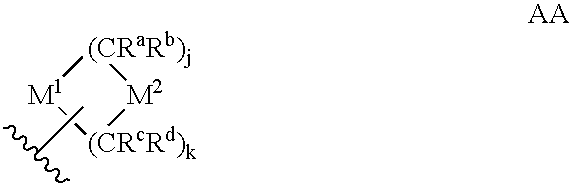Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
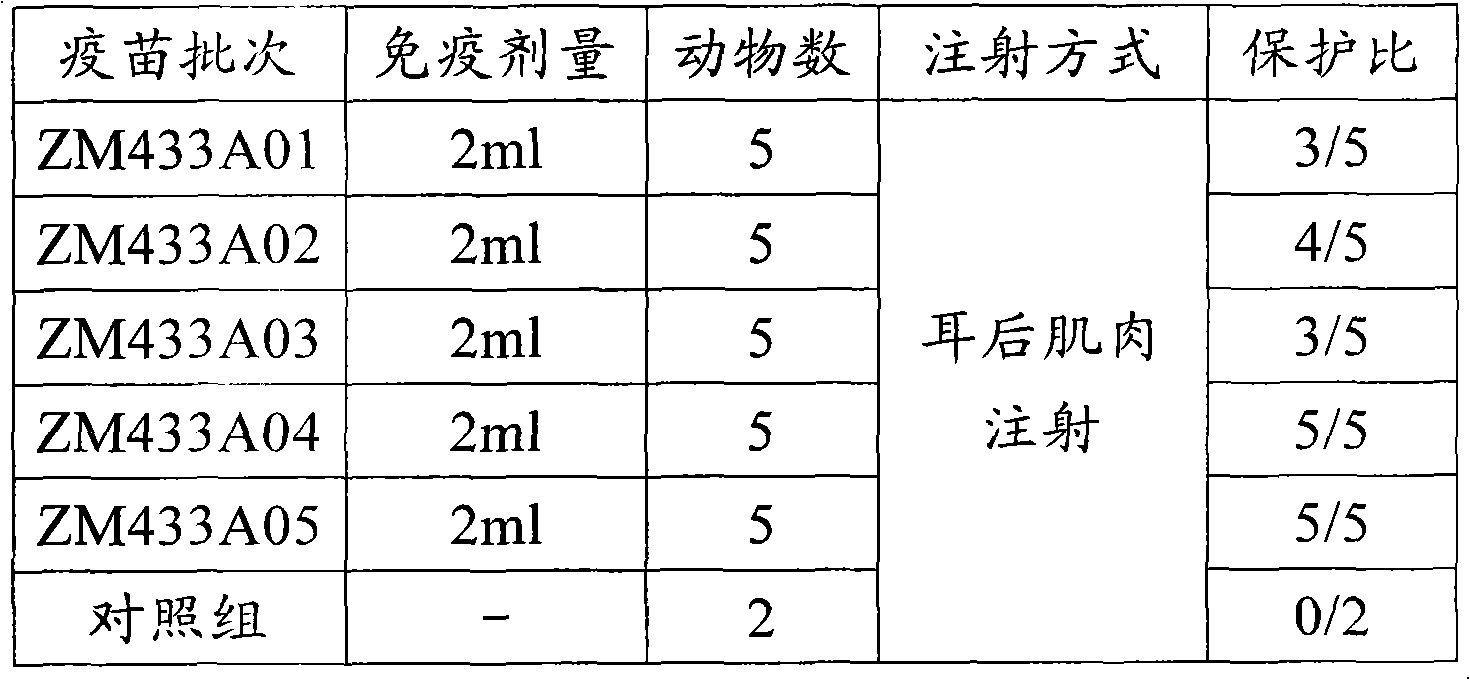
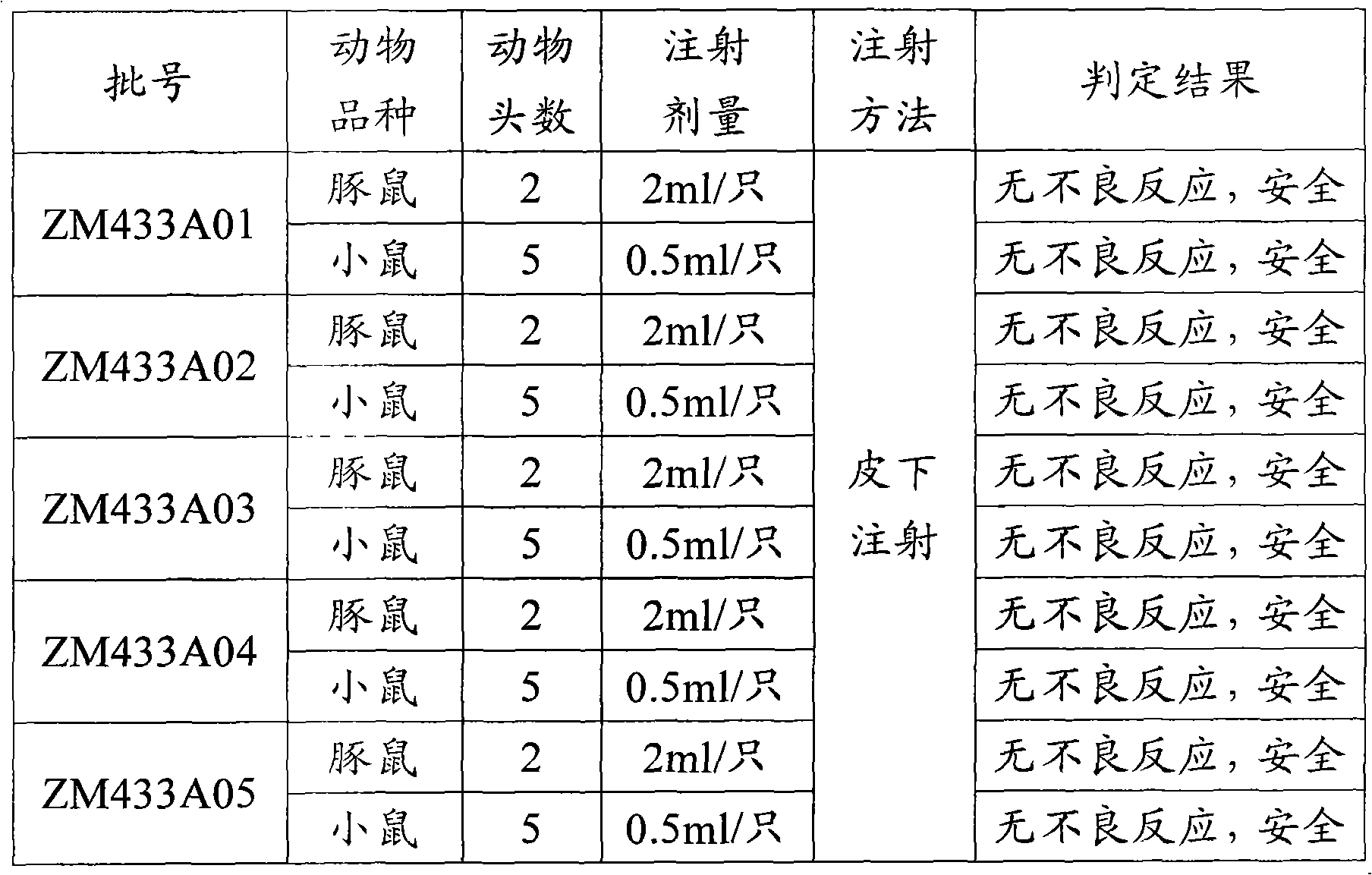
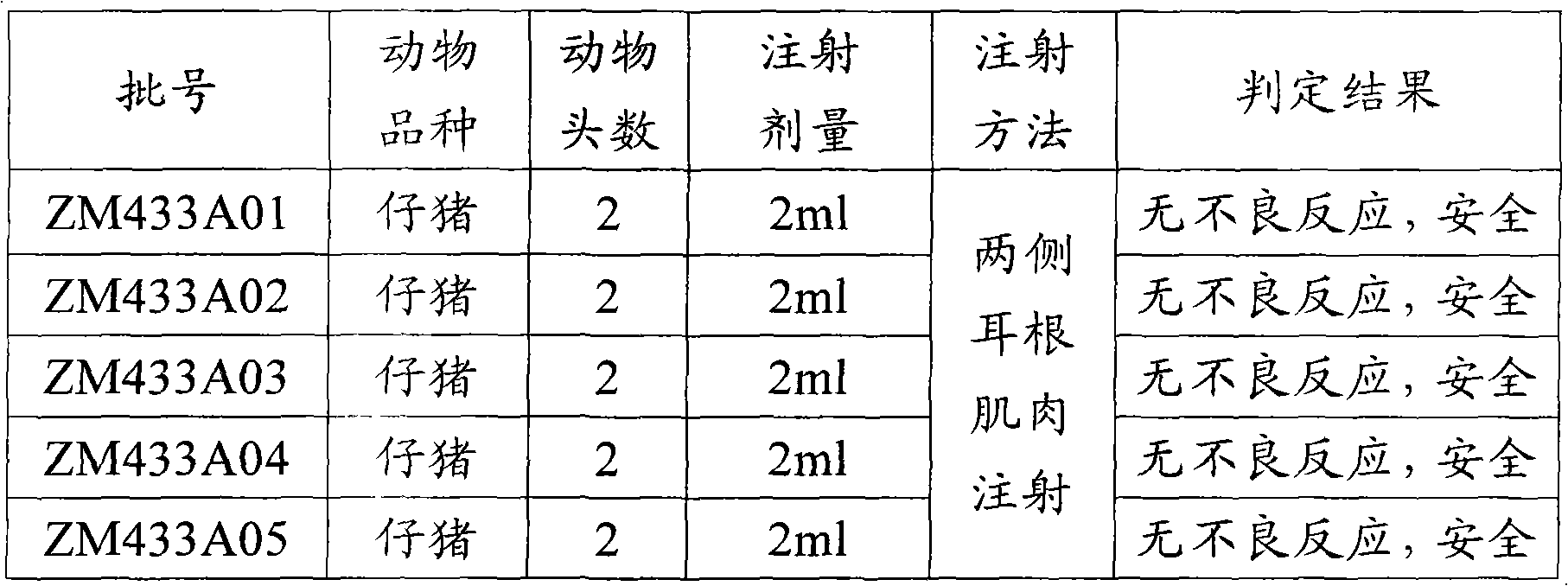
317 results about "Animal testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Animal testing, also known as animal experimentation, animal research and in vivo testing, is the use of non-human animals in experiments that seek to control the variables that affect the behavior or biological system under study. This approach can be contrasted with field studies in which animals are observed in their natural environments or habitats. Experimental research with animals is usually conducted in universities, medical schools, pharmaceutical companies, defense establishments and commercial facilities that provide animal-testing services to industry. The focus of animal testing varies on a continuum from pure research, focusing on developing fundamental knowledge of an organism, to applied research, which may focus on answering some question of great practical importance, such as finding a cure for a disease. Examples of applied research include testing disease treatments, breeding, defense research and toxicology, including cosmetics testing. In education, animal testing is sometimes a component of biology or psychology courses. The practice is regulated to varying degrees in different countries.
