Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "DEAE Sephadex" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DEAE Sephadex can be used in ion exchange chromatography for purifying and isolating proteins. In has been used to identify and label an insulin activated nitric oxide synthase inhibitor (protein) in acute myocardial infarction (AMI) patients.
Method for extracting and purifying calf serum protein-removing extract
ActiveCN1843376ASmall molecular weight rangeEfficient killingMetabolism disorderMammal material medical ingredientsVenous bloodUltrafiltration
The invention discloses a method for extracting and purifying calf serum protein-removing extract, which comprises extracting calf venous blood, separating plasma, removing protein with ethanol, removing ethanol through decompression, charging composite proteolytic enzyme, subjecting supernatant fluid to ultrafiltration, desalinizing ultrafiltrate with gluglucosan G-15 gel chromatography, collecting elution portion with specific absorption at 280nm, then separating with DEAE-Sephadex-50 gel chromatography, collecting elution portion with specific absorption at a second 280nm, then carrying out virus deactivation by means of microporous membrane filtration and ultraviolet irradiation.
Owner:JINZHOU AHON PHARM CO LTD
Spirulina phatensis polysaccharide and extraction method thereof
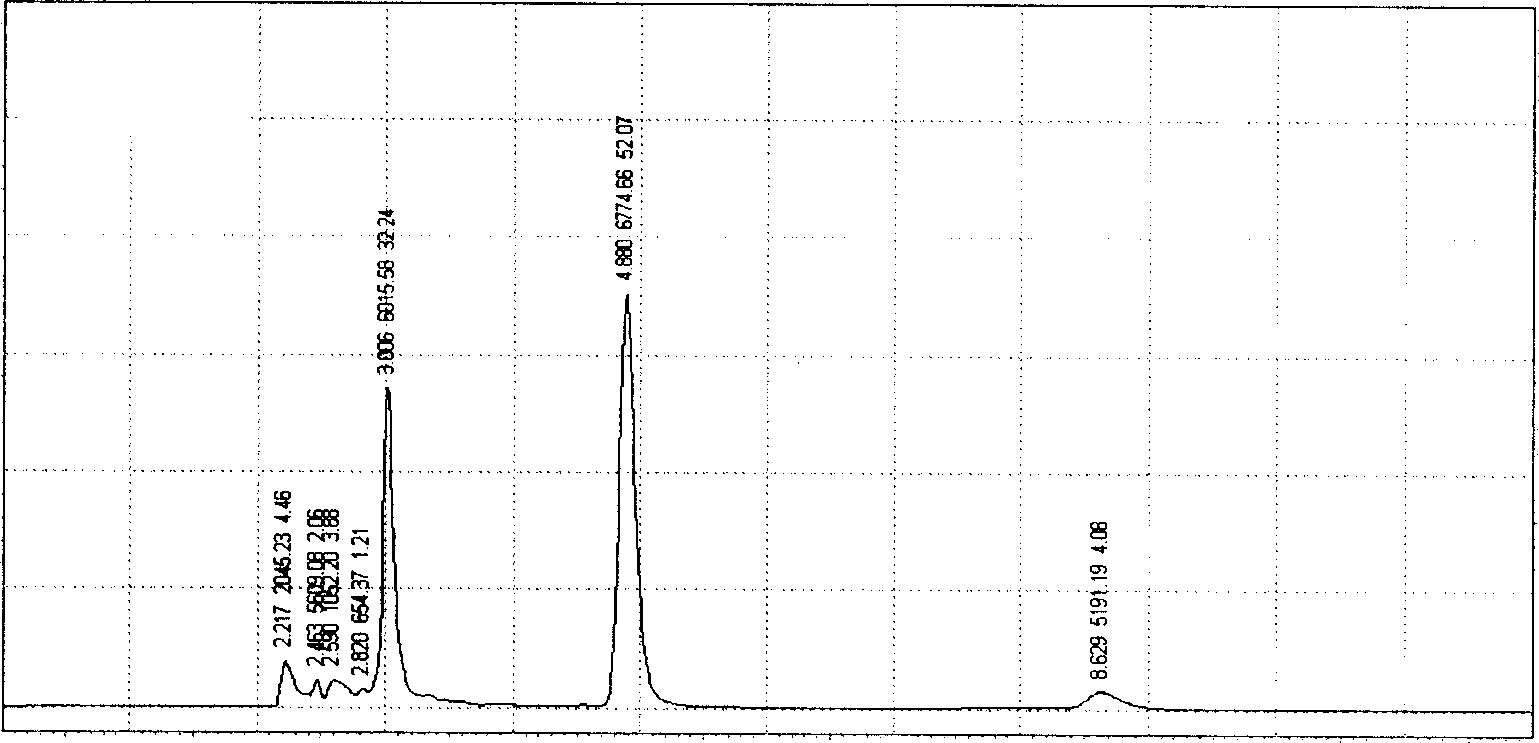
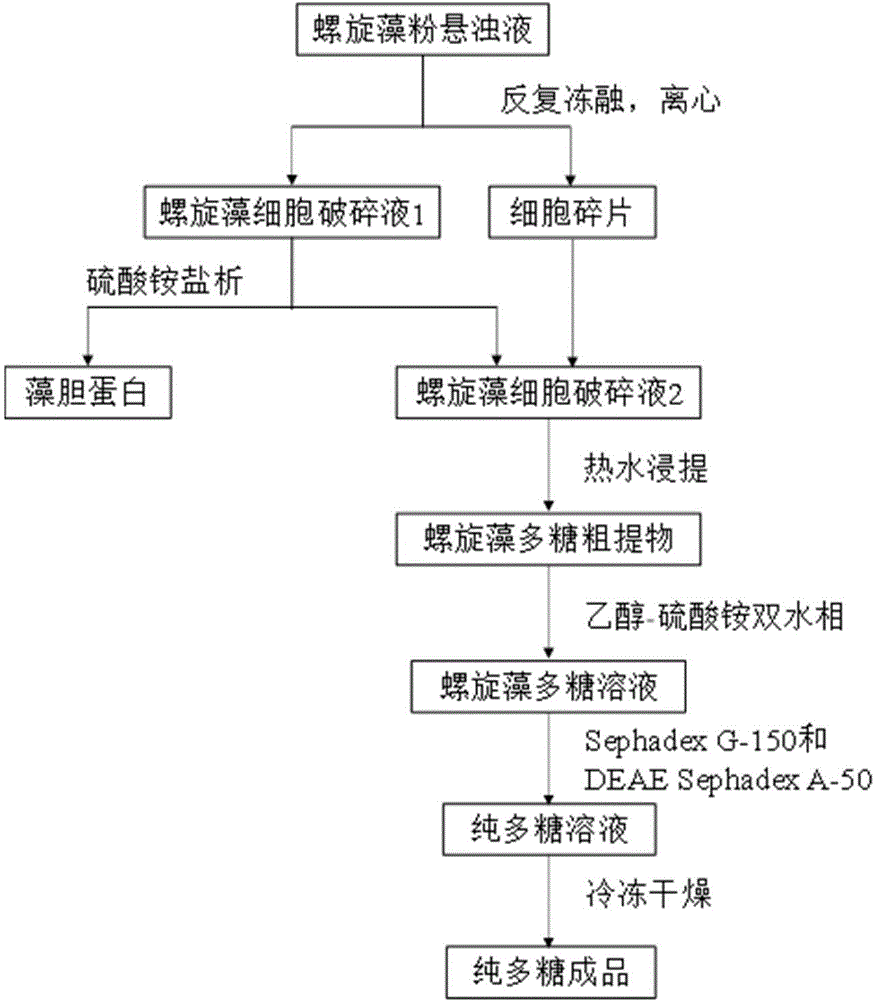
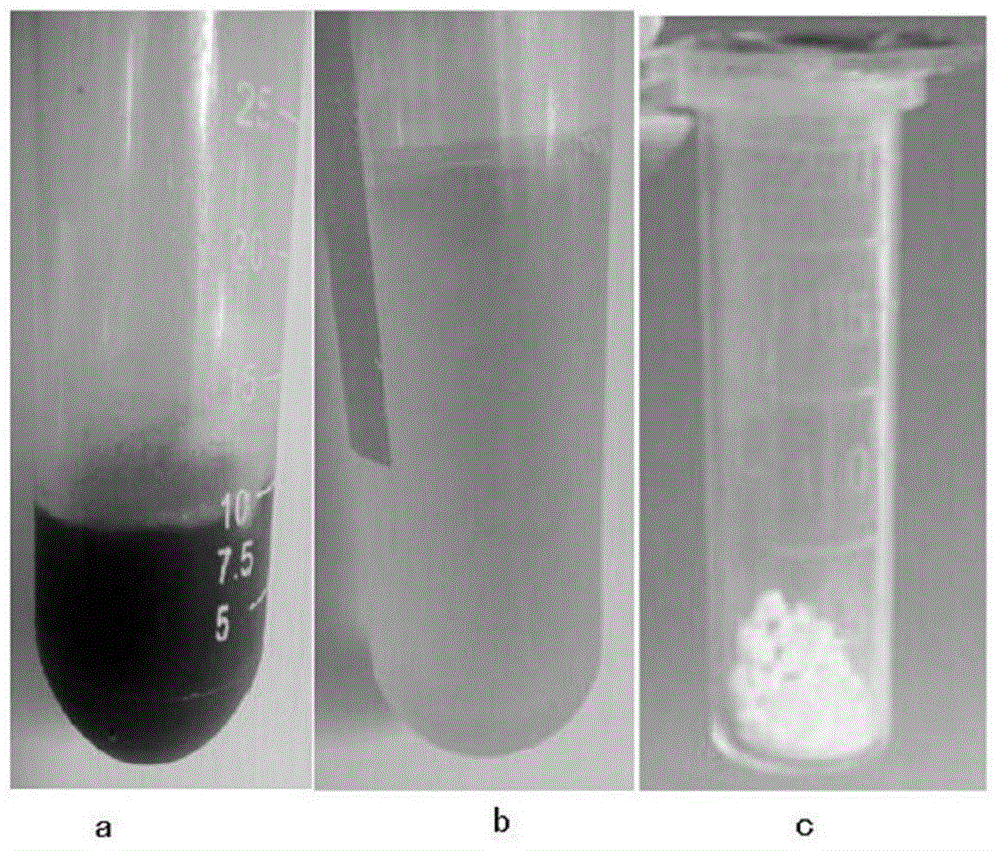
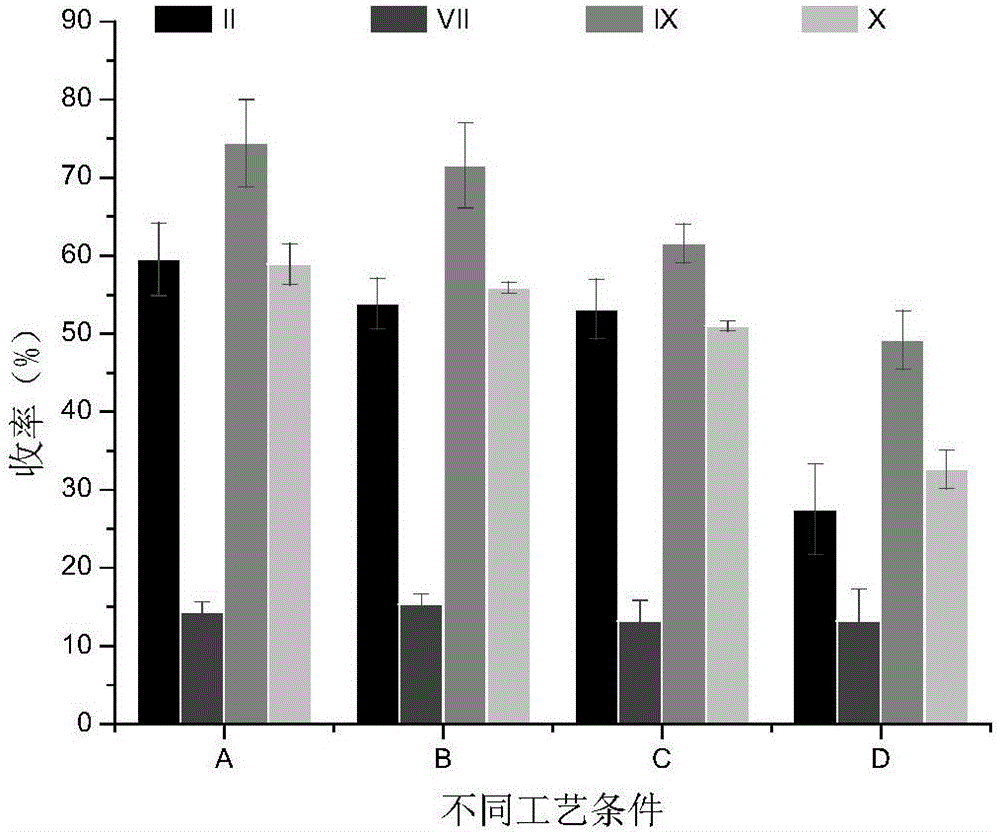
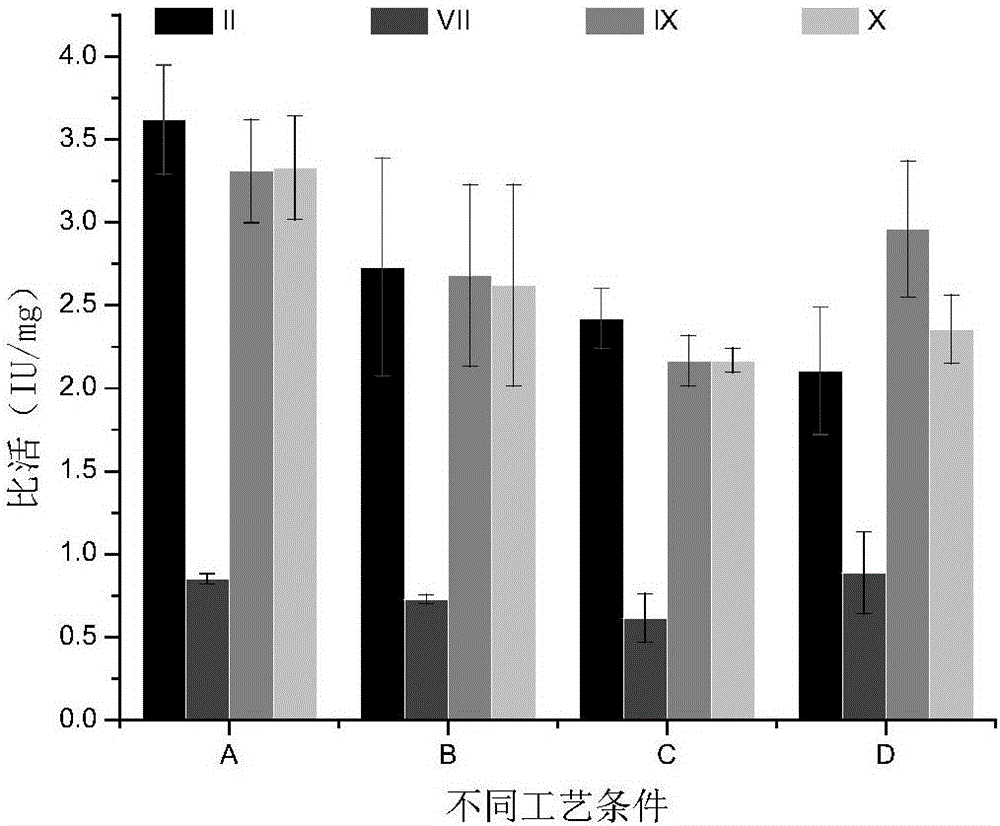
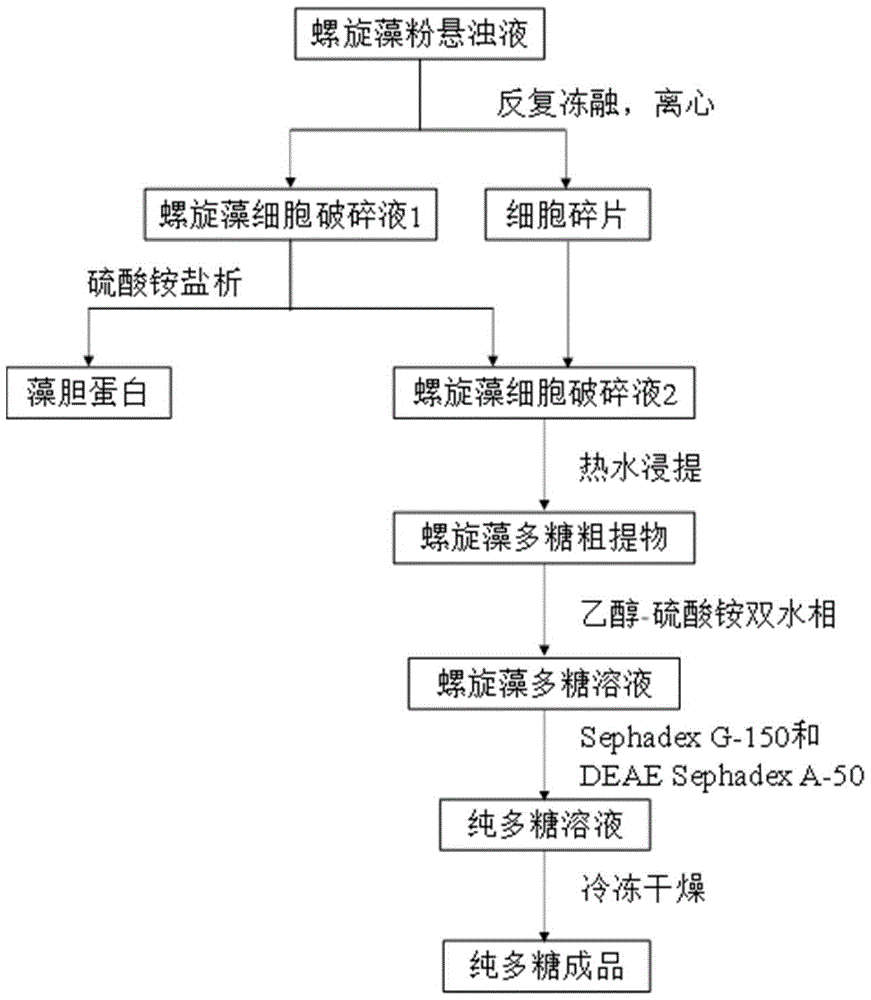
