Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117 results about "Thromboembolic disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ANSWER. Chronic Thromboembolic Disease: In rare cases, a blood clot to the lungs (pulmonary embolism) is never reabsorbed by the body. Instead, a reaction occurs in which multiple small blood vessels in the lungs also become diseased. The process occurs slowly, and slowly affects a large part of the pulmonary arterial system.
Thrombolysis and chronic anticoagulation therapy
InactiveUS7308303B2Sufficient amountAvoid the needElectrocardiographyMedical devicesDiseaseLife quality
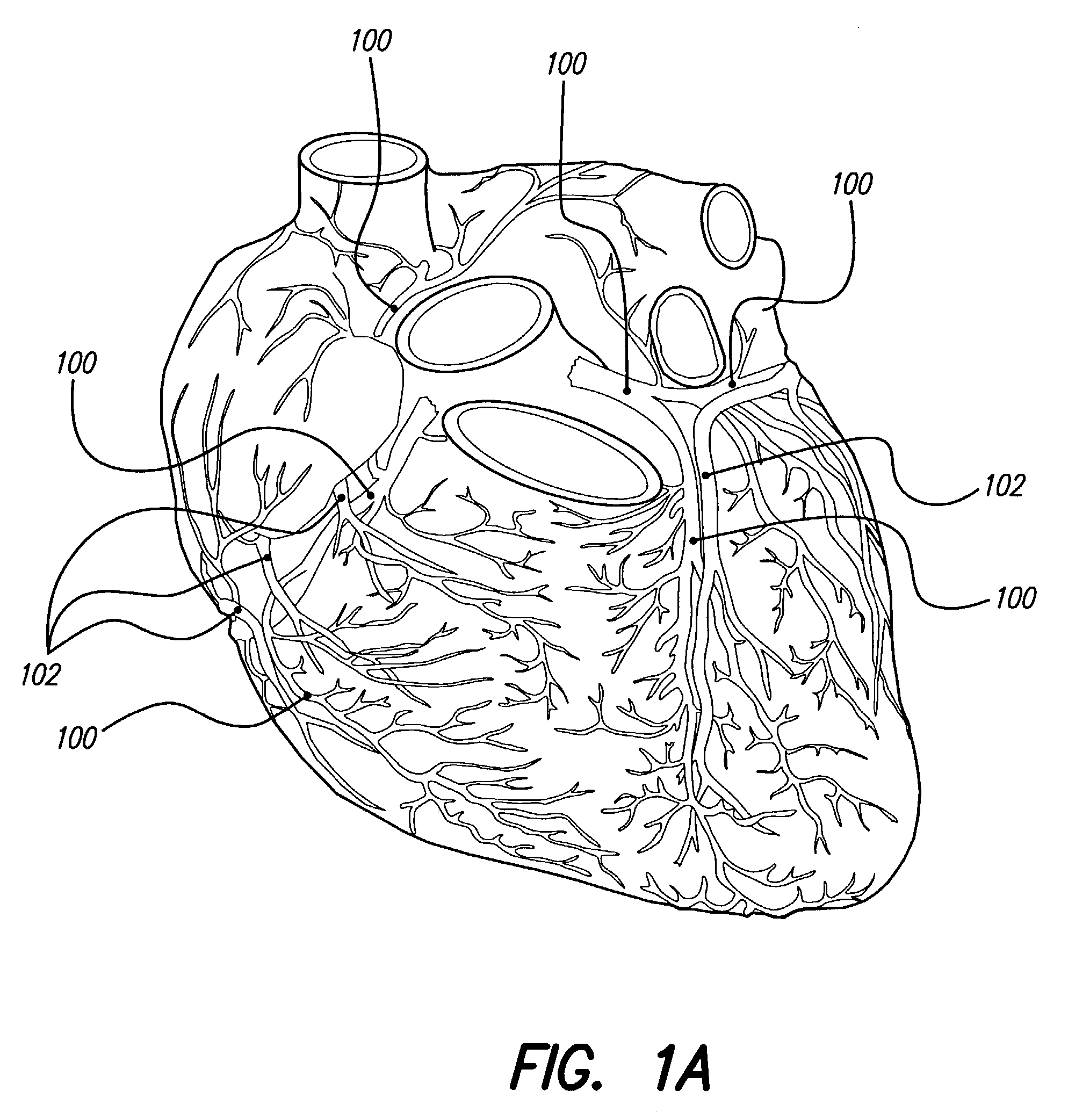
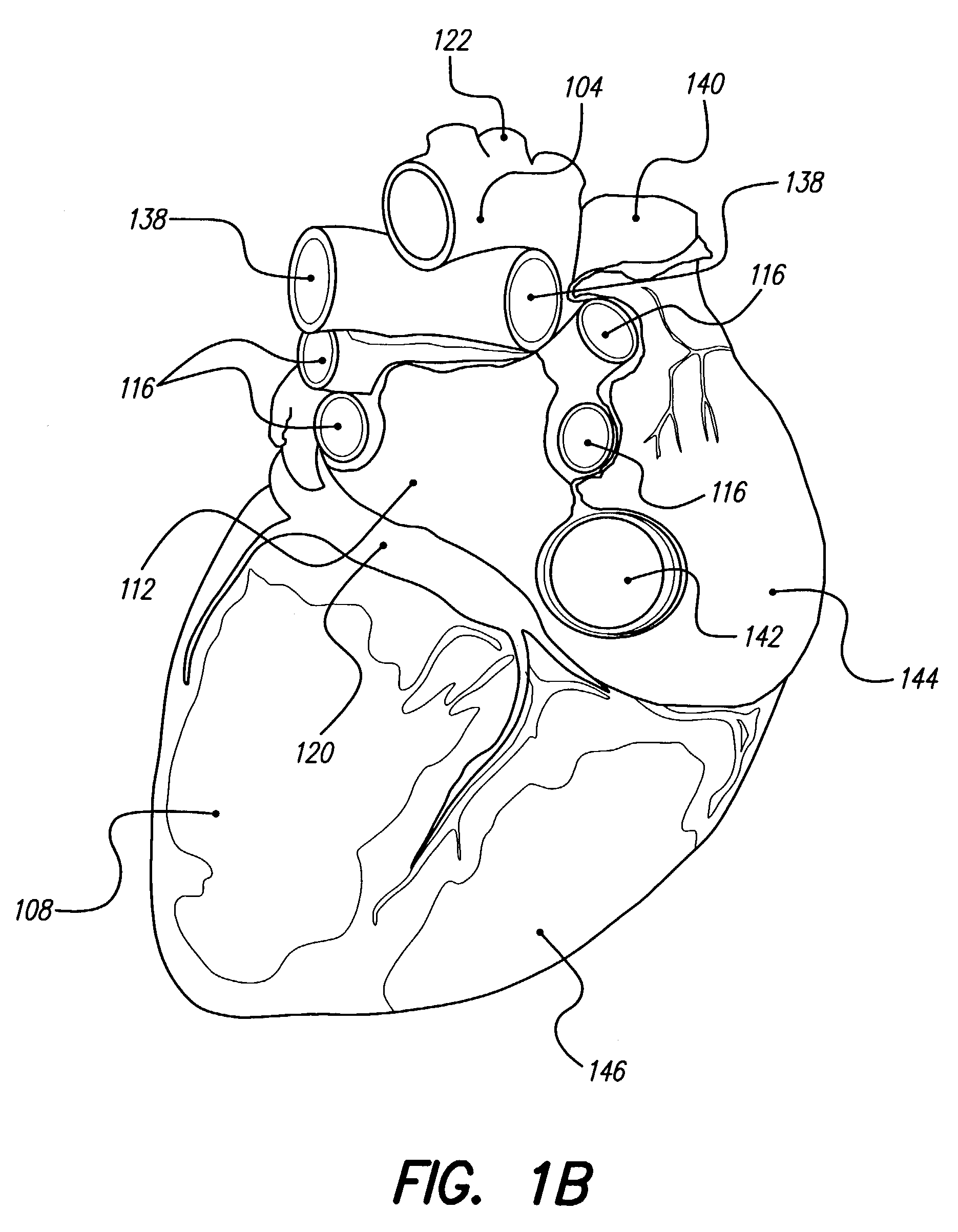
Thrombolytic and / or anticoagulation therapy of the present invention includes implantation of the discharge portion(s) of a catheter and, optionally, one or more electrodes on a lead, adjacent tissue(s) to be stimulated. Stimulation pulses, i.e., drug infusion pulses and optional electrical pulses, are supplied by a stimulator implanted remotely, and through the catheter or lead, which is tunneled subcutaneously between the stimulator and stimulation site. Stimulation sites include the coronary arteries, coronary veins, cerebral arteries, other blood vessels, chambers of the heart, mesenteric vessels, deep vessels of the leg, and other locations. Disclosed treatments include drugs used for chronic treatment and / or prevention of thromboembolic disease, for acute treatment of thromboembolic disease, for acute treatment of thrombosis, and combinations of these. The invention reduces or eliminates the incidence of thromboembolic disease and related morbidities, improve symptoms resulting from thromboembolic disease, and improve patient quality of life.