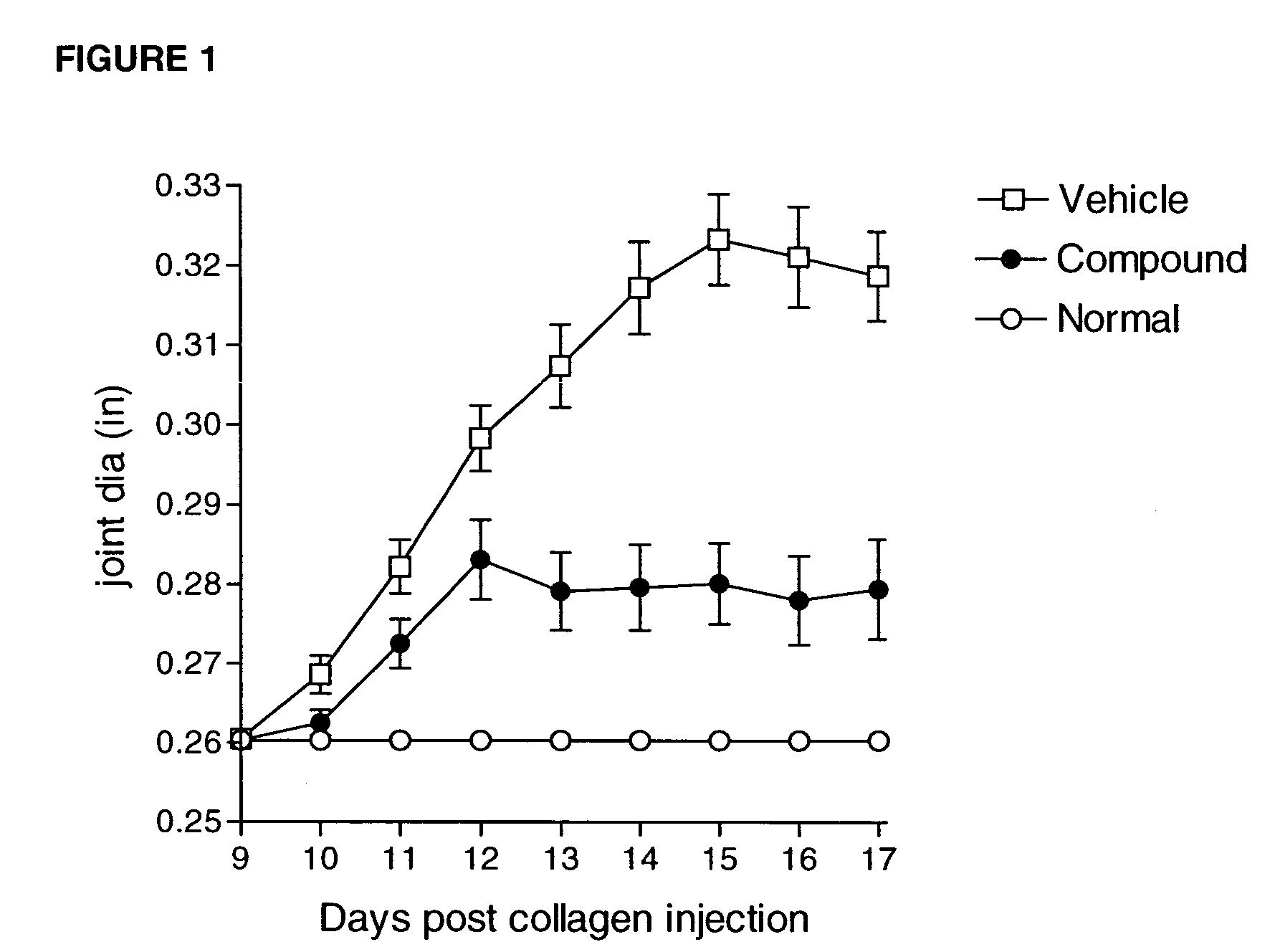
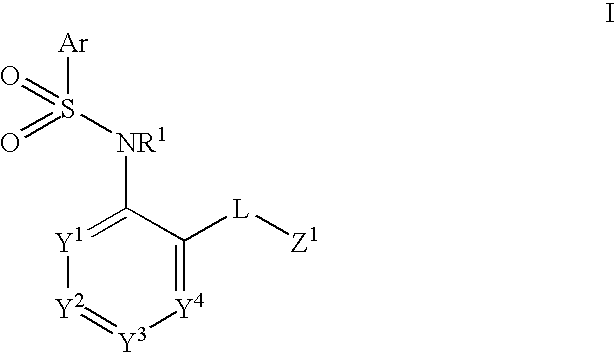
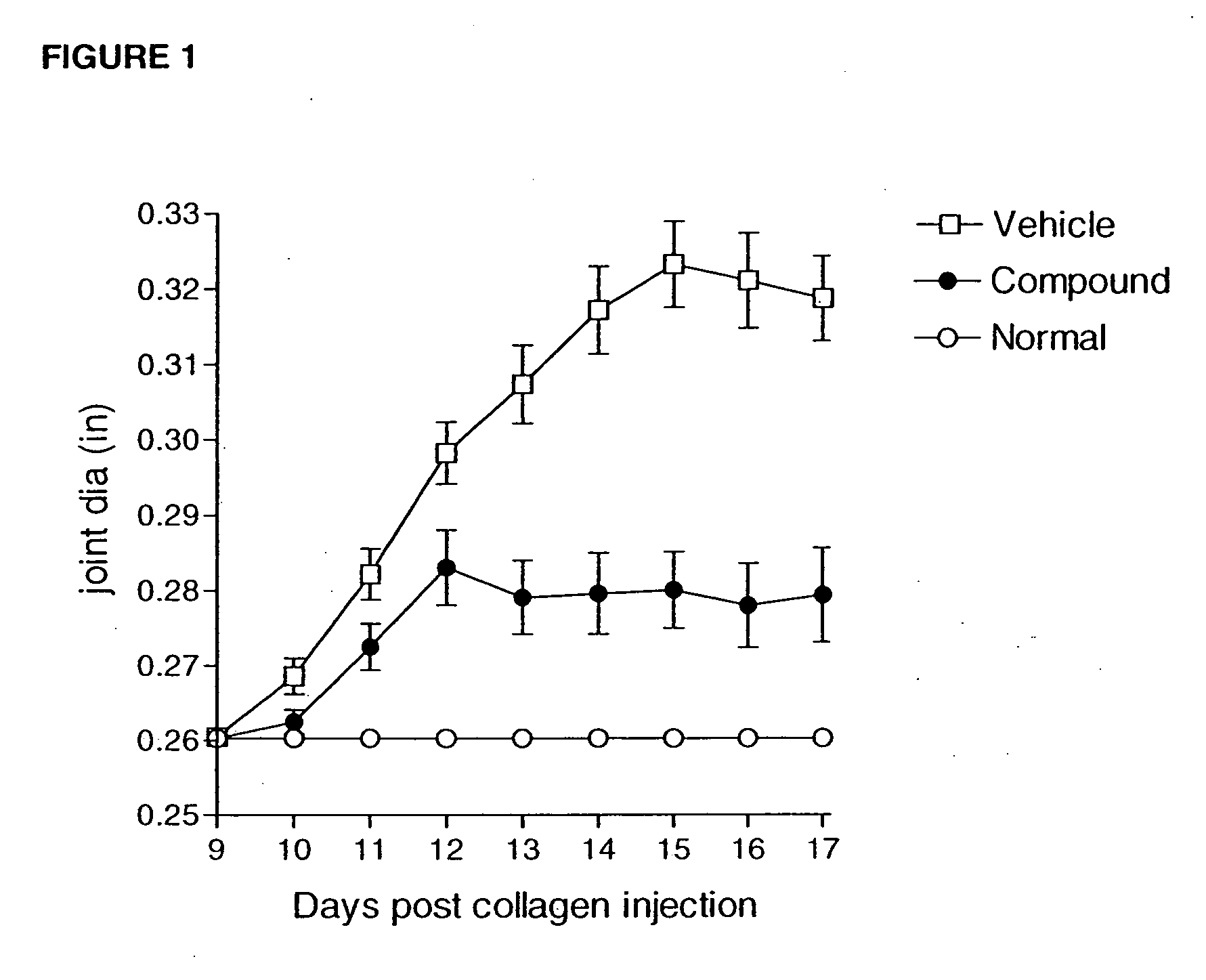
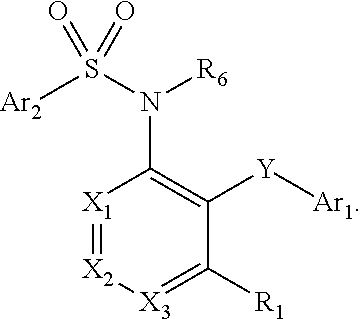
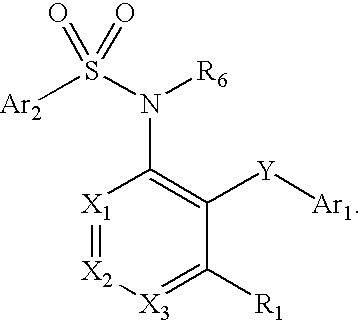
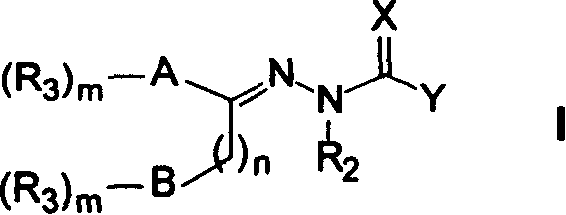
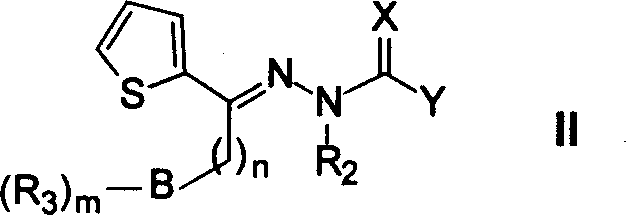
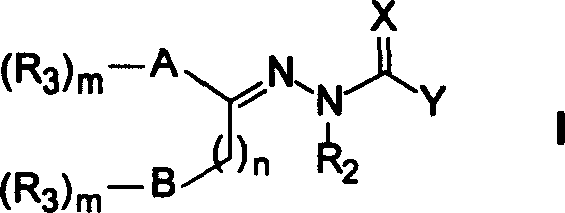
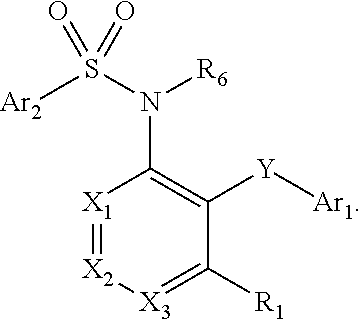
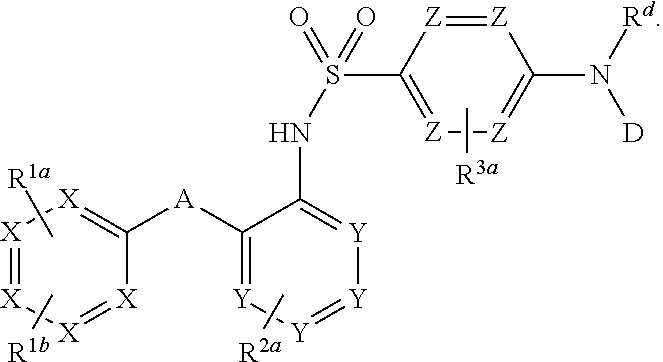
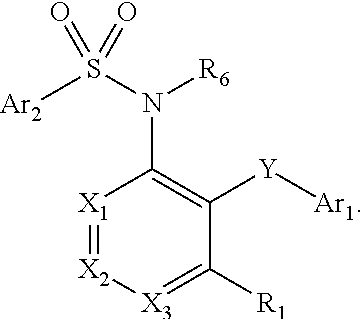
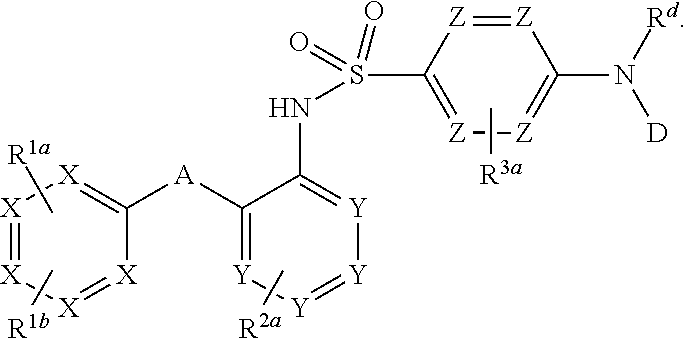
Heteroaryl sulfonamides and CCR2
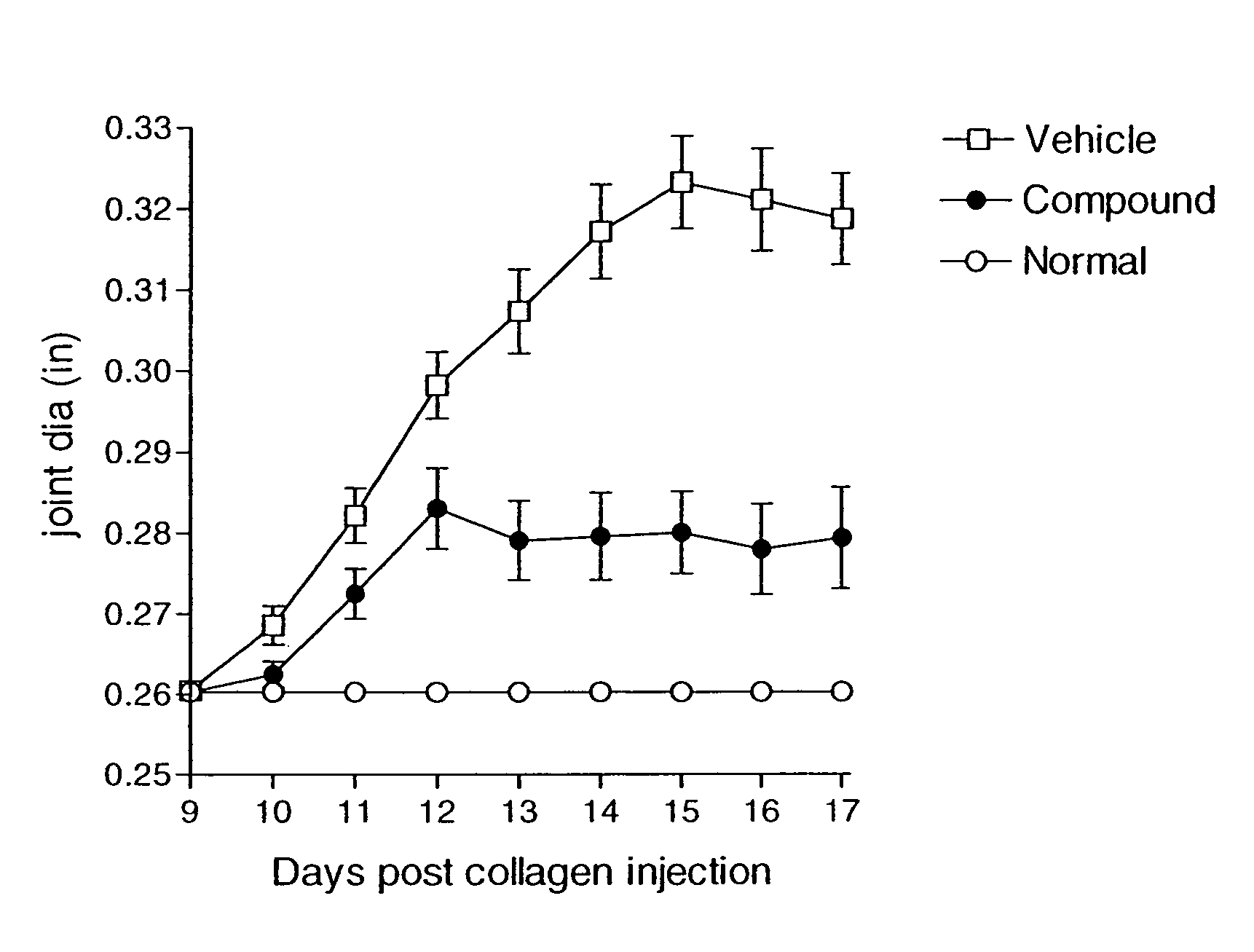
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Heteroaryl sulfonamides and CCR2/CCR9
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
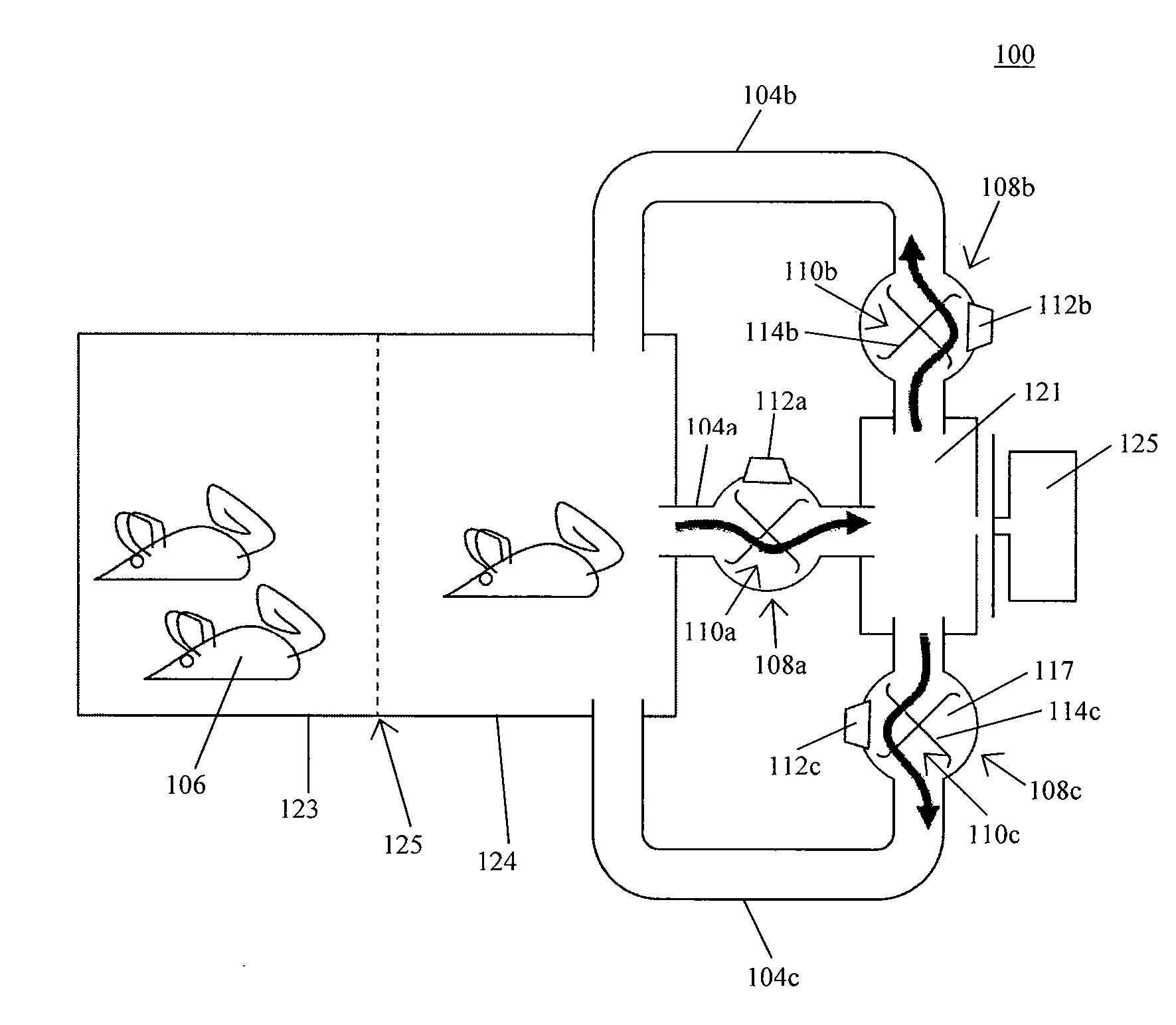
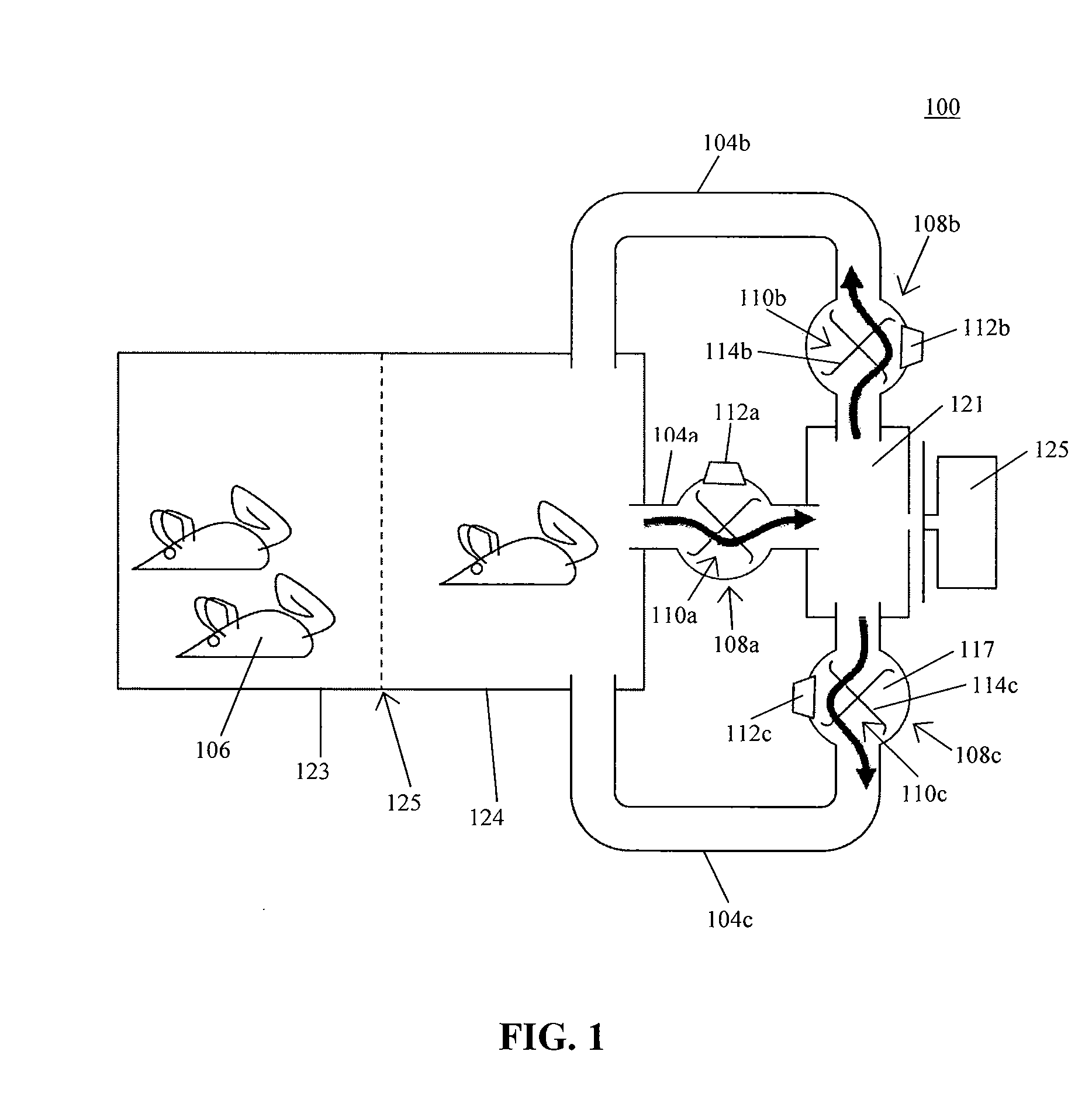
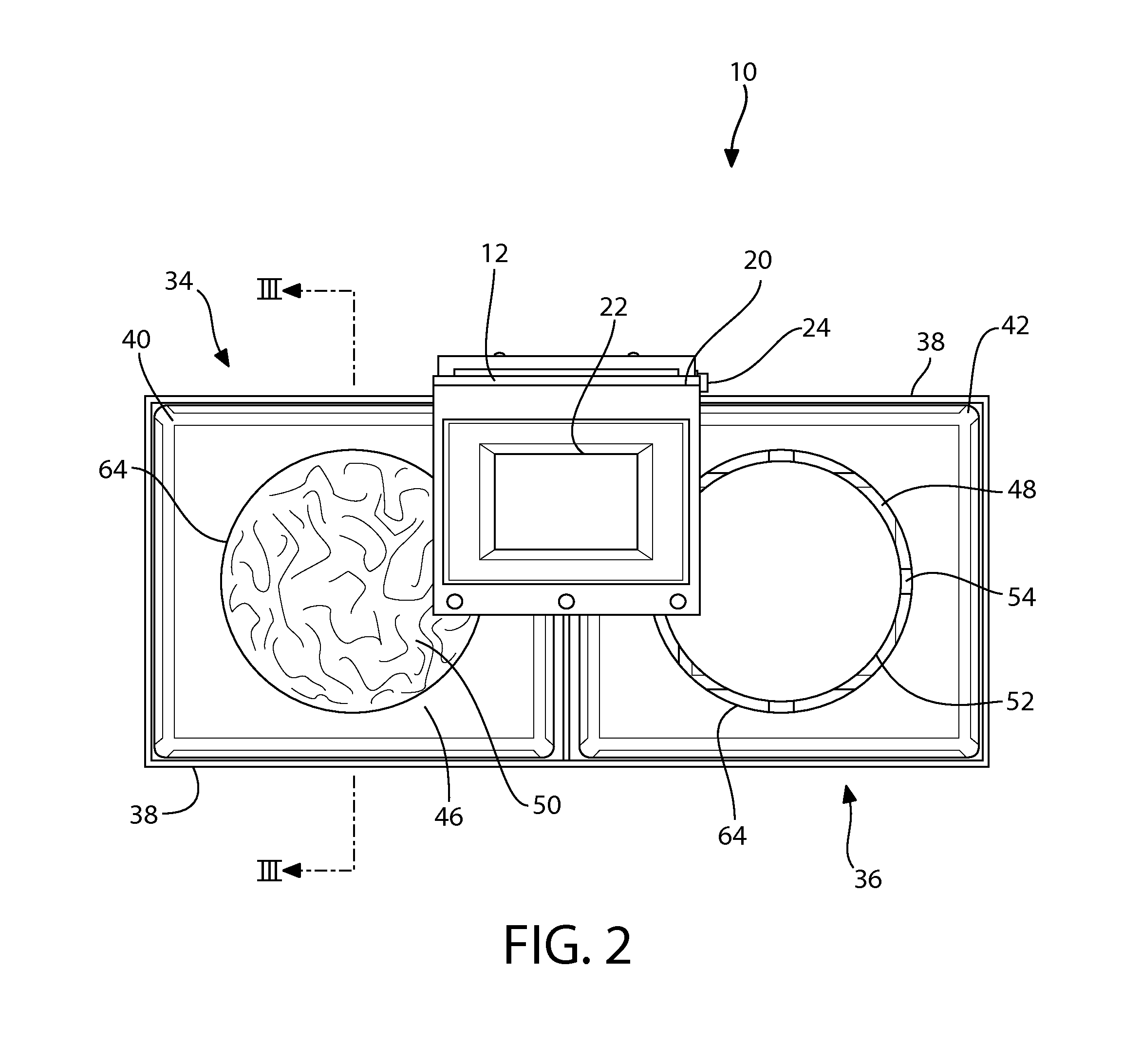
System for automating animal testing protocols
ActiveUS20120180731A1Improve throughputEasy to completeAnimal housingOther apparatusSmall animalAnimal testing
In one aspect, the present invention provides a housing system for conducting high throughput animal experiments. The housing system includes a home cage, at least one rotatable turnstile enclosed by housing to form two or more isolation chambers, a means for animal identification, and one or more action stations functionally coupled to one or more isolation chambers. The turnstile includes a plurality of one or more separation members rotatable about a vertical axis, each isolation chamber bounded by one or more separation members. The action stations contain one or more devices facilitating completion of at least one animal-directed or experimentor-initiated action. In a preferred embodiment, the home cage is sufficiently sized to house a plurality of small animals, such as mice. Tunnel passageways may be connected to the home cage, including one or more tunnel passageways containing a rotatable turnstile. Additional embodiments include rotatable turnstiles, rotatable turnstile assemblies, and methods of conducting high throughput animal experiments using the devices and systems described herein.
Owner:PURDUE RES FOUND INC
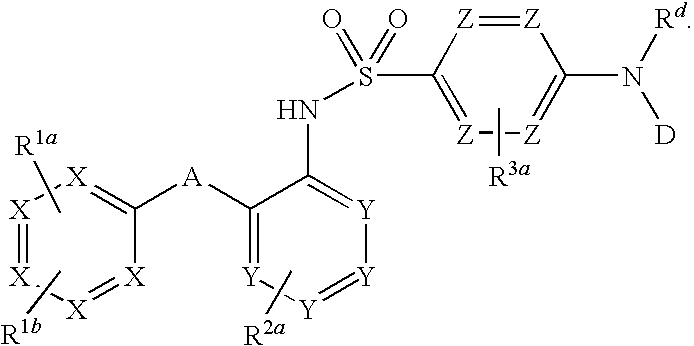
Triazolyl phenyl benzenesulfonamides
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
Heteraromatic ring thiosemicarbazone compound, and its derivatives and their use forpreparing antitumour medicine
The invention discloses thiosemicarbazon compounds, their derivatives and their applications for the preparing of antineoplastic drugs. The compound of the invention shows its good inhibitory effect on tumor through the experiments on animals. For example, the inhibition effect on the solid tumor S180 on mouse is up to 81.1%. The compounds can be made into tablet, capsule or injection agent in many forms.
Owner:卡南吉医药科技(上海)有限公司
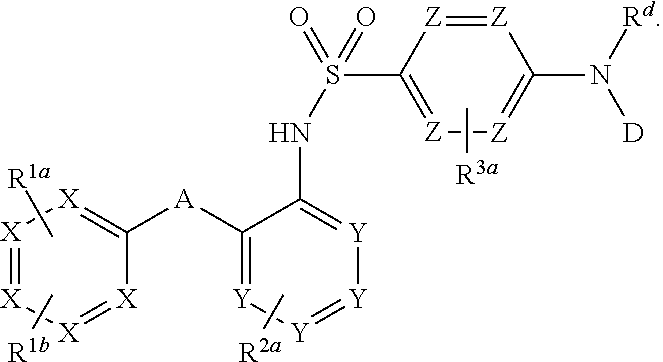
Heteroaryl sulfonamides and CCR2/CCR9
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Efficient and green diarrhea-preventing feed additive composite for weaned pigs
ActiveCN102228154AReduce usageAvoid the problem of drug resistance caused by abuseAnimal feeding stuffAccessory food factorsAnimal scienceAnimal testing
Owner:辽宁九州生物科技有限公司
O type foot and mouth disease virus-like particle and preparation method thereof and application
ActiveCN106479986AHigh expressionImprove assembly efficiencySsRNA viruses positive-senseViral antigen ingredientsAnimal testingStructural protein
The invention discloses an O type foot and mouth disease virus-like particle and a preparation method of the O type foot and mouth disease virus-like particle and an application. The O type foot and mouth disease virus-like particle is formed by assembling structural protein VP0, VP1 and optimized OP3 structural proteins of O type foot and mouth disease virus, wherein the gene sequence of the VP1 is shown as SEQ ID NO.1; the gene sequence of the VP0 is shown as SEQ ID NO.2; the gene sequence of the optimized VP3 is shown as SEQ ID NO.3. The invention tries to perform artificially missing on a part of section of the structural protein VP3 of O type foot and mouth disease virus; the result shows that the protein expression amount of the VP3 gene after the artificial missing is improved by 20% in comparison to that before mutation; the assembling efficiency of the virus-like particle is also improved by 15%; moreover, the animal test result shows that the immunogenicity thereof is good and free from significant difference with VLPs obtained through unmissed VP3 assembling. The invention provides a new technical manner for the research of the foot and mouth disease vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Heteroaryl sulfonamides and ccr2/ccr9