The invention relates to a spirulina phatensis polysaccharide and an extraction method thereof. The extraction method comprises the following steps: after multigelation and wall breaking of a spirulina phatensis powder suspension, centrifuging to obtain a cell lysate 1 and cell debris; adding ammonium sulfate in the cell lysate 1 until the saturation is 50%, after salting-out precipitation, centrifuging to remove phycobiliprotein to obtain a supernatant, and evenly mixing the supernatant and the cell debris to obtain a cell lysate 2; obtaining a spirulina phatensis polysaccharide crude extract by using a hot water extraction method; adding the crude extract to an ethanol / ammonium sulfate aqueous two-phase system, and extracting to obtain a bottom-phase solution of spirulina phatensis polysaccharide with higher purity; after the bottom-phase solution is desalted by dialysis, eluting by using a Sephadex G-150 chromatographic column and a DEAE Sephadex A-50 anion exchange column to obtain a pure spirulina phatensis polysaccharide solution; and freeze-drying, thereby obtaining a pure spirulina phatensis polysaccharide finished product. The extraction method can be used for avoiding the traditional complicated protein removal operation steps, has low cost, high yield, and high purity and activity of polysaccharide, and is suitable for intermittent and large-scale production and processing.
Owner:SHANTOU UNIV
Process for preparing tetra-hexose ganglioside monosialate
InactiveCN1379034ASolve problemsImprove permeabilitySugar derivativesSugar derivatives preparationDEAE SephadexPurification methods
A process for preparing monosialic acid tetra-hexose ganglioside preparation from pig's brain includes such steps as ultrafilter membrane separation, chromatography by Iatrobeads column and DEAE sephadex A-25 column, and efficient liquid-phase chromatography technique. In the preparation process, it retains the GMI amphipathy physico-chemical characteristic of ganglioside. Its advantages are highpurity (more than 95%), low cost, environment protection and high curative effect.