Owner:BOSTON SCI NEUROMODULATION CORP
Use of gamma-tocopherol and its oxidative metabolite LLU-alpha in the treatment of disease
InactiveUS6242479B1Suppress high blood pressureReduced immune system responseBiocideSenses disorderScavengerNitrogen oxides
The present invention is generally related to the discovery of the therapeutic benefit of administering gamma-tocopherol and gamma-tocopherol derivatives. More specifically, the use of gamma-tocopherol and racemic LLU-alpha, (S)-LLU-alpha, or gamma-tocopherol derivatives as antioxidants and nitrogen oxide scavengers which treat and prevent high blood pressure, thromboembolic disease, cardiovascular disease, cancer, natriuretic disease, the formation of neuropathological lesions, and a reduced immune system response are disclosed.
Owner:LOMA LINDA UNIV MEDICAL CENT
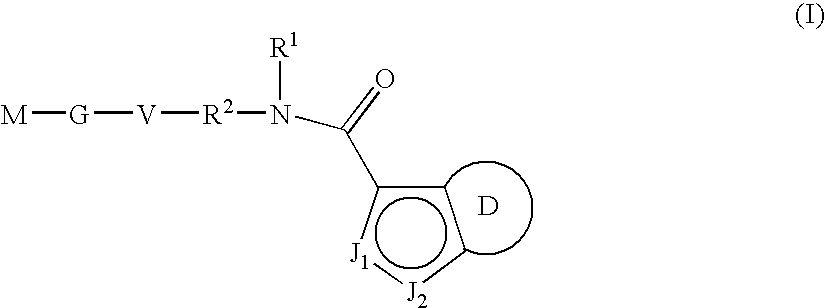
Azaindole-derivatives as factor Xa inhibitors
The present invention relates to compounds of the formula Iwherein R0, R1, R2, R3, Q, V, G and M are as defined herein. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUT GMBH
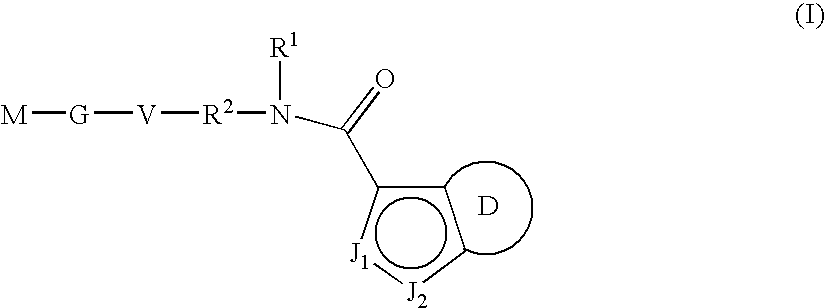
Indazole-derivatives as factor Xa inhibitors
The present invention relates to a compound of the formula Iwherein J1, J2, R0, R1, R2, Q, V, G and M are as defined herein. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUT GMBH
Use of dipyridamole in combination with antithrombotics for treatment and prevention of thromboembolic diseases
InactiveUS20090075949A1Good coagulationEnhancing thrombin formationBiocideSalicyclic acid active ingredientsAntithrombotic AgentDipyridamole
The invention relates to a method of treating and preventing thromboembolic disorders, comprising administering dipyridamole in combination with an antithrombotic selected from direct thrombin inhibitors, factor Xa inhibitors and combined thrombin / factor Xa inhibitors to a patient, pharmaceutical compositions suitable for this method of treatment as well as the use of dipyridamole for the manufacture of these pharmaceutical compositions.