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Preparation method and device of duramater/spinal dural transplanting substitute
ActiveCN102727935ASimple Surface Functional StructureWidely sourced and cheapProsthesisAntigenDefect repair
The invention provides a preparation method of a duramater / spinal dural transplanting substitute which is obtained by repeated freezing and thawing of dural tissue, rolling and cracking of cells, crosslinking fixed protection, accellular antigen extraction, dense surface fibrosis modification, packaging and sterilization, and has the advantages of simple method, wide raw material sources, cheap raw materials, and low cost. The prepared dural substitute completely removes components of cells and other antigen components simultaneously when protecting dural tissue natural structure and properties, is good in biocompatibility, free of immune rejection, safe and reliable, good in mechanical performance, and easy in clinical operation, can meet the needs of defect repair, has the function of promoting tissue regeneration as a loose surface is beneficial to the tissue fluid adsorption, active factor enrichment, and growth of blood vessels and cells, and has the advantages of rapidness in fusion with a host, biodegradable absorption, and good repair effect. The animal test shows that the defect can be completely repaired without brain or spinal fluid leakage, or adhesion with brain tissue, and significant rejection is not found.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
MntC recombinant protein of staphylococcus aureus and preparation method and application thereof
ActiveCN103694323AHigh expressionEasy to separate and purifyAntibacterial agentsBacteriaPurification methodsStaphylococcus aureus
The invention belongs to the field of biotechnology, and relates to a MntC recombinant protein of staphylococcus aureus (SA), a carrier comprising the recombinant protein, a host, a composition or a kit, application, preparation, fermentation and purification method of the protein. The MntC recombinant protein prepared by the method has strong immunogenicity, is safe and non-toxic, and is proved by animal tests to be able to effectively stimulate an organism to generate high efficient humoral immune response and good immune protection.
Owner:CHENGDU OLYMVAX BIOPHARM +1
One-bacterium multiple-enzyme bacterial strain as well as screening method and application thereof
The invention relates to a one-bacterium multiple-enzyme bacterial strain as well as a screening method and an application thereof. The bacterial strain is bacillus subtilis (Bacillus subtilis 1.1111) and is collected in the China center for type culture collection with the collection number of CCTCC (China center for type culture collection) No: M2011286. The bacillus subtilis (Bacillus subtilis 1.1111) can be used for preparing the bacterial strains of nine enzymes, i.e. xylanase, protease, phytase, pectinase, lipase, sweet dew glucanase, glucoamylase and the like, and the yields of the protease, the sweet dew glucanase, amylase and the glucoamylase are very high. Meanwhile, the bacterial strain is proved to have strong endurance capacity on cholate, artificial gastric juice and artificial intestinal juice by simulating the internal cholate environment, the artificial gastric juice, artificial intestinal juice and the animal test, safety and growth simulation capability are shown to a tested animal, and a foundation is laid for effectively improving the enzyme production capability of the bacterial strain, simultaneously generating the xylanase, the protease, the phytase, the pectinase, the lipase, the sweet dew glucanase and the glucoamylase and realizing one-bacterium multiple-enzyme fermentation in the fermentation process. The mutual synergistic effect among various enzymes generated by the bacterial strain is strong, and the bacterial strain can be used as a feed additive to be applied to agricultural production for livestock, fowls, aquatic livestock and the like.