Owner:LITUO DEV GUANGZHOU CITY
Acidic oligosaccharide having weight-reducing and lipid-lowering effects and application thereof
InactiveCN101613378AImprove solubilityRaw materials are easy to getOrganic active ingredientsSugar derivativesChromatographic separationLipid formation
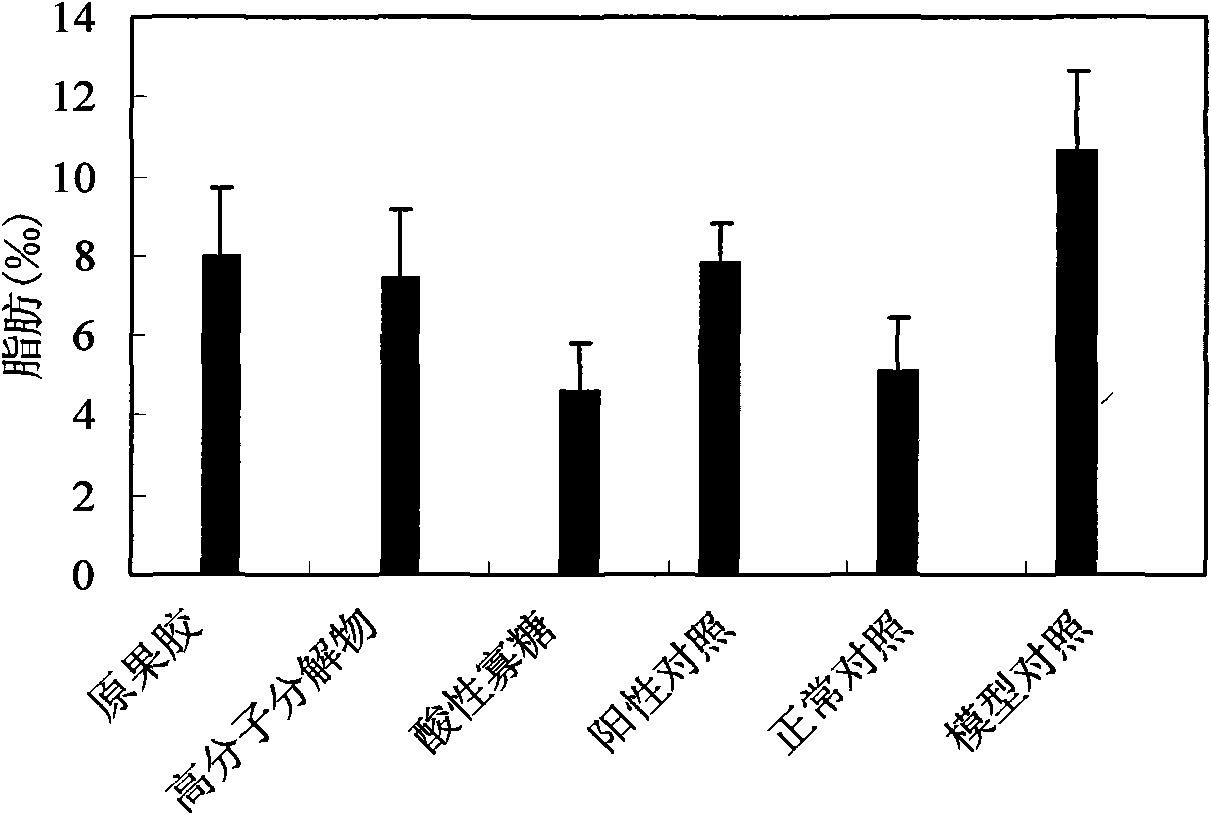
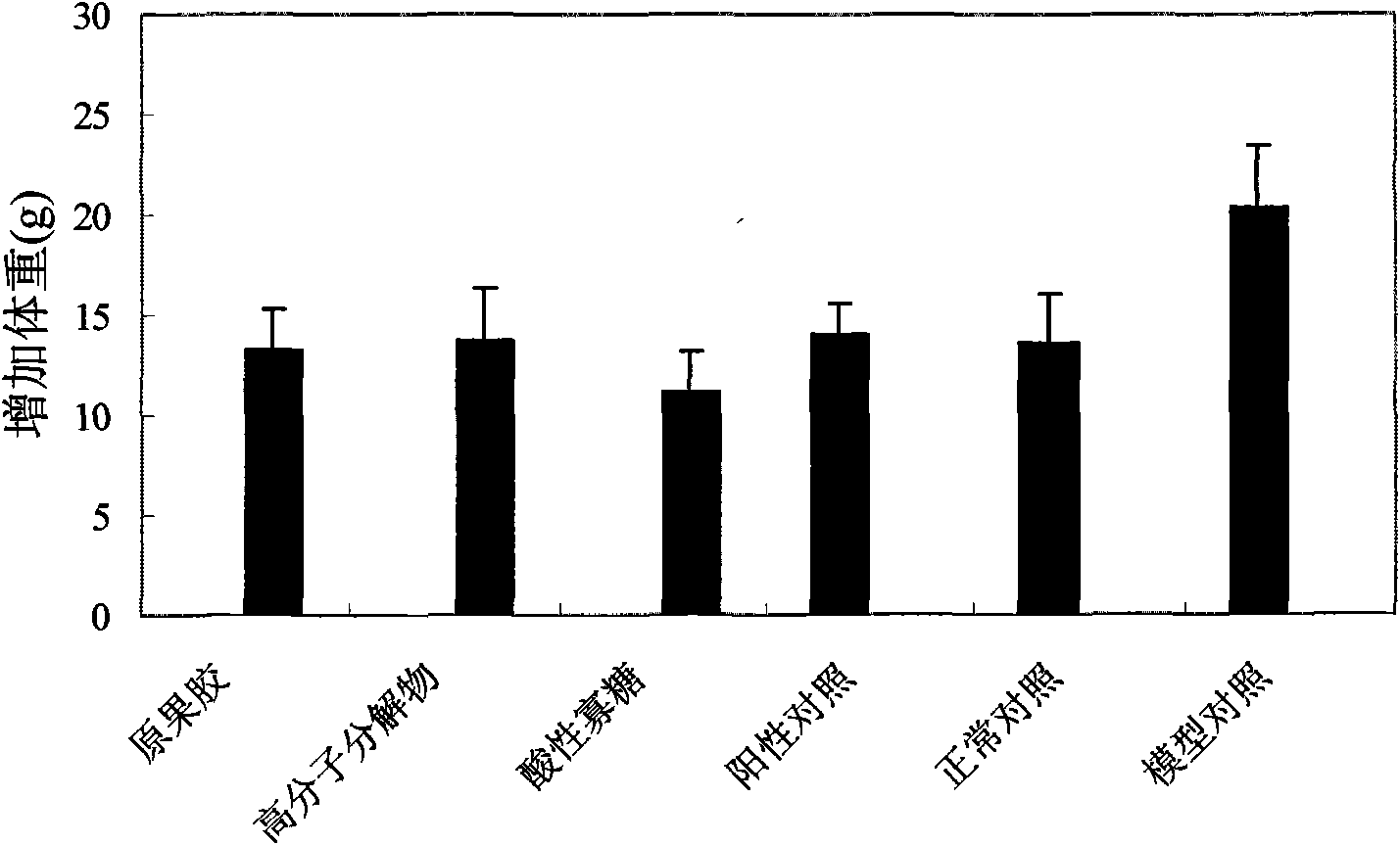
The invention relates to an acidic oligosaccharide having weight-reducing and lipid-lowering effects and application thereof. The adopted technical scheme is that a preparation method of the acidic oligosaccharide is as follows: taking pectin water solution with the weight percentage concentration of 1 percent to 2 percent, regulating pH to 2 to 4, decomposing and reacting the solution for 15 minutes to 24 hours at 90 to 120 DEG C to obtain pectin decomposer, treating the pectin decomposer by a hollow fiber membrane or a ceramic filter membrane, recovering oligosaccharide mixtures with the molecular weight of lower than 6000 daltons, recovering the oligosaccharide mixtures with the molecular weight of 200 to 6000 daltons through a nano-filter membrane, carrying out chromatographic separation by utilizing Bio-gel P-2 gel column or SephadexG-25 gel column or DEAE-Sephadex A-25 anion exchange resin, and carrying out gradient elution by utilizing inorganic salt water solution to obtain the pectin acidic oligosaccharide with the contact ratio of 2 to 20. The acidic oligosaccharide prepared by the invention can be applied to weight-reducing and lipid-lowering health food.
Owner:LIAONING UNIVERSITY
Method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen
InactiveCN105315360AHigh yieldIncrease productivityFactor VIIFibrinogenDEAE SephadexVirus inactivation
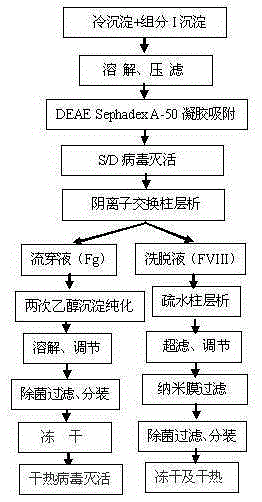
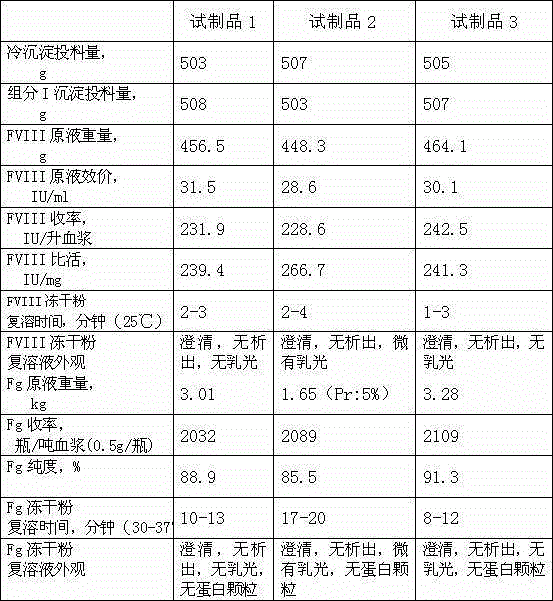
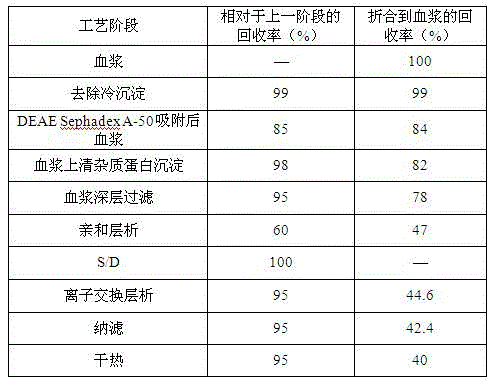
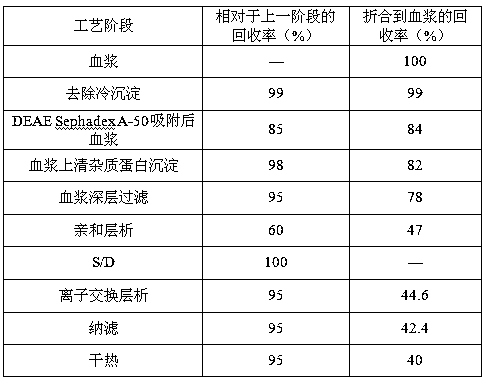
The invention discloses a method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen by cryoprecipitate and component I precipitation, mixing and feeding. The method comprises the following steps: (1) simultaneous feeding and dissolution of a cryoprecipitate and a component I; (2) DEAE Sephadex A-50 gel adsorption; (3) S / D virus inactivation; (4) anion exchange column chromatography; (5) two-step low-temperature ethanol precipitation and purification, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a chromatographic penetration liquid to obtain a human fibrinogen; (6) further hydrophobic column chromatography of a chromatographic eluant; (7) ultrafiltration, nanofilm filtration, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a hydrophobic eluant to obtain a high-purity human coagulation factor VIII. By the adoption of the process, FVIII and Fg in the two raw materials are extracted simultaneously, so that the yields of the two products are greatly improved, the yield of the human coagulation factor VIII can reach 200,000 IU / ton plasmas, the yield of the human fibrinogen exceeds 2,000 bottles / ton plasmas, and the yields are both far higher than those of a traditional process.