Owner:EISERT WOLFGANG
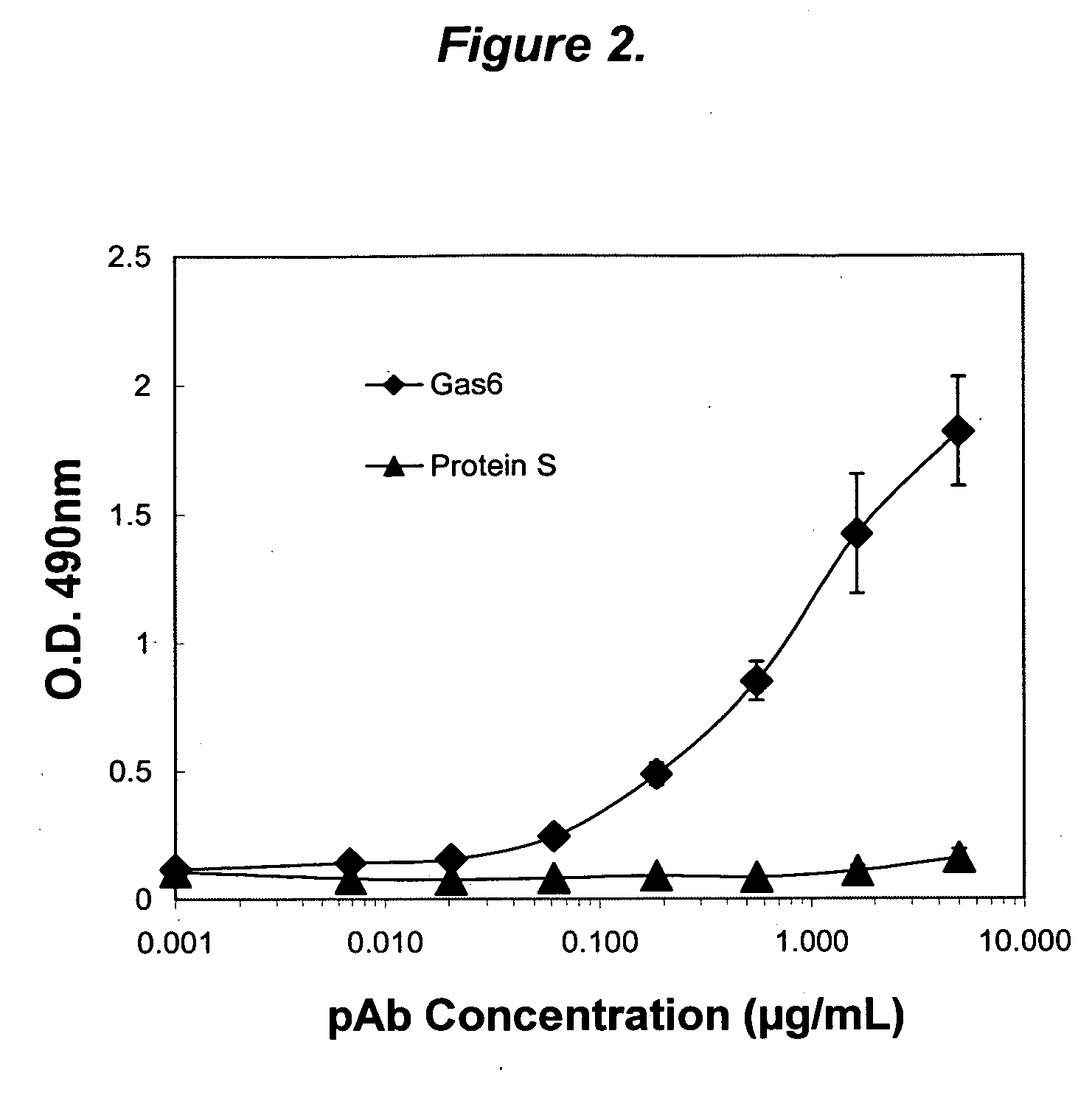
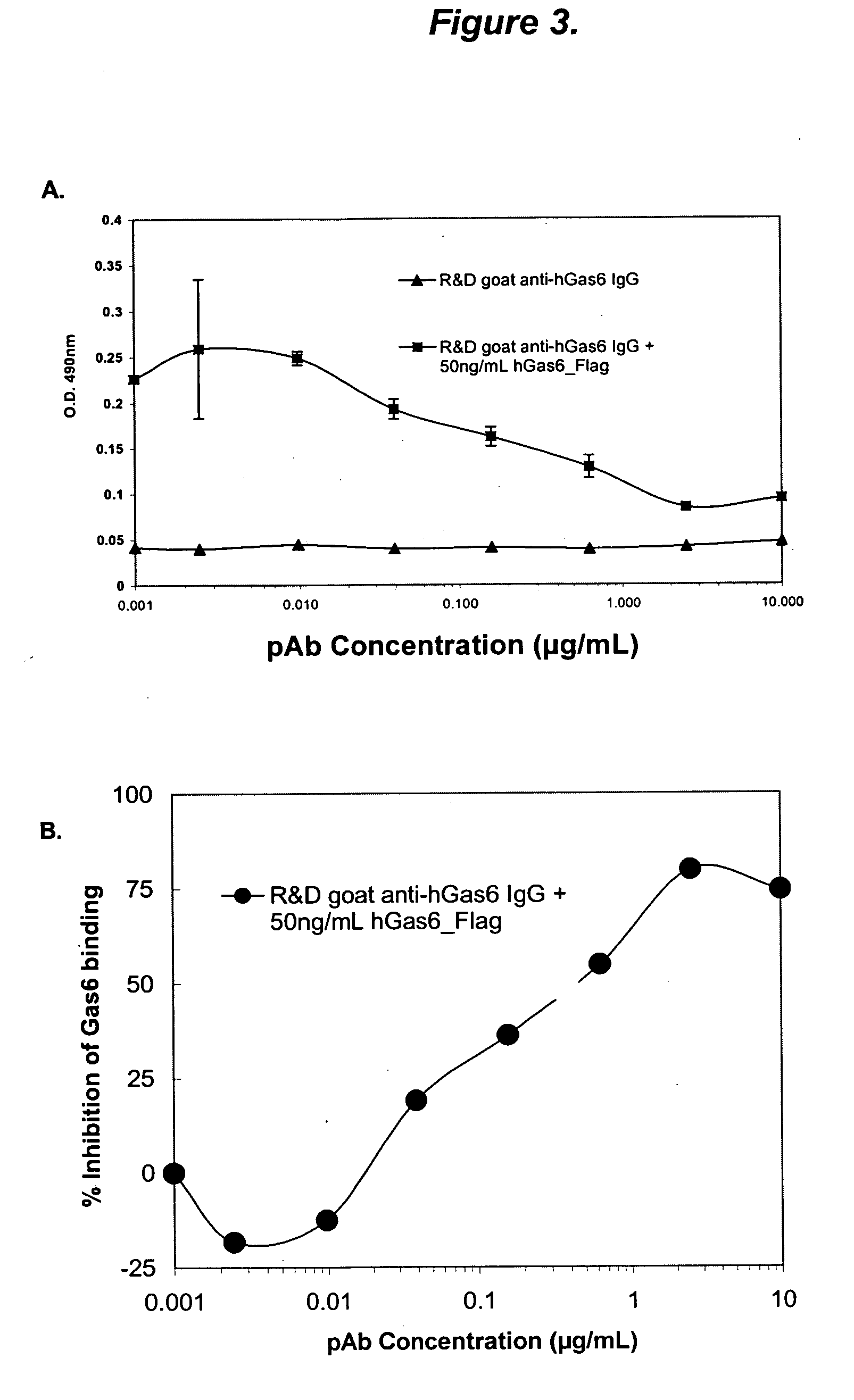
Growth arrest specific gene 6 peptides, antibodies, compositions, methods and uses
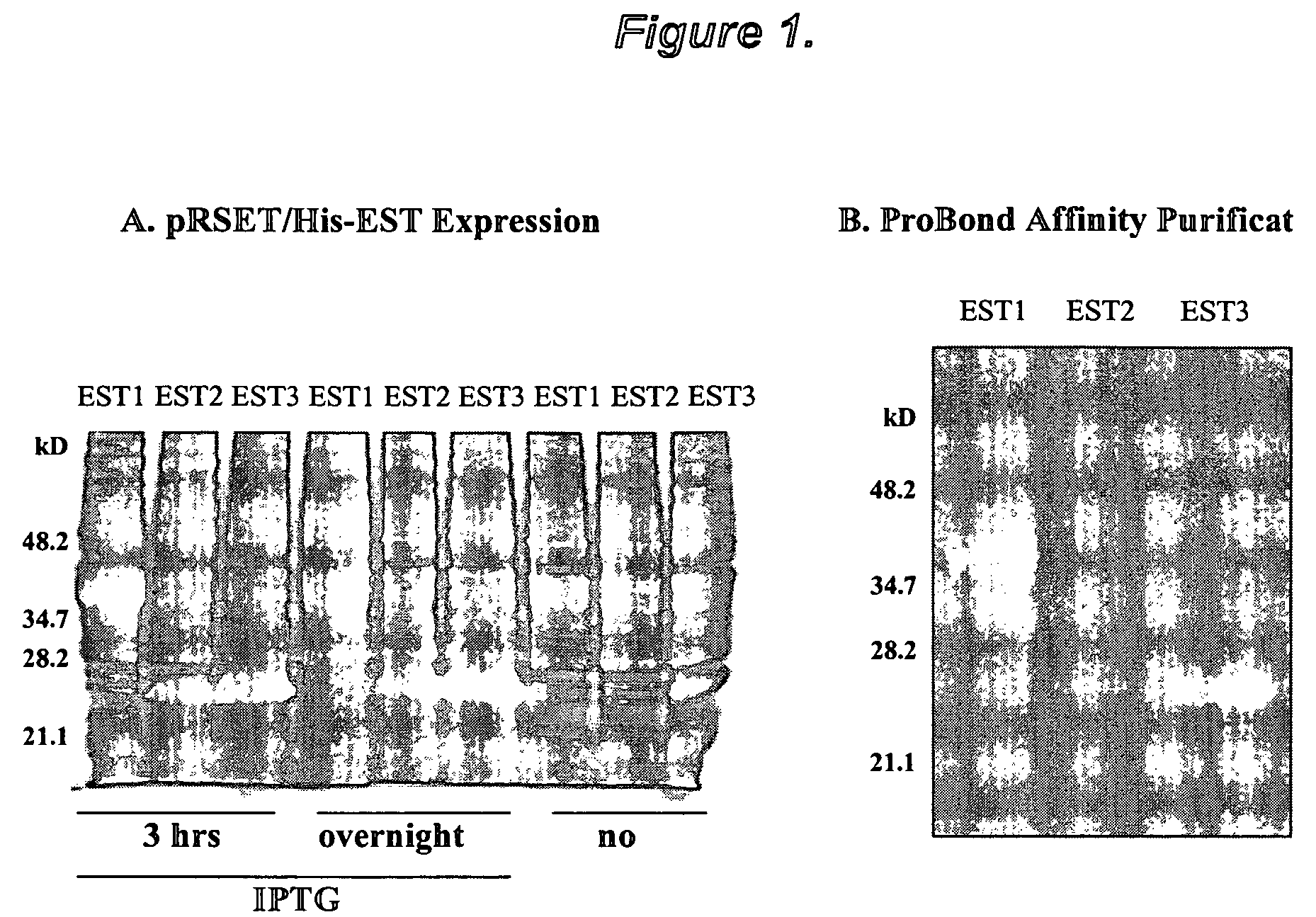
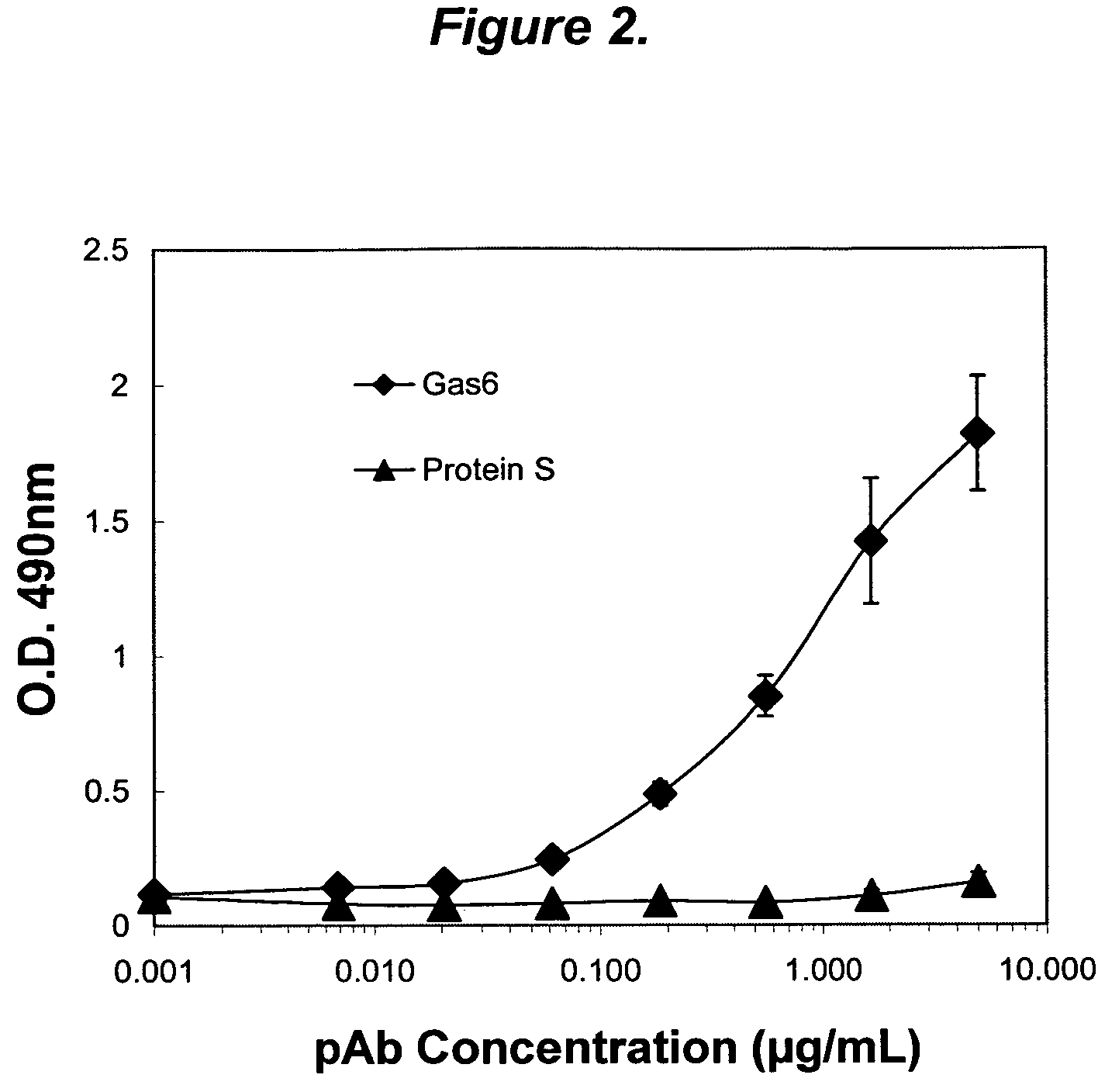
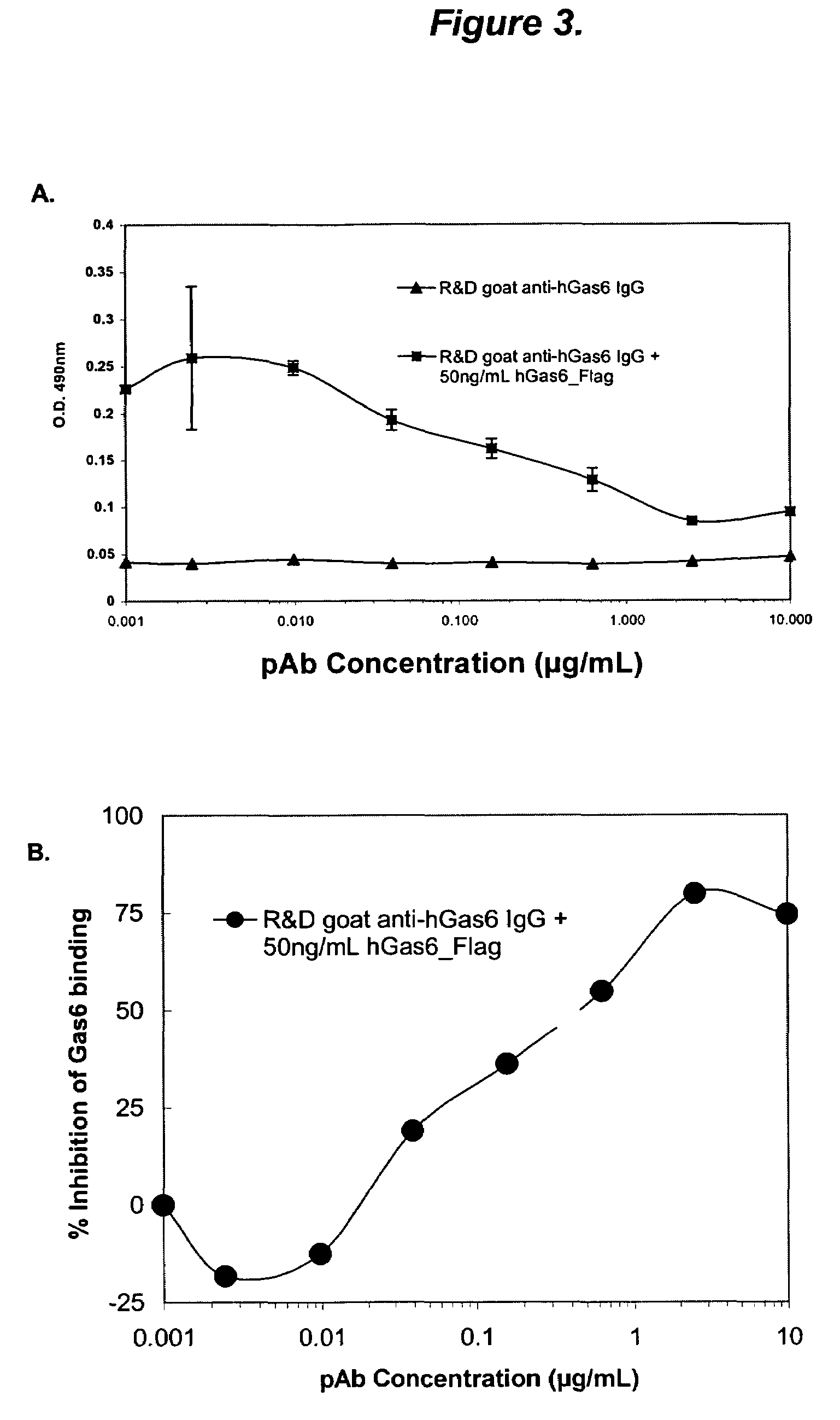
The present invention provides novel proteins and peptides from the receptor binding region of human Growth Arrest Specific Gene 6 (Gas6) and antibodies, including specified portions or variants, specific for at least one such Gas6 peptide or fragment thereof. The aforesaid peptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-Gas6 antibodies. The invention also provides for the nucleic acids encoding such peptides and anti-Gas6 antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, including therapeutic formulations, administration and devices. Fifteen novel peptide sequences from the Gas6 G domain that are implicated in Gas6 interactions with its receptors are identified, isolated, and synthesized so as to allow generation of anti-Gas6 antibodies. The peptide sequences include three ESTs that encompass regions predicted to contribute to receptor binding or that can raise anti-Gas6 antibodies. This invention provides for such antibodies to be used in modulating or treating at least one Gas6-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, thromboembolic disease, ischemic disease, venous thromboembolism, arterial or venous thrombosis, pulmonary embolism, restenosis, diabetic angiopathy and allograft atherosclerosis.
Owner:CENTOCOR ORTHO BIOTECH
Growth arrest specific gene 6 peptides, antibodies, compositions, methods and uses