Owner:HENAN UNIV OF SCI & TECH
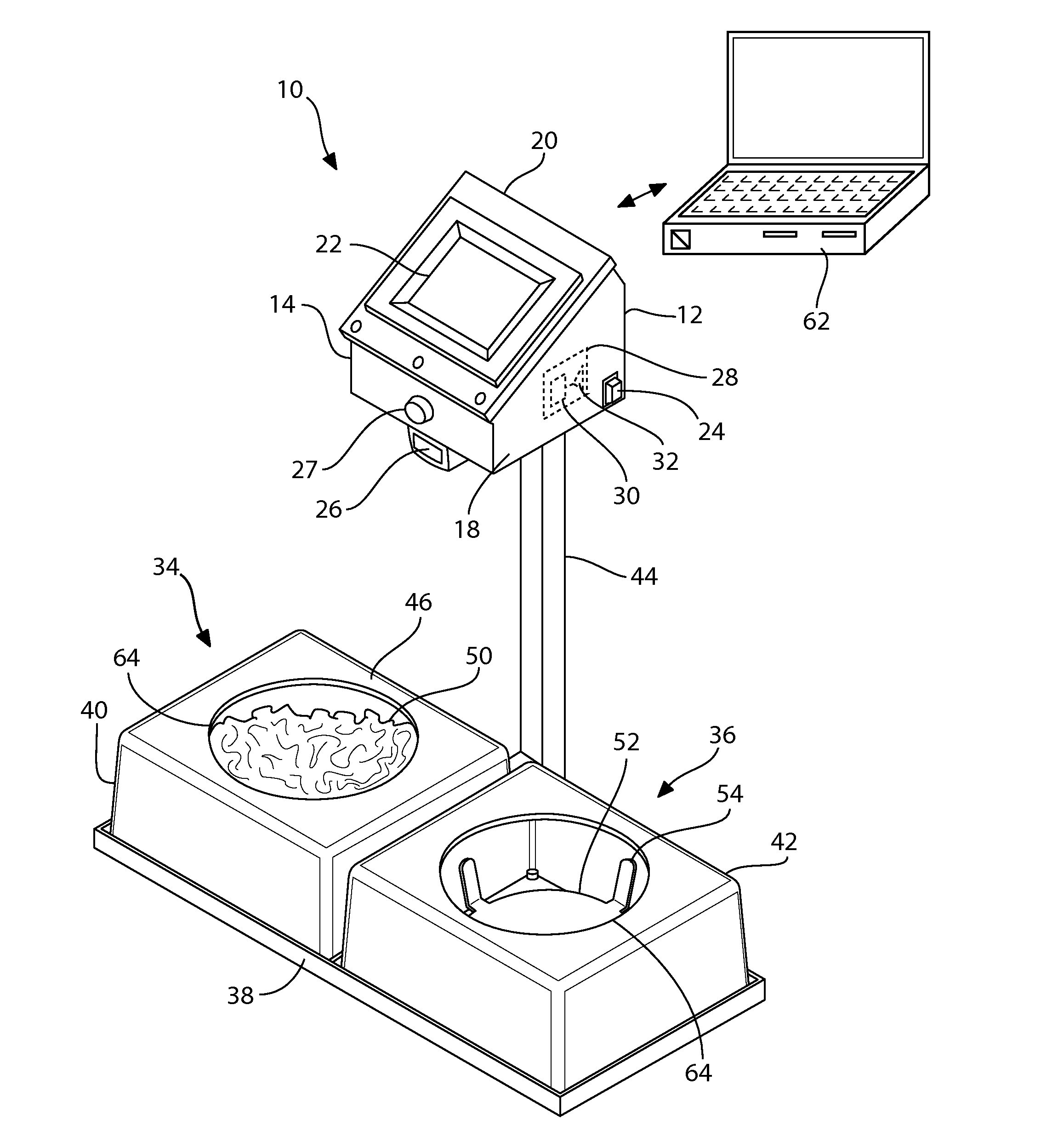
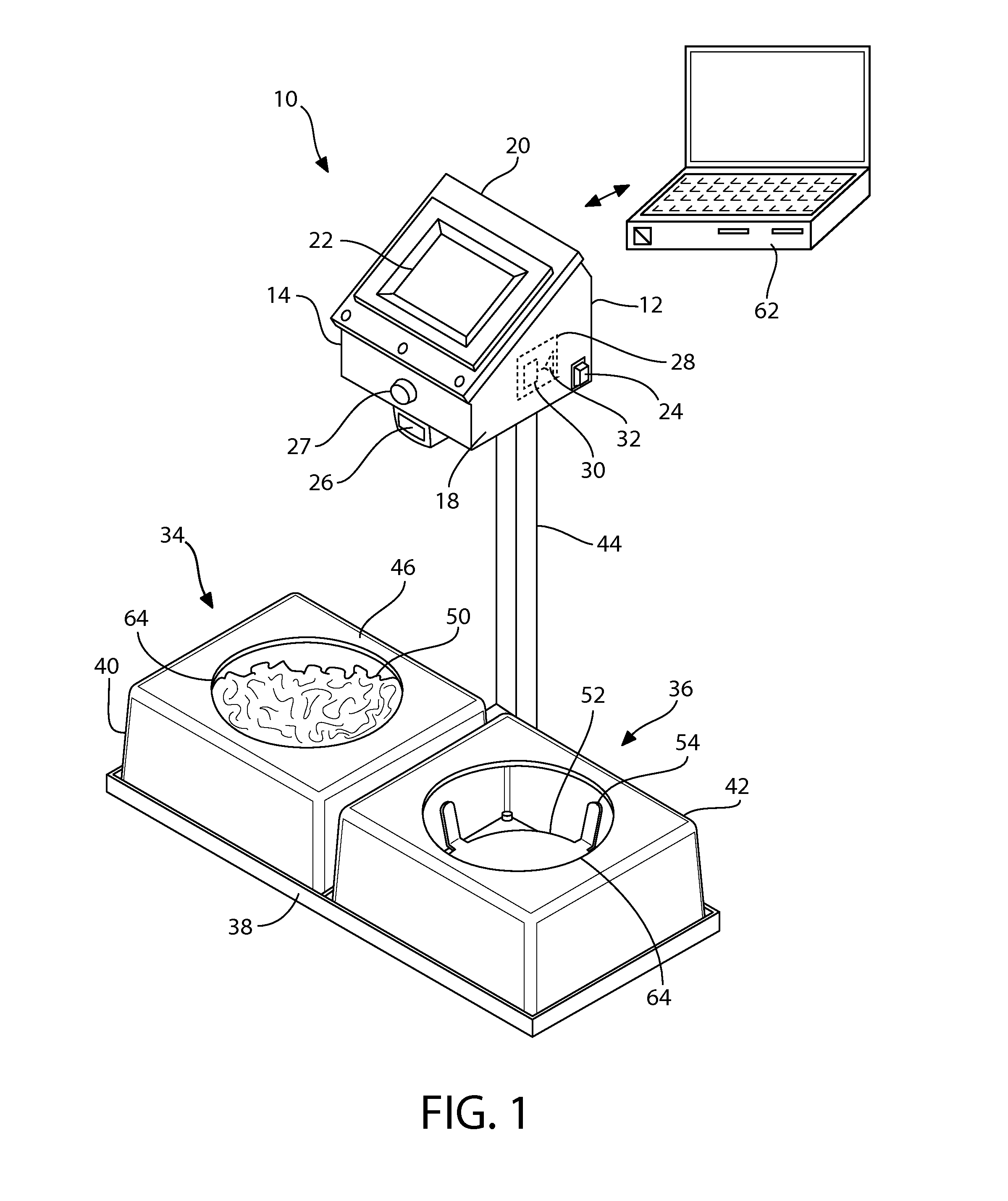
Automated feeding station for in-house companion animal testing
InactiveUS20120199076A1Amount of timeMinimizes human transcription errorAnimal feeding devicesAnimal housingDistractionAnimal science
An automated feeding station for in-home testing away from unfamiliar laboratory settings test the acceptance of pet food by monitoring the reaction of the companion animals or pets in their comfortable in-home environment where the animals make their preferred choice of food without distractions caused by an unfamiliar environment. A pet owner scans appropriate food identifiers to verify the proper diet is being distributed into the appropriate food-receiving region(s). Once the food bowls are placed in appropriate food receiving regions within the feeding station, the testing system begins collecting data and transmits this data, preferably wirelessly, to the testing investigators. The preferred feeding station can measure: intake for the companion animal, information pertaining to the rate of food consumption, the animal's first approach to food, and the food first tasted by the animal. This information is provided via appropriate sensors and algorithms designed for this purpose within the system.
Owner:HILLS PET NUTRITION INC
Method for minim hepatic tissue in vitro incubation and detecting CYP450 enzymatic activity
InactiveCN101308119AHigh simulationSave costsComponent separationMicrobiological testing/measurementMetaboliteCytochrome p450 enzyme
Disclosed is a method for detecting CYP450 enzymatic activity by means of micro-liver tissue incubation in vitro, which is characterized in that: a liver trace puncturing method used in clinic takes micro-liver tissues of 0.1 to 0.2g, or kills a mouse to take out the liver in an animal experiment, and builds a micro-liver tissue in vitro incubation system, then uses a one-probe medicine to perform the liquid phase chromatography tandem mass spectrometry for detecting probe substrates and concentration of corresponding metabolites, and obtains the activity of P450 enzyme according to metabolite ratios thereof. The method has the advantages of: 1. micro scale: only micro-liver tissues of 0.1 to 0.2g are needed for detecting cytochrome P450enzyme activity, and can be used for animal experiment and checking clinic micro-liver tissue liver drug enzyme activity; 2. time saving and convenience: costly ultracentrifugation equipment used in the conventional method is saved, costs and time for detection are greatly saved, and liver enzyme metabolic condition simulation is better; and 3. establishing indexes for evaluating the enzyme metabolic activity: metabolite ratios.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY
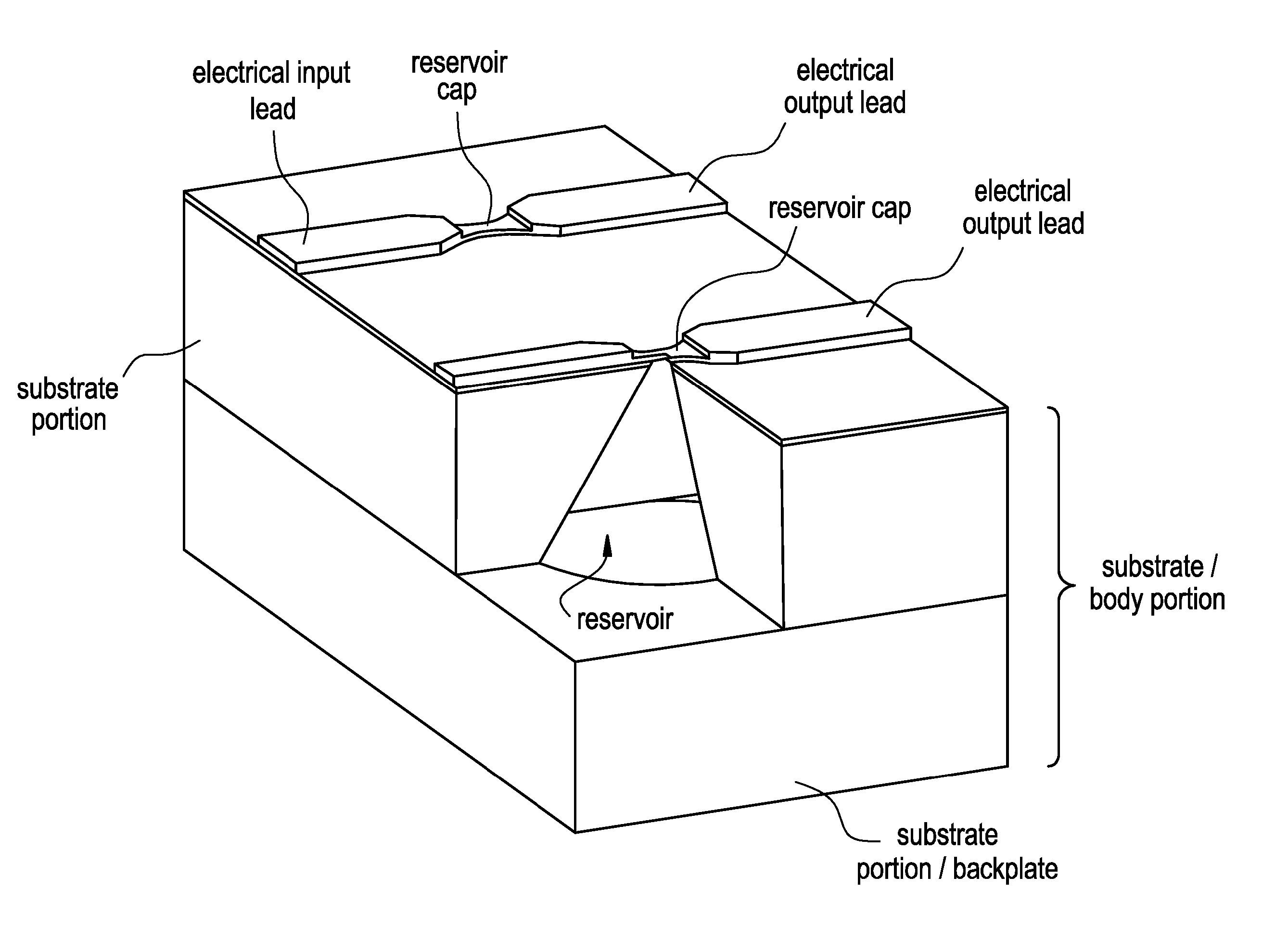
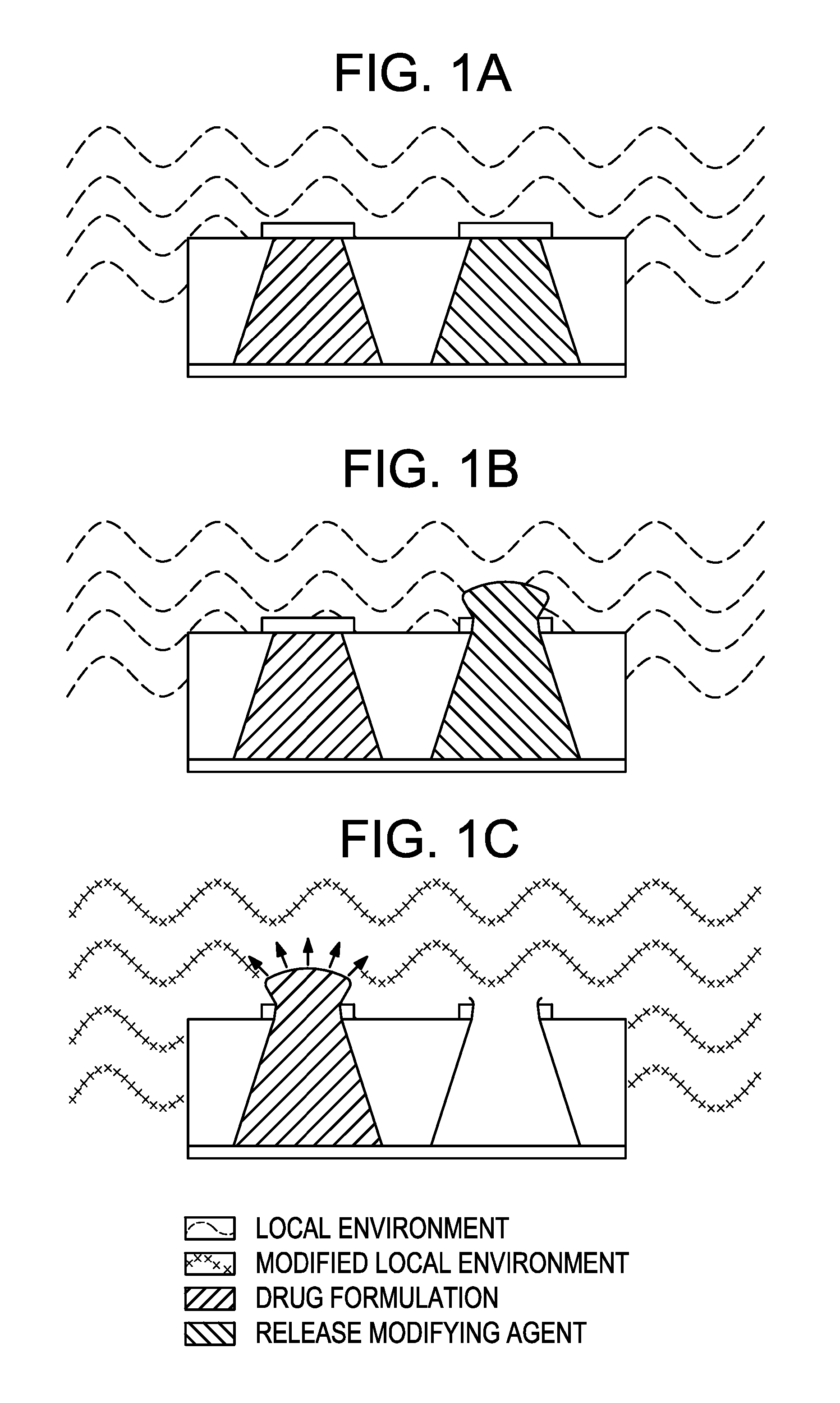
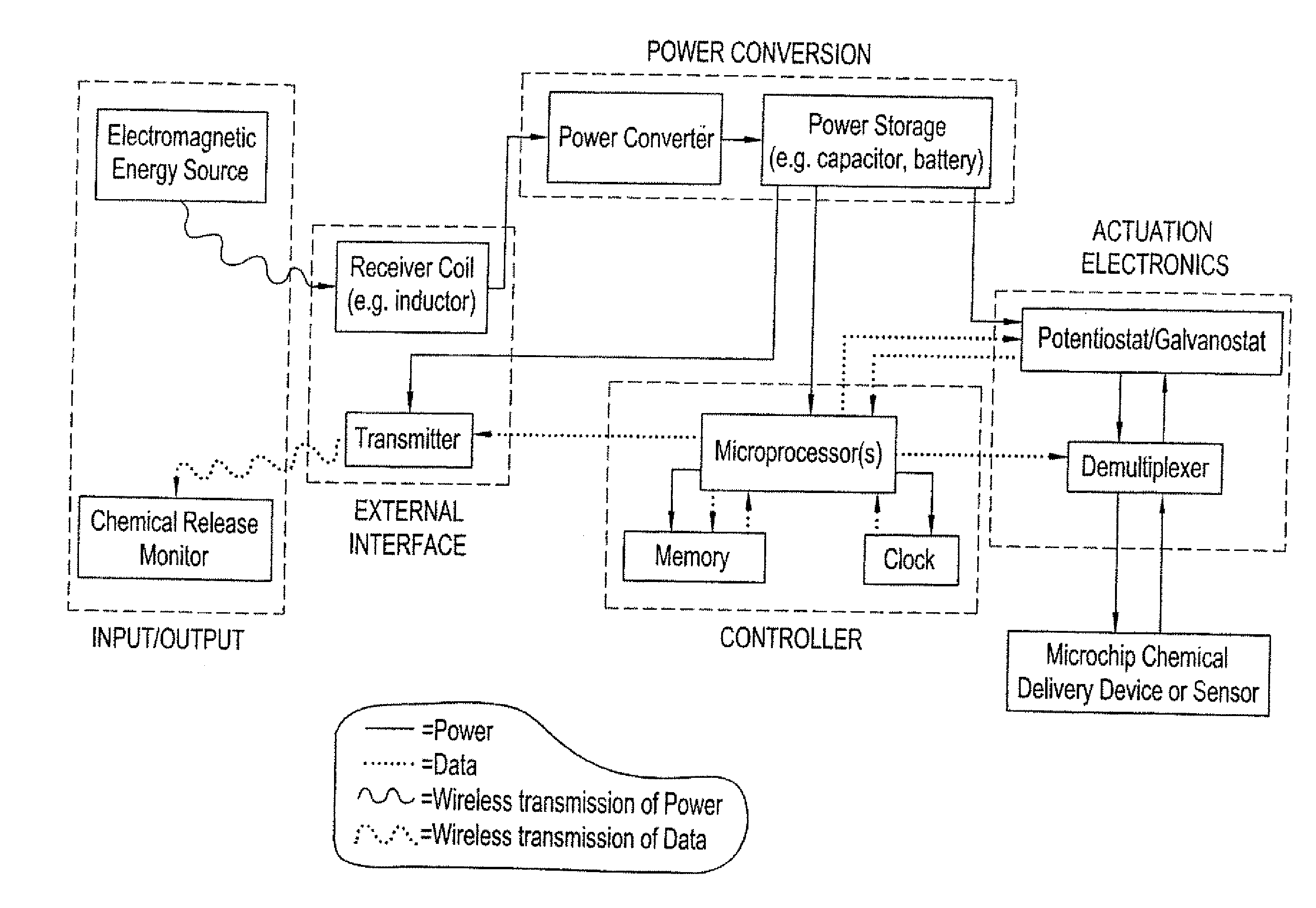
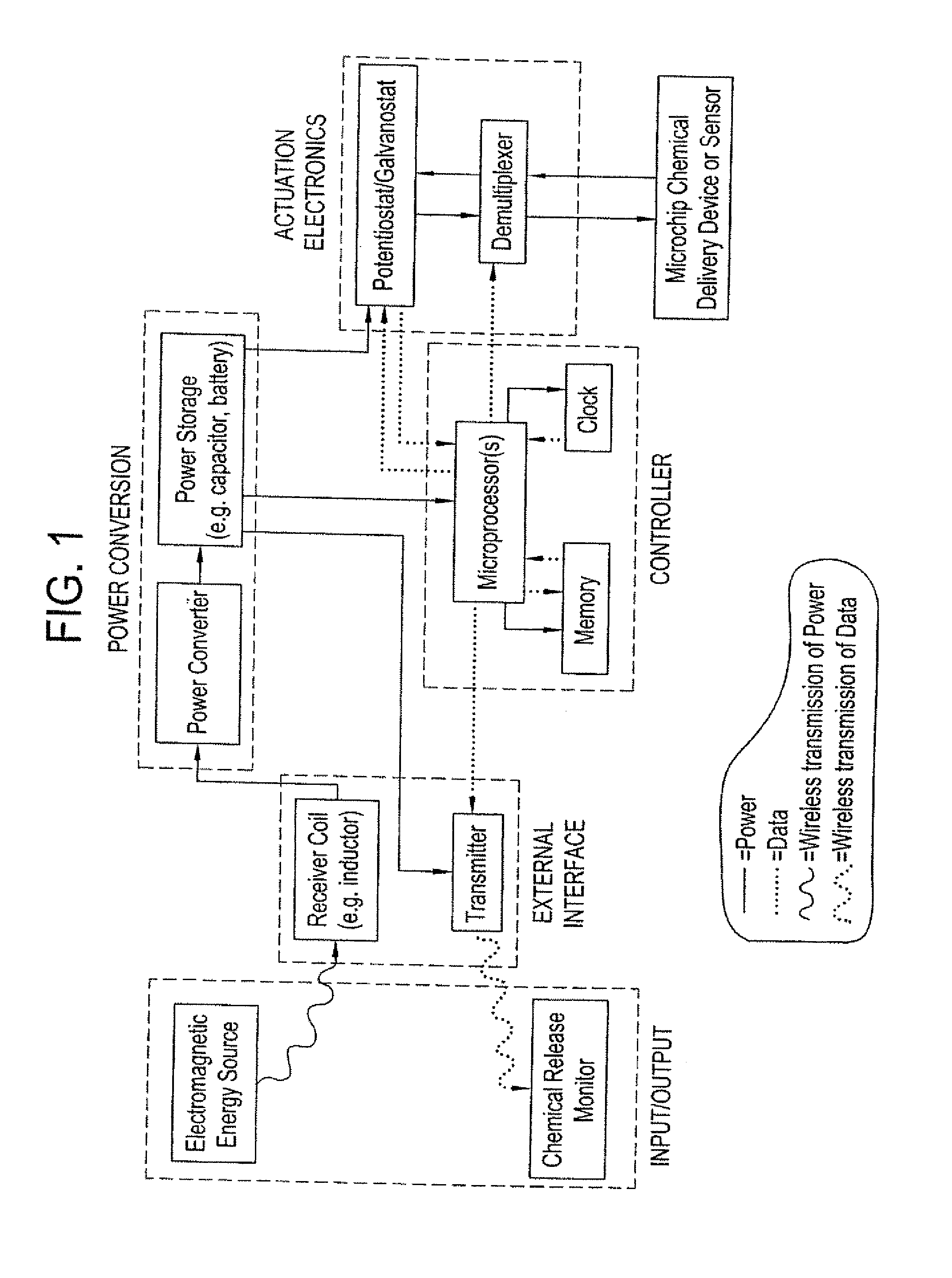
Method and implantable device with reservoir array for pre-clinical in vivo testing
InactiveUS20080076975A1Facilitated releasePrevent oxidationStentsPeptide/protein ingredientsPhysiological fluidPhysiology
Methods of pre-clinical animal testing to monitor physiological parameters of test animals following exposure of molecules sealed in reservoirs on implanted devices. The test molecules are exposed to physiological fluid of the animal. The molecules can be configured as a sensor chemistry that reacts with the physiological fluid. The molecules can be a drug or drug candidate that is released into the animal. The test animals are non-human mammals.
Owner:MICROCHIPS INC
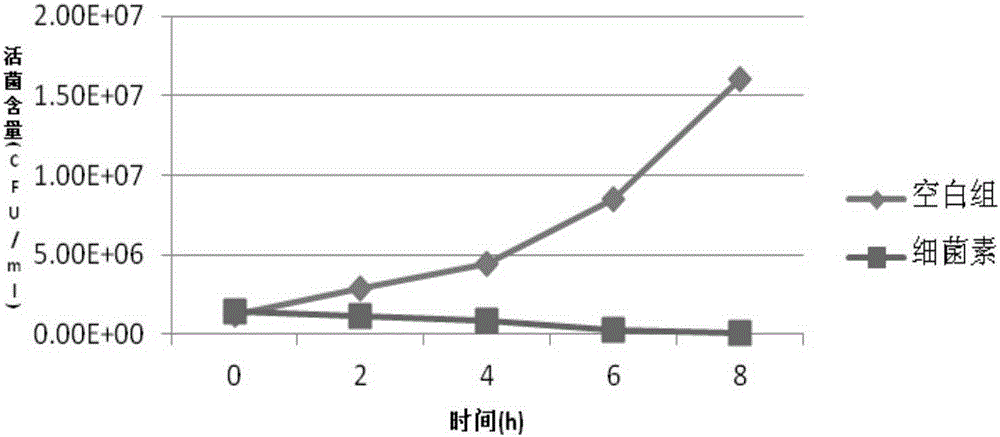
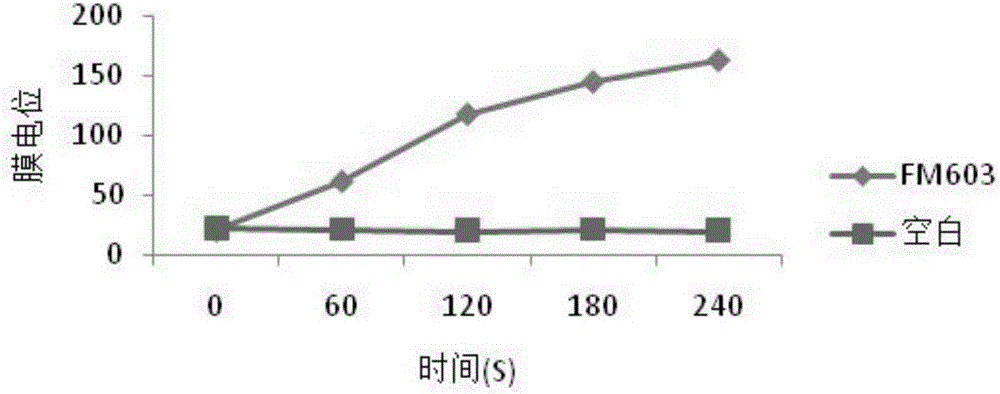
Bacillus coagulans FM 603 and application thereof
ActiveCN105176874AReduce or substituteIncrease production capacityBacteriaMicroorganism based processesFeed conversion ratioAnimal testing
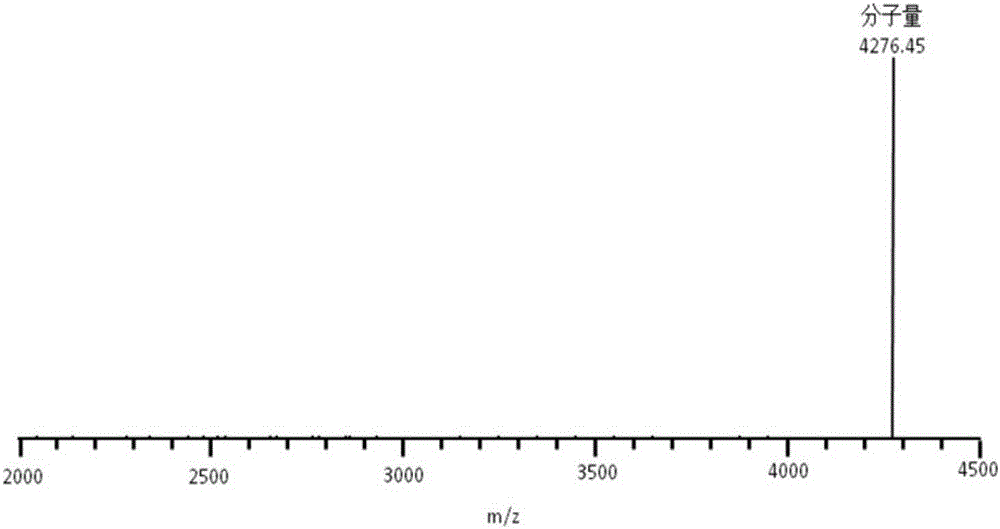
The invention provides a new strain FM 603 of bacillus coagulans, and the preservation number of the strain is CGMCC NO.10221. The strain can generate bacteriocin antibacterial substances and achieves antibacterial activity for Gram-positive pathogenic bacteria such as Listeria monocytogenes, staphylococcus aureus, methicillin-resistant staphylococcus aureus, clostridium perfringens and clostridium difficile. The molecular weight of bacteriocin is 4276.45 Da, the partial amino acid sequence is Ala-Gly-His-Dhb-Phe-Val-Dhb-Gly-Pro, and the bacteriocin is stable under treatment of heat, acid, and pepsase or trypsin and can be degraded easily by pronase to be inactivated. The invention further provides an FM603 fermentation product which can be used as a microbiological feed additive. Animal test results show that the fermentation product can increase the laying rate of laying hens, reduce the feed-egg ratio and improve egg quality; the feed intake and daily gain of piglets are increased, and the feed conversion ratio is reduced; the daily gain and the content of lysozyme in serum of broiler chickens are increased, and the feed conversion ratio and the death rate are reduced.