Owner:上海洲跃生物科技有限公司
Production method of human antithrombin III
ActiveCN104672328AHigh purityIncrease capacityPeptide preparation methodsProtease inhibitorsDEAE SephadexVirus inactivation
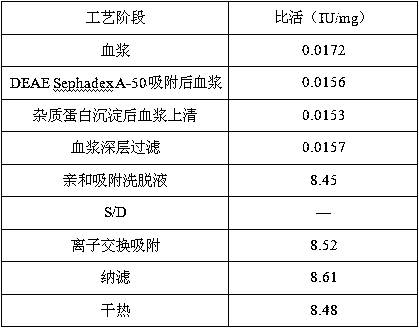
The invention relates to a production method of human antithrombin III. The production method comprises the following steps: removing cryoprecipitate from fresh frozen human plasma to obtain a plasma supernatant, and carrying out adsorption treatment on the plasma supernatant by using a DEAE Sephadex-A50 gel; precipitating impurity protein of the plasma supernatant, and deeply filtering plasma; carrying out Capto Heparin affinity chromatography; carrying out S / D virus inactivation and Capto Q ion exchange chromatography, and removing an S / D reagent; and filtering by virtue of a nanometer film to remove viruses, preparing, freeze-drying and dry-heating. The process is capable of preparing the antithrombin III by virtue of affinity chromatography with only one step. According to the production method disclosed by the invention, the recovery rate can reach above 40 percent, and the specific activity can reach 8-10 IU / mg.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Extraction of polysaccharose of three bristle cudrania
Cudrania tricuspidata polysaccharide is extracted at 70deg.C by: immersing raw materials for 6-8 hours, centrifugalizing, filtrating, incorporating filtrated liquids, passing them through macro-porous adsorbing resin-3520 with pH=4, decompressed concentrating, alcohol separating out, deproteinizing by Sevag method, dialyzing to pass gel column DEAE-Sephadex A-50, cleansing with water and NaCl solution, freezing dialyzed liquid and drying to obtain the products.
Owner:ZHONGAO SCI & TECH SHANDONG LUKANG GROUP
Method for preparing antiviral active oligomeric peptide
InactiveCN1814775AHigh activityEnhanced inhibitory effectPeptide preparation methodsFermentationDEAE SephadexFiltration
This invention relates to a method for preparing antivirus active oligomerized peptides by step directional enzymolyzing oysters with a compound enzyme characterizing in adding Tris-HCL buffer solution into the oyster meat to be beated, the increased number of the buffer is 2-3 times of kilograms of the meat, trypsase is added according to the proportion of 60-65U / g meat to be mixed under constant temperature and enzymolyzed for 3-4 hours under 40-50deg.C then to eliminate the enzymes and cool, regulating pH value to 5-6 with an acid and adding pineappleproteinase in the meat proportion of 70-75U / g to be mixed and enzymolyzed for 5-6 hours under the 45-55deg.C constant temperature to eliminate the enzymes and cool, centrifuge to take the supernatant fluid to pass through super-filtration, Sephadex G-25 gel chromaphoresis, DEAE Sephadex A-25 ionic exchange post chromaphoresis and counter-phase high efficient liquid phase chromqtogram separation and purification to be frozen and dried.
Owner:OCEAN UNIV OF CHINA
Water-soluble low-molecular-weight cordyceps polysaccharide with anti-tumor activity, preparation method and application thereof
InactiveCN101805412AGrowth inhibitionEnhanced inhibitory effectOrganic active ingredientsImmunological disordersWater soluble polysaccharidesEthanol precipitation
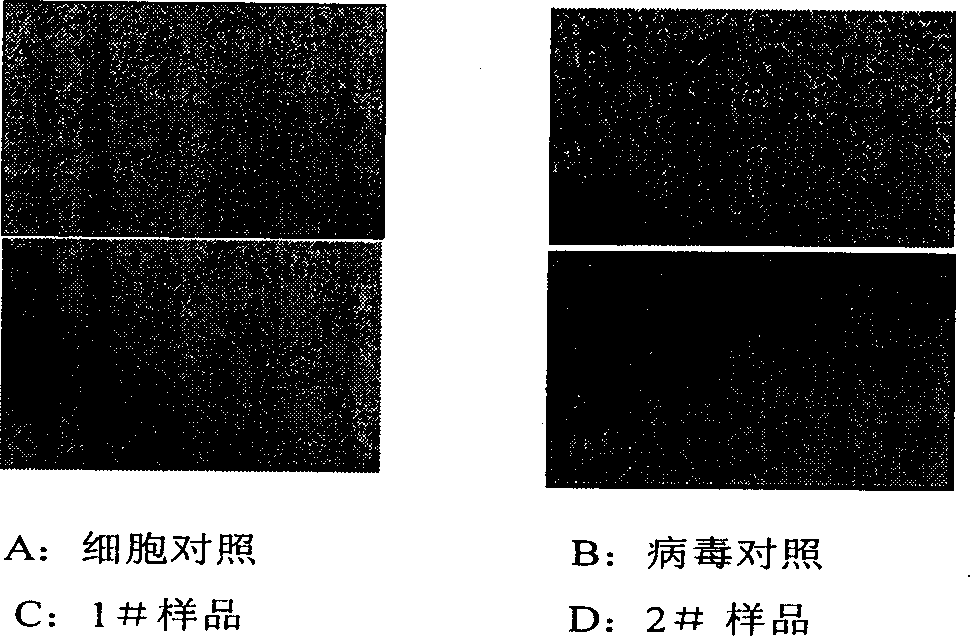
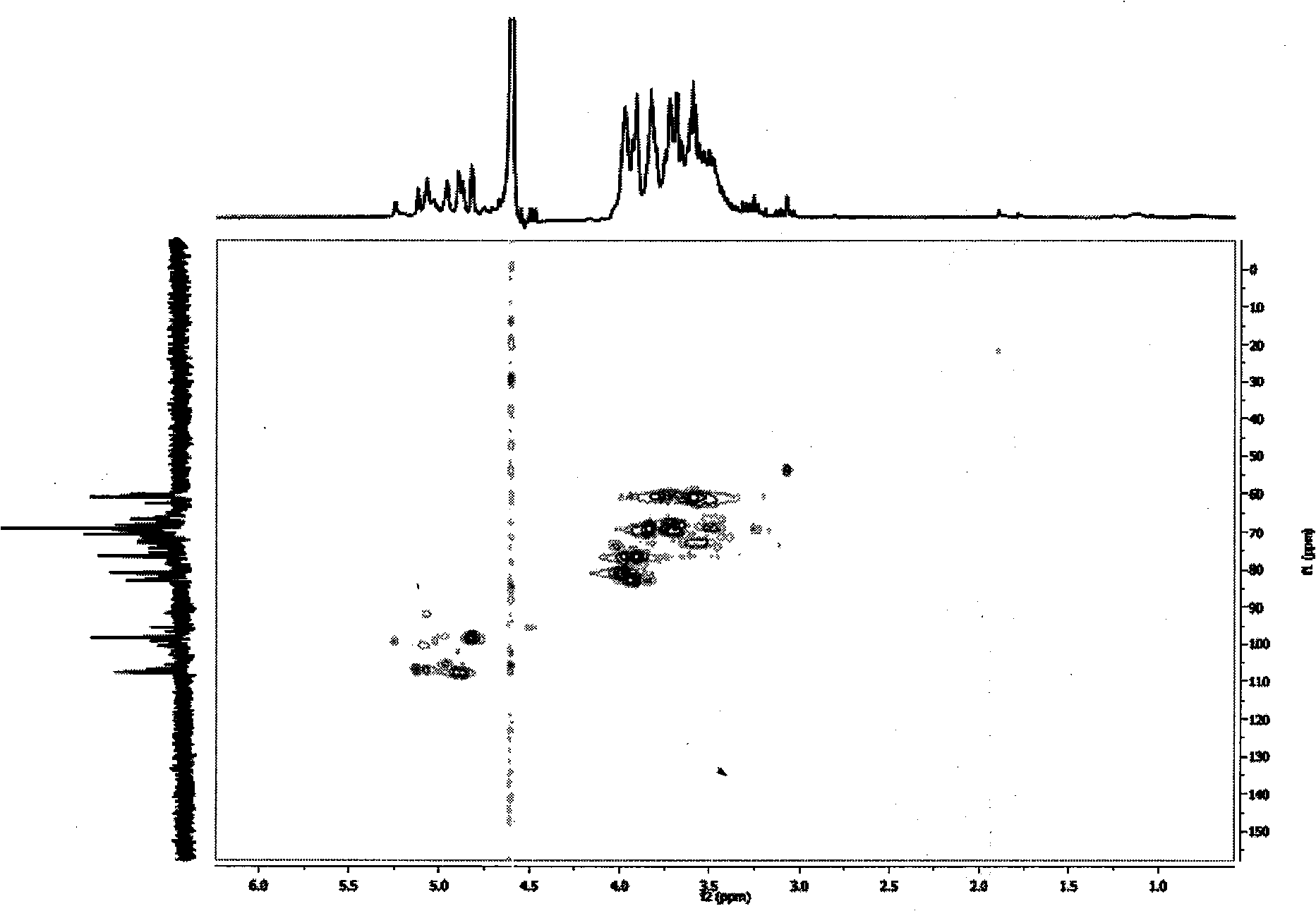
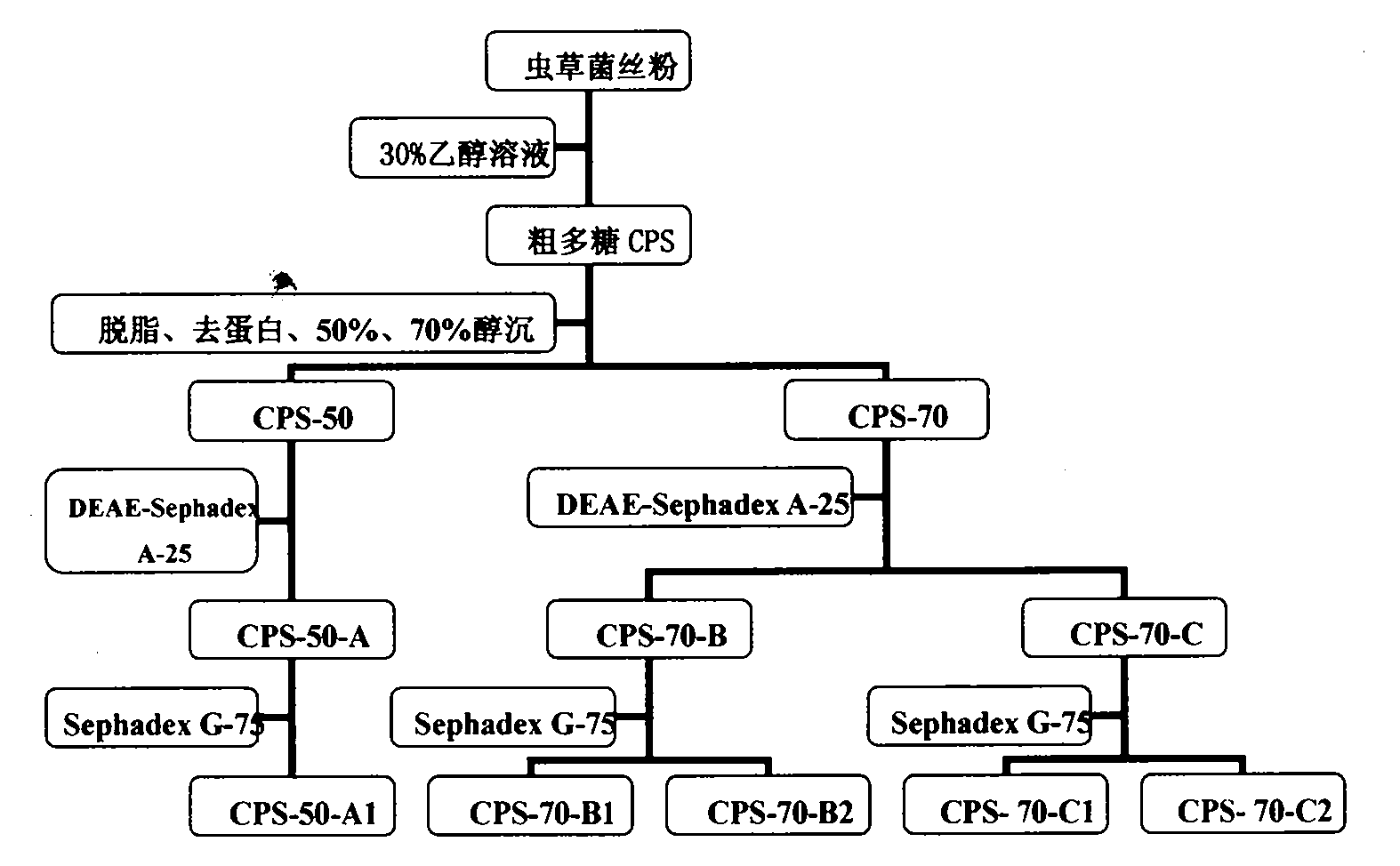
The invention discloses water-soluble low-molecular-weight polysaccharide with anti-tumor activity for artificially cultured cordyceps mycelium, a preparation method and an application thereof. 30 percent ethanol is used as extractant, and the best extraction process of the polysaccharide has the temperature of 70 DEG C, material-liquid ratio of 1 to 20, and extracting time of 2 hours. The polysaccharide is subjected to grease removal, protein removal, 50 percent ethanol precipitation and 70 percent ethanol precipitation to prepare crude polysaccharide after freezing and drying. The crude polysaccharide is purified grade by grade through a DEAE Sephadex A-25 column and a Sephadex G-75 column to prepare the water-soluble low-molecular-weight polysaccharide. The polysaccharide has higher purity, wherein the polysaccharide content is 94.57 percent, and relative molecular mass is about 9,874. The water-soluble polysaccharide has obvious inhibiting effect on proliferation of liver cancer cell line Bel-7204 and gastric cancer cell line SGC-7901 of human beings, and has obvious inhibiting effect on growth of S180 tumor cells implanted in a mouse body. The water-soluble low-molecular-weight cordyceps polysaccharide has clear active components and controllable quality, and can be used as an anti-tumor medicament or a health-care product for improving body immunity.
