtained. The preparation method of the human fibrinogen is simple, easy to operate, low in cost and capable of increasing the comprehensive utilization ratio of blood plasma, and the obtained product is high in purity, activity and safety.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Method for preparing human fibrinogen
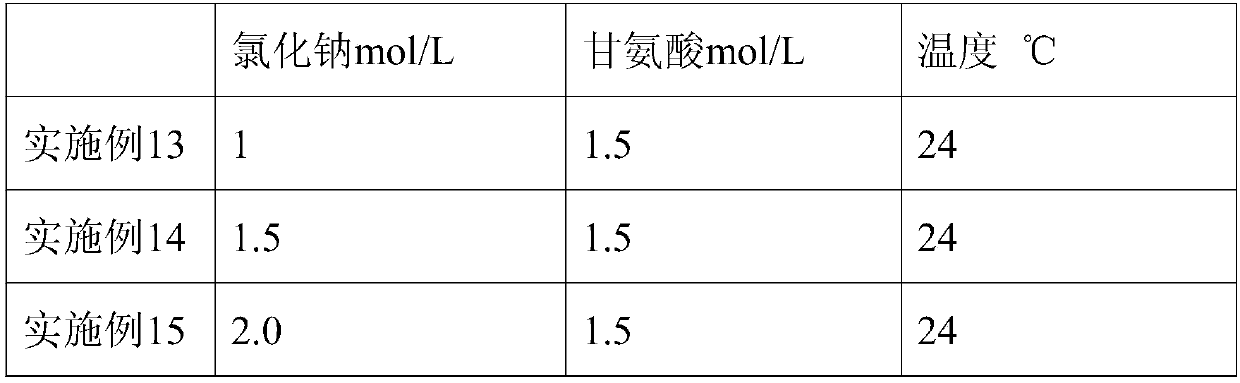
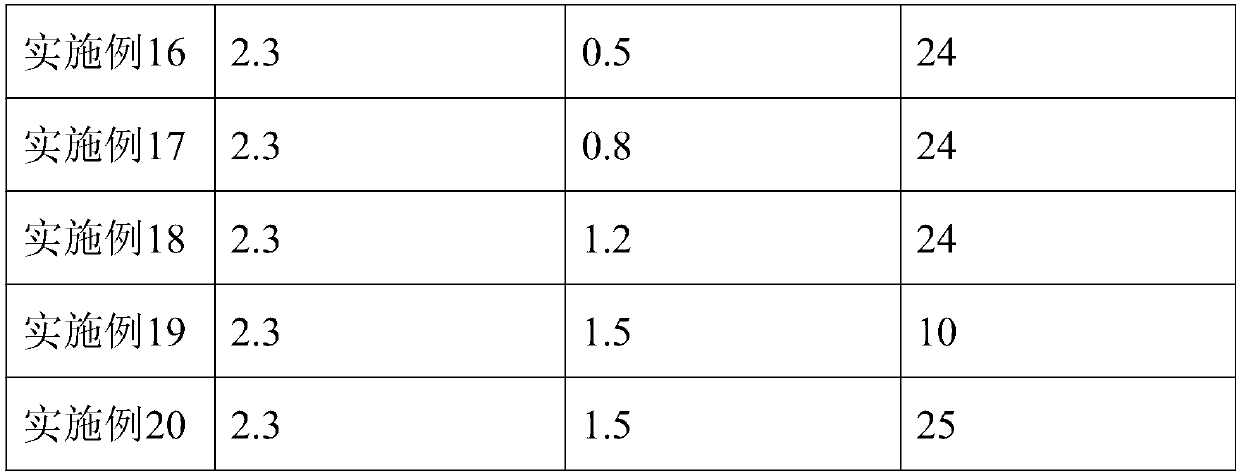
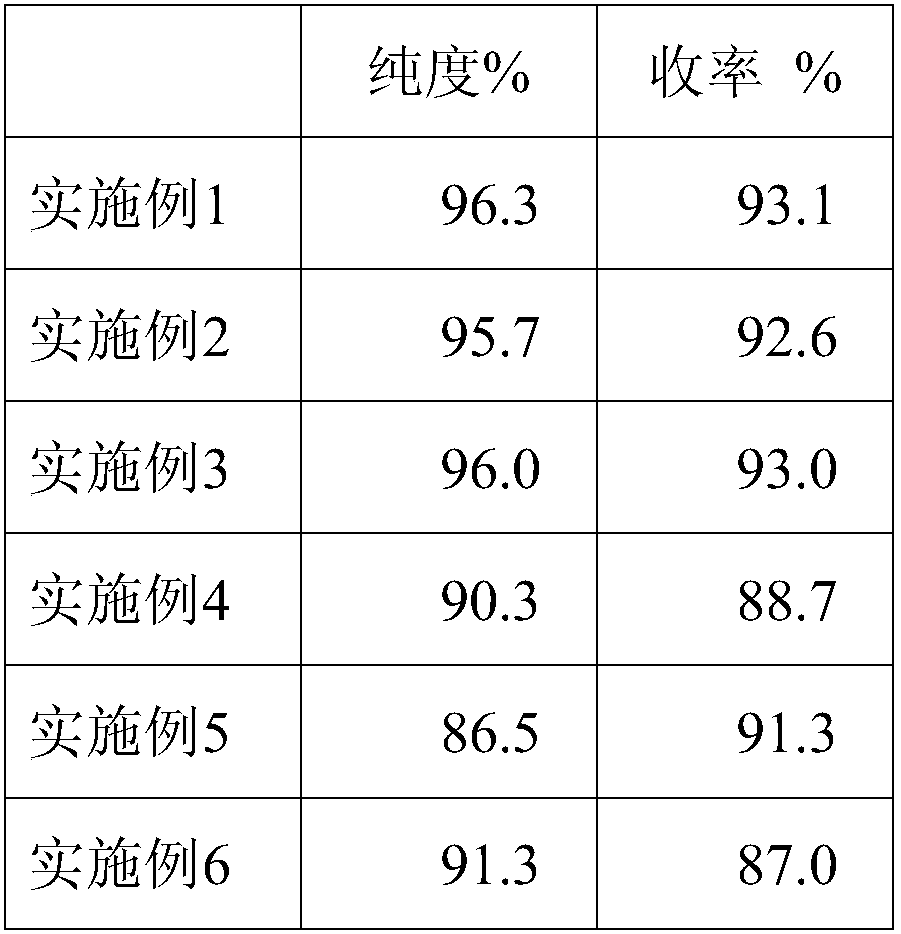
ActiveCN108017710AGood removal effectHigh purityFibrinogenPeptide preparation methodsFiberForeign matter
The invention belongs to the technical field of biopharmaceuticals and blood products and particularly relates to a method for preparing human fibrinogen. According to the method, optimal conditions are obtained through optimizing ethanol precipitation and first-time extracting conditions, optimal dissolving conditions are obtained through screening a dissolving solution 1, thus, impurities can bebetter removed during subsequent adsorption, the Fg purity and yield of running-through liquid are relatively high and reach up to 96.3%. During glycine and sodium chloride precipitation, glycine andsodium chloride of different concentrations are adopted during twice precipitation, and dissolving is carried out at different temperatures, and thus, the yield of the obtained Fg is relatively high;further carrying out a freeze-drying protectant formula and a freeze-drying process, obtained freeze-dried products are uniform and loose in appearance and are free of macrocrystals, redissolution time of the products is 5 to 6 minutes, solutions are clarified and transparent and are free of visible foreign matters after redissolution, and the moisture content of the products is in line with regulations of pharmacopoeia.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Method for preparing human fibrinogen by bilayer chromatography
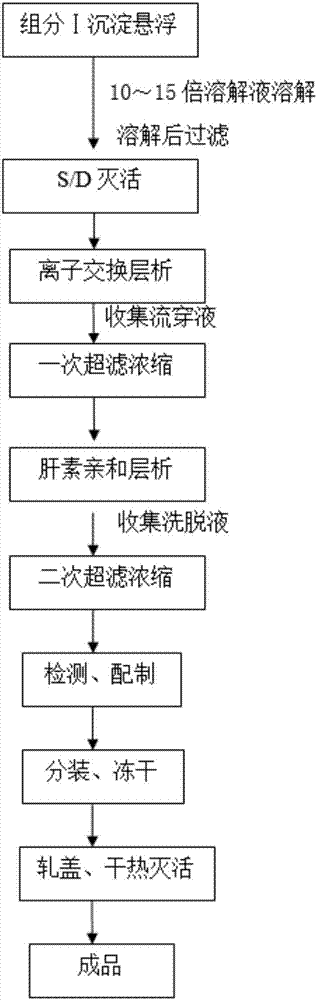
PendingCN107540743AHigh activityImprove appearance qualityFibrinogenHydrolasesUltrafiltrationFiltration
