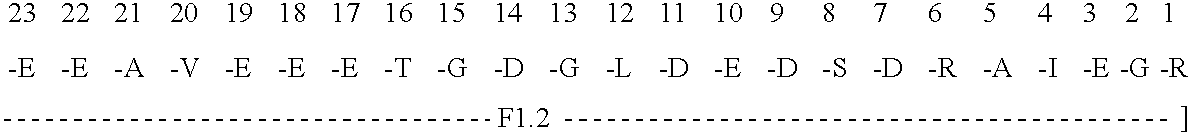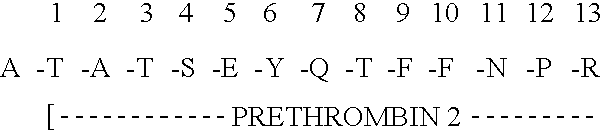Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
216 results about "Prothrombinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
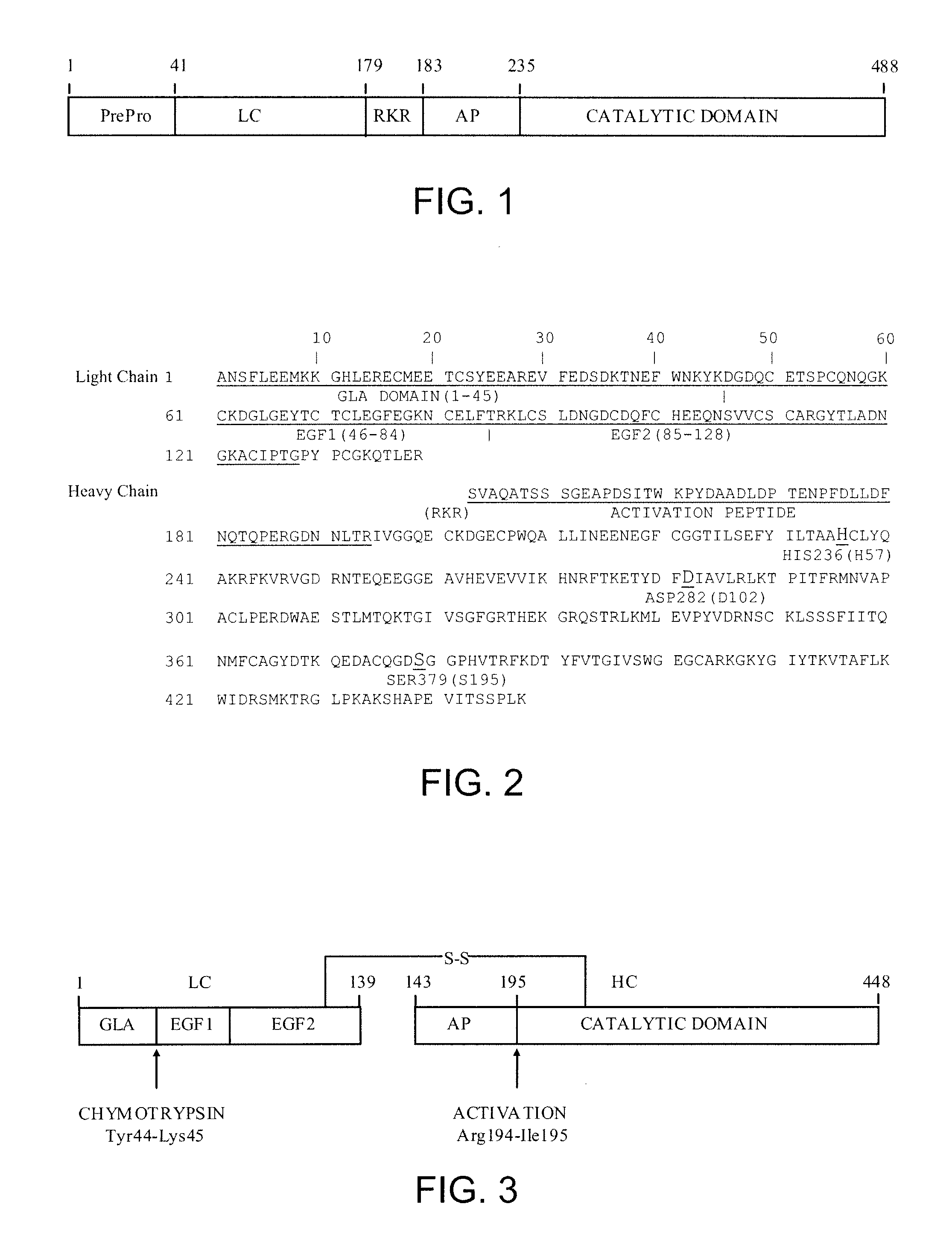
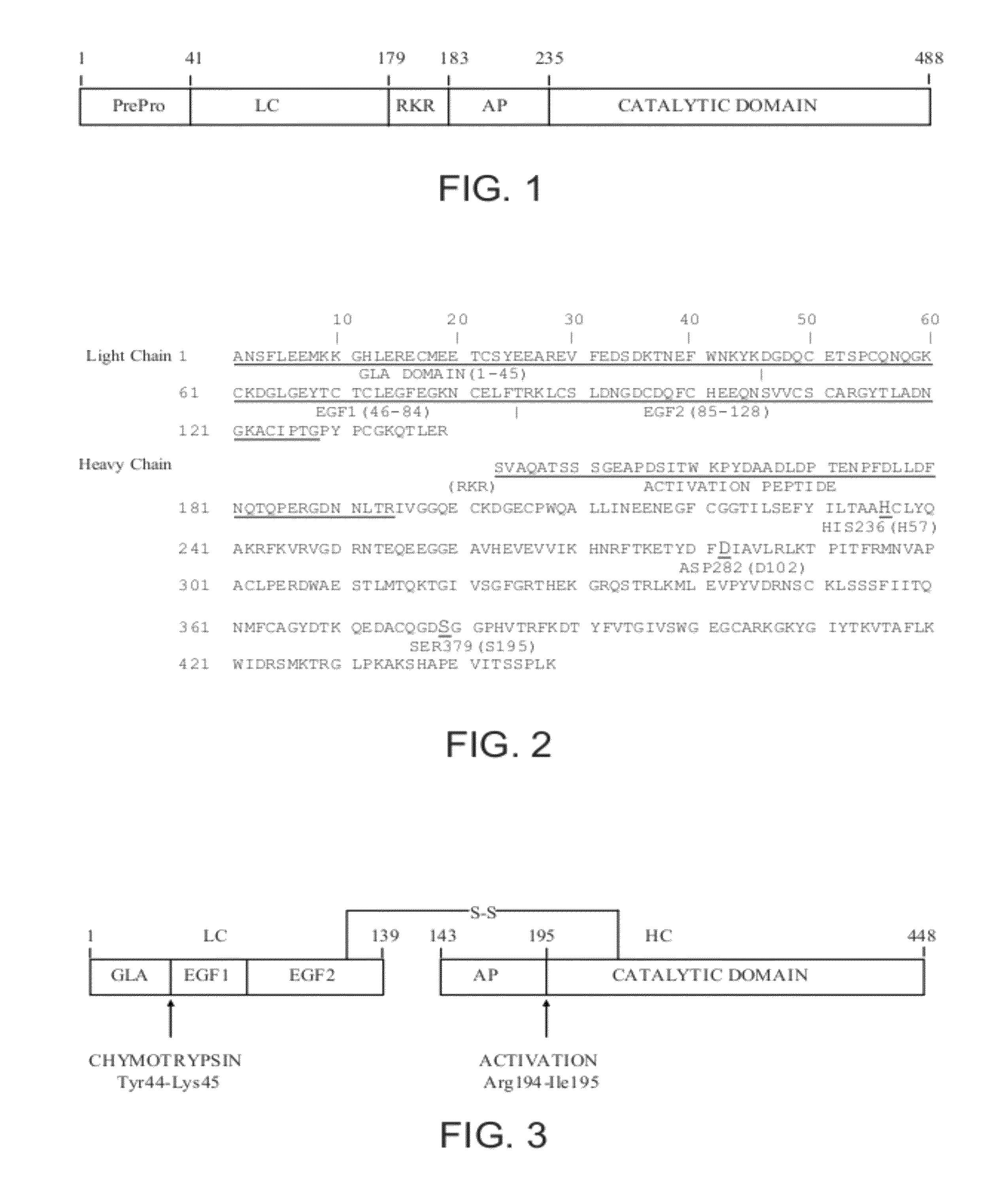
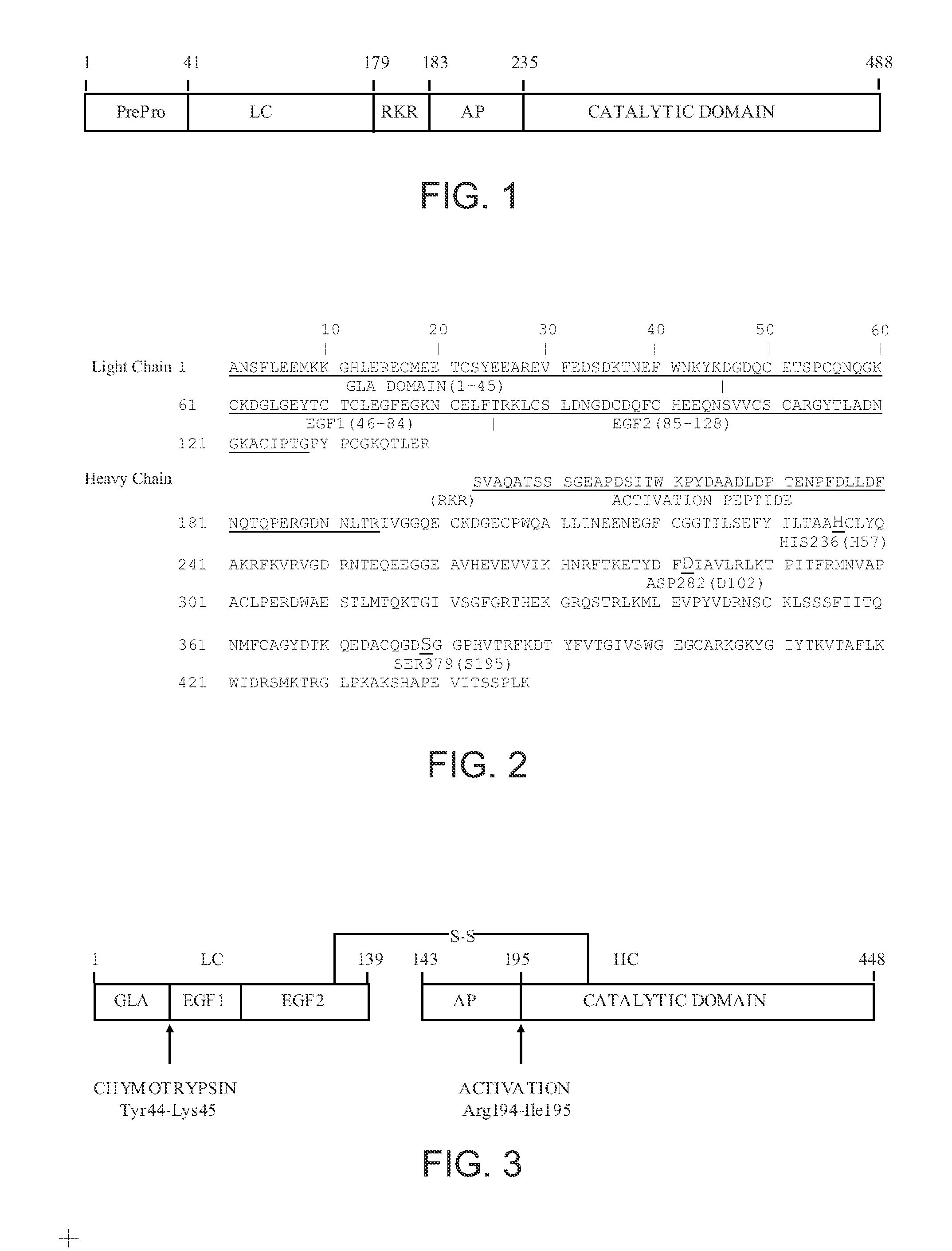
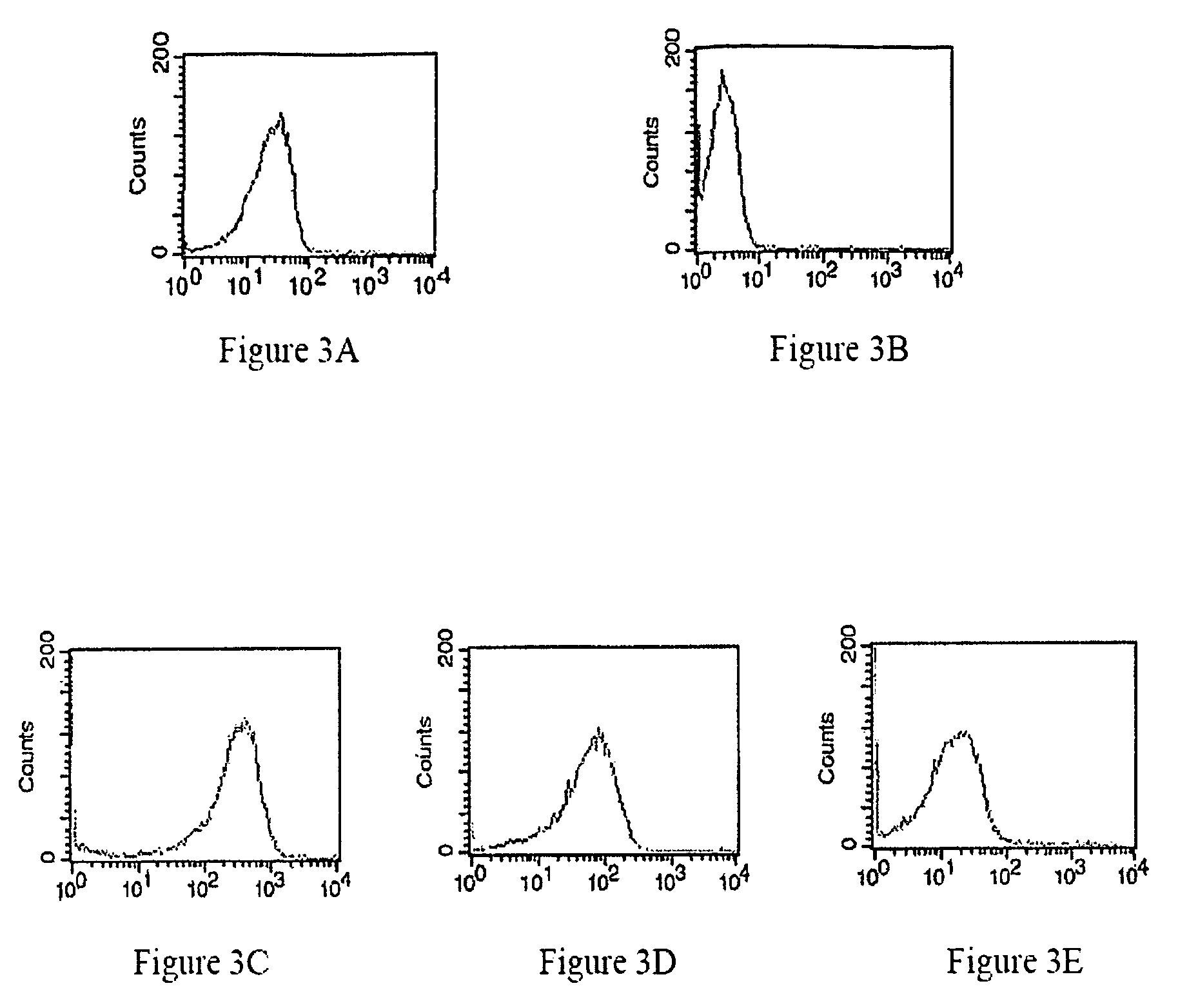
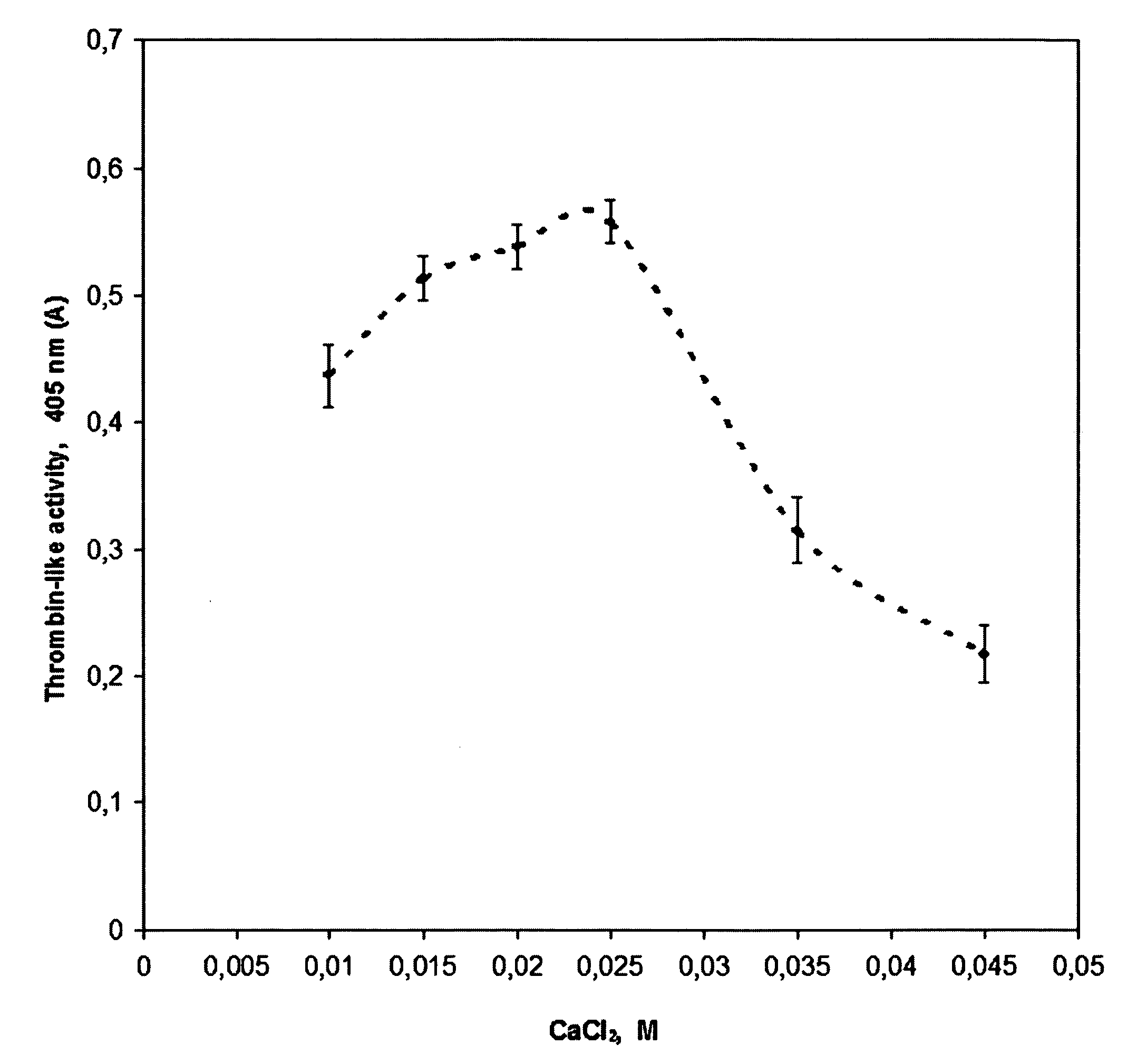
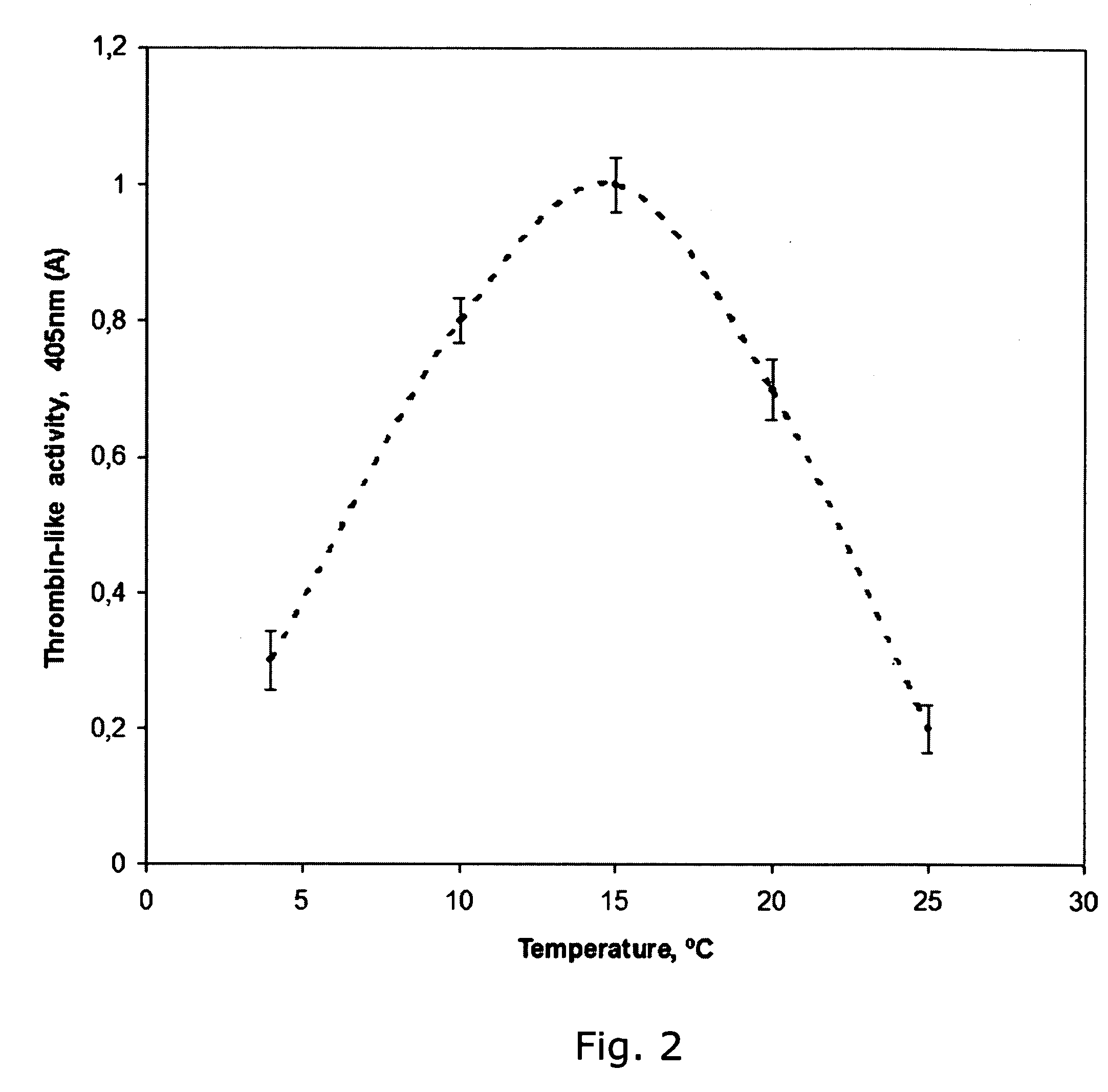
The prothrombinase complex consists of the serine