Owner:TIANJIN UNIV OF SCI & TECH
Purification method of deproteinized calf blood serum extract
InactiveCN102805754ASmall molecular weight rangeEfficient killingMammal material medical ingredientsDrug compositionsChromatographic separationDeproteinized calf blood serum
The invention belongs to the technical field of biotechnology and provides a purification method of deproteinized calf blood serum extract. The purification method includes collecting calf venous blood, separating to obtain serum, deproteinizing with ethanol, decompressing to remove the ethanol, adding compound protease for hydrolization, centrifugally separating after hydrolization and reserving supernatant, and ultra-filtrating the supernatant; subjecting ultrafiltrate to gel chromatography with Sephadex G-15 for desalting, and collecting specific absorption elution at 280nm position; performing gel chromatographic separation with DEAE-Sephadex-50, and collecting specific absorption elution at the second 280nm position; and filtering with microporous membranes and inactivating viruses by ultraviolet irradiation so as to obtain the deproteinized calf blood serum extract. A great many of inactive ingredients are removed, molecular weight range of products is reduced, probability of adverse reactions is reduced, product purity, safety and bioactivity are improved, and the purification method is widely applicable.
Owner:FUDAN UNIV
Fritillariae cirrhosae bulbus polysaccharide extraction, separation and purification technology
ActiveCN106397622AOvercoming the low extraction rateOvercome purityBulk chemical productionDEAE SephadexPyridinium
The invention discloses a fritillariae cirrhosae bulbus polysaccharide extraction, separation and purification technology. As a novel supercritical CO2 extraction technology is utilized, the defects that a general method is low in extraction rate and not high in purity are overcome; quaternary ammonium salt cetyl pyridinium chloride monohydrate and fritillariae cirrhosae bulbus polysaccharide can precipitate in water solution with low ionic strength; when the ionic strength is high, precipitate can be dissolved, dissociated and released, and the purification effect is good; DEAE-Sephadex ionic exchange column chromatography is utilized to further remove neutral impurities and impurities with positive charges, and the separation and purification effect is better.
Owner:CHONGQING THREE GORGES MEDICAL COLLEGE
Method for preparing methyl parathion degradation bacterium and enzyme preparation thereof
InactiveCN101168731AWill not affect the use effectEasy to useBacteriaPesticide residueMethyl parathion
The invention relates to methyl parathion degradation fungus and the preparing method of the enzyme preparation thereof, and belongs to the biological high technical field. The methyl parathion degradation fungus strain is plesiomus shigelloides, and the production process of liquid enzyme preparation adopts the steps of slant culture, seed liquid shaking, seed fermenter, fermenter, bacterial collection, mechanical cell crushing, clear liquid collection, (NH4)2SO4 fractional precipitation, HEPES buffer solution suspension, dialysis, and crude enzymes production. Enzyme dry powder preparation which is easy to be stored or transported can also be further refined, and processing steps added on the basis of the crude enzymes production are as follows: DEAE-Sephadex-A50 anion column chromatography, CM Sepharose Fast Flow cation column chromatography, dialysis, cryodesiccation and enzyme dry powder production. The direct application of the liquid crude enzymes preparation can ensure the organic phosphorus pesticide residue quantity in agricultural crops to be reduced by more than 90 percent, and the residual on the surface of garden spgarden stuff washed to be reduced by about 98 percent; after the enzyme dry powder preparation is diluted according to a specific proportion, the pesticide residue quantity can be reduced by more than 95 percent. The enzyme preparation product resolves the problem that the pesticide residue is over-standard in the agricultural production.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
Preparation method of plasma human immunoglobulin
ActiveCN109456407AReduce contentLow costSerum immunoglobulinsPeptide preparation methodsUltrafiltrationBlood plasma
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Process for purifying prothrombin compound
ActiveCN106497903AHigh purityImprove balanceFibrinogenPeptide preparation methodsDEAE SephadexCentrifugation
The invention provides a process for purifying a prothrombin compound. The process comprises the following steps: subjecting qualified detected plasma of a healthy person to centrifugation for removal of sediments so as to a raw plasma material; subjecting the raw plasma material to adsorption with DEAE-Sephadex A-50 gel and carrying out washing and elution so as to obtain crude extract of the prothrombin compound; and subjecting the crude extract of the prothrombin compound to further adsorption by using Capto DEAE gel column chromatography and carrying out washing and elution so as to obtain the liquid prothrombin compound. According to the invention, the prothrombin compound is prepared through two-step operations via the DEAE-Sephadex A-50 gel and Capto DEAE gel; and under the designed process conditions, the prepared prothrombin compound has a purity far higher than the purity of a prothrombin compound product prepared by using a traditional process, and blood coagulation factors II, IX and X are good in equalization.
Owner:DAAN PHARMA +1
A kind of spirulina polysaccharide and its extraction method
The invention relates to a spirulina phatensis polysaccharide and an extraction method thereof. The extraction method comprises the following steps: after multigelation and wall breaking of a spirulina phatensis powder suspension, centrifuging to obtain a cell lysate 1 and cell debris; adding ammonium sulfate in the cell lysate 1 until the saturation is 50%, after salting-out precipitation, centrifuging to remove phycobiliprotein to obtain a supernatant, and evenly mixing the supernatant and the cell debris to obtain a cell lysate 2; obtaining a spirulina phatensis polysaccharide crude extract by using a hot water extraction method; adding the crude extract to an ethanol / ammonium sulfate aqueous two-phase system, and extracting to obtain a bottom-phase solution of spirulina phatensis polysaccharide with higher purity; after the bottom-phase solution is desalted by dialysis, eluting by using a Sephadex G-150 chromatographic column and a DEAE Sephadex A-50 anion exchange column to obtain a pure spirulina phatensis polysaccharide solution; and freeze-drying, thereby obtaining a pure spirulina phatensis polysaccharide finished product. The extraction method can be used for avoiding the traditional complicated protein removal operation steps, has low cost, high yield, and high purity and activity of polysaccharide, and is suitable for intermittent and large-scale production and processing.
Owner:SHANTOU UNIV
Preparation method of high purity serum gonadotrophin
InactiveCN1919864AImprove the production processImprove purification efficiencyPeptide preparation methodsAnimals/human peptidesSodium acetateForeign matter
The invention discloses a preparing method of high-purity lobulantina, which is characterized by the following: adopting chromatography medium of DEAE-Sephadex A-50 as ionic exchange chromatography column, using PH as 6.5-8.5 and density is 0.005-0.07mol / L sodium acetate buffer solution to dissolve PMSG semifinished product and up-sample, using PH is 6.5-8.5 and density is 0.005-0.07mol / L sodium acetate buffer solution to break so as to remove foreign matter, using PH is 6.5-8.5 and density is 0.15-0.3mol / L sodium acetate buffer solution to elute and check at the some time, assembling eluent to get collecting solution, dialyzing and freeze-drying collecting solution to get elaboration product.
Owner:NINGBO SANSHENG PHARMA
Method for extracting and purifying calf serum protein-removing extract
ActiveCN100374123CSmall molecular weight rangeEfficient killingMetabolism disorderMammal material medical ingredientsVenous bloodUltrafiltration
The invention discloses a method for extracting and purifying calf serum protein-removing extract, which comprises extracting calf venous blood, separating plasma, removing protein with ethanol, removing ethanol through decompression, charging composite proteolytic enzyme, subjecting supernatant fluid to ultrafiltration, desalinizing ultrafiltrate with gluglucosan G-15 gel chromatography, collecting elution portion with specific absorption at 280nm, then separating with DEAE-Sephadex-50 gel chromatography, collecting elution portion with specific absorption at a second 280nm, then carrying out virus deactivation by means of microporous membrane filtration and ultraviolet irradiation.
Owner:JINZHOU AHON PHARM CO LTD
Preparation method for human antithrombin III
InactiveCN105315365AReduce the probability of activationImprove pass ratePeptide preparation methodsProtease inhibitorsUltrafiltrationPolyethylene glycol
The invention discloses a preparation method for human antithrombin III (AT-III). The preparation method comprises the following steps: (1) precipitation and dissolution of a blood plasma fraction IV; (2) polyethylene glycol (PEG) precipitation and impure protein removing; (3) DEAE Sephadex A-50 gel adsorption; (4) S / D virus inactivation; (5) heparin affinity column chromatography; (6) ultrafiltration and concentration; (7) addition of a stabilizer and regulation; (8) nanofilm virus-removing filtration; (9) sterilizing filtration; (10) freeze-drying; (11) dry and fever virus inactivation. According to the preparation method, blood coagulation factors, which remain in the raw materials and are depended by vitamin K, are removed, so as to greatly lower the possibility of protein activation in the production process, and improve the product qualification ratio; PEG is adopted for the impure protein removing, so as to reduce the load of a chromatographic column; a liquid obtained from lyophilized powder re-dissolution is clear, transparent, and free of protein precipitation and opalescence; through adoption of a three-step virus inactivation method, the safety of human AT-III for clinical use can be greatly improved.