Owner:沈阳丰美生物技术有限公司
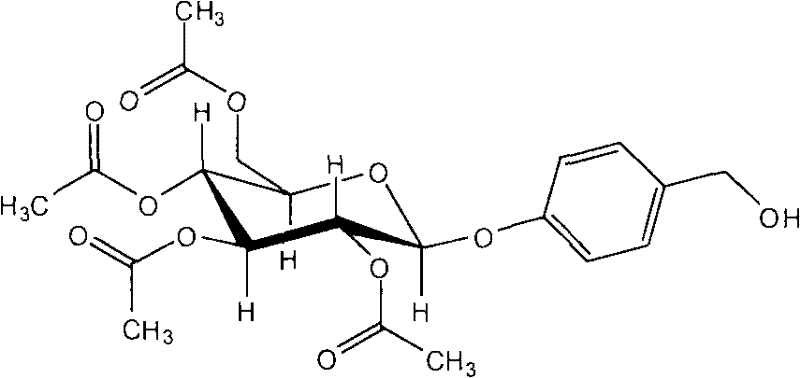
Application of acetagastrodine in preparing medicines to prevent and treat vascular dementia (VD) and Alzheimer disease (AD)
Relating to the medicine field, the invention discloses the use of acetagastrodine in preparing medicines to prevent and treat vascular dementia and Alzheimer disease. The invention also provides a medicinal composition for preventing and treating senile dementia and its capsule as well as sustained release agent. Animal tests show that: acetagastrodine can substantially enhance the learning and memory abilities of rats with vascular dementia, reduce the activity of AchE in brains, enhance the vitality of ChAT in brains, and remarkably reduce Glu content; acetagastrodine has significant protective effect on damaged PC12 cells caused by H2O2 by enhancing the vitality of SOD and total ATP enzyme in PC12 cells and reducing the content of MDA and LD; acetagastrodine can substantially enhance the learning and memorizing abilities of AD model rats, enhance the CAT content in brains of the model rats, reduce the MAO vitality and simultaneously prevent the decrease of neurotransmitters. Acetagastrodine is better than gastrodin in terms of the treatment effects on VD and AD, and enjoys good clinical application prospect.
Owner:KPC PHARM INC
Production method of feed additive 'Acremonium terricola culture'
ActiveCN103734482AShorten the production cycleReduce lossFungiMicroorganism based processesBiotechnologyFeed conversion ratio
The invention discloses a production method of a feed additive 'Acremonium terricola culture'. The production method has the beneficial effects that by researching growth characteristics of Acremonium terricola strains and improving a production process of the Acremonium terricola culture, the production period of the Acremonium terricola culture is shortened from original 11-15 days to 5 days, the production cost of the Acremonium terricola culture is substantially lowered, and the production method has an important significance to the market promotion of the high-end feed additive; meanwhile, the energy loss of water, gas and electricity in the production process is greatly reduced. According to the production method, the production process of the Acremonium terricola culture mainly comprises the steps of 'liquid strain fermentation' and 'solid fermentation'. According to an animal test result, by utilizing the feed additive, the immunities of animals are improved, the feed conversion ratio is lowered, and the meat quality is improved. The production method has the advantages of low cost, short production period, controllability in control, good application effect and the like.
Owner:合肥河川生物医药科技有限公司
Pre-Clinical Animal Testing Method
Devices and methods are provided for wirelessly powering and / or communicating with implanted medical devices used for the controlled exposure and release of reservoir contents, such as drugs or sensors. The device may include a substrate having a plurality of reservoirs containing reservoir contents for release or exposure; and a rechargeable or on-demand power source comprising a local component which can wirelessly receive power from a remote transmitter wherein the received power can be used, directly or following transduction, to activate the release or exposure of the reservoir contents.
Owner:MICROCHIPS INC
Brain-invigorating traditional Chinese medicine composite and preparation method and detection method thereof
ActiveCN101926950AGood brain effectGood effectHeavy metal active ingredientsNervous disorderCistancheAdemetionine
The invention relates to a brain-invigorating traditional Chinese medicine composite and a preparation method thereof. The traditional Chinese medicine composite of the invention comprises the traditional Chinese medicine raw materials in parts by weight: 20-40 parts of Chinese angelica, 5-15 parts of Tabasheer, 15-35 parts of cistanche, 5-15 parts of dens draconis, 15-35 parts of Chinese yam, 5-15 parts of amber, 10-25 parts of Schisandra chinensis, 3-10 parts of gastrodia elata, 2-8 parts of semen boitae, 3-10 parts of Salvia miltiorrhiza, 10-25 parts of fructus alpiniae oxyphyllae, 3-10 parts of ginseng, 5-15 parts of polygala tenuifolia, 3-10 parts of chrysanthemum, 5-15 parts of Irkutsk Anemone Rhizome, 5-15 parts of ochre, 5-15 parts of arisaema cum bile, 35-60 parts of spina date seed and 15-30 parts of barbary wolfberry fruit. The invention also provides a detection method for the composite according to the components of the traditional Chinese medicine composite. The traditional Chinese medicine composite of the invention has good brain-invigorating effect. The animal testing proves that the traditional Chinese medicine composite of the invention has the functions of accelerating sleep, resisting fatigue and strengthening humoral immunity, can be used for invigorating brain, improving intellectual development, nourishing heart and soothing the nerves, and can be used for treating brain overstrain, fading memory, neurasthenia, light-headedness, fright insomnia, feeling of oppression over the chest, easy tiredness and Senile Dementia. The detection method has good ruggedness and high sensitivity.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Vaccine adjuvant
InactiveCN101444623AExcellent allergenicityExcellent securityAntibody medical ingredientsZinc hydroxideBody fluid
The invention relates to a vaccine adjuvant, in particular to the application of compound zinc hydroxide in the aspect of vaccine adjuvant preparations and further the application in the aspect of VAOTA adjuvant preparation.s The zinc hydroxide has a chemical formula of Zn(OH)2 and a chemical formula weight of 99.4046 Dalton. The zinc hydroxide working as an adjuvant is jointly applied with vaccine, so as to effectively strengthen body fluid immune response of the vaccine, and the immune strength effect is superior to that of aluminum adjuvant. In addition, according to the animal experiment result, both the sensitization and the safety of zinc hydroxide are superior to aluminum adjuvant.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
O-type aftosa synthetic peptide vaccine
ActiveCN101659695AImprove protectionFree from attackAntiviralsPeptide preparation methodsChemical synthesisPeptide vaccine
The invention provides O-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3. The O-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites ofaftosa and combining with computer assistant to carry out antigen site analytical prediction. Candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments and aftosa virus antigen sites are optimized according to the screening result; and T cell epitope and B cell epitope are effectively combined to improve the immune effects of the polypeptide antigens. TheO-type aftosa synthetic peptide vaccine can effectively cope with the antigen variation of aftosa virus and has ideal biosafety and easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
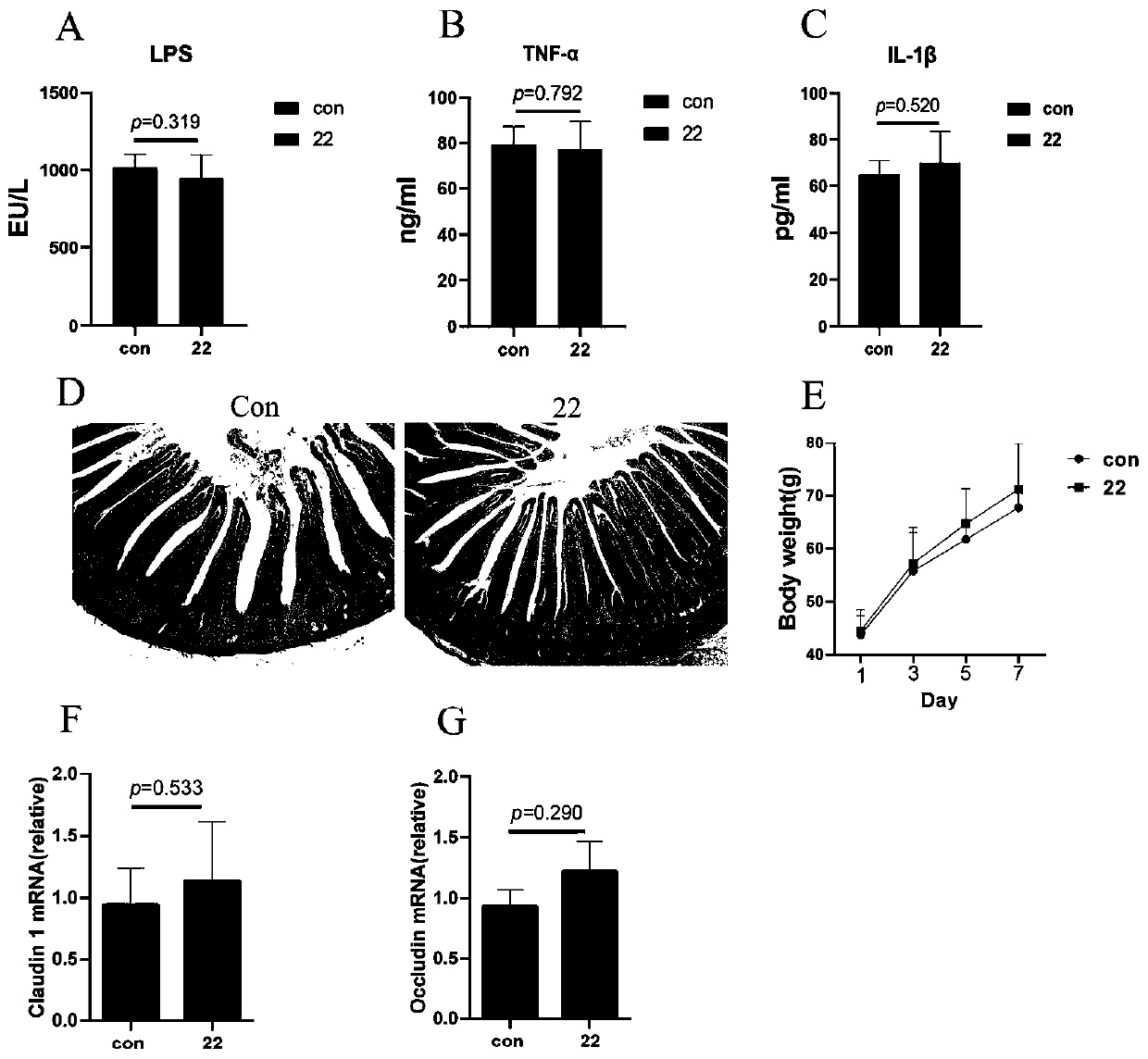
Lactobacillus reuteri 22 and application thereof
ActiveCN110540950AGrowth inhibitionImprove adhesionBacteriaMicroorganism based processesInflammatory factorsBiotechnology
The invention discloses a lactobacillus reuteri 22 and application thereof. The lactobacillus reuteri 22 disclosed by the invention is separated from healthy chicken flocks, is classified and named aslactobacillus reuteri, and has a preservation number of CGMCC No.17932. By detecting the performance of probiotics, the invention finds that the lactobacillus reuteri 22 has good acid resistance, cholate resistance and adhesion performance, and can inhibit growth of pathogenic bacteria. Through Animal assays, in a healthy physiological state, the lactobacillus reuteri 22 does not influence the level of inflammatory factors, but can increase the number of goblet cells and promote the increase of expression levels of related genes such as compact protein, defensin and lysozyme, can increase thelength of intestinal villi and the depth of crypt, and has the potential of maintaining intestinal mucosa barriers. Therefore, the strain is expected to be developed into probiotics for maintaining achicken intestinal mucosa barrier and guaranteeing green and healthy chicken breeding.
Owner:NANJING AGRICULTURAL UNIVERSITY
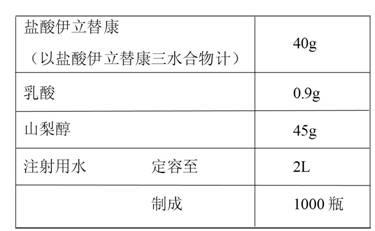
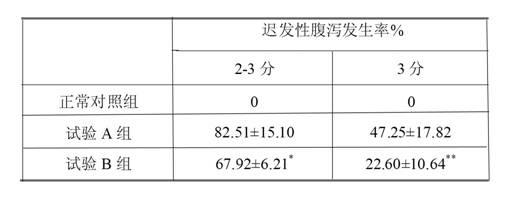
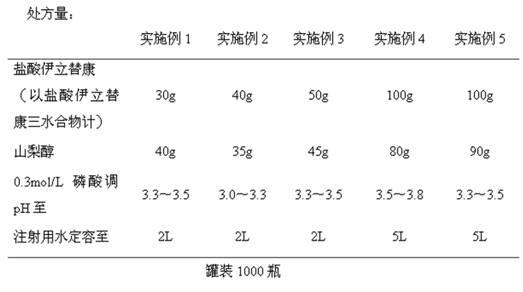
Irinotecan hydrochloride injection and preparation method thereof
ActiveCN102670500AReduce adverse reactionsPromote drug dependenceOrganic active ingredientsPharmaceutical delivery mechanismO-Phosphoric AcidAnimal testing
The invention relates to an irinotecan hydrochloride injection consisting of irinotecan hydrochloride (15-25g / L), sorbierite (17.5-22.5g / L), and the balance of phosphoric acid and injection water, wherein the phosphoric acid adjusts the pH of the injection to 3.0-3.8. The invention also relates to a preparation method of the irinotecan hydrochloride injection. Compared with the prior art, the irinotecan hydrochloride injection disclosed by the invention has the following beneficial effects: (1) the untoward effect is reduced; as shown in an animal test, the irinotecan hydrochloride injection disclosed by the invention can greatly reduce the untoward effect of delayed-onset diarrhea caused by irinotecan, which has an active promotion effect on the drug dependency and treatment effect of cancer patients; (2) the stability is high, as shown in an acceleration test, the character, acidity, solution clarity and color, insoluble particles, visible foreign matters, moisture, related substance and content of the irinotecan hydrochloride injection meet the regulations; and (3) the preparation method is simple and is favorable to the industrial mass production.