The present invention provides novel proteins and peptides from the receptor binding region of human Growth Arrest Specific Gene 6 (Gas6) and antibodies, including specified portions or variants, specific for at least one such Gas6 peptide or fragment thereof. The aforesaid peptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-Gas6 antibodies. The invention also provides for the nucleic acids encoding such peptides and anti-Gas6 antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, including therapeutic formulations, administration and devices. Fifteen novel peptide sequences from the Gas6 G domain that are implicated in Gas6 interactions with its receptors are identified, isolated, and synthesized so as to allow generation of anti-Gas6 antibodies. The peptide sequences include three ESTs that encompass regions predicted to contribute to receptor binding or that can raise anti-Gas6 antibodies. This invention provides for such antibodies to be used in modulating or treating at least one Gas6-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, thromboembolic disease, ischemic disease, venous thromboembolism, arterial or venous thrombosis, pulmonary embolism, restenosis, diabetic angiopathy and allograft atherosclerosis.
Owner:CENTOCOR ORTHO BIOTECH
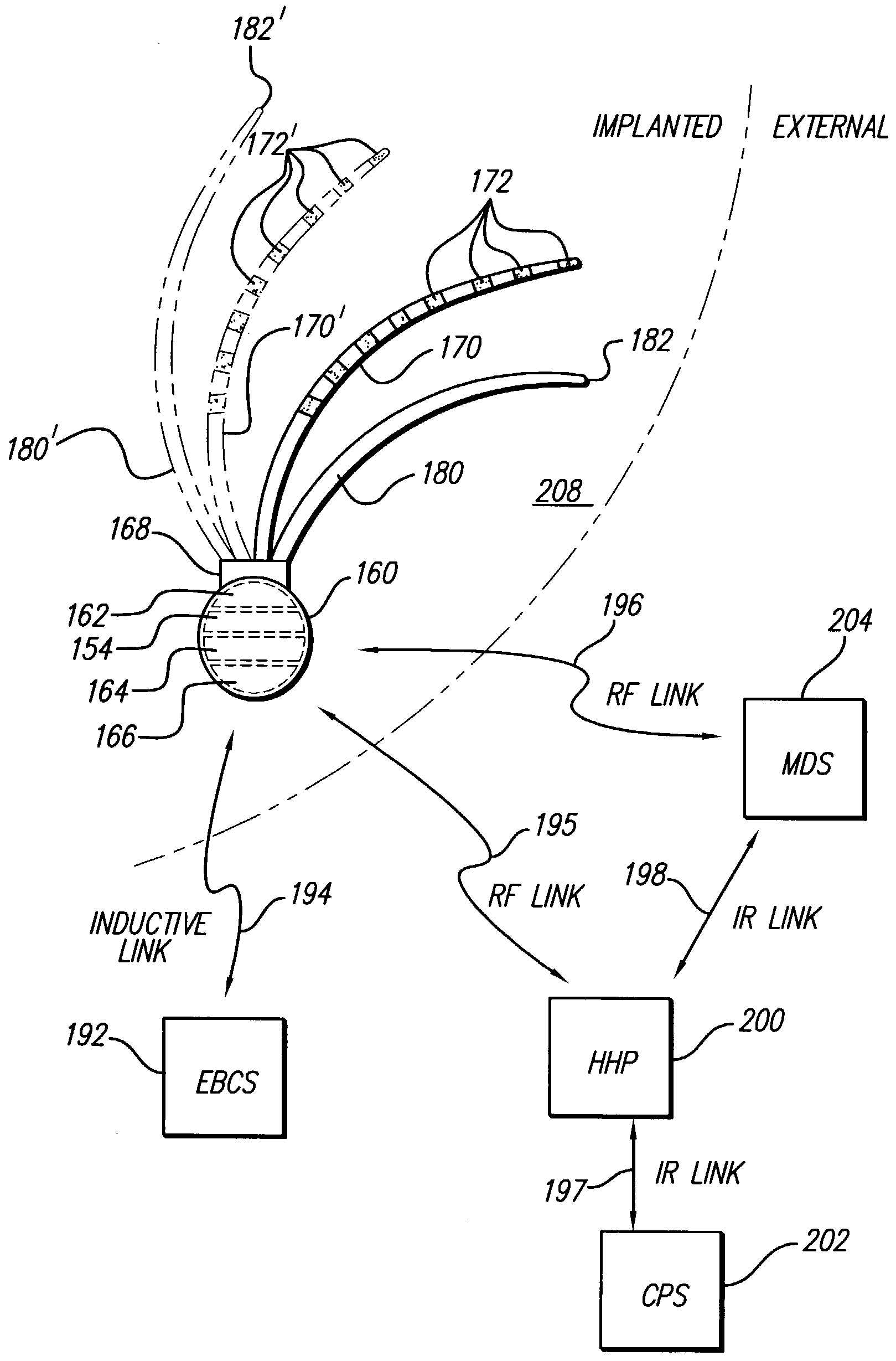
Catheter system for treating thromboembolic disease
ActiveUS20210315596A1Improve responseFacilitate flushing the catheter and/or dilute the contrast agentMulti-lumen catheterGuide wiresVeinThrombus
A vacuum aspiration system may be used to treat thromboembolic disease, such as deep vein thrombosis or pulmonary embolism. The system includes a housing, and a fluid flow path extending through the housing. A first catheter is in fluid communication with the flow path, and a connector is configured to place a source of aspiration in communication with the flow path. A clot container is carried by the housing. A hemostasis valve is provided in the housing, and configured to receive a second catheter and direct the second catheter through the first catheter.