Owner:上海洲跃生物科技有限公司
A kind of production method of human antithrombin III
ActiveCN104672328BIncrease capacityDoes not affect productionPeptide preparation methodsProtease inhibitorsDEAE SephadexVirus inactivation
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Extraction method of polysaccharide with activities of kidney warming and Yang tonifying from Aspongopus chinensis Dallas insects of Pentatomidae and application of polysaccharide
ActiveCN109280090ARestore immune function activityHigh cure rateAntinoxious agentsUrinary disorderFiltrationYang deficiency
The invention discloses an extraction method of polysaccharide with activities of kidney warming and Yang tonifying from Aspongopus chinensis Dallas insects of Pentatomidae and application of the polysaccharide. The extraction method comprises the following steps: (1) drying Aspongopus chinensis Dallas insect bodies, performing pulverization, performing reflux extraction with petroleum ether, andconducting filtration liquid-solid separation so as to obtain drug residues with removal of grease; (2) evaporating the drug residues, performing reflux extraction with distilled water, performing filtration, combining the filtrate, and performing concentration so as to obtain total water extract; (3) adding distilled water to the total water extract for slight dilution, performing precipitation on polysaccharide with an alcohol solution, performing stirring while the alcohol solution is added, and performing still standing and filtration so as to obtain an insect polysaccharide alcohol precipitate; and (4) adding water to the insect polysaccharide alcohol precipitate for dilution and dissolution, performing chromatography separation and purification by using a DEAE-Sephadex ged cellulosecolumn, and performing rinsing with distilled water and a sodium chloride solution so as to obtain the Aspongopus chinensis Dallas polysaccharide. The invention also relates to the application of thepolysaccharide in preparation of drugs for treating or preventing Yang deficiency, impotence and erectile dysfunctions, the method is easy has simple and convenient operation, and the obtained polysaccharide compound with a purity of up to 95% has strong effects of kidney warming, Yang tonifying, fatigue resistance and body immunity recovery.
Owner:HUBEI UNIV OF CHINESE MEDICINE
Preparation of kilogram-grade scale high-purity monosialotetrahexosylganglioside
ActiveCN101177439BHigh content of main ingredientsLittle side effectsSugar derivativesUnknown materialsDEAE SephadexPig brain
The invention discloses a manufacturing technique for extracting, separating and purifying kilogram-grade scale high purity GM1 using fresh pig brain tissue as raw material, which is characterized in that large sized extraction pot and adsorption resin with large holes, DEAE Sephadex A-25 and silica gel layer are utilized to build a set of manufacturing technique. The invention has the advantagesof elimination of toxic solvent chloroform in mobile phase in traditional method, less pollution for whole process, simple process, low cost and applicability to industrialized production.
Owner:LUNAN PHARMA GROUP CORPORATION
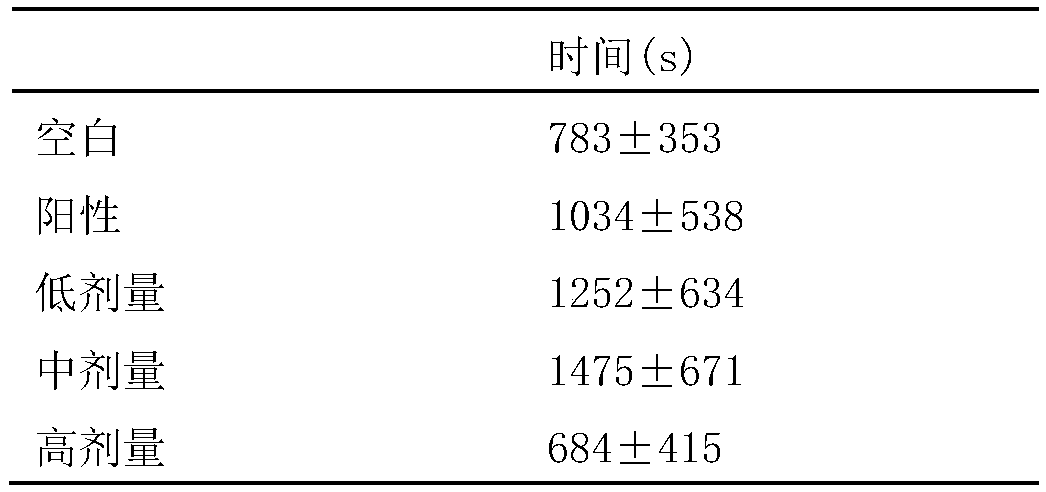
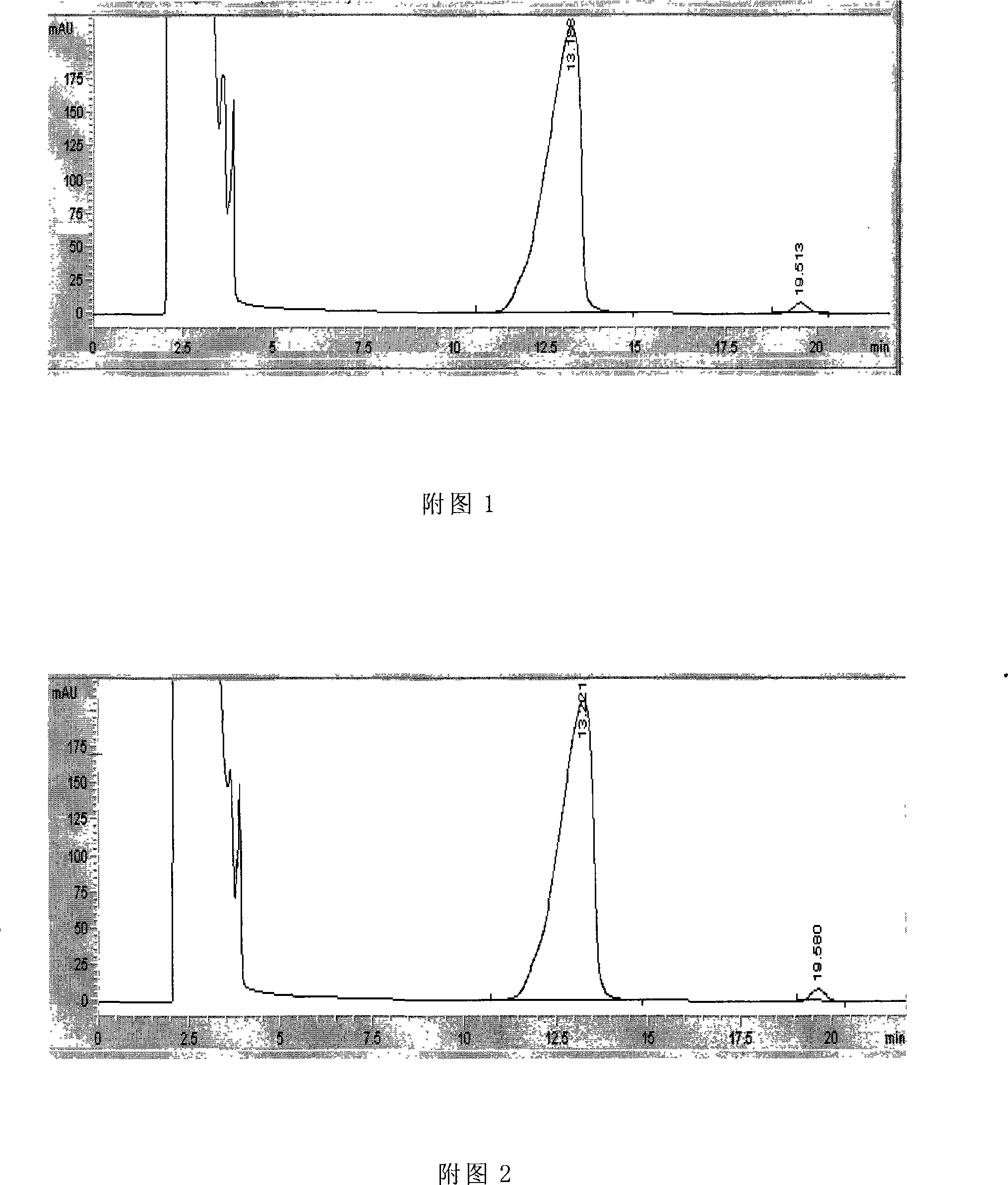
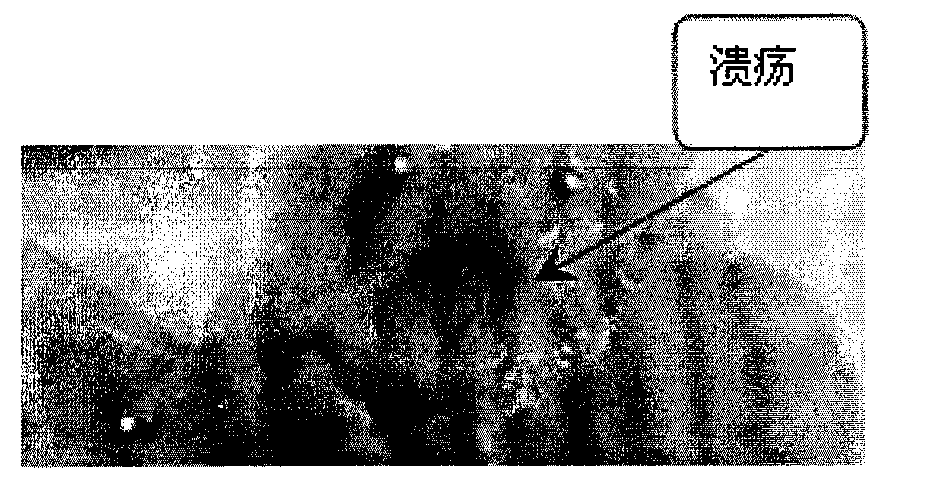
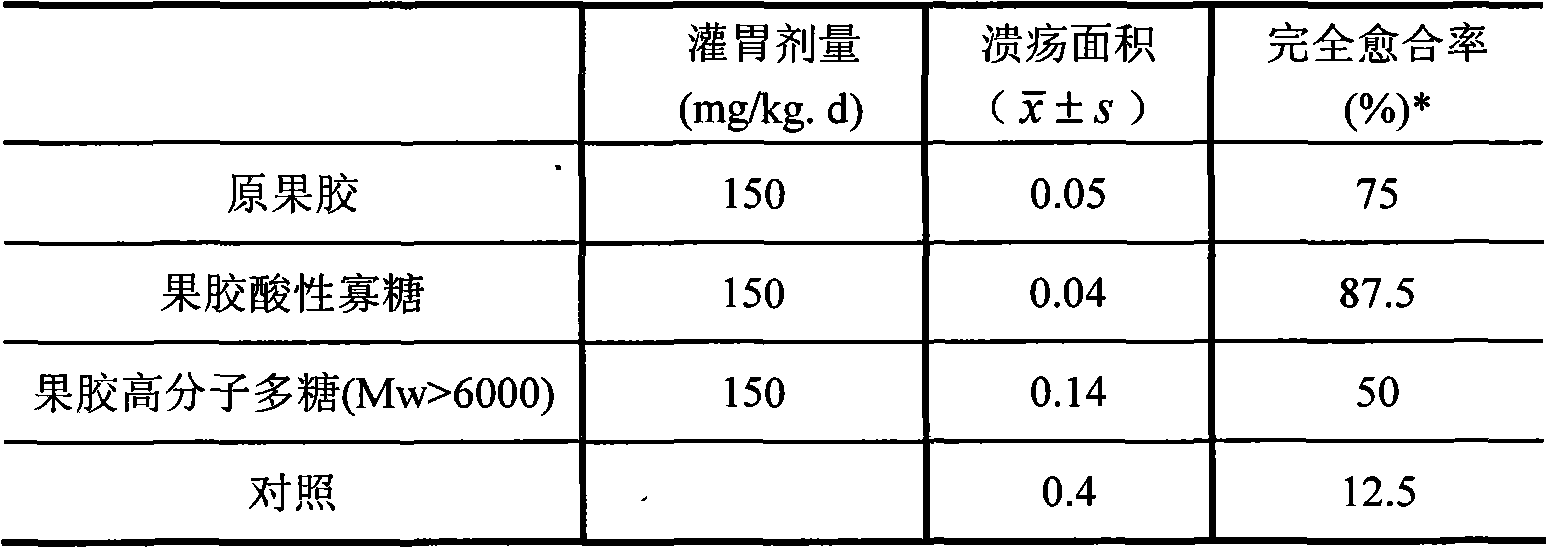
Application of pectic acid oligosaccharide in preparing health-care product treating gastric ulcer
InactiveCN101632459ASignificant effectImprove solubilityOrganic active ingredientsDigestive systemHollow fibreFiber
The invention relates to an application of pectic acid oligosaccharide in preparing a health-care product treating gastric ulcer. The invention adopts the technical scheme that firstly, pectin is dissolved in water, and a pectin decomposer is prepared by a decomposition reaction; the pectin decomposer is processed by using a hollow fibre film or a ceramic filtration film, an oligosaccharide mixture with the molecular weight of 200-6000 daltons is processed and recovered by using a nano filtration film after an oligosaccharide mixture with the molecular weight being below 6000 daltons is recovered, and the pectic acid oligosaccharide with the superposition degree of 2-20 is obtained by the chromatographic separation of a Bio-gel P-2 gel column and / or a Sephadex G-25 gel column or the filtration of DEAE-Sephadex A-25 anion exchange resin. The prepared pectic acid oligosaccharide and auxiliary materials are matched to prepare health-care products with various forms and components for treating the gastric ulcer. The invention adopts a congeneric theory of medicines and foods, has no toxic or side effect and can be taken for a long time. By verification, the invention has obvious curative effect to sour burning type gastric ulcer.