The invention discloses a method for preparing human fibrinogen by bilayer chromatography. The method comprises the following steps: precipitation, dissolution and filtration of a component I; inactivation of S / D virus; ion exchange chromatography; primary ultrafiltration concentration; heparin affinity chromatography; secondary ultrafiltration concentration; detection and preparation; packaging and freeze drying; capping and dry heating deactivation. According to the method disclosed by the invention, the ion exchange chromatography is combined with the heparin affinity chromatography, and the whole process is produced in a normal-temperature environment; the method has the advantages of short process flow, high purity of a product, high yield and short redissolving time.
Owner:NANYUE BIOPHARMING
Kit for detecting immune synchronous scattering spectrum of human serum fibrinogen and use method thereof
InactiveCN101718782ASimple and fast operationStrong anti-interferenceScattering properties measurementsBiological testingDiseasePolyethylene glycol
The invention relates to a kit for detecting immune synchronous scattering spectrum of human serum fibrinogen and a use method thereof. The kit comprises three reagents, wherein the reagent 1 is a fibrinogen standard solution, the reagent 2 (R1) contains 24.6-122mmol / L OF Na2HPO4, 56-175.4mmol / L of NaH2PO4 and 100-400g / L of polyethylene glycol 6000, and the reagent 3 (R2) contains a stabilizer and 1.00mg / ml of goat anti-human fibrinogen antibody. The specific use method comprises the following steps: preparing a standard series according to a defined ratio, measuring the synchronous scattering intensity, drawing a synchronous scattering intensity-concentration standard curve, then measuring the synchronous scattering intensity of a sample to be detected, and calculating the fibrinogen content according to the standard curve. The kit has simple and fast and operation, high sensitivity and strong anti-interference; and compared with the measure result of the Clauss method, the consistency of the method is better so as to provide reliable experimental data for the clinical diagnosis and treatment of diseases such as coronary disease, myocardial infarction and cerebrovascular disease.