Owner:IMPERATIVE CARE INC
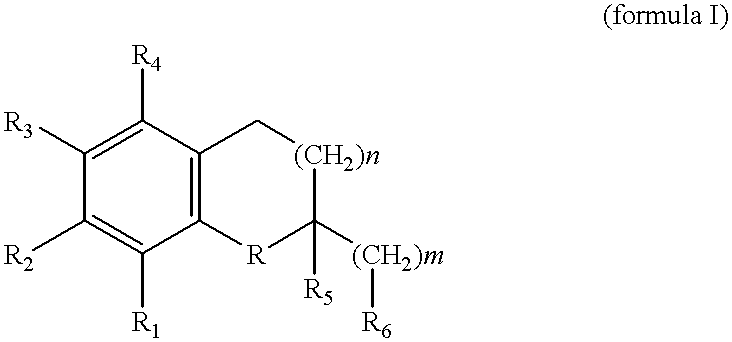
Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof
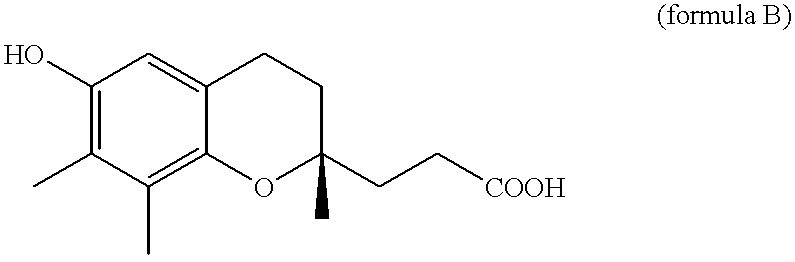
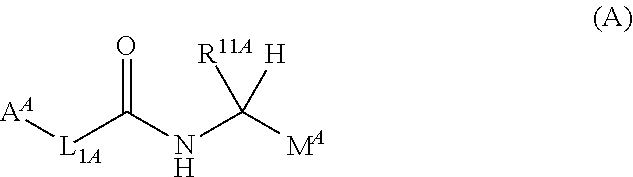
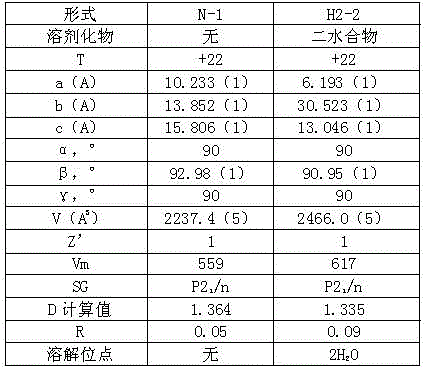
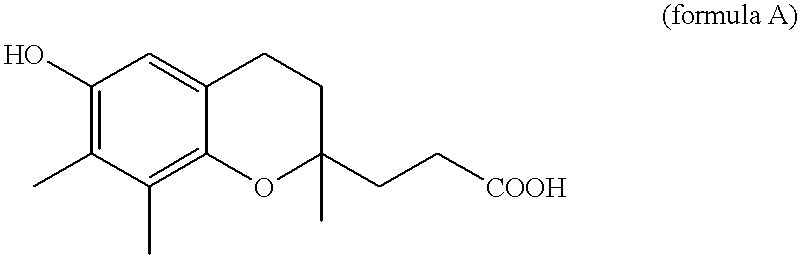
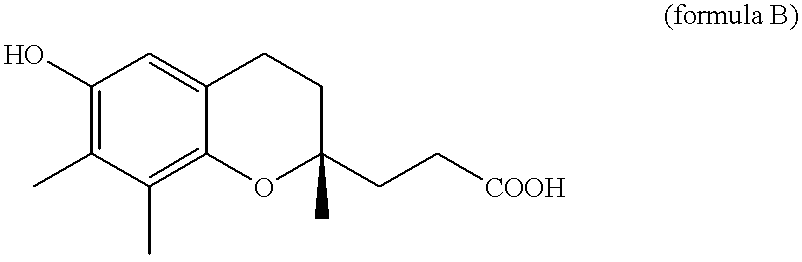
Belonging to the technical field of medicine, the invention relates to a 4, 5-dihydro-1H-pyrazolo[3, 4-c]pyridine-7-one containing derivative shown as general formula I, and pharmaceutically acceptable salt, hydrate or prodrug thereof, wherein the substituents A, R1 and R2 have meanings given in the specification. The preparation also relates to a preparation method of the general formula I compound and its pharmaceutically acceptable salt or prodrug, medicinal compositions containing the compound and application of the compound as an Xa factor inhibitor, especially application in preparation of drugs for treatment and / or prevention of thromboembolic diseases. (formula I).
Owner:SHENYANG PHARMA UNIVERSITY
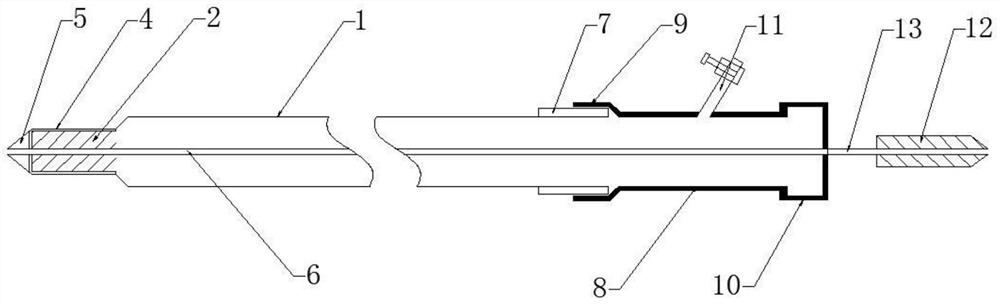
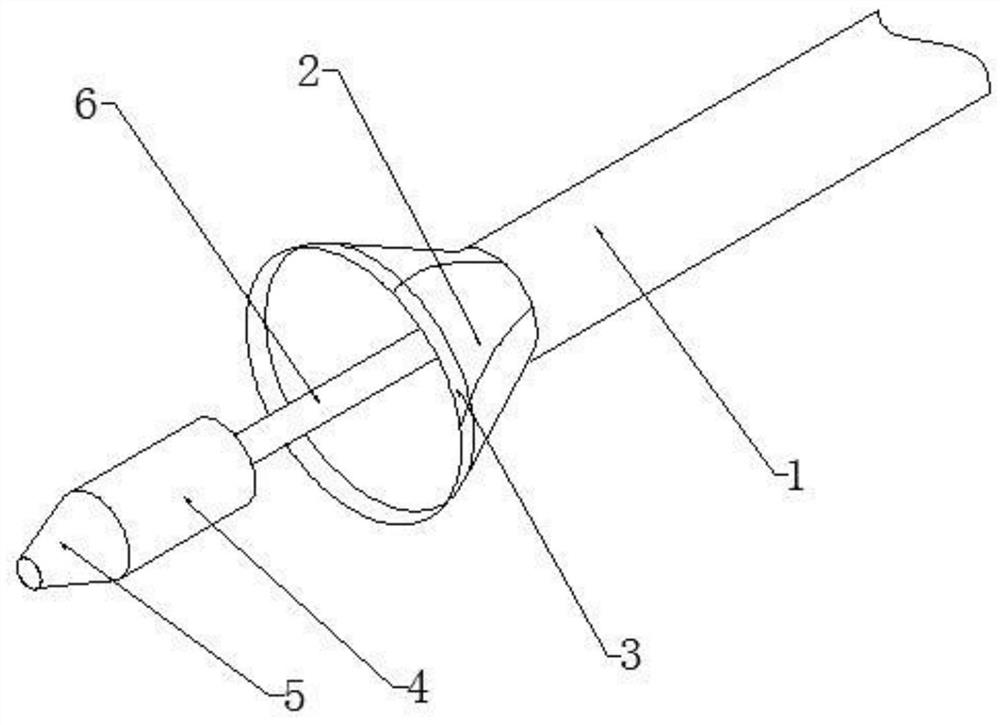
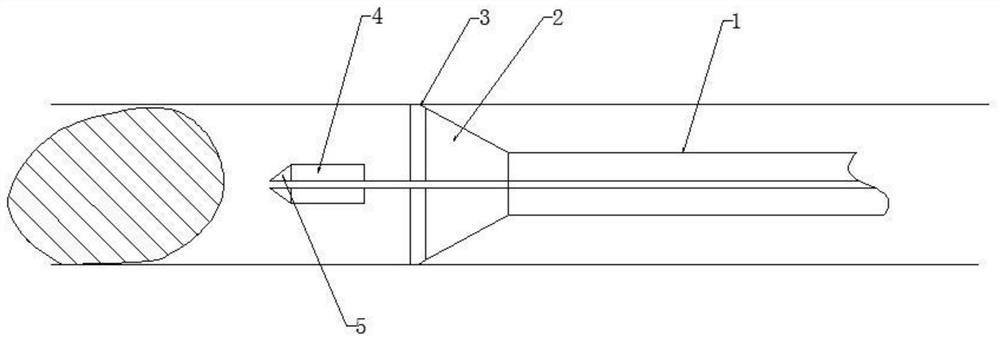
Thrombus removing sleeve and thrombus removing balloon catheter assembling kit
The invention discloses a thrombus removing sleeve and a thrombus removing balloon catheter assembling kit for treating thromboembolic diseases in a percutaneous puncture cavity. The thrombus removingsleeve comprises a thrombus removing passage catheter with a thrombus leading-in umbrella and an umbrella cover releasing assembly, wherein the umbrella cover releasing assembly is used for controlling the folding compression or release of the thrombus leading-in umbrella. A thrombus removing balloon catheter is a single-cavity balloon catheter, and is matched with a guide wire through a side wall guide wire hole of a guide section, so that the cross section area of the thrombus removing balloon catheter is reduced; the thrombus removing balloon catheter is matched with the thrombus removingsleeve, so that thrombus in arteries and veins can be effectively and rapidly removed; and the thrombus removing sleeve is particularly suitable for use under the condition of high-load thrombus embolism of the arteries and veins of limbs, so that a patient is benefited.