Owner:LIAONING UNIVERSITY
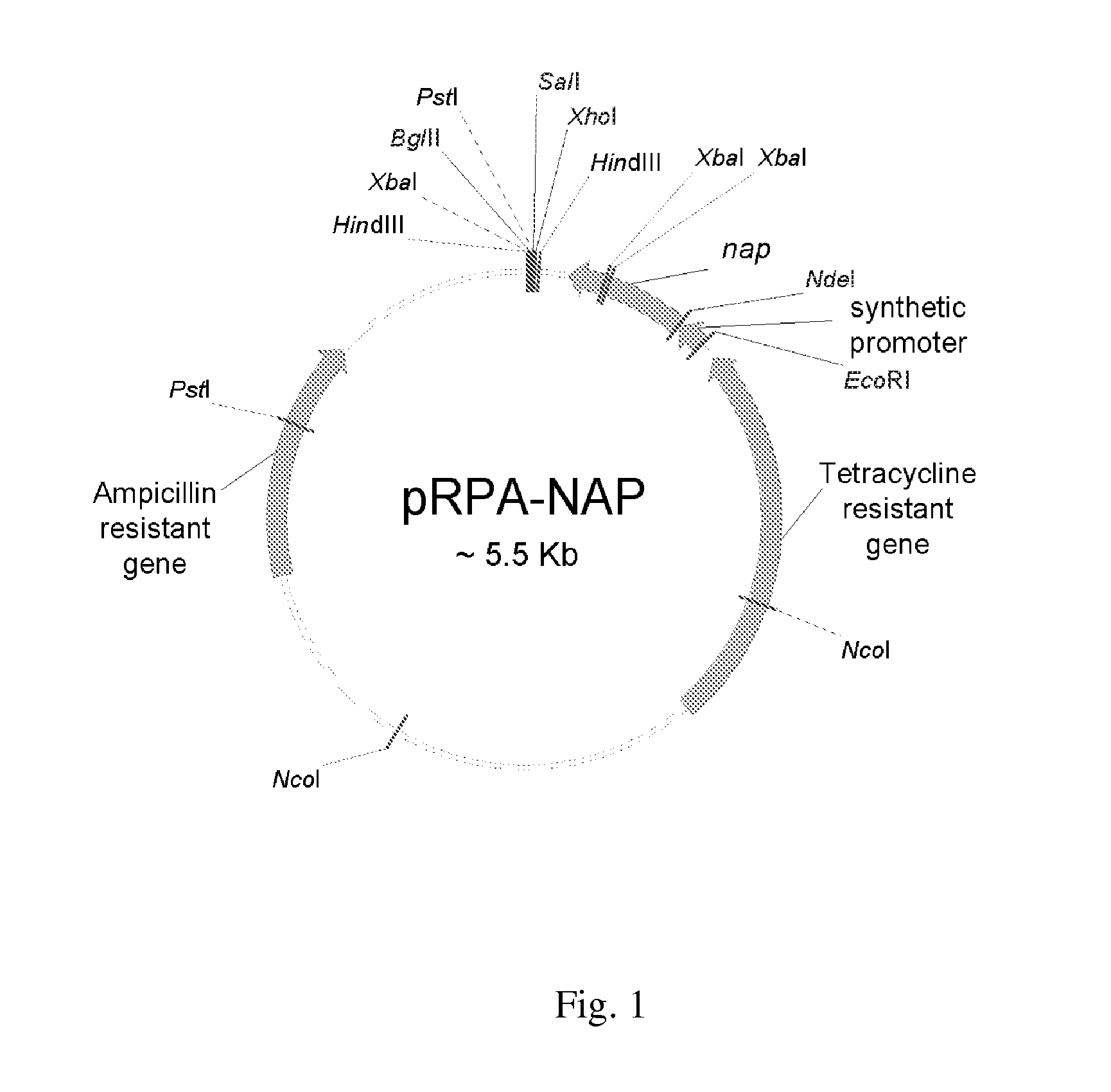
Method for one-step purification of recombinant helicobacter pylori neutrophil-activating protein
ActiveUS20130190482A1Reduce endotoxinFold preciselyDepsipeptidesPeptide preparation methodsEscherichia coliPurification methods
Helicobacter pylori is closely associated with chronic gastritis, peptic ulcer disease, and gastric adenocarcinoma. Helicobacter pylori neutrophil-activating protein (HP-NAP), a virulence factor of Helicobacter pylori, plays an important role in pathogenesis of Helicobacter pylori infection. Since HP-NAP has been proposed as a candidate vaccine against Helicobacter pylori infection, an efficient way to obtain pure HP-NAP needs to be developed. In the present invention, recombinant HP-NAP expressed in Bacillus subtilis and Escherichia coli was purified through a single step of DEAE Sephadex ion-exchange chromatography with high purity. Also, purified recombinant HP-NAP was able to stimulate neutrophils to produce reactive oxygen species. Thus, recombinant HP-NAP obtained from our Bacillus subtilis expression system and Escherichia coli expression system is functionally active. Furthermore, this one-step negative purification method should provide an efficient way to purify recombinant HP-NAP expressed in Bacillus subtilis and Escherichia coli for basic studies, vaccine development, or drug design.
Owner:NATIONAL TSING HUA UNIVERSITY
Method for preparing fermented glutinous rice Chinese yeast microorganism sourced chymosin
The invention relates to a method for preparing wine drug microbial origin chymosin of glutinous rice wine. The method of the invention comprises: utilizing glutinous rice flour as culture medium, producing the glutinous rice wine through fermenting wine drug, adopting an ammonium sulfate graded precipitation method to extract chymosin from the glutinous rice wine, further utilizing Sephadex G-100 and DEAE-Sephadex A-50 to purify, and obtaining the chymosin with high purity. The method is used to produce the chymosin, sources of raw materials are wide, the cost is low, the operation is simple, the fermentation period is short, the chymosin which is produced has high activity, the milk-clotting activity after purifying can reach 4360+-50U / mg, and the protein recovery is 16.42+-1.98%.
Owner:上海瀚厨餐饮管理有限公司
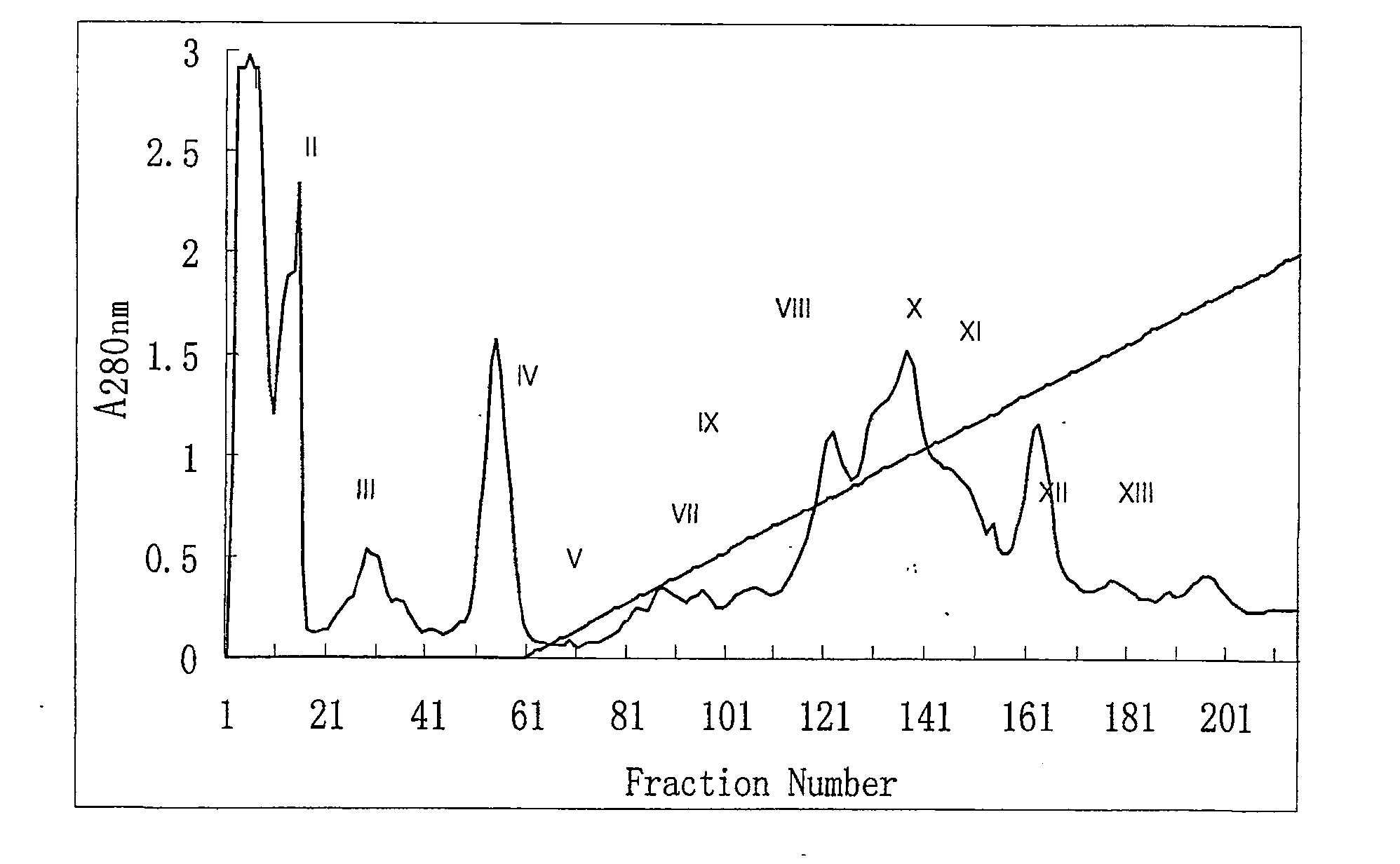
Technique of enzyme
The invention relates to a technique of an enzyme. The technique of the enzyme comprises the following steps: (1) carrying out a DEAE-sephadex A50 balance method; and (2) carrying out a linear elution method, wherein nine peaks are separated in total, and qualified peaks are combined. The technique provided by the invention has the advantages that a technique for extracting defibrase by virtue of snake venom is adopted, and compared with the original technology, separation time is short, usage amount of a reagent is small, process is simple, yield is high, and quality is stable.