Owner:GUANGXI NORMAL UNIV
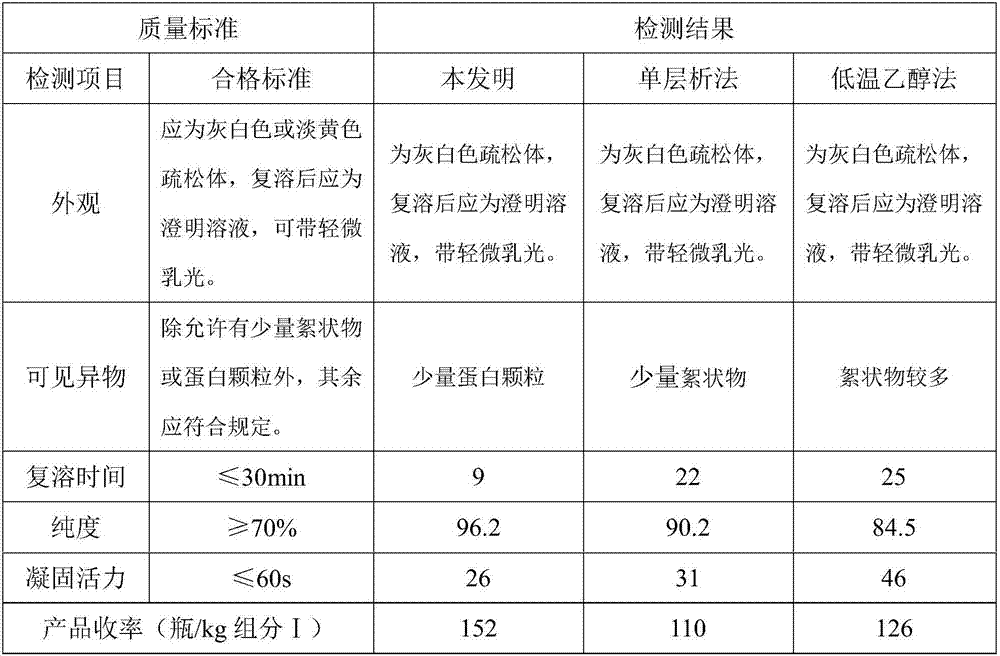
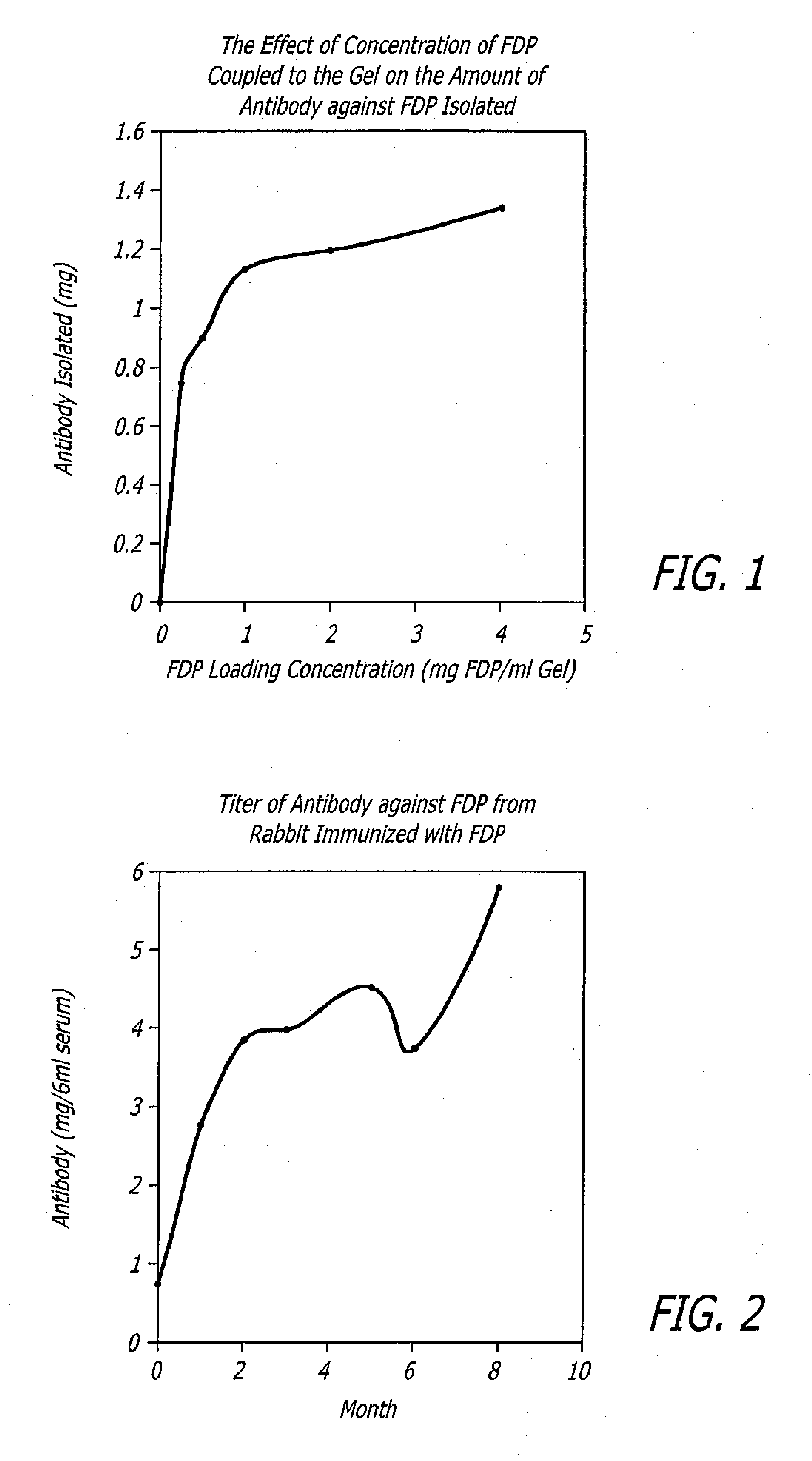
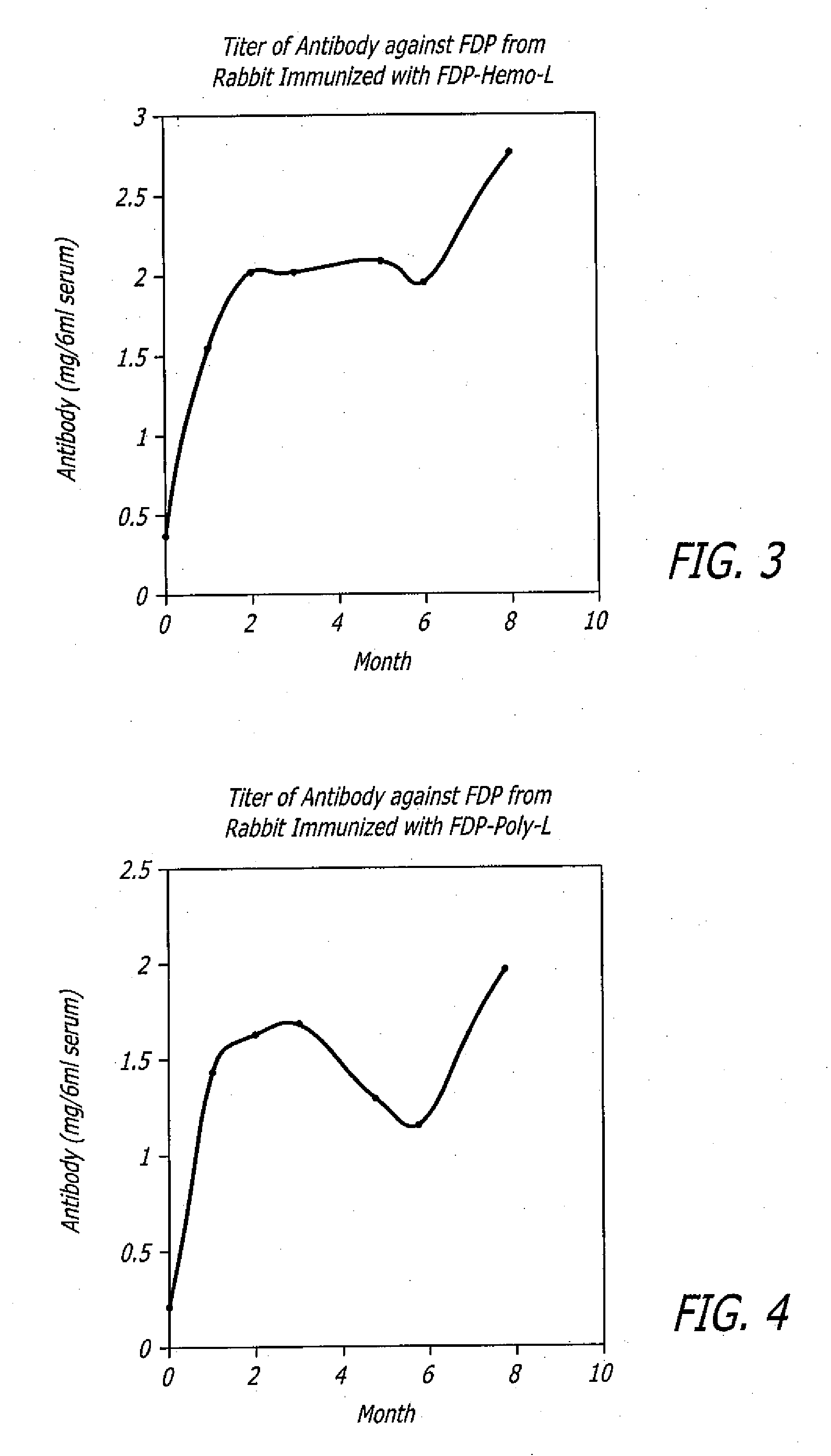
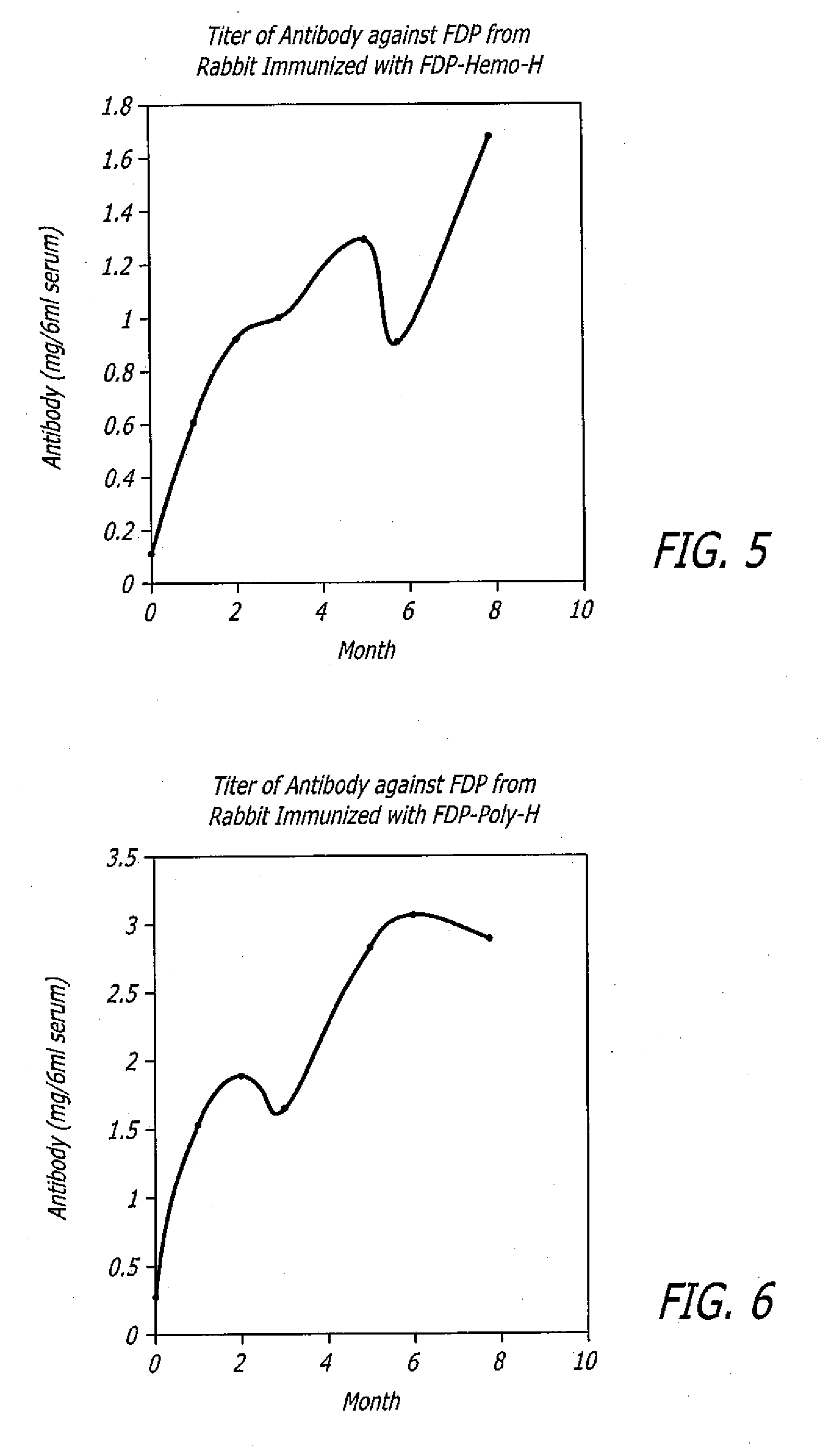
Polyclonal antibodies against fibrinogen degradation products and associated methods of production and use
InactiveUS20080118936A1Increase production levelsReduce effortSerum immunoglobulinsBiological material analysisScreening cancerSerum ige
Monospecific polyclonal fibrinogen degradation product antibodies, their method of use, the methods to detect cancer and for monitoring the progress of anticancer treatment by immunochemically measuring the quantity of serum FDP in serum are disclosed. The present invention teaches that monospecific polyclonal FDP antibodies that bind to human fibrinogen degradation products (“FDP”) can be obtained by inoculating a laboratory animal with human FDP or human FDP derivatives to induce the production in the inoculated laboratory animal of at least one monospecific polyclonal antibody that binds to human FDP and isolating the monospecific polyclonal antibody. By generating anti-serum to FDP from immunogens and purifying said immunogens using affinity chromatography, increased levels of production of FDP antibodies over the prior art are achieved. A method for screening cancer is disclosed comprising contacting biological sample obtained from a patient with at least one monospecific polyclonal FDP antibody that binds to mammalian FDP. A method is also disclosed for producing a quantitative enzyme linked immunosorbent assay (ELISA) for serum FDP by using monospecific polyclonal antibodies that bind to human FDP.