Owner:华俊 +1
System and method for transdermal delivery of an anticoagulant
InactiveUS20050273047A1Precise dose controlOrganic active ingredientsElectrotherapyMedicineBenzamidine
A device for transdermally delivering an anticoagulant agent by electrotransport. Preferably, the anticoagulant comprises a benzamidine or a naphthamidine derivative. A particularly preferred benzamidine derivative is a 2-[3-[4-(4-piperidinyloxy)anilino]-1-propenyl]benzamidine derivative. The devices are configured to maintain a plasma concentration of 20-80 ng / mL or providing a flux in the range of approximately 20-40 mg / day. Suitable current densities include 0.050 and 0.10 mA / cm2. Methods of the invention include delivering the anticoagulants to precisely maintain the desired plasma concentrations. The invention also comprises treating thromboembolic disease and inhibiting Factor Xa.
Owner:ALZA CORP
Gas micro-liposome compound
Formulations comprising a gas microsphere liposome composite suspended in a medium, wherein the gas mcirosphere liposome composite comprises: a gas-filled microsphere; at least one of a lipid and a surfactant adsorbed onto the surface of the gas-filled microsphere; and liquid-filled liposomes attached to the lipid or surfactant are described. Methods of preparing the same and using them in ultrasound imaging are also described. The present invention also comprises used of the same in treating heart disease, inflammation, infection, cancer or thromboembolic disease in a patient.
Owner:BRISTOL MYERS SQUIBB PHARMA CO
Use of gamma-tocopherol and its oxidative metabolite LLU-alpha in the treatment of disease
The present invention is generally related to the discovery of the therapeutic benefit of administering gamma-tocopherol and gamma-tocopherol derivatives. More specifically, the use of gamma-tocopherol and racemic LLU-alpha, (S)-LLU-alpha, or gamma-tocopherol derivatives as antioxidants and nitrogen oxide scavengers which treat and prevent high blood pressure, thromboembolic disease, cardiovascular disease, cancer, natriuretic disease, the formation of neuropathological lesions, and a reduced immune system response are disclosed.
Owner:LOMA LINDA UNIV MEDICAL CENT
Remediation of functional cardiac mitral valve regurgitation
Owner:COLORADO STATE UNIVERSITY
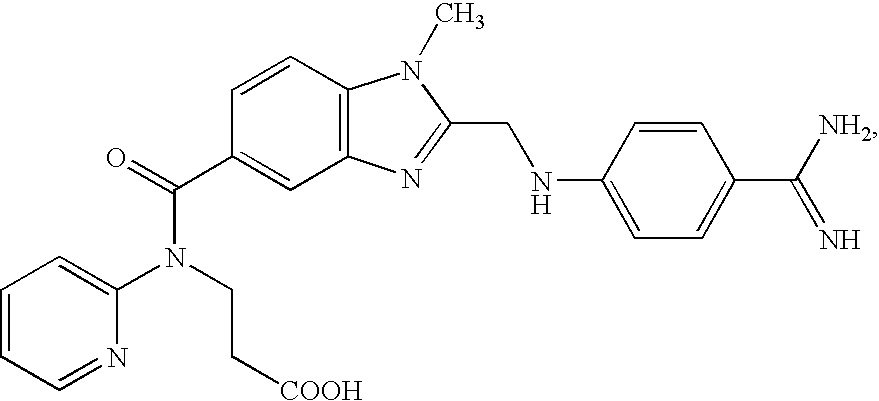
Chlorothiophene-amides as inhibitors of coagulation factors xa and thrombin
The present invention relates to compounds of the formula I,wherein R1; R2; R3; R4; R5, R13, R16, X and M have the meanings indicated in the claims. The compounds of formula I are valuable pharmacologically active compounds. They exhibit a strong anti-thrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and thrombin and can in general be applied in conditions in which an undesired activity of factor Xa and / or thrombin are present or for the cure or prevention of which an inhibition of factor Xa and thrombin are intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:BEIJING LIANXIN PHARMA CO LTD
Peptides derived from plasminogen activator inhibitor-1 and uses thereof
InactiveUS20100215636A1Higher neuroprotective activityReduce mortalityNervous disorderPeptide/protein ingredientsPlasminogen activator inhibitor-1Peptide
The present invention relates to isolated 18-mer peptides corresponding to amino acid residues 369-386 of human plasminogen activator inhibitor 1 (PAI-1) and fragments thereof, compositions that include such peptides, and uses of such compositions for treating thromboembolic diseases and pathological conditions associated with neurological damage.
Owner:D PHARMA LTD
Kinase inhibitor and method for treatment of related diseases
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +1
Chelants and macrocyclic metal complex radiopharmaceuticals thereof
InactiveUS20050010038A1Group 5/15 element organic compoundsRadioactive preparation carriersImaging agentEnzyme inhibitor
Chelants and macrocyclic metal complexes thereof, methods of preparing the chelants and macrocyclic metal complexes, and radiopharmaceutical compositions comprising the macrocyclic metal complexes are disclosed. Methods of using the macrocyclic metal complexes as radiopharmaceuticals for the diagnosis of cardiovascular disorders, infectious diseases and cancer are also disclosed. Chelants as bifunctional chelators (BFCs) for the radiolabeling of target-specific biomolecules, such as proteins, peptides, peptidomimetics, non-peptide receptor ligands, enzyme inhibitors, and enzyme substrates are disclosed. Methods of using macrocyclic metal complexes containing the chelant-biomolecule conjugates as target-specific diagnostic radiopharmaceuticals that selectively localize at sites of disease and allow an image to be obtained of the loci using gamma scintigraphy are disclosed. Methods of use of the radiopharmaceuticals as imaging agents for the diagnosis of cardiovascular disorders, such as thromboembolic disease or atherosclerosis, infectious disease and cancer are further disclosed.
Owner:LANTHEUS MEDICAL IMAGING INC
Unsaturated Fatty Acids as Thrombin Inhibitors
InactiveUS20090234007A1Positive inotropic effectInhibiting thrombinBiocideOrganic chemistryChain lengthTriglyceride
A preparation having at least one unsaturated fatty acid and the use of at least one unsaturated fatty acid having a chain length of 18 or 20 carbon atoms and of plant drugs containing the unsaturated fatty acid as a free fatty acid or a fatty acid radical of a triglyceride. The fatty acid has a chain length of 18 carbon atoms which are provided with 1 to 3 double bonds and the fatty acid has a chain length of 20 carbon atoms which are provided with 1 to 4 double bonds. The double bond or one of the double bonds are located in position 9 or 11 of the carbon chain. The unsaturated fatty acid is supplied in the all-cis configuration. The at least one unsaturated fatty acid prevents and / or treats or is used for producing a preparation for preventing and / or treating thrombosis and thromboembolic diseases.