protein, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (Factor II), an inactive zymogen, to thrombin (Factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg²⁷¹ and the other after Arg³²⁰. Although it has been shown that Factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 10⁵-fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
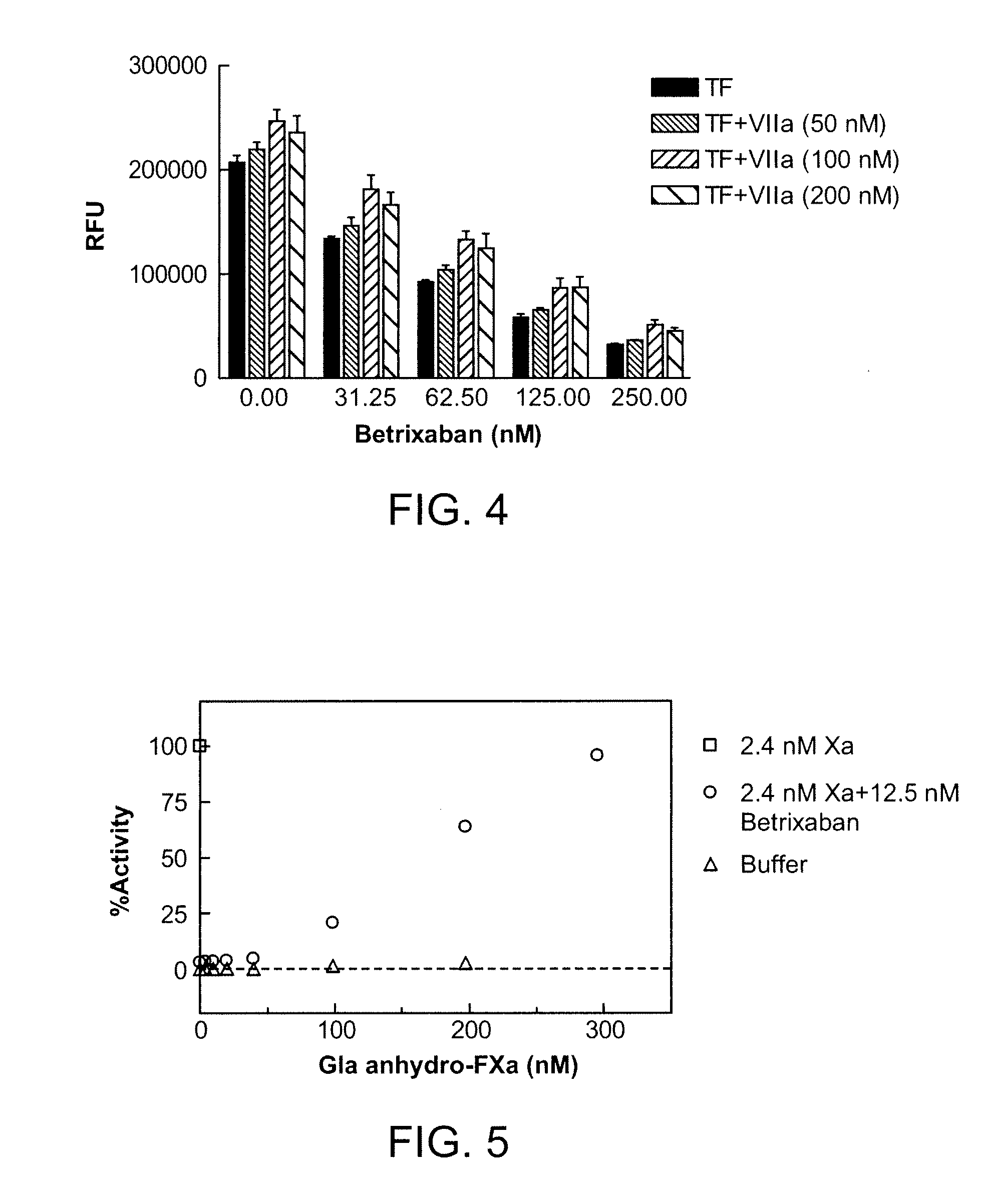
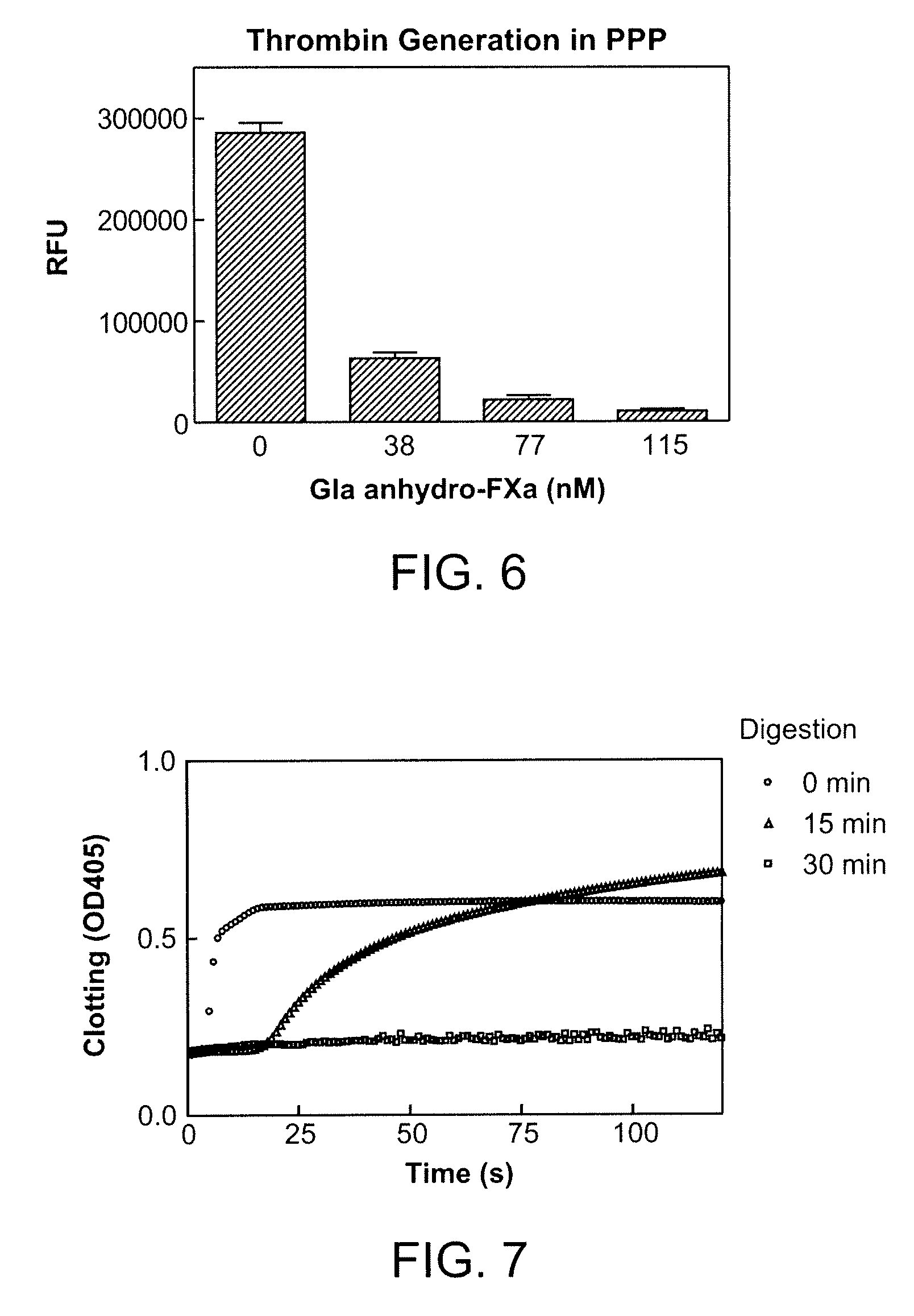
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
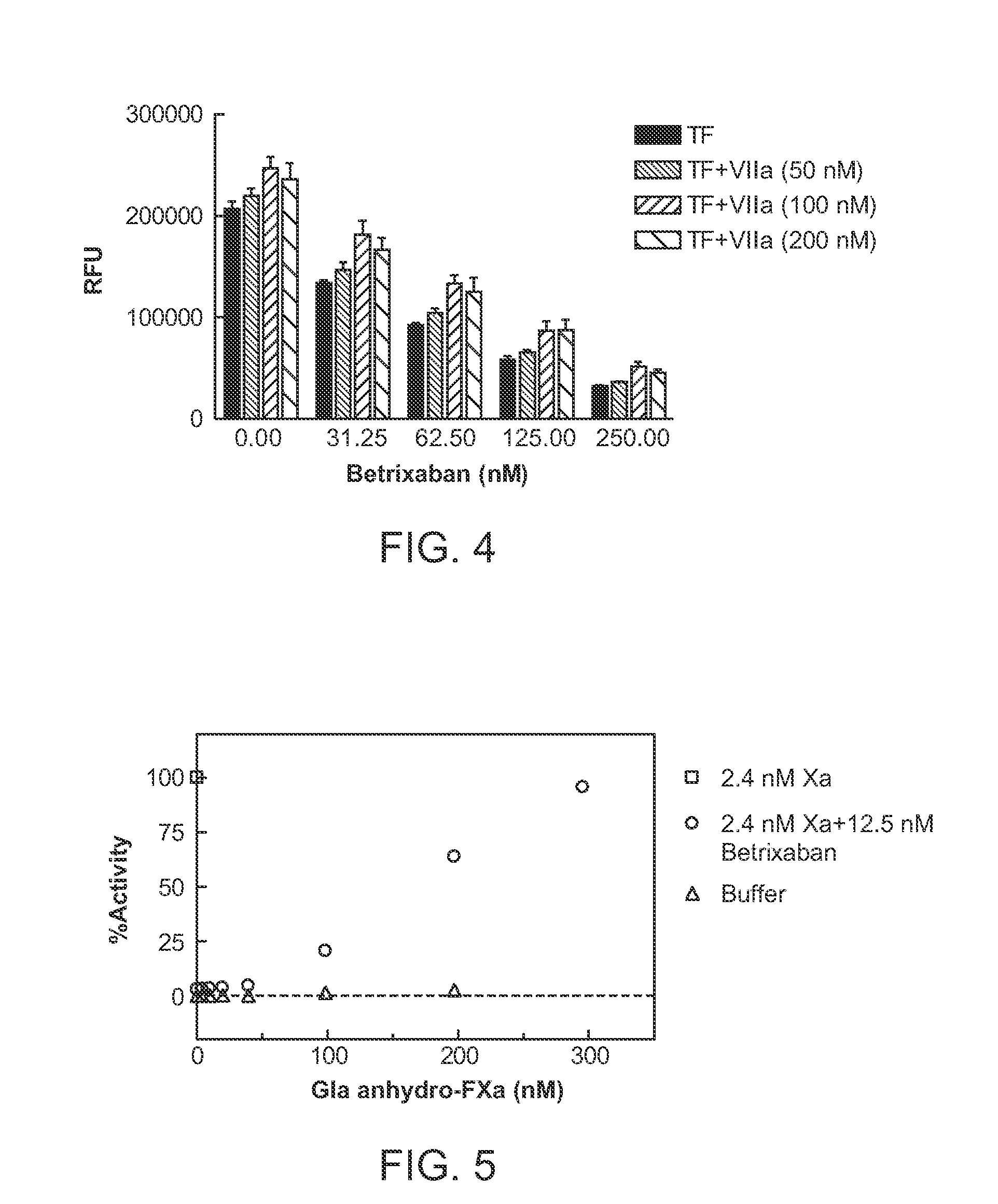
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
