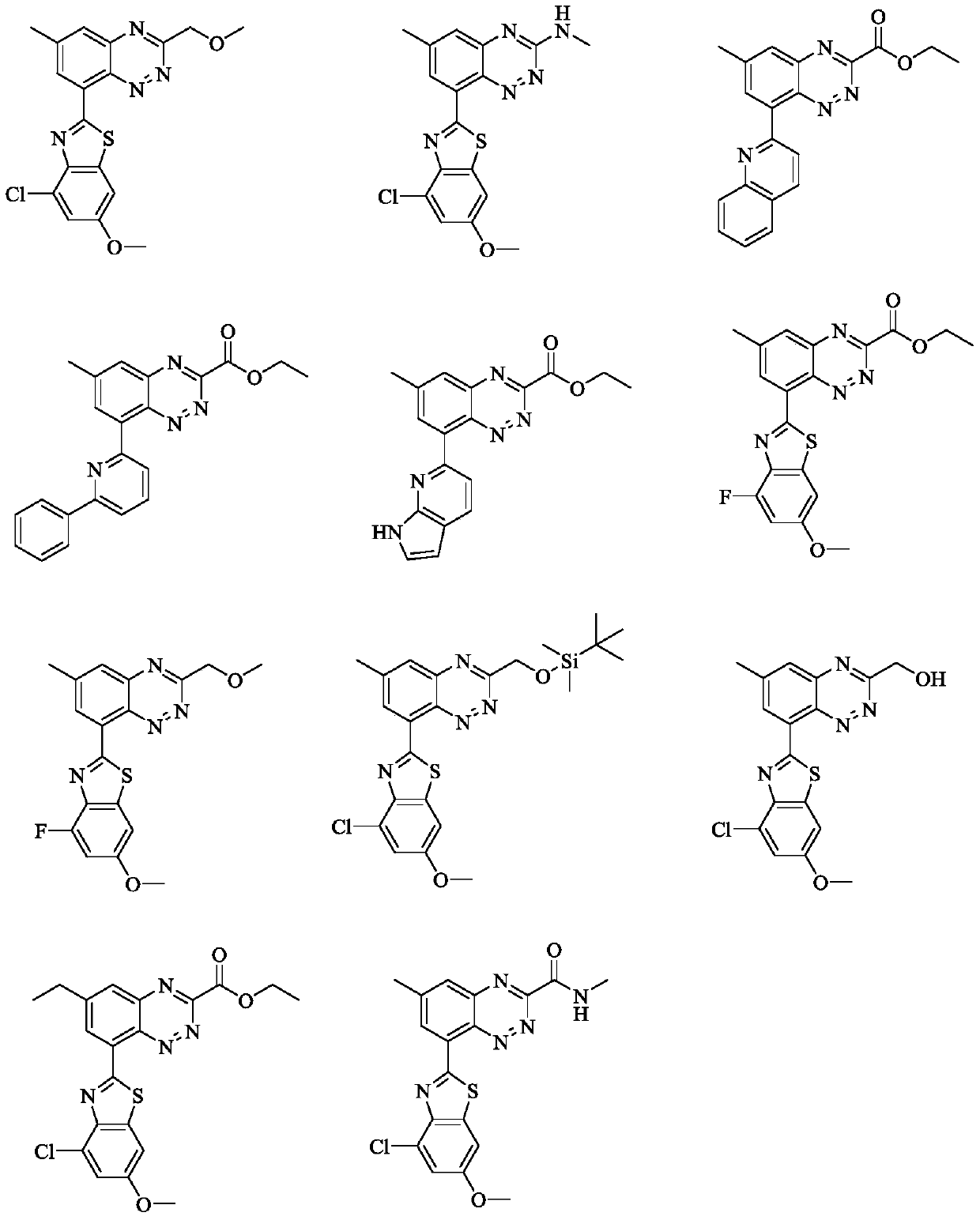
Benzotriazine compound with PAR4 antagonistic activity and application thereof
A compound and solvate technology, applied in the field of chemical drugs, can solve problems such as marketed and oral small-molecule PAR4 antagonists.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 4-Chloro-6-methoxy-2-(3-(methoxymethyl)-6-methylbenzo[e][1,2,4]triazin-8-yl)benzo[d ]thiazole (Compound 1)
[0061]
[0062] Compound 1-1 (1.00 g, 5.70 mmol) was dissolved in THF (10 mL), and 40% sodium methoxide in methanol (2 mL) was slowly added dropwise at room temperature, and stirred at room temperature for 2.5 h. The reaction was monitored by TLC. After the raw materials were reacted, saturated ammonium chloride (20 mL) was added to quench the reaction, and ethyl acetate (20 mL×3) was added for extraction. The organic phase was washed with saturated sodium bicarbonate (50 mL), washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated. Silica gel column chromatography, eluting with PE / EA=20 / 1-10 / 1, gave compound 1-2 (1.04 g) as light yellow solid, yield 94%, Rf=0.5 (PE / EA=3 / 1).
[0063]Compound 1-2 (1.00g, 5.33mmol) was dissolved in methanol (25mL), zinc powder (3.5g, 53.3mmol) and ammonium chloride (5.72g, 106.6mmol) were added, an...
Embodiment 2
[0076] 8-(4-chloro-6-methoxybenzo[d]thiazol-2-yl)-N,6-dimethylbenzo[e][1,2,4]triazin-3-amine ( Compound 2)
[0077]
[0078] At room temperature, compound 1-10 (1.0 g, 3.39 mmol) was dissolved in THF (20 mL), and aqueous sodium hydroxide solution (1 M, 7 mL) was slowly added dropwise, stirred for 1 h, and the reaction was monitored by TLC. After the reaction of the raw materials, dilute hydrochloric acid (1.0M, 10mL) was slowly added dropwise, extracted with EA (20mL×3), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, silica gel column chromatography, DCM / MeOH Rinse with / AcOH=10 / 1 / 1 to obtain the crude compound 2-1 as a brown-black oil. Rf=0.2-0.4 (DCM / MeOH / AcOH=5 / 1 / 1, severe tailing).
[0079] Under nitrogen protection, compound 2-1 (900mg, 3.36mmol) was dissolved in anhydrous tert-butanol (5mL), added activated 4A molecular sieves and stirred at room temperature for 2h, and diphenylphosphoryl azide (1.02...
Embodiment 3
[0085] 8-(4-Chloro-6-methoxybenzo[d]thiazol-2-yl)-6-methylbenzo[e][1,2,4]triazine-3-carboxylic acid ethyl ester ( Compound 4)
[0086]
[0087] Compound 1-10 (500mg, 1.69mmol), pinacol diboronate (860mg, 3.38mmol) and anhydrous potassium acetate (340mg, 3.38mmol) were added to anhydrous 1,4-dioxane (10mL), Under strict nitrogen protection, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (75mg, 0.1mmol) was added, and the temperature was raised to 120°C for 2h under reflux. The reaction was monitored by TLC. After the raw materials have been reacted, the reaction is cooled to room temperature, and EA / H 2 O was separated, and the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated. Wet sample loading, flash silica gel column chromatography, eluting with DCM / MeOH=50 / 1, the crude product containing compound 4-1 was obtained as a dark oily substance. It was used in the next reaction without further purification.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com