Owner:NANJING CHENGONG PHARM CO LTD
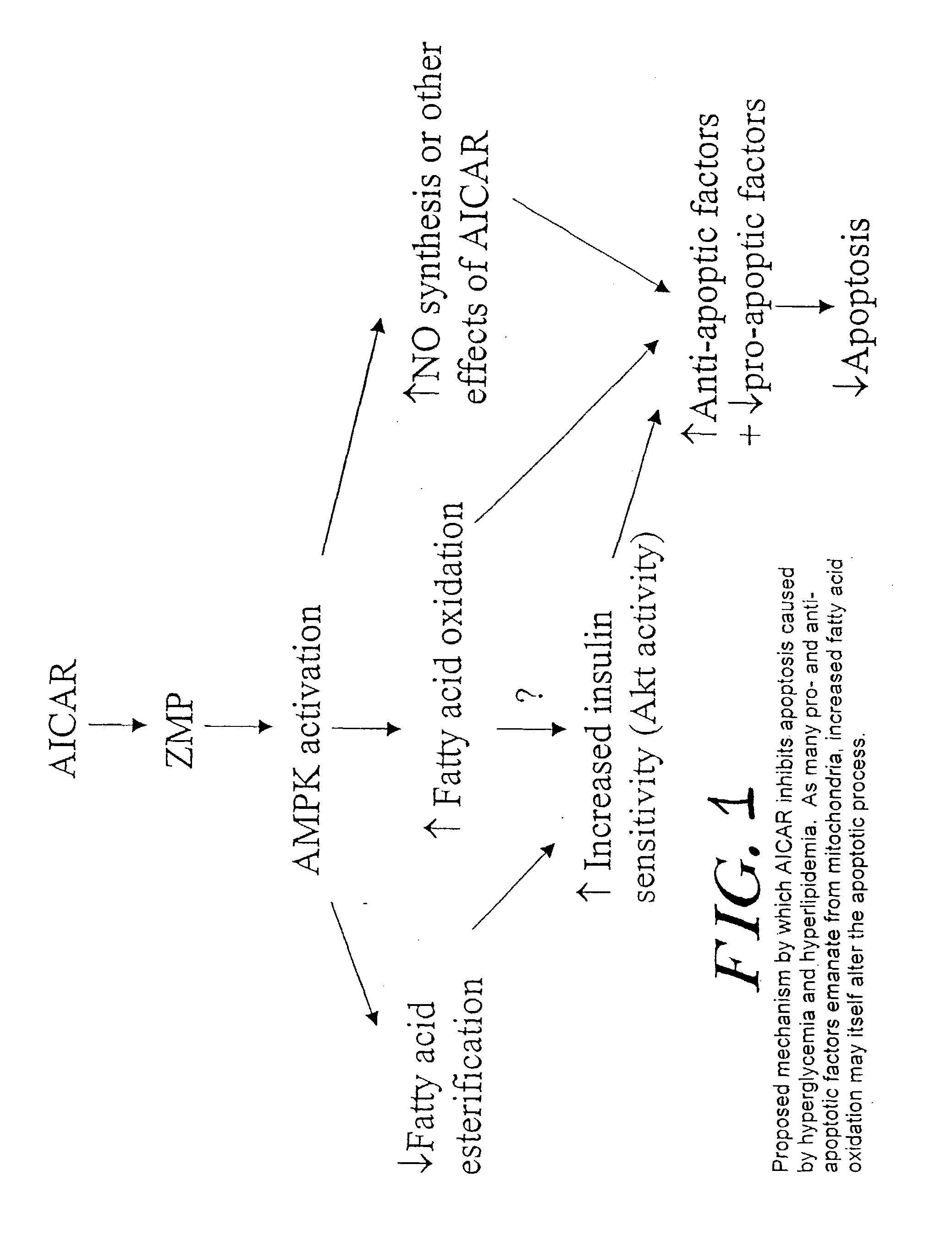
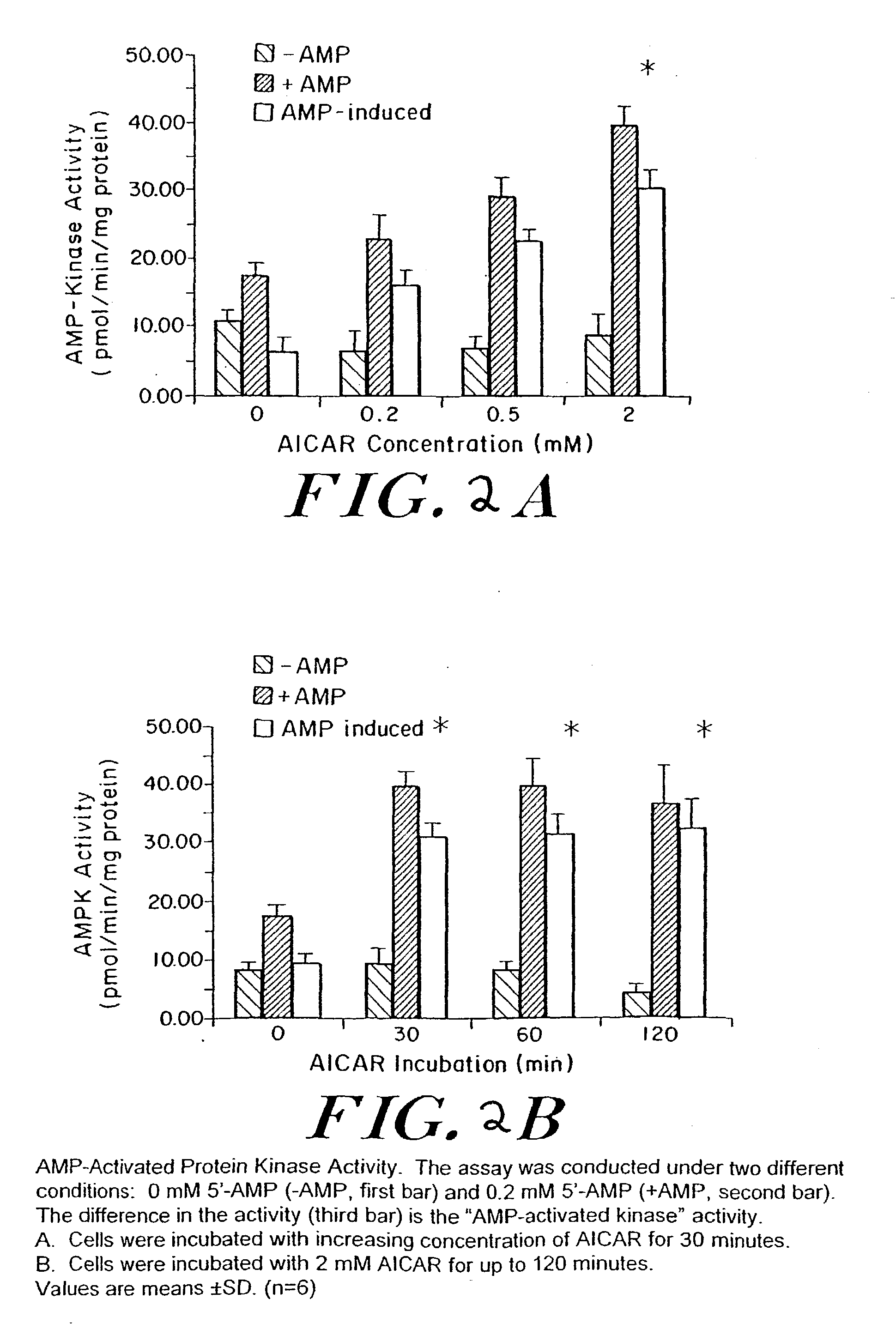
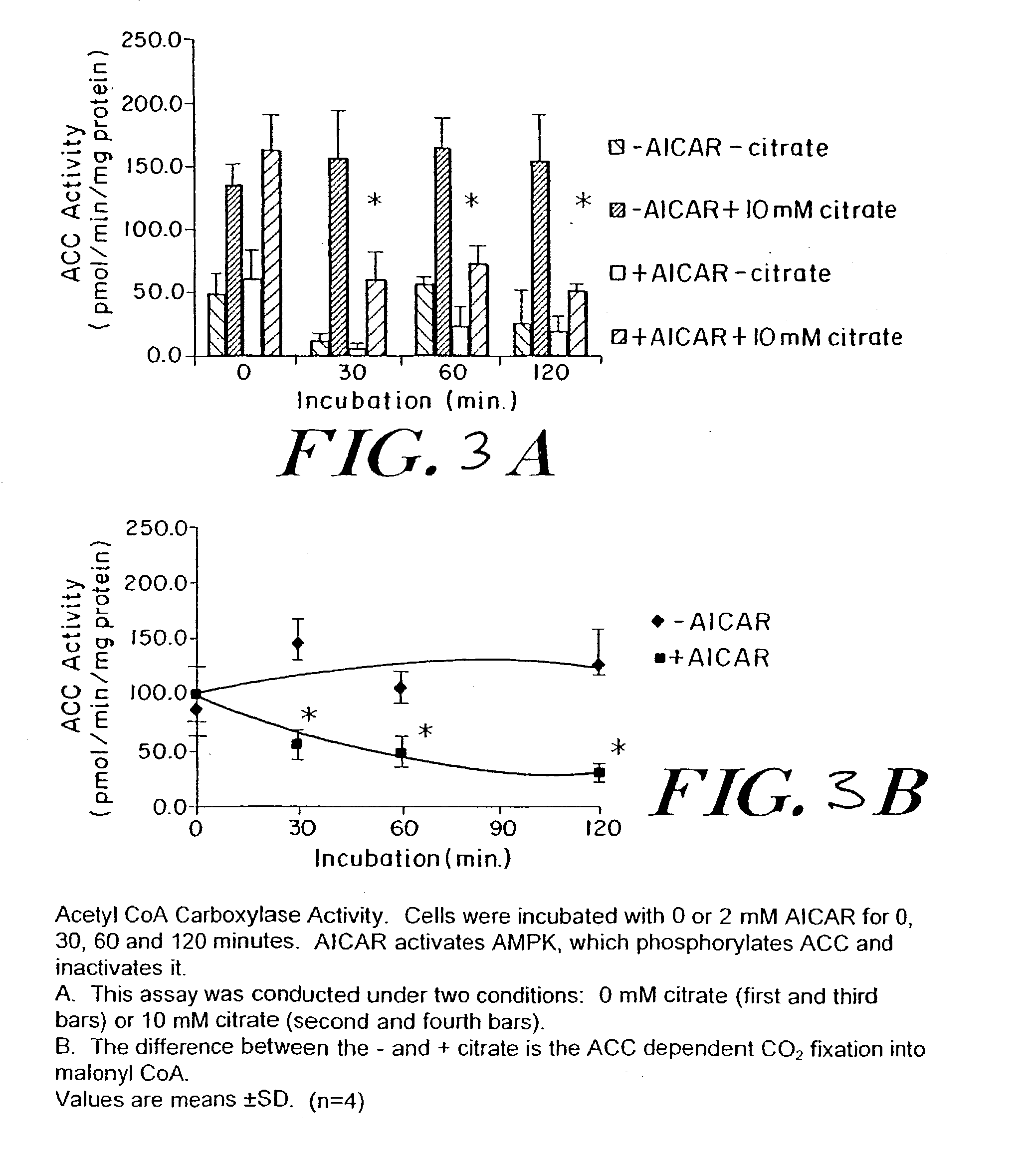
Methods fo treating conditions associated with insulin resistance with aicar, (5-amino-4-imidazole carboxamide riboside) and related compounds
InactiveUS20030212014A1Positive impact in reducing obesityIncrease insulin sensitivityBiocideCompound screeningMammalDisease cause
The long-term usage of AICR (5-aminio, 4-imidazole carboxamide riboside) to produce sustained metabolic and biological changes in mammals that overcome insulin resistance, i.e., increase insulin sensitivity, and result in benefits in diseases and conditions such as diabetes, hypertension, atherosclerosis, polycystic ovary syndrome and gallstones is described long-term usage of AICAR, particularly intermittent administration, e.g., three days per week, appears to have some of the positive effects of exercise, having an impact on the amount Of food consumed by a subject and resulting in reduced fat build-up and increase in muscle mass. Therefore, AICAR administration has a positive impact in reducing obesity. AICAR can also Prove useful in preventing or treating vascular diseases associated with hyperglycemia, high plasma levels of free fatty acids (FFA) and triglyceride, and insulin resistance by virtue of the fact that this agent activates fatty acid oxidation. Animal tests have Shown that chronic intermittent treatment with AICAR has not resulted in any noticeable toxic effects. AICAR and related compounds are activators of AMP-activated protein kinase (AMPK) and, furthermore, are effective at decreasing malonyl CoA levels in the animal.