Owner:LTS LOHMANN THERAPIE-SYST AG
Substituted pyrrolidines as factor xia inhibitors for the treatment thromboembolic diseases
ActiveUS20150152048A1Effective treatmentEnhanced inhibitory effectAntibacterial agentsBiocidePyrrolidineFactor XIa
The present invention provides compounds of the general formula (I), their salts and N-oxides, and solvates and prodrugs thereof (wherein the substituents are as defined in the description). The compounds of the general formula (I) are inhibitors of factor XIa, and are useful in the prevention of and / or therapy for thromboembolic diseases.
Owner:ONO PHARMA CO LTD
Apixaban composition and preparation method thereof
InactiveCN104644593AImprove solubilityPromote dissolutionOrganic active ingredientsBlood disorderMedicineThromboembolic disease
Owner:TIANJIN HANKANG PHARMA BIOTECH
Ginkgo dipyridamole composition and preparation method of preparation thereof
ActiveCN102626427AImprove stabilityImprove securityOrganic active ingredientsGinkgophyta medical ingredientsDipyridamoleCoronary heart disease
The invention discloses a novel ginkgo dipyridamole composition and a preparation method of a preparation thereof. The ginkgo dipyridamole composition mainly comprises the following ingredients by weight: 25-35% of ginkgo total flavonoid glycol glycosides, 10-15% of dipyridamole and 10-18% of ginkgo total lactones, wherein the ginkgo total lactones are composed of bilobalide, ginkgolides A, ginkgolides B, ginkgolides C and ginkgolides M according to the weight ratio of 22-30: 15-18: 5-8: 8-22: 4-8. The ginkgo dipyridamole composition is high in active ingredient content and low in volume of contained impurities of ginkgo biloba phenolic substances (such as ginkgolic acid), proteins, tannins, pyrogen materials and the like. The pharmaceutic preparation, especially injection prepared by theginkgo dipyridamole composition is suitable for preventing and treating coronary diseases, thromboembolic diseases and the like, has obvious curative effects, and is safe in clinic use.
Owner:通化谷红制药有限公司
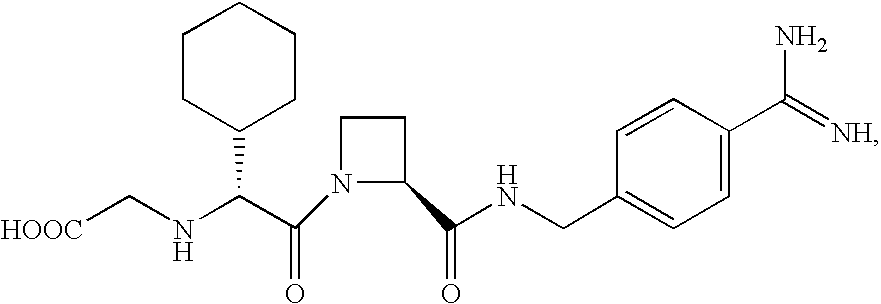
Pyrrole derivatives as p2y12 antagonists
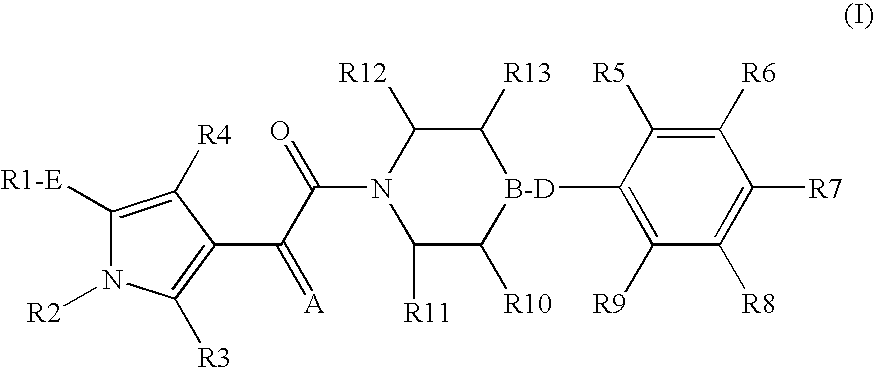
The present invention relates to compounds of the formula I,in which R1; R2; R3; R4; R5; R6; R7; R8; R9; R10; R11; R12; R13; A; B, D and E have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong anti-aggregating effect on platelets and thus an anti-thrombotic effect and are suitable e.g. for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible antagonists of the platelet ADP receptor P2Y12, and can in general be applied in conditions in which an undesired activation of the platelet ADP receptor P2Y12 is present or for the cure or prevention of which an inhibition of the platelet ADP receptor P2Y12 is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
Benzotriazine compound with PAR4 antagonistic activity and application thereof
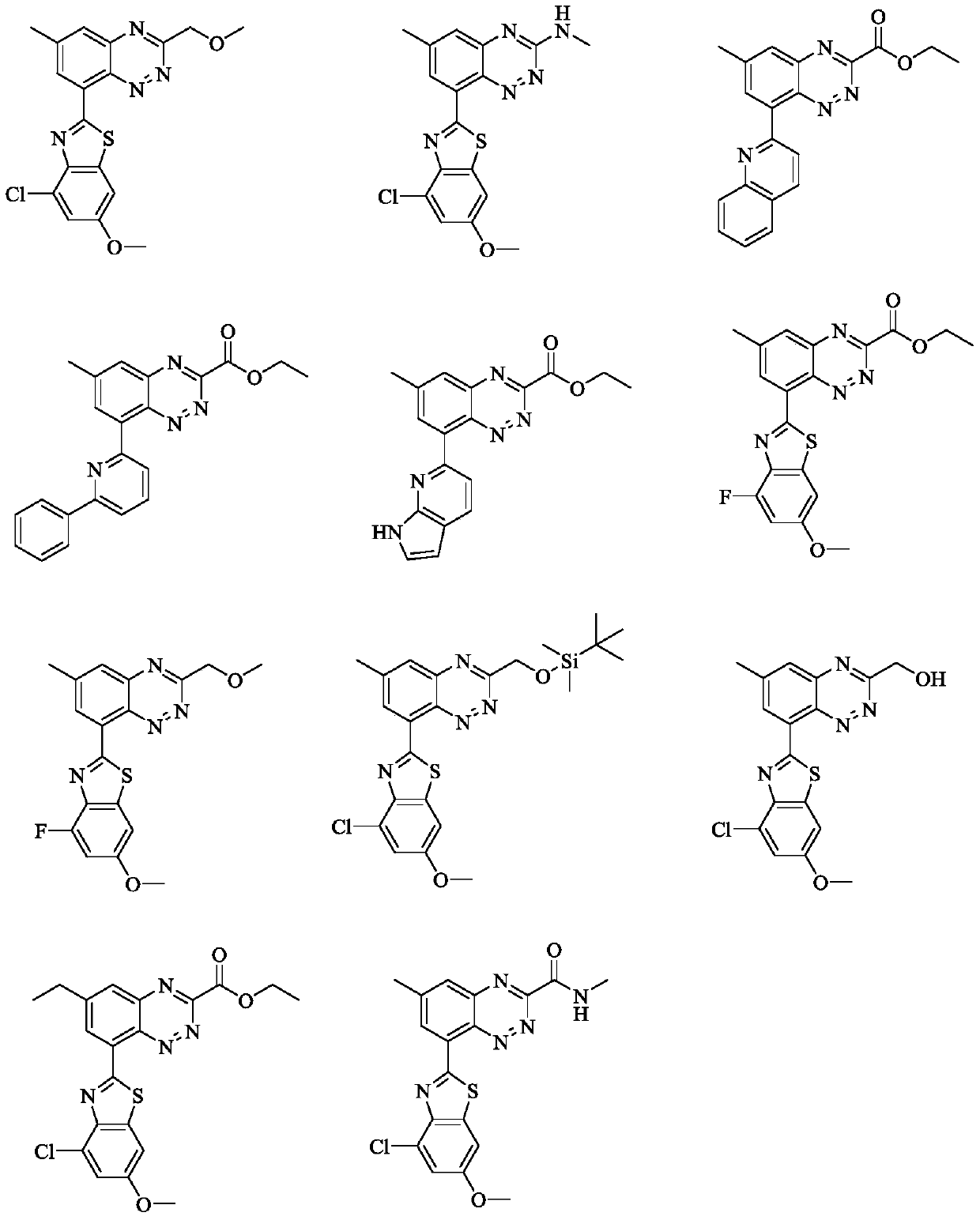
ActiveCN111440146AHigh antagonistic activityInhibits platelet aggregationSilicon organic compoundsSilicon compound active ingredientsThrombusPharmaceutical drug
The invention discloses a benzotriazine compound with PAR4 antagonistic activity and application thereof. The present invention relates to a compound of formula (I) or a stereoisomer, tautomer, pharmaceutically acceptable salt, ester, solvate or prodrug thereof, and the compound of the present invention has significant antagonistic activity on PAR4, thereby effectively inhibiting platelet aggregation, and thus can be used for the preparation of a medicament for preventing or treating a variety of thromboembolic diseases.