Owner:NGO THAT T
Mass production of ready-to-use suspensions of fibrinogen-coated albumin spheres for the treatment of thrombocytopenic patients
InactiveUS20140030347A1Improve the situationEfficiently formedPowder deliveryFibrinogenMedicineHuman albumin
A composition and a method effective in the production of the composition. The composition is a ready-to-use aqueous suspension in large and small quantities comprising human-fibrinogen-coated human-albumin spheres and the supernatant, said suspension being useful for the treatment of thrombocytopenic patients.
Owner:PTL PHILANTHROPY
Marker proteins for diagnosing liver disease and method of diagnosing liver disease using the same
InactiveUS20090275059A1FibrinogenMaterial analysis by observing effect on chemical indicatorDiseaseSerum ige
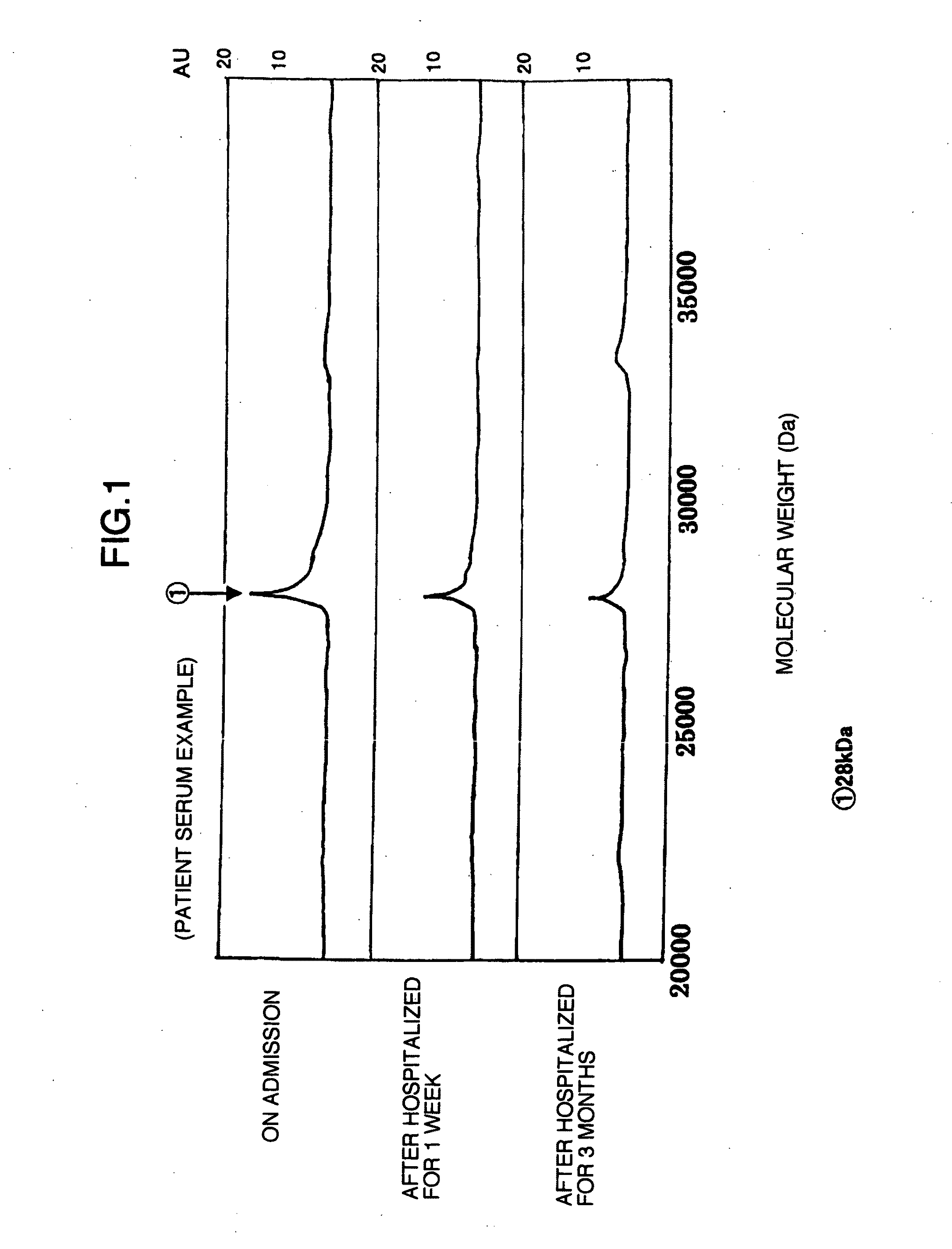