Owner:UNIV BOSTON TRUSTEES OF THE +1
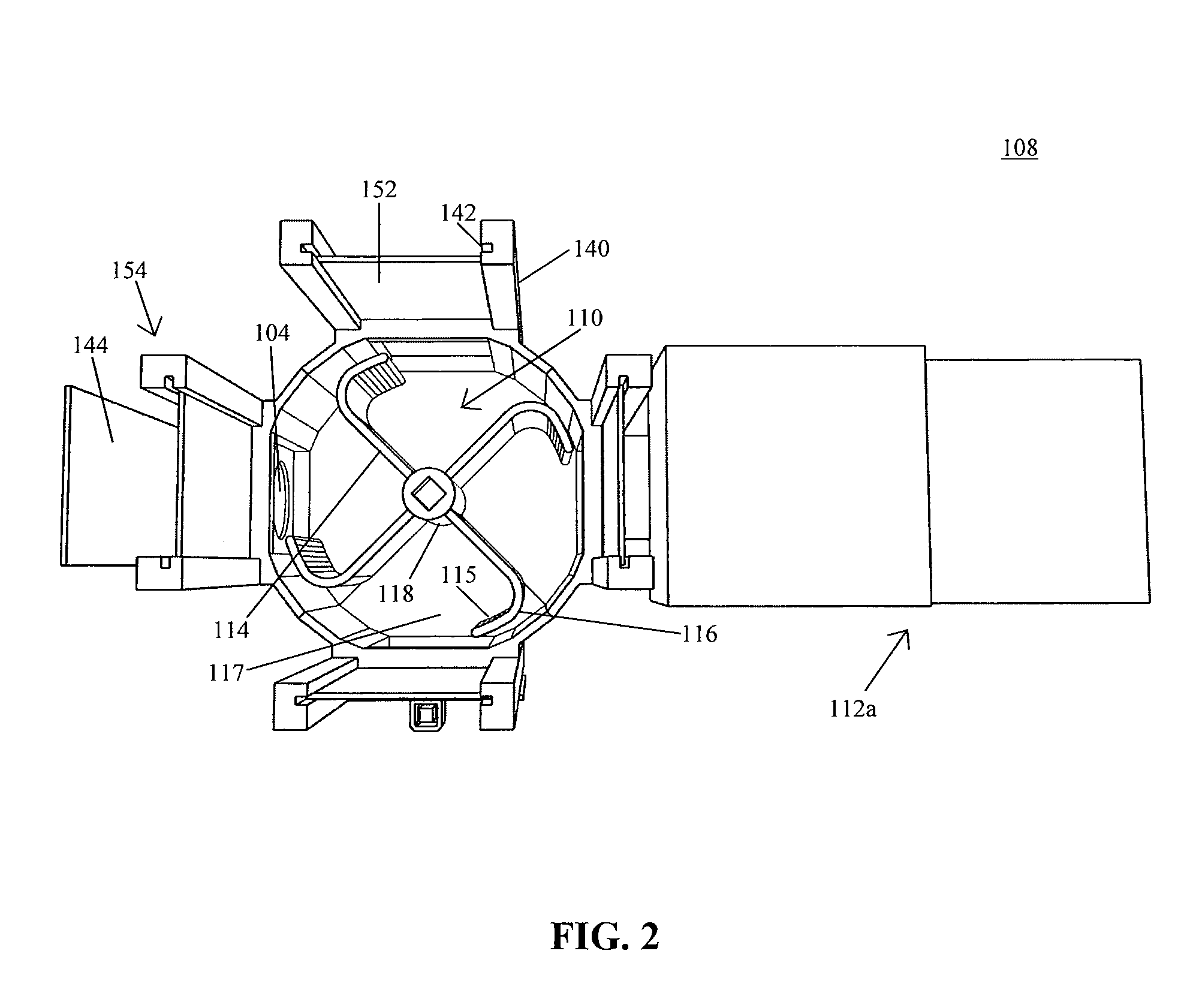
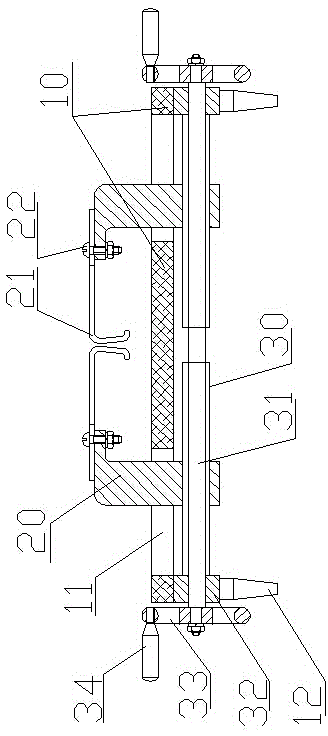
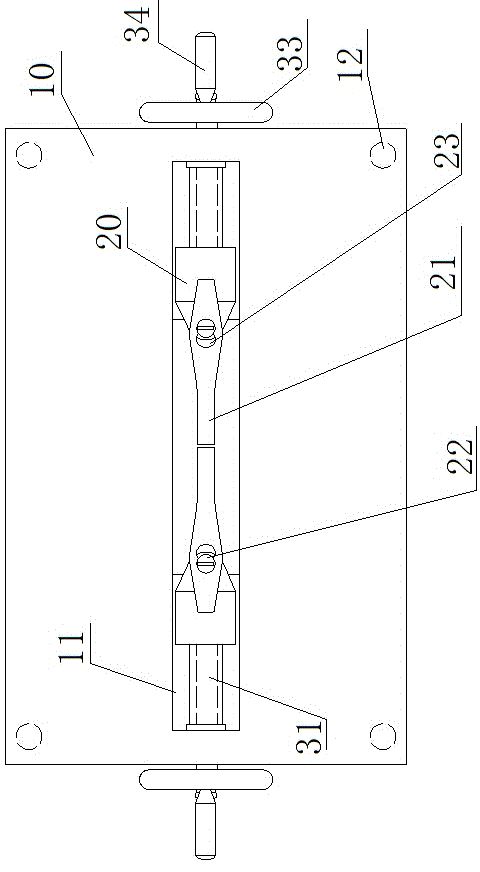
Novel retractor of animals such as rabbits and mice
InactiveCN104146791AReduce bleedingReduce mortalitySurgeryAnimal fetteringAnimal scienceAbdominal cavity
The invention belongs to the field of animal experiment instruments and particularly relates to a novel retractor of animals such as rabbits and mice. The retractor comprises an operation table plate (10). Column feet (12) are arranged at the bottom of the operation table plate (10). The two ends of the operation table plate (10) are respectively provided with a guiding groove (11) at the same horizontal line. The guiding grooves (11) are in sliding connection with a support (20). A retractor hook (21) is arranged above the support (20). According to the novel retractor, mechanical transmission is used for replacing manpower for fixing animal thoracic cavities and abdominal cavities for opening, manpower is saved, stability is good, an operation view field is large, animal bleeding amount is low, and the death rate of animals during operation is lowered.
Owner:HUANGHE S & T COLLEGE
Synthetic peptide vaccine and preparation method thereof
InactiveCN101565457AImprove protectionImprove immunityVirus peptidesAntiviralsChemical synthesisSite analysis
The invention provides aftosa synthetic peptide vaccine, more specifically to polypeptide or polypeptide polymer used for O type aftosa synthetic peptide vaccine, vaccine containing the polypeptide orpolypeptide polymer and a preparation method thereof. The polypeptide has amino acid sequence represented by SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3. By sequencing of recent aftosa strain in China an d combination of aftosa vaccine strain OS / 99 and OZK / 93 sequence, the invention researches variation case of main antigen site, carries out antigen site analysis and forecast assisted by computer, andcarries out chemical synthesis to possible antigen site peptide segment. The invention screens polypeptide antigen through a large amount of animal tests, optimizes aftosa virus antigen site accordin g to screening result, effectively combines T cell epitope and B cell epitope, and enhances immunization effect of the polypeptide antigen. The O type aftosa synthetic peptide vaccine can effectively reply antigenic variation of aftosa virus, has good biological safety, is easy for large scale production, and has better application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Extraction method and application of moringa oleifera leaf total flavones
The invention belongs to the chemical field of natural products and in particular relates to an extraction method and an application of moringa oleifera leaf total flavones. The extraction method adopts microwave and ultrasound to synergistically extract the moringa oleifera leaf total flavones, and has the advantages of high efficiency, time saving, energy conservation, low energy consumption, simple operation and good repeatability. Besides, an animal test proves that the extracted moringa oleifera leaf total flavones have a significant treatment effect on hyperuricosuria and Alzheimer's disease. The total flavones provided by the invention can reduce uric acid, and can also reduce cholesterol, triacylglycerol, urea nitrogen, creatinine and xanthine oxidase, reduce the generation of uric acid, directly decompose uric acid, improve the renal function, promote uric acid excretion, and protect multiple levels of blood vessels to reduce uric acid, so that the moringa oleifera leaf total flavones are beneficial for healing of lithemia patients and have a broad medical application prospect.
Owner:萧丽雅
Method for producing medicine using silkworm expressed numan epidermal growth factor
InactiveCN1405310ADetermine therapeutic functionPeptide/protein ingredientsMicroencapsulation basedDiseaseCuticle
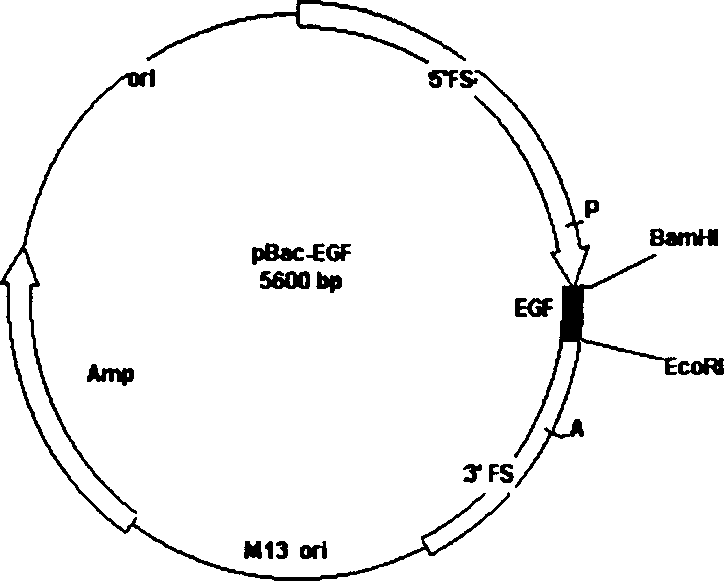
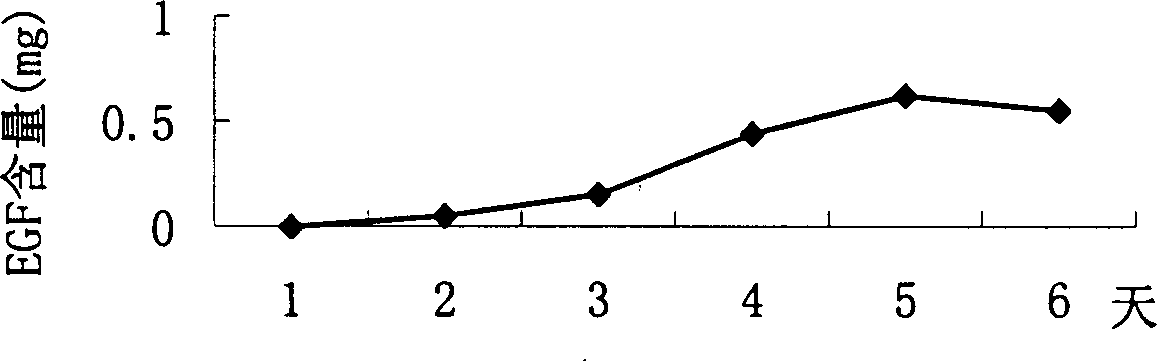
The invention belongs to the technique field of producing multi-peptide drugs by gene engineering. It clones human EGF gene into the silkworm bacilliform virus (Bombyx mori Nuclear polyhedrosis Virus) transferring carrier pBacPAK8 and makes them carry through homologous reformation in the silkworm cells to obtain the reformed virus BmBacEGF with human-coat growing gene. Use the reformed virus to to inocualte the silkworm grub and chrysalis by acupuncture in order to make the human-coat growing gene efficient expressed. Then make the grub and chrysalis into oral drugs which has remarkable action on the digestible ulcer by examination on animal.
Owner:正源堂(天津)生物科技有限公司
New strain of pseudoalteromonas flavipulchra and use thereof
InactiveCN102304484AAchieve antibacterial effectImmune boosterBacteriaMicroorganism based processesBiotechnologyAnimal testing
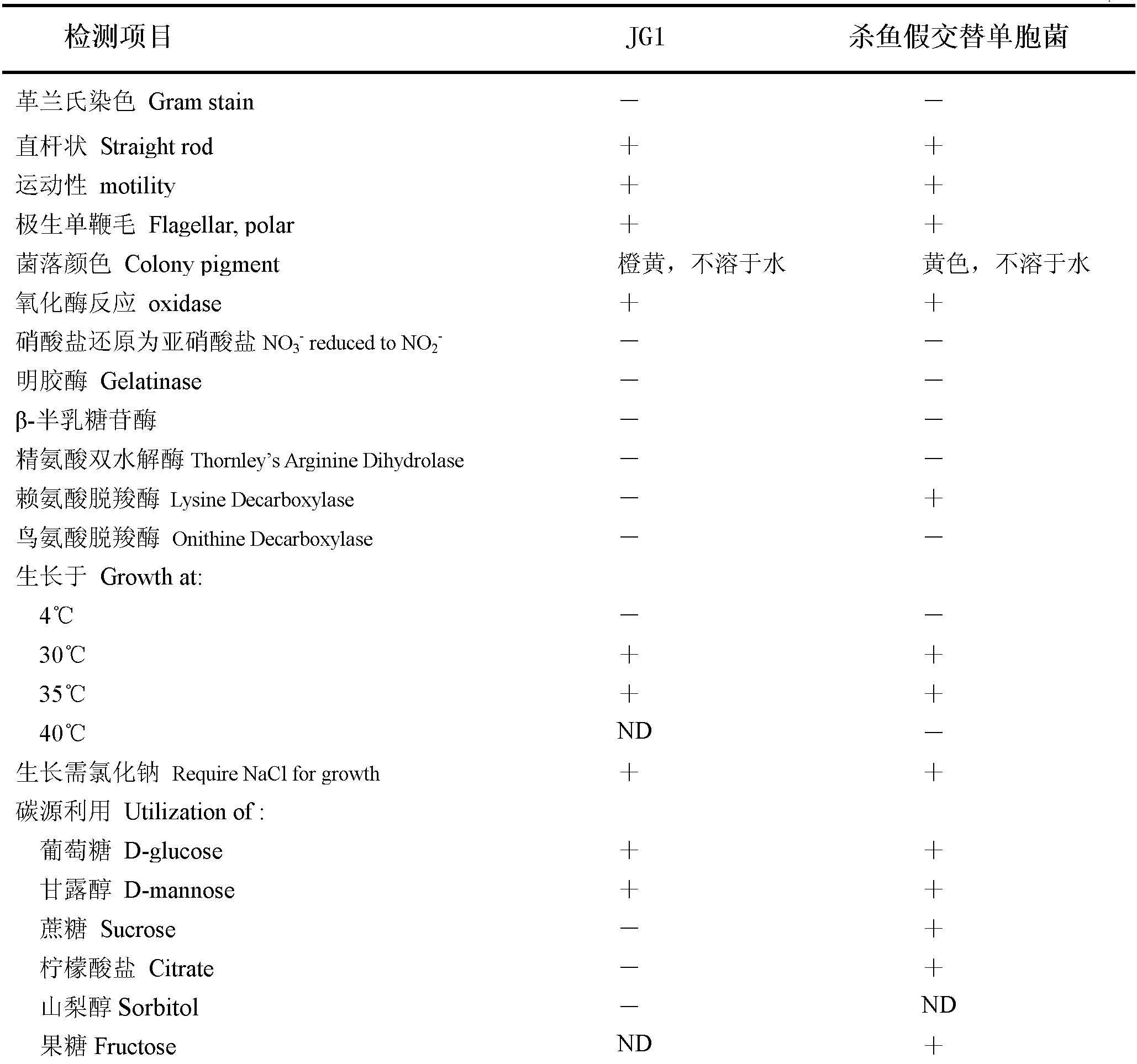
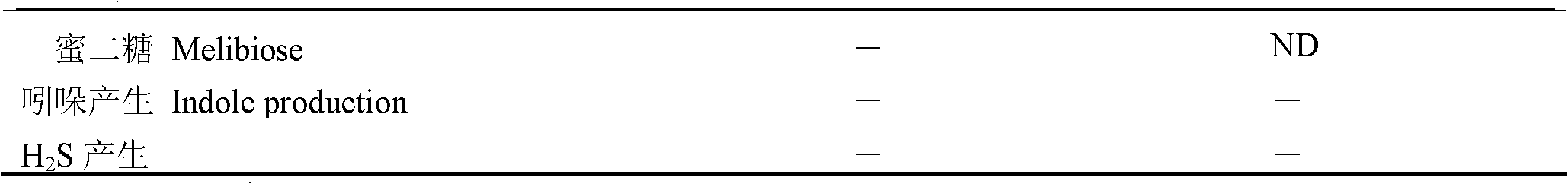
The invention relates to a new strain of pseudoalteromonas flavipulchra and use thereof. The new strain is the JG1 strain of the pseudoalteromonas flavipulchra, and the collection number of the JG1 strain of pseudoalteromonas flavipulchra is CGMCC No.5009; and the JG1 strain produces Pfa protein during metabolism. The JG1 strain of pseudoalteromonas flavipulchr, which is disclosed by the invention, can achieve antibacterial effects by means of bacteriocin protein, small molecular compounds and the like and can inhibit various common pathogenic bacteria in aquaculture animals; and the results of animal tests on zebra fish, mantis shrimps, scallops, clams and the like indicate that JG1 has neither toxic nor side effects on animals and that the bacterial preparation prepared by using the JG1 can be effectively used in actual production as a synergist in aquiculture. The Pfa protein produced in the metabolism of the JG1 strain has an obvious antibacterial effect and can be used as a preparation for improving the immunity in aquaculture animals.
Owner:OCEAN UNIV OF CHINA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com