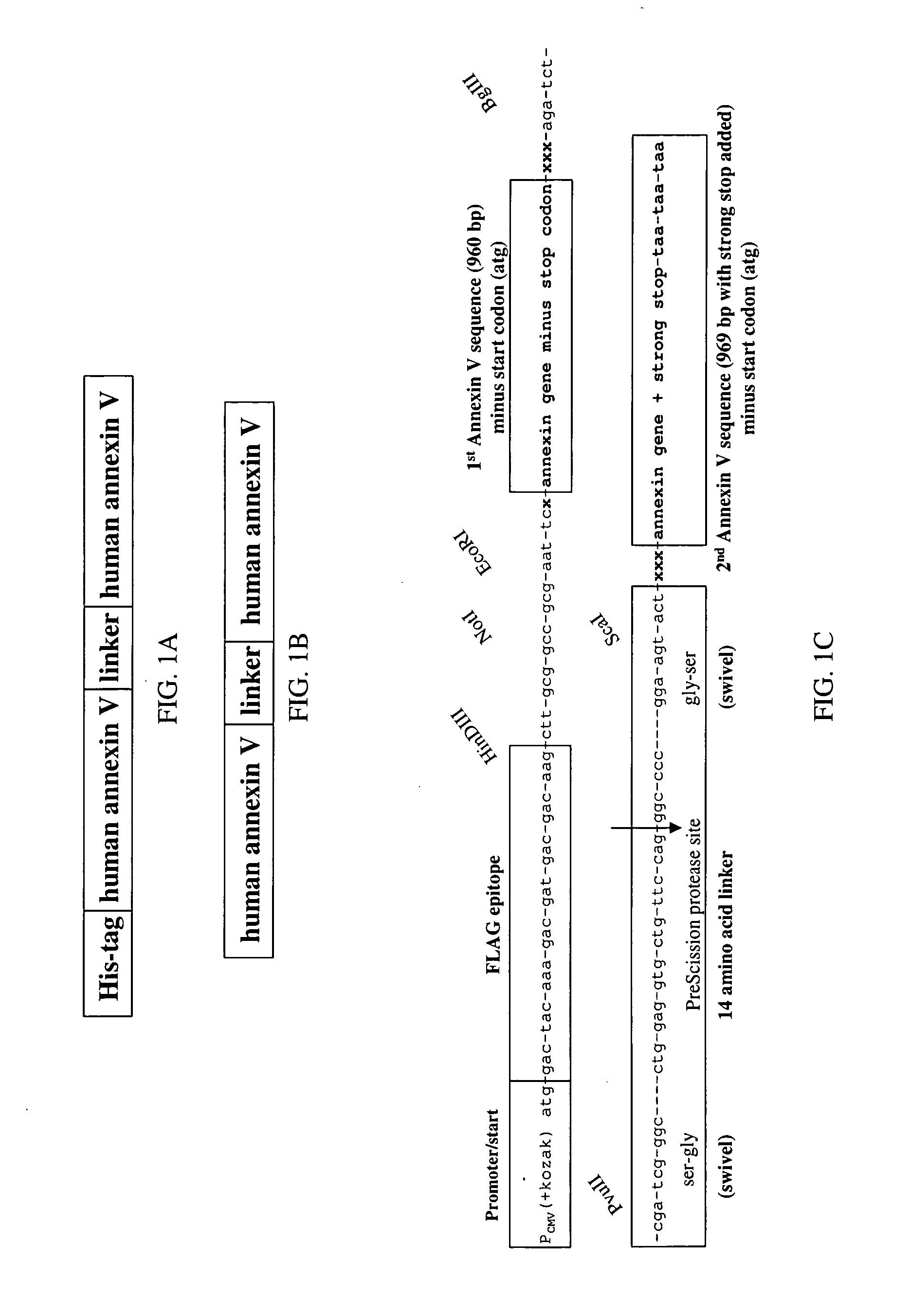
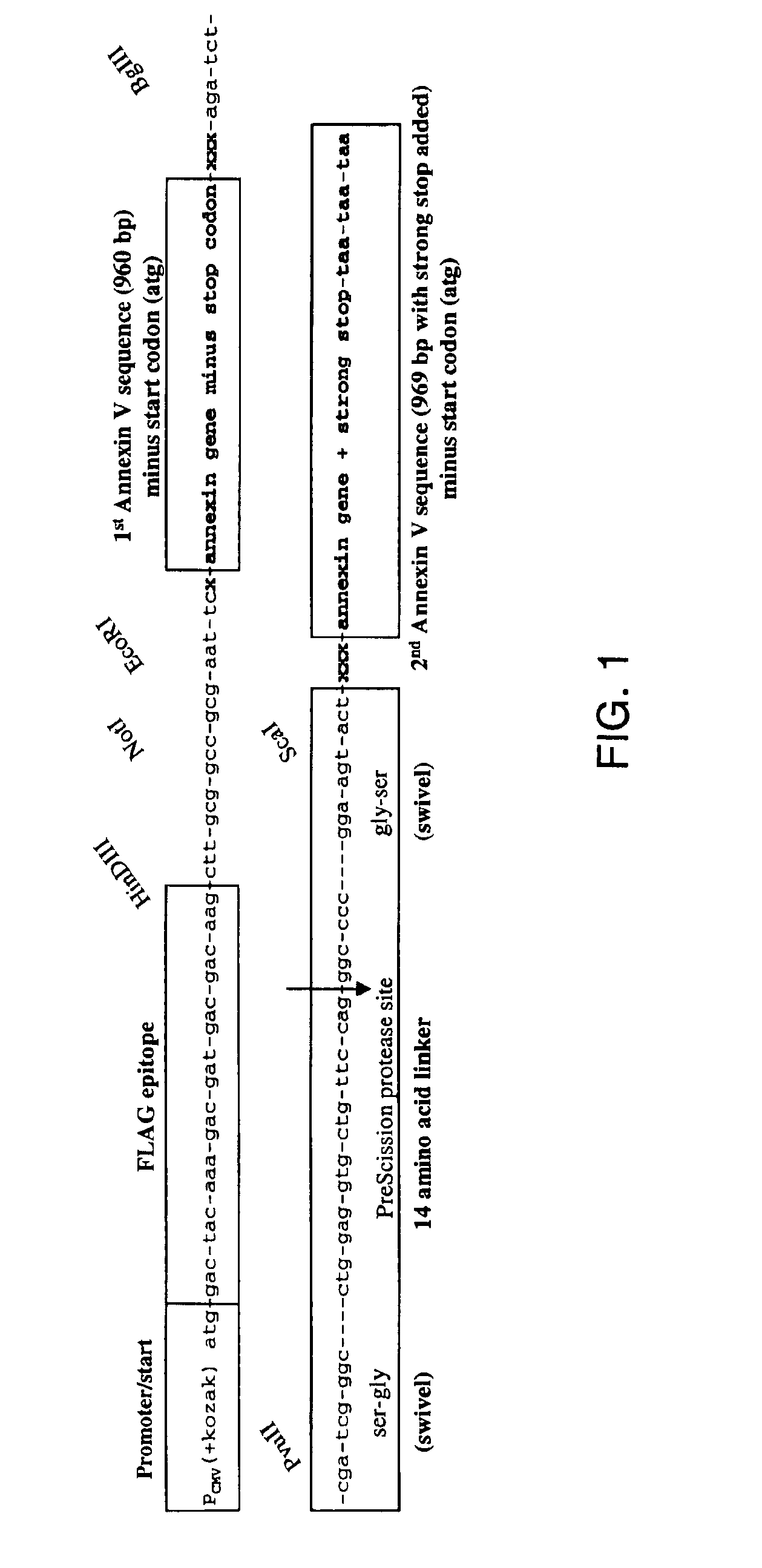
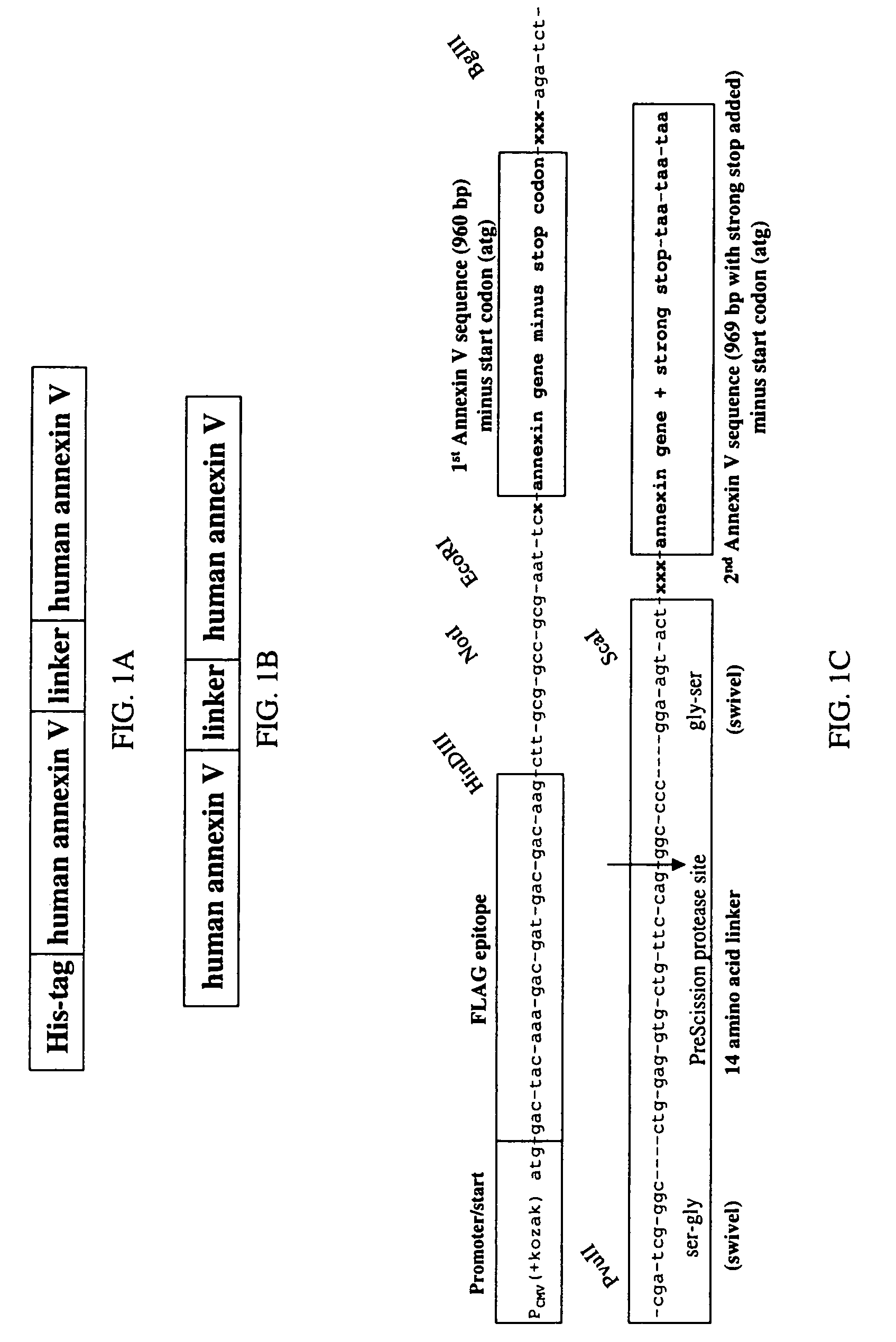
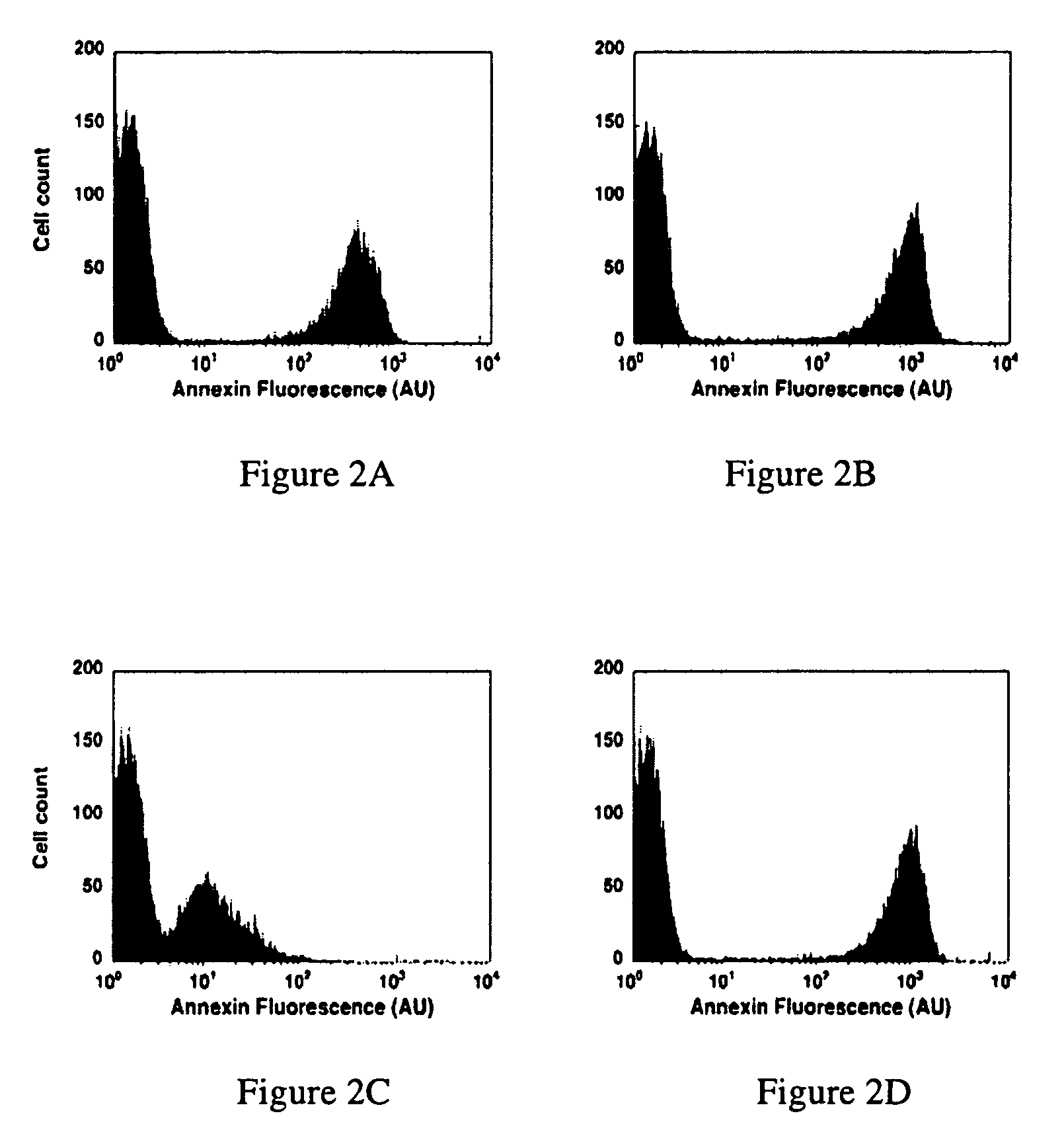
Modified annexin proteins and methods for preventing thrombosis
InactiveUS20050222030A1Inhibition of attachmentReducing endothelial cell damagePeptide/protein ingredientsAntibody mimetics/scaffoldsTreatment effectSufficient time
A modified annexin protein, preferably annexin V, is used to prevent thrombosis without increasing hemorrhage. Annexin binds to phosphatidylserine on the outer surface of cell membranes, thereby preventing binding of the prothrombinase complex necessary for thrombus formation. It does not, however, affect platelet aggregation necessary for hemostasis. The modified annexin molecule can be a homodimer of annexin, an annexin molecule coupled to one or more polyethylene glycol chains, or an annexin molecule coupled to another protein. By increasing the molecular weight of annexin, the modified annexin is made to remain in circulation for sufficient time to provide a sustained therapeutic effect.