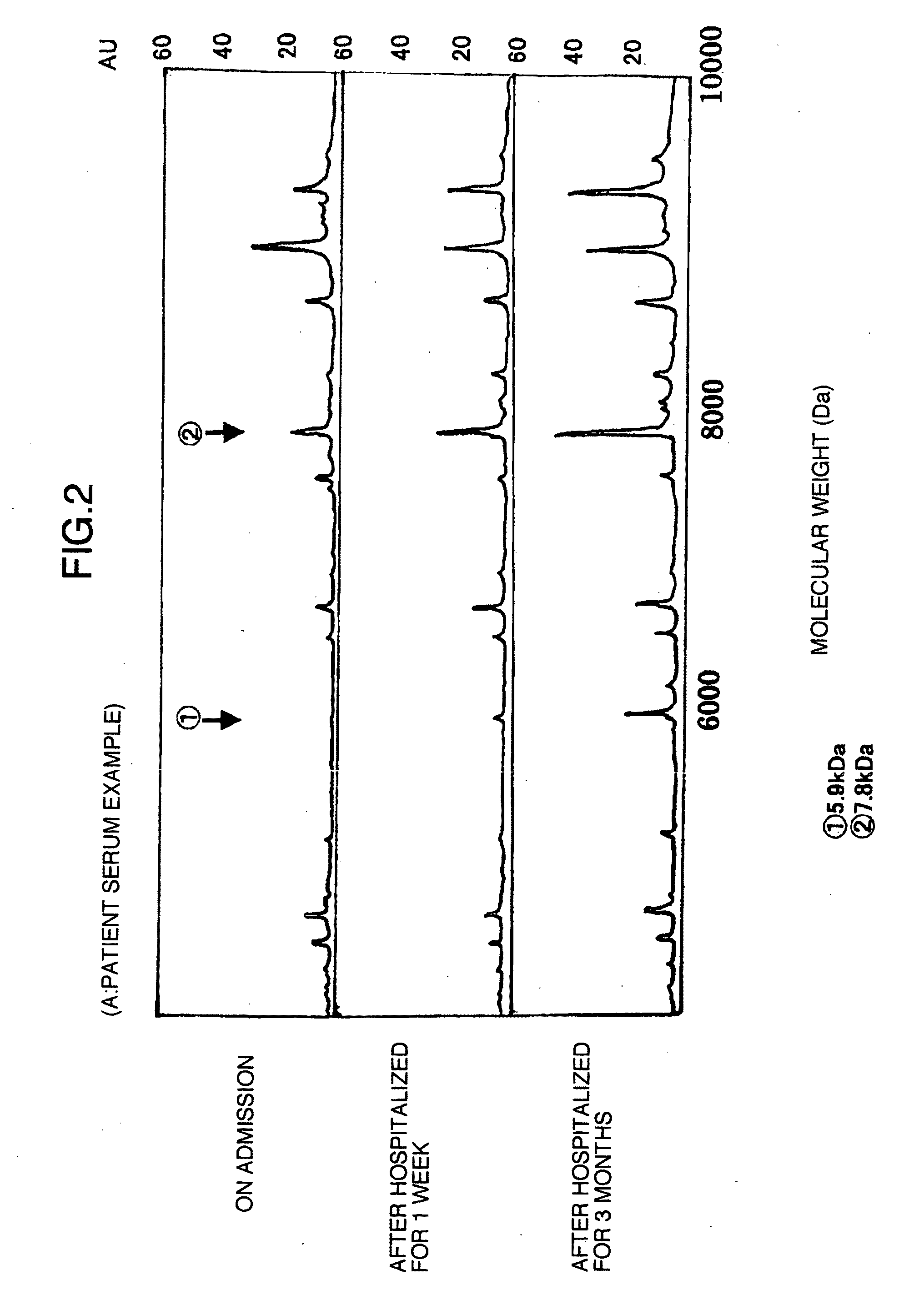
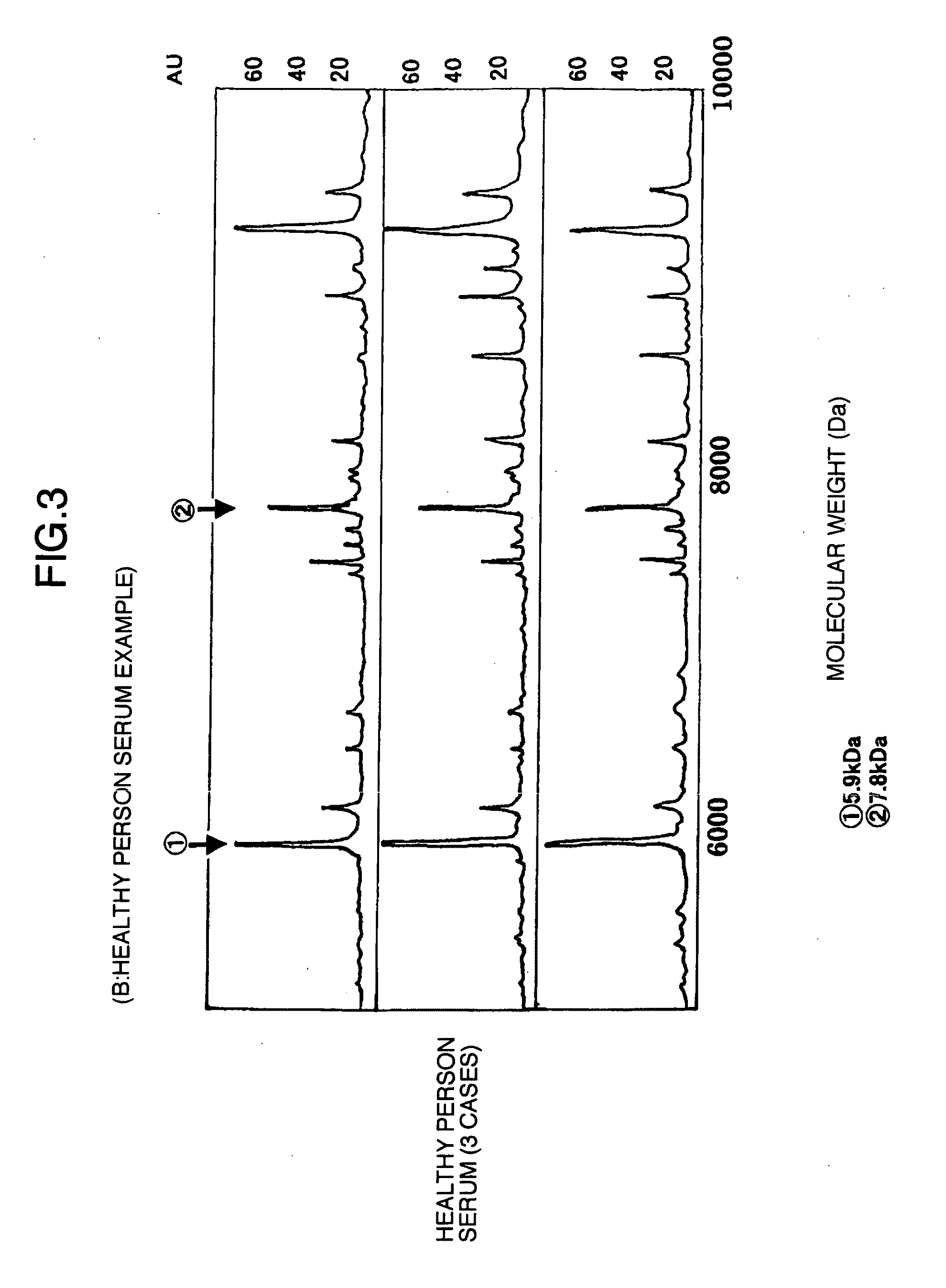
Using the protein chip technology, biological samples such as sera are subjected to proteome analysis. Thus, a protein which is a human fibrinogen α-E chain decomposition product and has a molecular weight of 5,900, a protein which is an apolipoprotein AII decomposition product and has a molecular weight of 7,800, and a protein which is an apolipoprotein AI decomposition product and has a molecular weight of 28,000, each showing an increase or a decrease with the habit of drinking, are newly found out. By detecting or quantifying these proteins, a liver disease in a subject such as one having a problem of drinking can be diagnosed at the early stage.
Owner:NITTO BOSEIKI CO LTD
Monoclonal antibody against D-dimer and diagnosis agent for detecting D-dimer, crosslinked fibrin and its derivatives containing D-dimer by using the antibody