Owner:芜湖通全科技有限公司
Technology for extracting hyaluronic acid from squid eyes
The invention discloses a technology for extracting hyaluronic acid from squid eyes. The technology comprises the following steps: 1) cleaning the fresh squid eyes and smashing; 2) subcritical water extraction: putting a processed raw material into an extraction kettle, adding sodium acetate, injecting deoxidized deionized water into the kettle, controlling an extraction temperature as 120 to 130DEG C, pressure as 5 to 8 MPa and extraction time as 20 to 30 minutes, extracting the squid eyes, releasing pressure after extraction finishes, making extract liquor flow into a collection tank to becooled to room temperature to obtain the extract liquor; 3) adding an isometric chloroform and n-butyl alcohol mixed solution into the extract liquor obtained in the last step, evenly stirring and standing, wherein a volume ratio of the chloroform to the n-butyl alcohol is equal to 4 to 1; 4) purification: taking supernatant after standing and layering to pass through a DEAE-SephadexA-25 ion column and eluting by a NaCl solution to obtain eluant; 5) drying: concentrating the eluant, dialyzing to remove salt and vacuum drying to obtain a finished product.
Owner:桂林华诺威生物科技有限公司
Process for extracting hyaluronic acid from cockscombs
The invention discloses a process for extracting hyaluronic acid from cockscombs. The process comprises the following steps: (1) cleaning and crushing fresh cockscombs; (2) performing subcritical water extraction: putting the treated raw material into an extraction kettle, adding sodium acetate, injecting deoxygenated deionized water into the kettle, extracting the cockscombs at extraction temperature of 120 to 130 DEG C and pressure of 5 to 8 Mpa for 20 to 30 min, relieving the pressure at the end of the extraction, and putting extraction liquid into a collection tank for cooling to room temperature, thus obtaining extraction liquid; (3) performing suction filtration: adding kieselguhr which accounts for 0.010 to 0.012 of the weight of the extraction liquid into the extraction liquid obtained in the previous step, and performing suction filtration at 0.1 MPa, thus obtaining filtrate; (4) performing purification: putting the filtrate obtained in the previous step through a DEAE-Sephadex A-25 ion column, and eluting the filtrate with a NaCl solution, thus obtaining eluent; (5) performing drying: concentrating the eluent, dialyzing the eluent to remove salt, and performing vacuum drying to obtain a finished product.
Owner:桂林华诺威生物科技有限公司
Preparation method of mono-sulfonated Lewis trisaccharide
PendingCN107522756AGenerate efficientlyEasy to prepareSugar derivativesOligosaccharidesDEAE SephadexHydrogen
The invention discloses a preparation method of mono-sulfonated Lewis trisaccharide. The preparation method of the mono-sulfonated Lewis trisaccharide comprises the following steps: (1) dissolving 300-350 mg of 8-p-methoxy-benzeneoctanoxy 2-acetamido 3-O-(b-D-galactopyranoside) 4-O-(2, 3, 4-tri-O-benzyl a-L-fucopyranoside) 2-deoxidation-b-D-glucopyranoside 0.3 mmol / L in 3-5 mL of anhydrous pyridine, then adding 85-100 mg of a sulfur trioxide pyridine complex 0.6 mmol / L, mixing and stirring for 14-15.5 hours at the temperature of 78-82 DEG C, adding methylbenzene, and evaporating a cosolvent to obtain original residues; (2) dissolving the original residues in 4-5 mL of a mixed solvent of methanol and acid, adding a palladium or carbon catalyst, stirring for 25-32 hours at the room temperature under the condition that hydrogen exists, and removing the catalyst and the mixed solvent to obtain residues; (3) placing the residues in DEAE-Sephadex A-50 columns, removing sulfated saccharide by elution with water, and eluting a mono-sulfonatedsaccharide component and a sulfated saccharide component by using a NaCl solution with the molar concentration of 5% and a NaCl solution with the molar concentration of 15% separately; and (4) loading residues obtained after a mono-sulfonated fraction is desolventized on C-18 columns, eluting the residues with water and then washing the residues with methanol to obtain the mono-sulfonated Lewis trisaccharide. The preparation method is the most effective method for generating sulfonic acid oligosaccharideuntil now.
Owner:FOSHAN UNIVERSITY
Trimeresurus albolabris defibrase preparation method
InactiveCN100584944CHigh activityPromote hydrolysisHydrolasesPeptide/protein ingredientsThrombusIon exchange
The invention relates to fibrinolytic enzyme of trimeresurus albolabris venom, and the preparation method and the application thereof in pharmaceutical industry, and belongs to the biomedical field. Trimeresurus albolabris venom fibrinolytic enzyme is metal protease obtained in the venom of the domestic trimeresurus albolabris through separation and purification, has the activity of simultaneously degrading fibrinogen Aalpha chain and fibrinogen Bbeta chain, and is 66300 dalton under the reducing and non-reducing conditions of the apparent molecular weight on polyacrylamide gel electrophoresis. The preparation method comprises the following steps: firstly, pretreatment of trimeresurus albolabris venom; secondly, DEAE-Sephadex A-25 ion-exchange chromatography; thirdly, DEAE-Sephadex G-100 gel chromatography; fourthly, CM-Sephadex C-25 cation-exchange chromatography. The fibrinolytic enzyme of the trimeresurus albolabris venom has higher activity of simultaneously degrading fibrinogen Aalpha chain and fibrinogen Bbeta chain, and can be applied for curing thromboembolic disease.
Owner:CHONGQING NORMAL UNIVERSITY
C1 esterase inhibitor and preparation method thereof
PendingCN114835801AHigh outputPlasma Resource ConservationPeptide preparation methodsProtease inhibitorsDEAE SephadexIon exchange
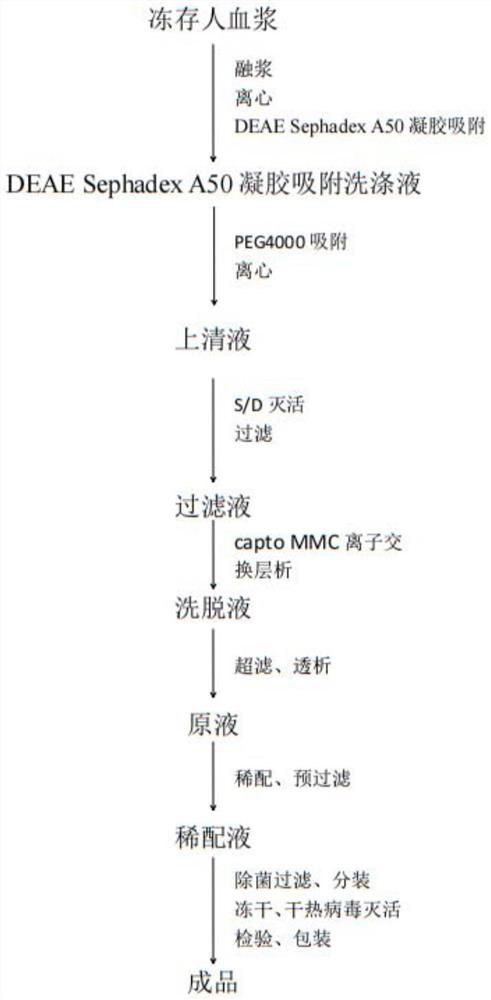
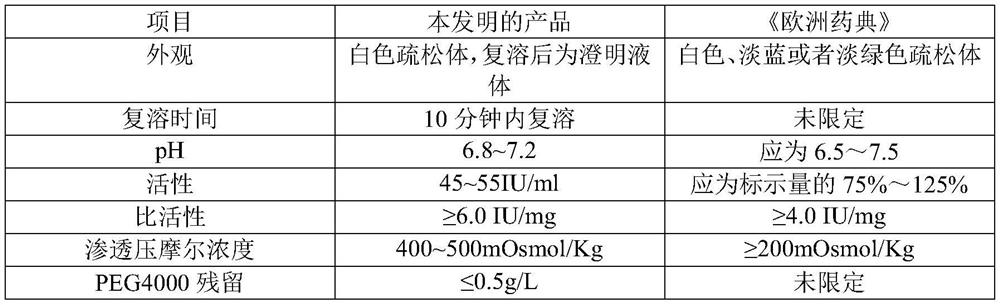
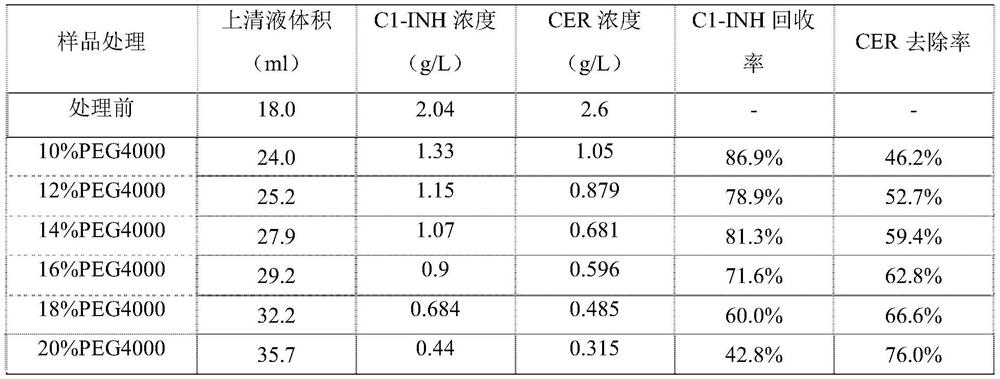
The invention relates to a C1 esterase inhibitor and a preparation method thereof, and belongs to the field of biological pharmacy. The C1 esterase inhibitor is prepared from a washing solution which flows out of a chromatographic column in a DEAE Sephadex A50 gel adsorption process and is a waste material obtained by preparing a human prothrombin complex from plasma, and is purified by a PEG precipitation method and refined by capto MMC ion exchange chromatography, and the specific activity of a final finished product is higher than 6IU / mg.
Owner:华润博雅生物制药集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com