Owner:ALAVITA PHARMA
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8153590B2Reduces and removes anticoagulant effectReduced activityPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8268783B2Reduces and removes anticoagulant effectReduced activityBiocidePeptide/protein ingredientsFactor XAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20100255000A1Preventing and reducing bleedingReduces and removes anticoagulant effectPeptide/protein ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
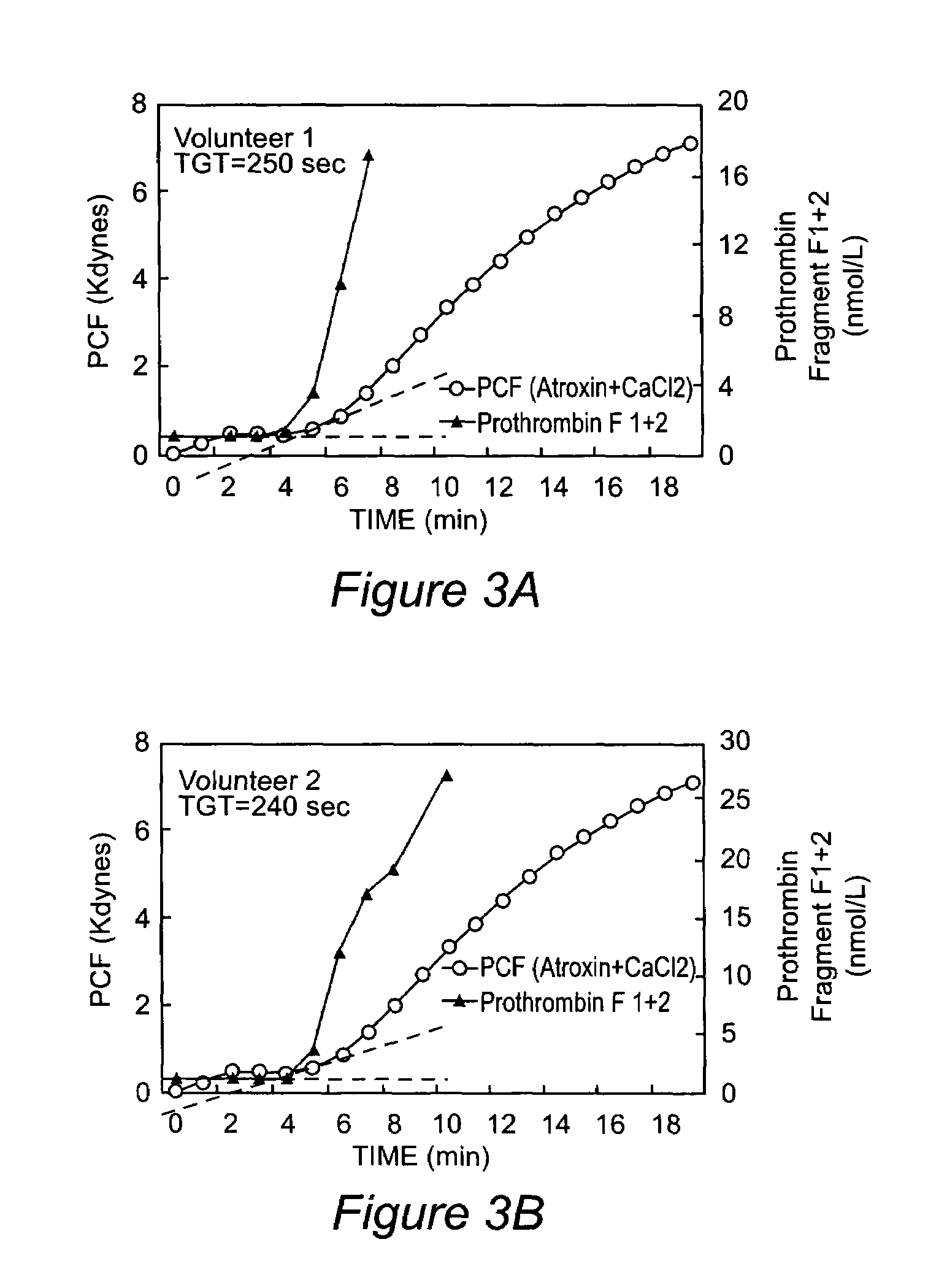
Onset of force development as a marker of thrombin generation
InactiveUS7202048B2Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseClotting factor deficiency
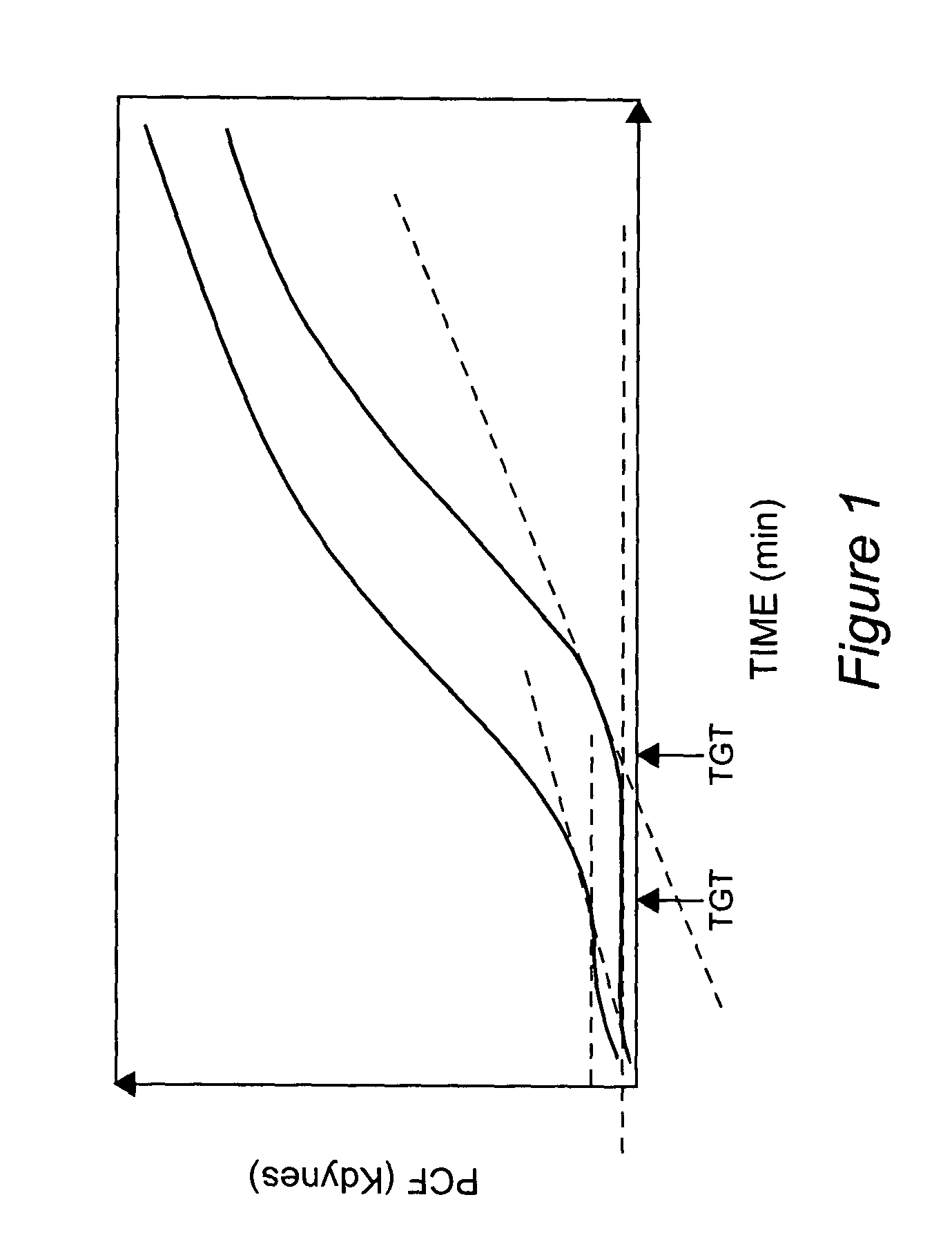
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
Preparation method of porcine thrombin
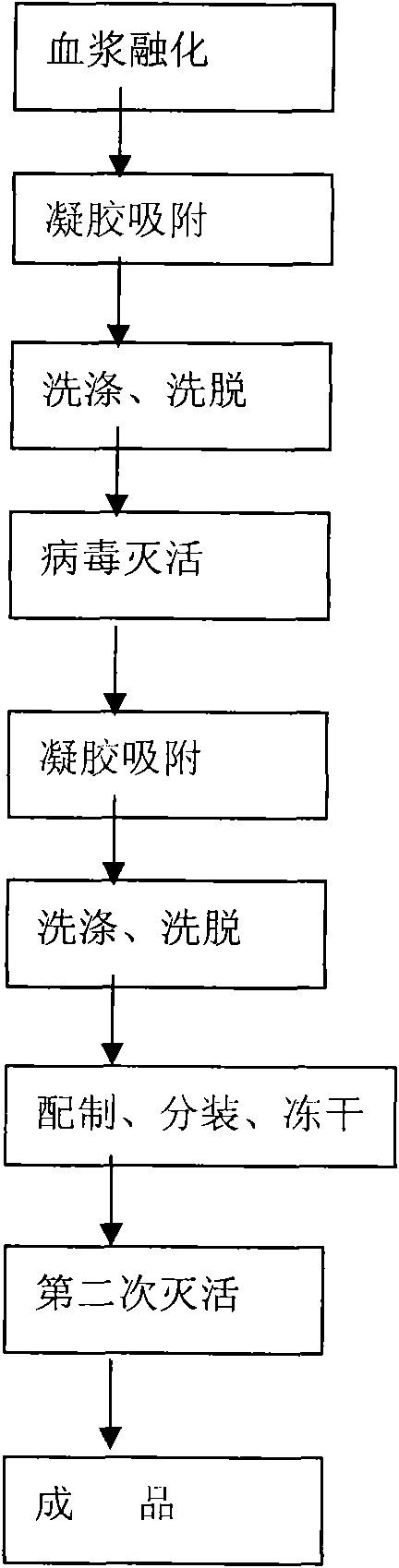
A preparation method of porcine thrombin relates to a preparation method of thrombin. The preparation method of porcine thrombin is used for solving the problems of low yield and unsuitability for large-scale production of the existing preparation method of porcine thrombin. The method provided by the invention comprises the following steps of: 1, separating porcine plasma; 2, inactivating virus by a chemical method; 3, absorbing prothrombin; 4, collecting the prothrombin; 4, nano-filtering; 6, activating the prothrombin; and 7, freeze-drying and storing the prothrombin. The preparation method provided by the invention is capable of improving the biosecurity of the thrombin product, and simultaneously, obtains higher thrombin yield rate by employing a special ion exchange resin extraction process; specifically, 80000 IU / L plasma can be obtained. According to the invention, the method of directly and proportionally feeding the ion exchange resin into the plasma is adopted, one ton to several tons of plasma can be processed each time; therefore, large-scale production is realized. The preparation method of porcine thrombin is applied to the industrial production field of thrombin.