ActiveUS8940489B2Avoid poor resultsImmunoglobulins against blood coagulation factorsAnimal cellsEpitopeCross-link
Disclosed are a monoclonal antibody against human D-dimer produced in a mouse and high molecular weight cross-linked fibrin including a corresponding epitope, a cell line secreting the monoclonal antibody, and methods for producing the same. The anti-D-dimer monoclonal antibody of the present invention may be effectively used as a diagnotic agent for screening and detecting in-vivo D-dimer, and high molecular weight cross-linked fibrin and its derivatives containing the D-dimer since the monoclonal antibody specifically reacts with D-dimer, and cross-linked fibrin and its derivatives containing the D-dimer, which do not bind to human fibrinogen or fibrin.
Owner:DOH HYUN JU +2
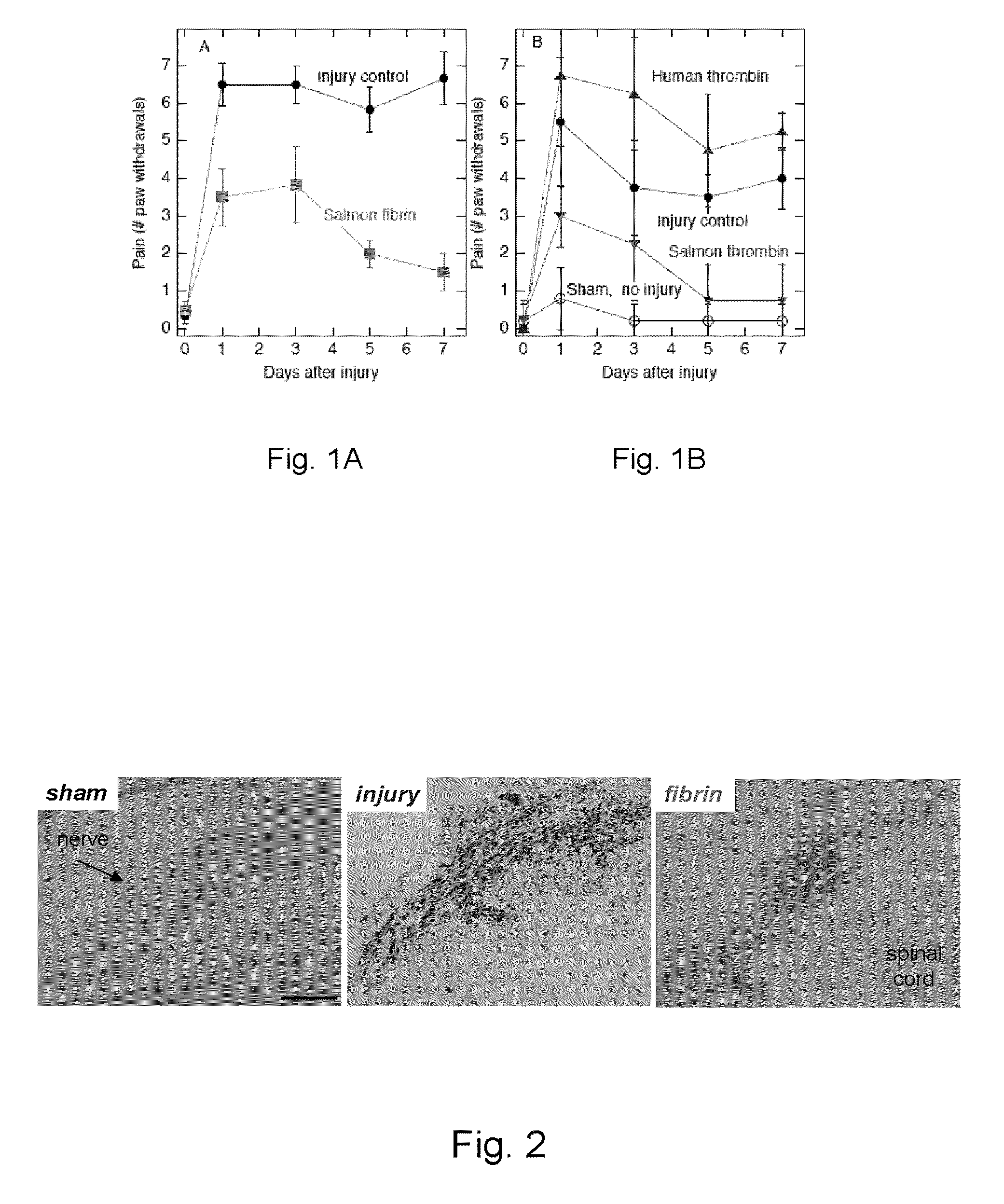
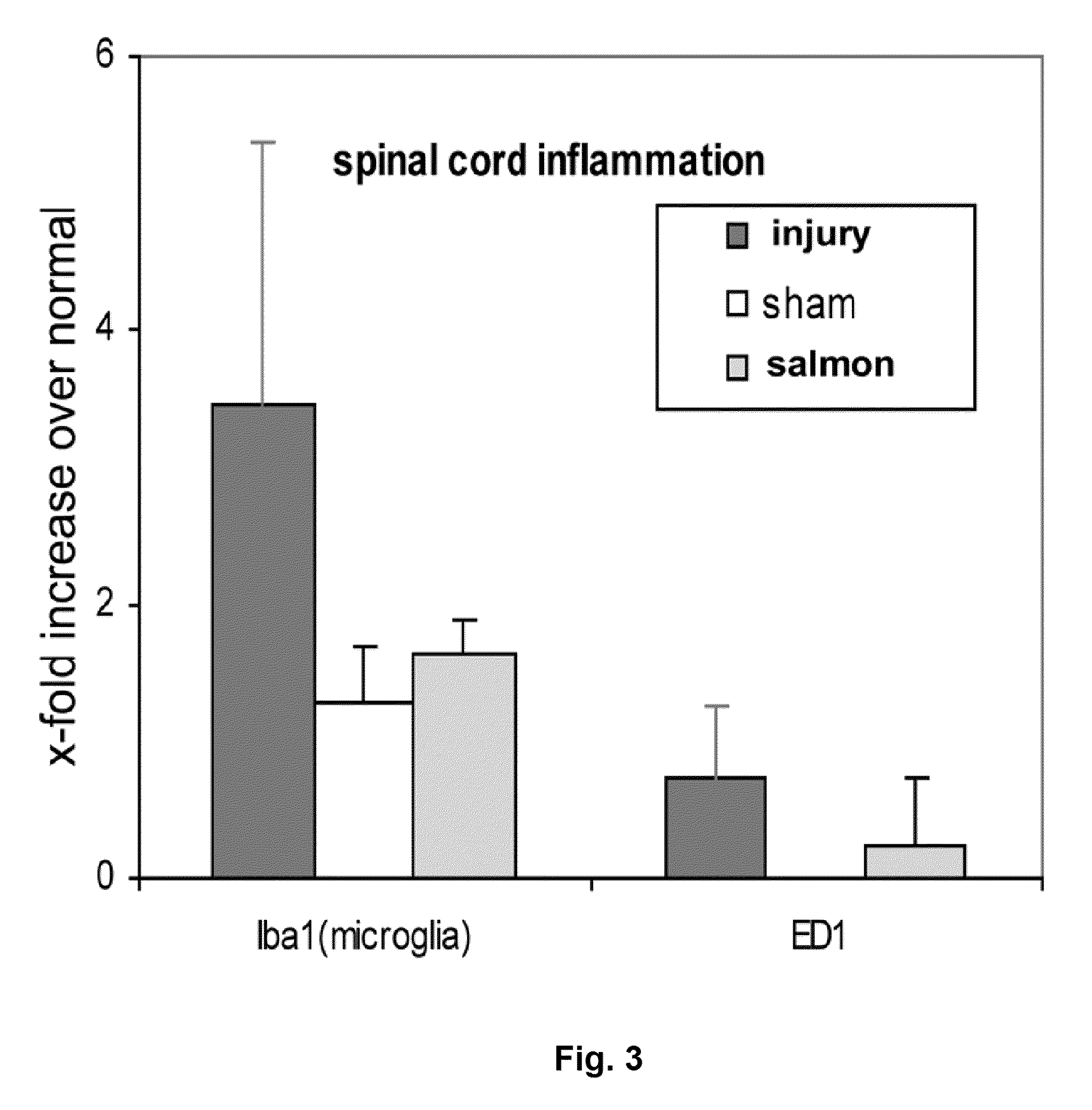
Method of Using Salmon Thrombin to Alleviate Central Nervous System-Mediated Pain
ActiveUS20100111926A1Lower Level RequirementsNervous disorderPeptide/protein ingredientsNervous systemFractionation
A method of alleviating central nervous system-mediated pain includes applying salmon thrombin at a neural injury site. Applying salmon thrombin can include applying a gel that includes salmon thrombin. The gel can also include fibrinogen, for example, salmon fibrinogen, human fibrinogen, or bovine fibrinogen. The salmon thrombin can be obtained from salmon plasma, or using recombinant technology, or by fractionation. A pain relief substance includes a gel that includes salmon thrombin.
Owner:SALMONICS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com