Owner:CHINA PHARM UNIV
Azaindole-derivatives as factor Xa inhibitors
The present invention relates to compounds of the formula I wherein R0, R1, R2, R3, Q, V, G and M are as defined herein. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders like thromboembolic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUT GMBH
Heterocyclic pyrazole-carboxamidesas P2Y12 antagonists
Owner:SANOFI SA
Pyrazole-carboxamide derivatives as P2Y12 antagonists
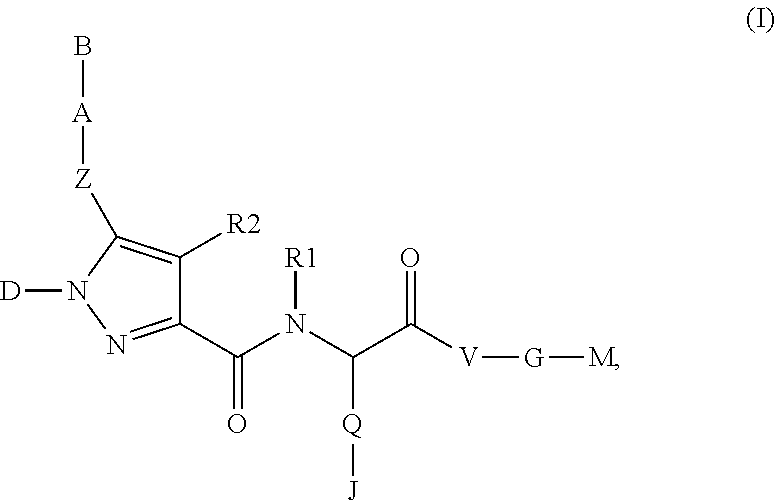
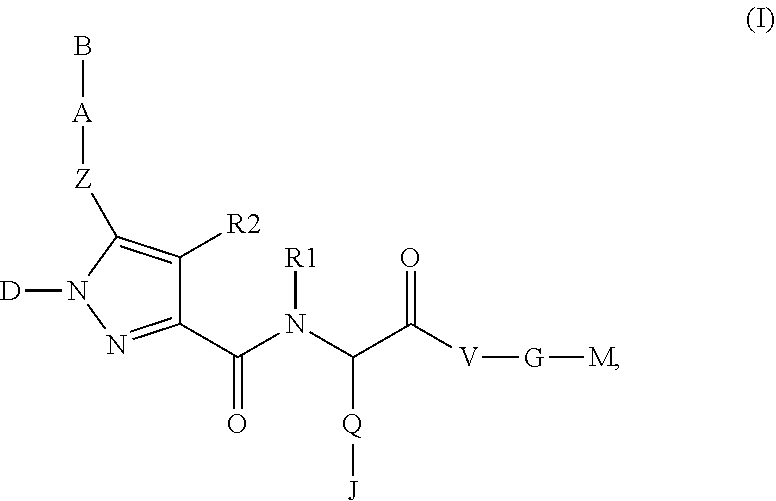
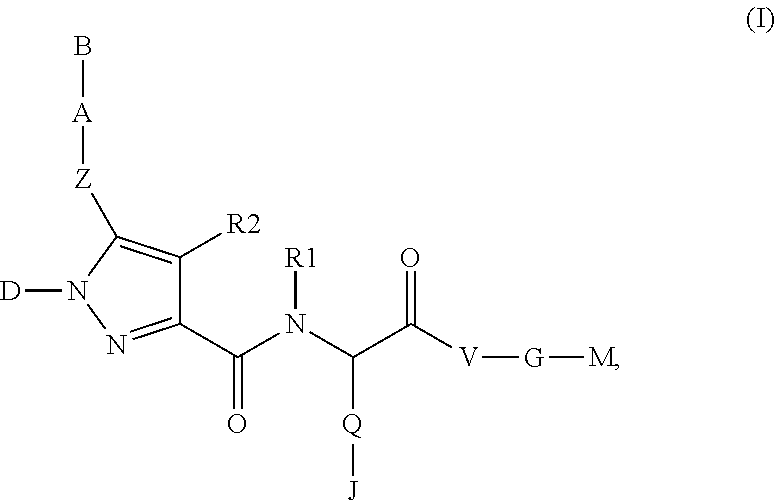
The present invention relates to compounds of the formula I,whereinR1; R2; Z; A; B; D; Q; J; V; G and M have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong anti-aggregating effect on platelets and thus an anti-thrombotic effect and are suitable, e.g., for the therapy and prophylaxis of cardio-vascular disorders like thromboembolic diseases or restenoses. They are reversible antagonists of the platelet ADP receptor P2Y12, and can in general be applied in conditions in which an undesired activation of the platelet ADP receptor P2Y12 is present or for the cure or prevention of which an inhibition of the platelet ADP receptor P2Y12 is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI SA
Peptides derived from plasminogen activator inhibitor-1 and uses thereof
InactiveUS8507436B2Reducing tPA-induced brain edemaReduce mortalityNervous disorderPeptide/protein ingredientsPlasminogen activator inhibitor-1Plasminogen Activator Inhibitors
Owner:D PHARMA LTD
Application of holothuria glycosaminoglcan in preparation of medicines for preventing and treating thromboembolism disease
InactiveCN104147040ADifferent anticoagulant activityImprove securityOrganic active ingredientsLyophilised deliveryDiseaseThrombus
The present invention discloses the use of sea cucumber glycosaminoglycan in preparing medicine; animal testing has shown that for a weight average molecular weight exceeding 54,500Da of depolymerized sea cucumber glycosaminoglycan or of one or more segments of natural molecular segments of sea cucumber glucosaminoglycan, the anti-coagulant activity thereof shows dose-dependency; in contrast to heparin and low molecular weight heparin, the dose-dependency thereof increases the incremental easing of blood coagulation, and, with an identical dose size, as weight average molecular weight increases, the onset time is delayed while the duration of efficacy is increased. For certain dosages, natural molecular segment sea cucumber glycosaminoglycan can have a duration of efficacy of up to 16 hours, and can be used for preparing medicine for preventing and treating arterial thromboembolic diseases. The present invention has a higher level of safety when compared with heparin-type drugs and vitamin K antagonist-type drugs. In clinical use, the present invention has a wide treatment window for thromboembolic disease, has a high level of safety, and has good research and development value.
Owner:SHANGHAI KAIRUN BIOLOGY MEDICINE LIMITED LIABILITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
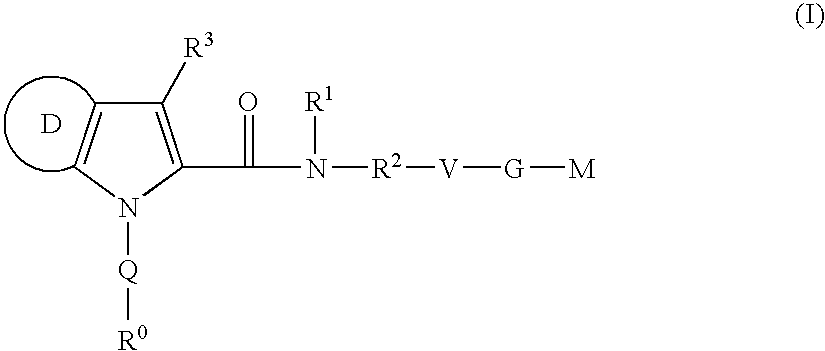
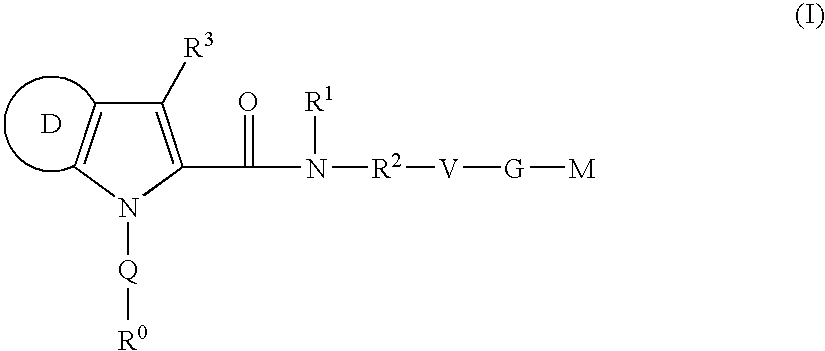
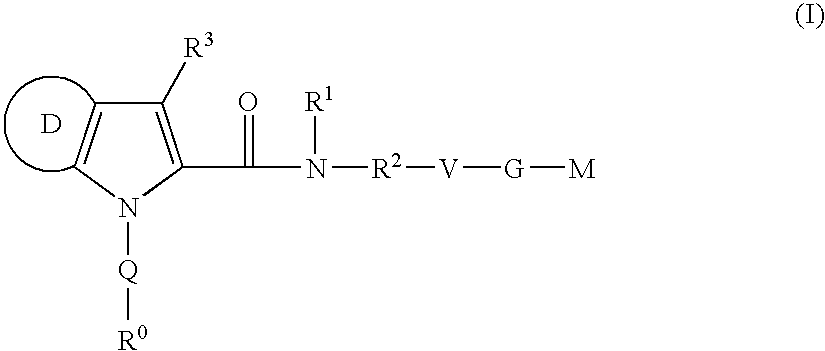





![Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ba01bb1d-6279-47d7-a61b-2f65d761156a/BDA0000629594000000021.PNG)
![Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ba01bb1d-6279-47d7-a61b-2f65d761156a/BDA0000629594000000022.PNG)
![Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof Pyrazolo[3, 4-c]pyridine-7-one compound and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ba01bb1d-6279-47d7-a61b-2f65d761156a/BDA0000629594000000032.PNG)