Owner:黑龙江迪龙制药有限公司
Modified annexin proteins and methods for preventing thrombosis
A modified annexin protein, preferably annexin V, is used to prevent thrombosis without increasing hemorrhage. Annexin binds to phosphatidylserine on the outer surface of cell membranes, thereby preventing binding of the prothrombinase complex necessary for thrombus formation. It does not, however, affect platelet aggregation necessary for hemostasis. The modified annexin molecule can be a homodimer of annexin, an annexin molecule coupled to one or more polyethylene glycol chains, or an annexin molecule coupled to another protein. By increasing the molecular weight of annexin, the modified annexin is made to remain in circulation for sufficient time to provide a sustained therapeutic effect.
Owner:SURROMED
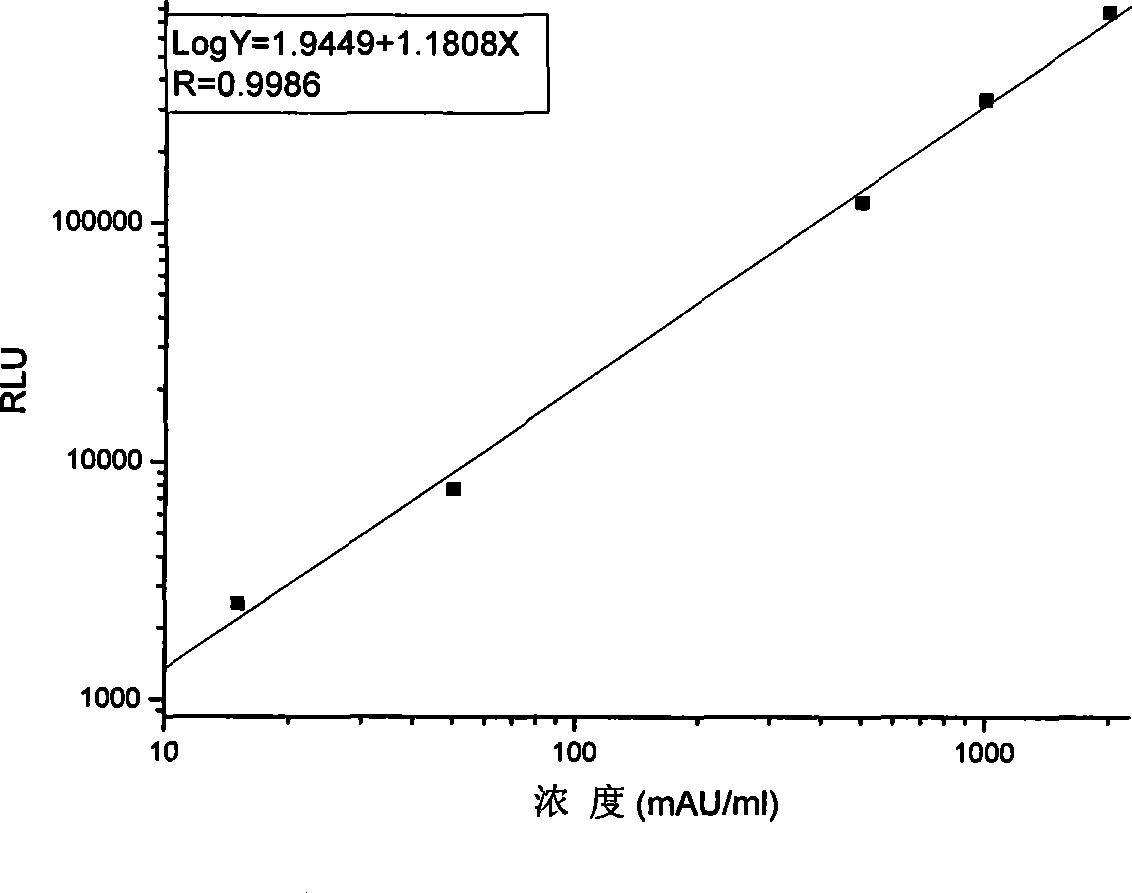
Des-gamma-carboxy-pro-thrombin microplate chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377505ASimple and fast operationFair priceChemiluminescene/bioluminescenceCelluloseThrombin activity
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for des-gamma-carboxyand-prothrombin (DCP) microporosity plate and a preparation method thereof, and realizes the rapid, sensitive and high-specificity serological detection of DCP with the one step chemiluminescent immunoassay method. The kit of the invention has the advantages of simplicity, convenience, rapidness, sensitivity, stability and the like, eliminates the interference of the prothrombin analogues and the serum cellulose and the analogues thereof, and has the advantage of high specificity.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
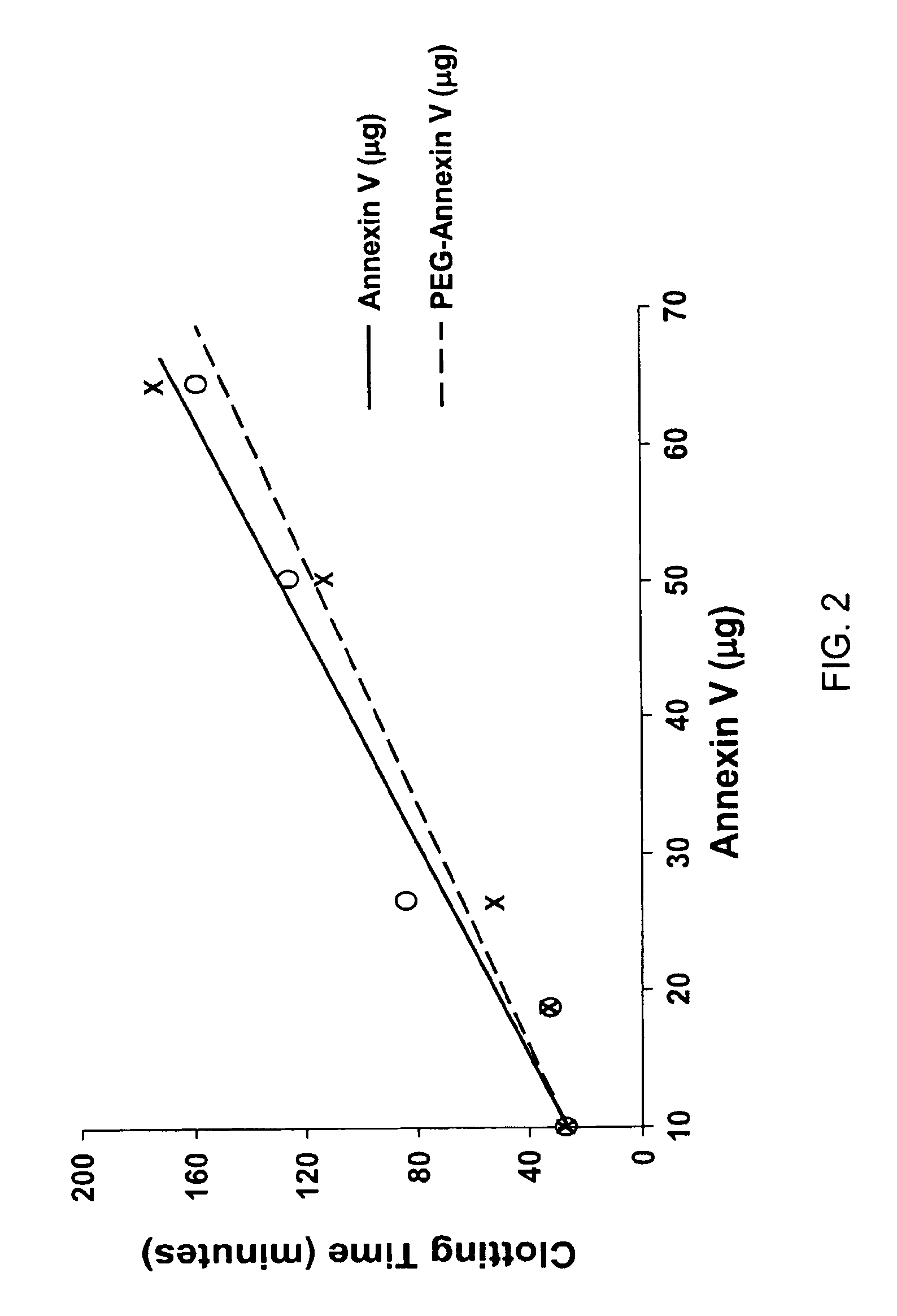
Modified annexin compositions and methods of using same
InactiveUS7645739B2Preventing arterial or venous thrombosisExtended half-lifePeptide/protein ingredientsAntinoxious agentsReperfusion injurySurvivability
Modified annexin proteins, including a homodimer of human annexin V, are provided. Methods for their use, such as to prevent thrombosis without increasing hemorrhage, enhancing the survivability of platelets during storage or transfusion and to attenuate ischemia-reperfusion injury (IPI), are also provided. The modified annexins bind phosphatidylserine (PS) on cell surfaces, thereby preventing the assembly of the prothromkinase complex. The modified annexin decreases the binding of leukocytes and platelets during post-ischemic reperfusion, thereby restoring microvascular blood flow and decreasing organ damage. In addition, the modified annexin prevents lipid loss from platelets during storage.
Owner:ALAVITA PHARMA
Method for adsorbing human prothrombin complex from plasma
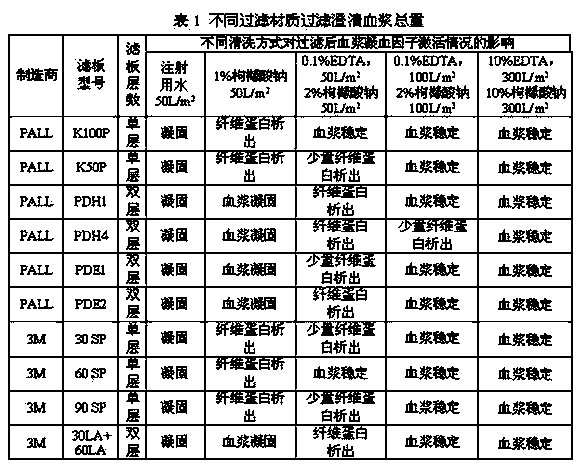
ActiveCN104109202AHigh yieldHigh activityPeptide preparation methodsPeptidasesProthrombin complex concentrateCellulose
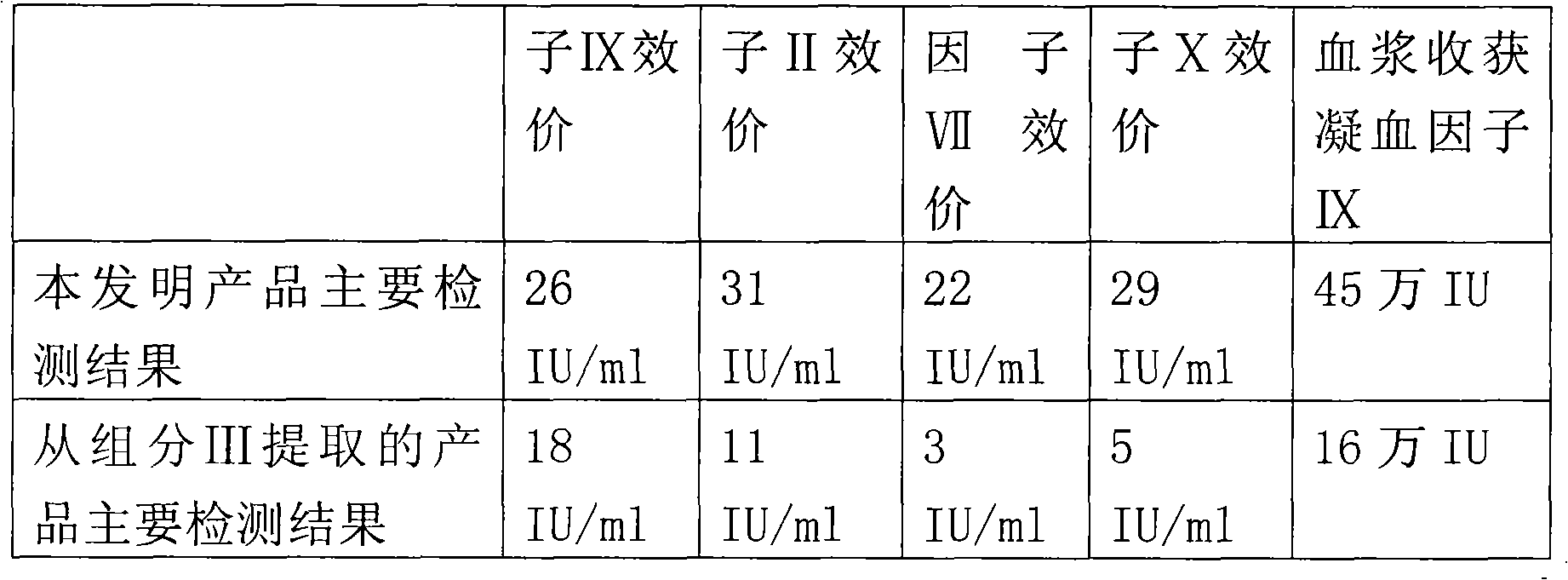
The invention relates to a production method for adsorbing a human complex from plasma by a fixed bed column chromatography technique, which comprises the following steps: (1) cryoprecipitation plasma removal: filtering by using a cellulose deep filter plate which is cleaned by an EDTA (ethylene diamine tetraacetic acid) solution and a sodium citrate solution; (2) filtering the plasma subjected to deep filtration through a 0.2 mu m filter element membrane while fixed bed loading; (3) balancing 2-5 column volumes in a fixed bed chromatographic column filled with anion exchange gel Capto DEAE by using a buffer solution A at the plasma loading flow rate of 60-120 cm / hour, washing the chromatographic column with a buffer solution B, and eluting the chromatographic column with a buffer solution C to obtain a PCC (prothrombin complex concentrate) product. When the calculation is based on coagulation factor IX, the yield of the PCC can reach 75-90%, and the specific activity can reach 5.5 IU / mg above.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Genetically modified ecarin and process for producing the same
A recombinant ecarin protein that specifically activates prothrombin, said protein being efficiently prepared by the genetic engineering technique comprising the steps: (1) culturing a transformant microorganism or animal cell transformed with an expression vector in which a gene encoding ecarin is incorporated to the downstream of a promoter so as to produce and accumulate ecarin in culture supernatant or within said transformant and recovering the produced ecarin; and (2) purifying a solution containing the recovered ecarin to obtain purified ecarin. The present invention allows for production of recombinant ecarin on an industrial scale.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Method for evaluating chemical composition of Rosa xanthina on basis of antithrombotic spectrum-effect relationship
ActiveCN108195989AComprehensive and accurate spectrum effect basisClear chemical compositionComponent separationMathematical modelSeparation technology
The invention discloses a method for evaluating chemical composition of Rosa xanthina on the basis of antithrombotic spectrum-effect relationship. The method comprises the following steps: preparing extract of different polar components of the Rosa xanthina with a modern separation technology; establishing fingerprint of extract of each component with high-performance liquid chromatography, and calibrating characteristic peaks; evaluating antithrombotic activity of different extract on the basis of platelet aggregation inhibition rate, prothrombin time, thrombin time and activated partial thromboplastin time as indexes; substituting fingerprint characteristic peak data and pharmacodynamical activity data into a mathematical model for spectrum-effect correlation analysis, and evaluating pharmacodynamical activity of the characteristic peaks. With adoption of the method for evaluating the chemical composition of the Rosa xanthina on the basis of the antithrombotic spectrum-effect relationship, the antithrombotic chemical composition in the Rosa xanthina can be evaluated rapidly and accurately, a scientific and effective method is provided for research of pharmacodynamic material basis and quality control of the Rosa xanthina, and reference is provided for further development of Rosa xanthina drugs or health care products for treating thrombotic diseases.
Owner:山西省医药与生命科学研究院
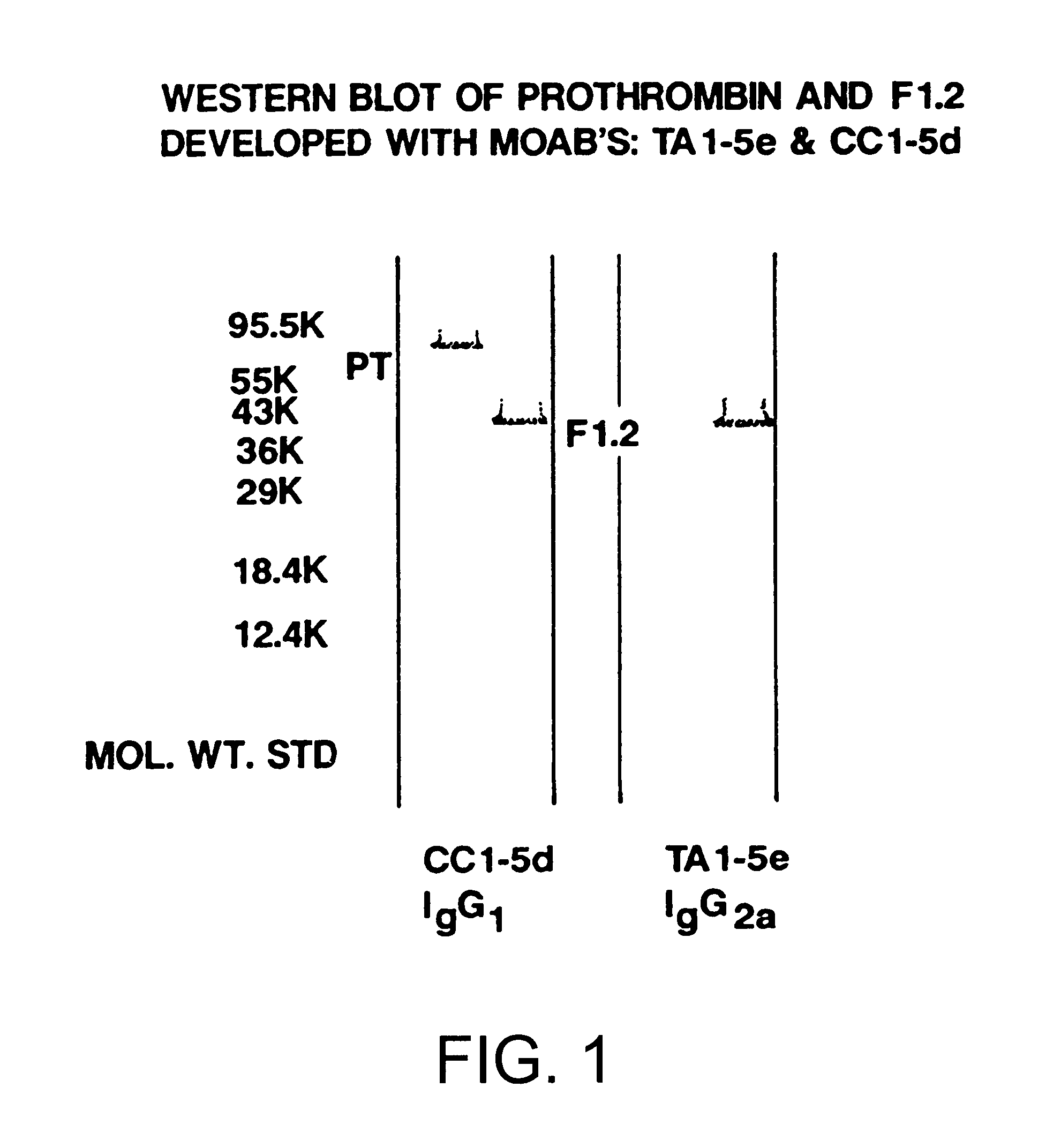
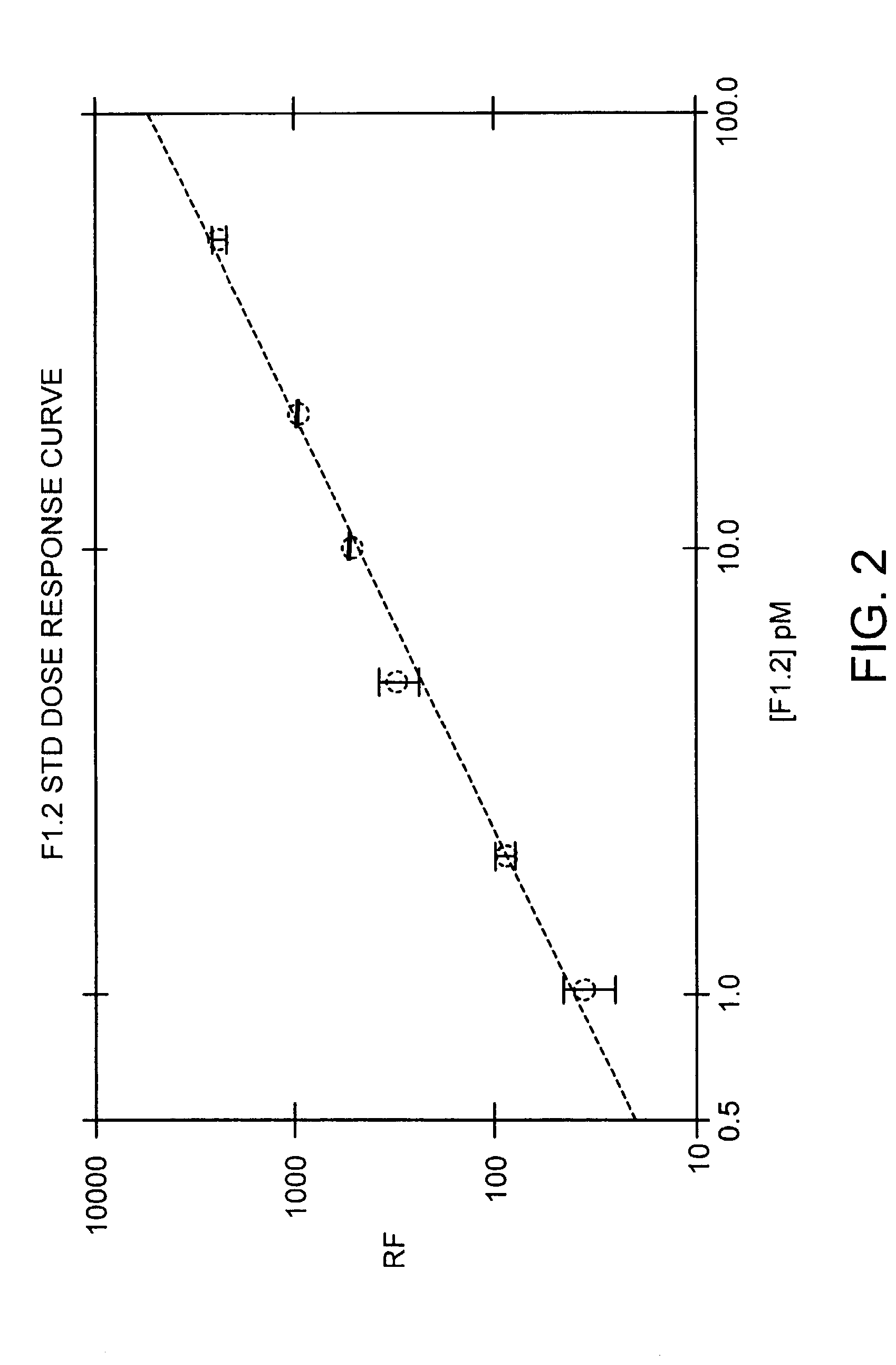
Immunoassay for F1.2 prothrombin fragment
According to the present invention highly specific-low affinity antibodies are generated which allow for the assay of F1.2 in bodily fluids that also contain prothrombin or other plasma proteins. Antibodies having the necessary properties for this assay are made using synthetic polypeptides which mimic the carboxy terminus of F1.2.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Thromboplastin reagents
A thromboplastin reagent includes tissue factor, Factor VIIa, and a net negatively charged phospholipid. The thromboplastin reagent is a synthetic thromboplastin reagent, and is in dried form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
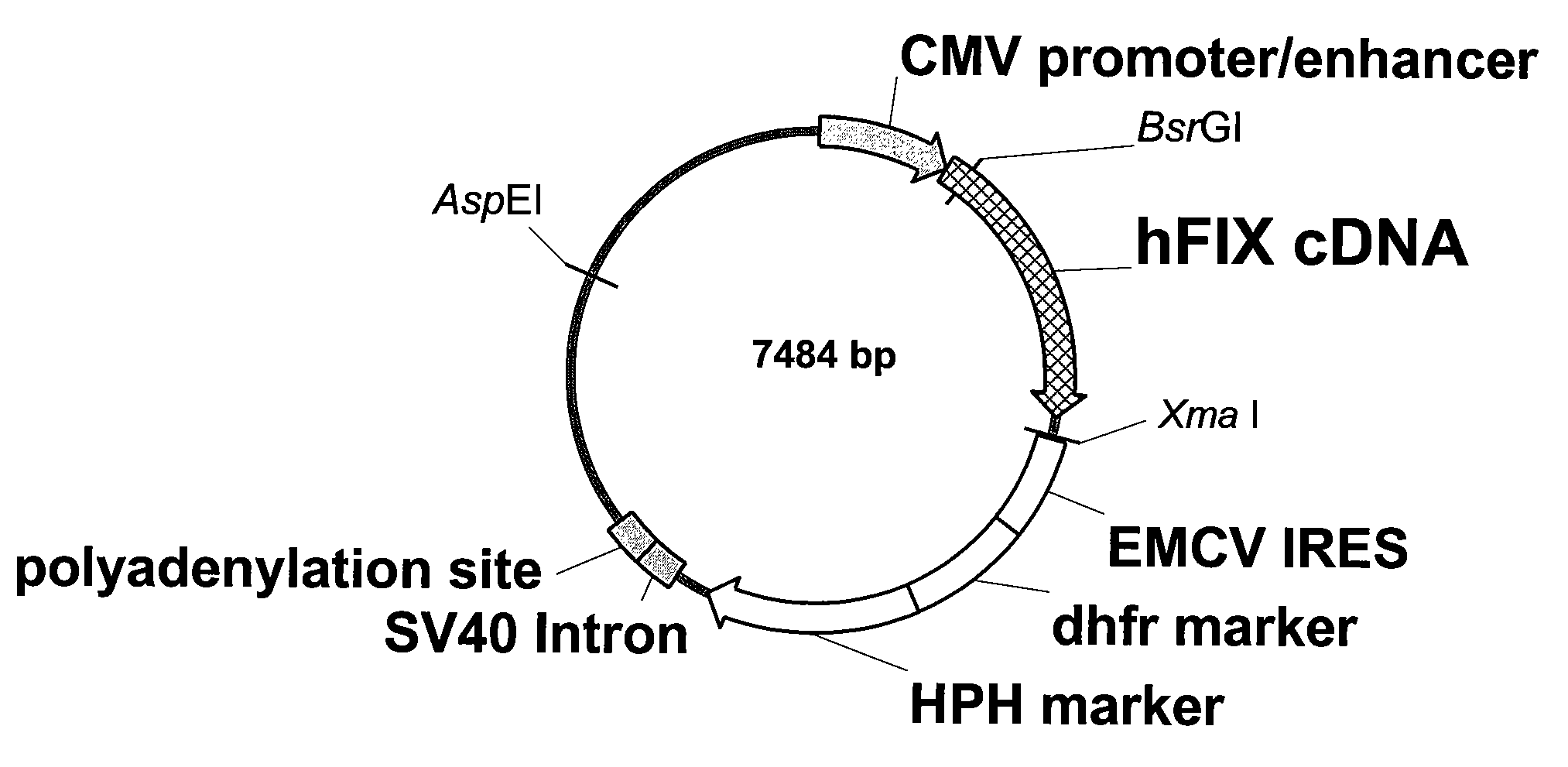
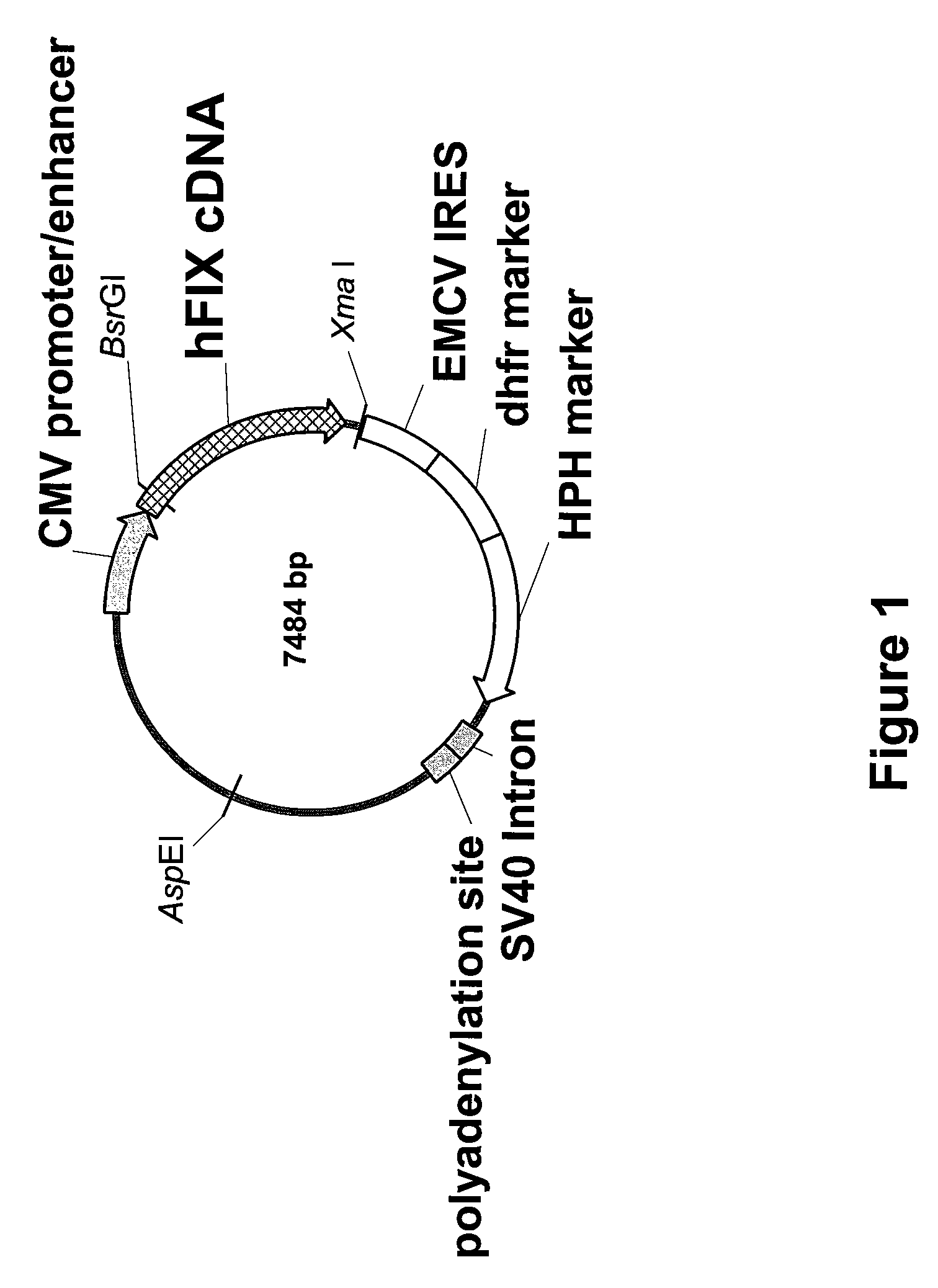
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
Immunoassay for F1.2 prothrombin fragment
Owner:RUIZ JUAN A +1
Hemostatic compositions, devices and methods
InactiveUS7094428B2Lower Level RequirementsReduce concentrationPowder deliveryFactor VIIFactor VIIaBiopolymer
A hemostatic composition which comprises at least one procoagulant metal ion, such as silver (I) or mercury (II), and at least one procoagulant biopolymer, such as collagen, thrombin, prothrombin, fibrin, fibrinogen, heparinase, Factor VIIa, Factor VIII, Factor IXa, Factor Xa, Factor XII, von Willebrand Factor, a selectin, a procoagulant venom, a plasminogen activator inhibitor, glycoprotein IIb-IIIa, a protease, or plasma. The composition in the form of a paste, dough, glue, liquid, lyophilized powder or foam, may be provided, for application to a wound. A hemostatic device is also described which comprises a hemostatic composition as described above. The device may be in the form of, for example, a plug, bandage, gauze, cloth, tampon, membrane or sponge. Methods are also provided for prophylaxis or treatment of bleeding at a site by application to the site of the composition or device as described.
Owner:RUTGERS THE STATE UNIV
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
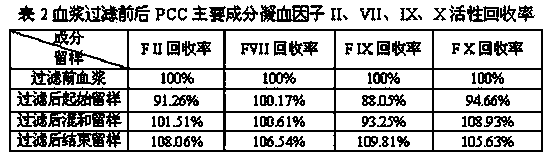
Method for producing human prothrombin complex
ActiveCN102151289AInhibition of activationHigh yieldPeptide/protein ingredientsMammal material medical ingredientsActivation methodVirus inactivation
The invention relates to a method for producing a human prothrombin complex. The method is characterized in that the following steps of direct separation and extraction from blood plasma, virus inactivation, refining and secondary virus inactivation are adopted to obtain finished human prothrombin complex. As the method adopts the step of direct separation and extraction from the blood plasma, the separation condition is mild, the batch-to-batch difference of the products is small, the blood coagulation factor activity is stable, the yield rate is high, and the activation phenomenon basicallydoes not exist. The virus inactivation process adopts a method of combining an S / D (organic solvent / detergent) method and a dry and thermal activation method and fully ensures that the virus safety of the human prothrombin complex.
Owner:哈尔滨派斯菲科生物制药有限公司
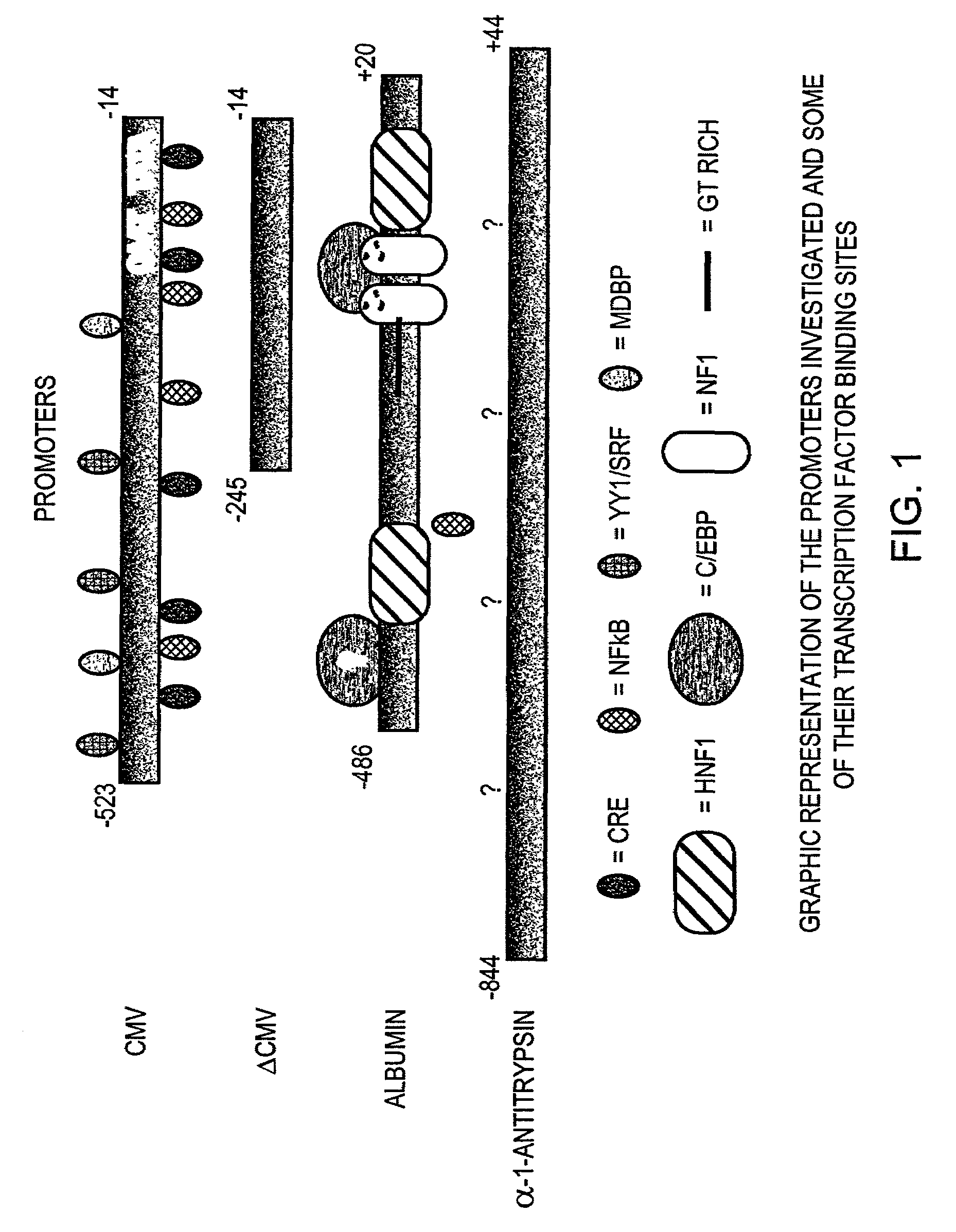
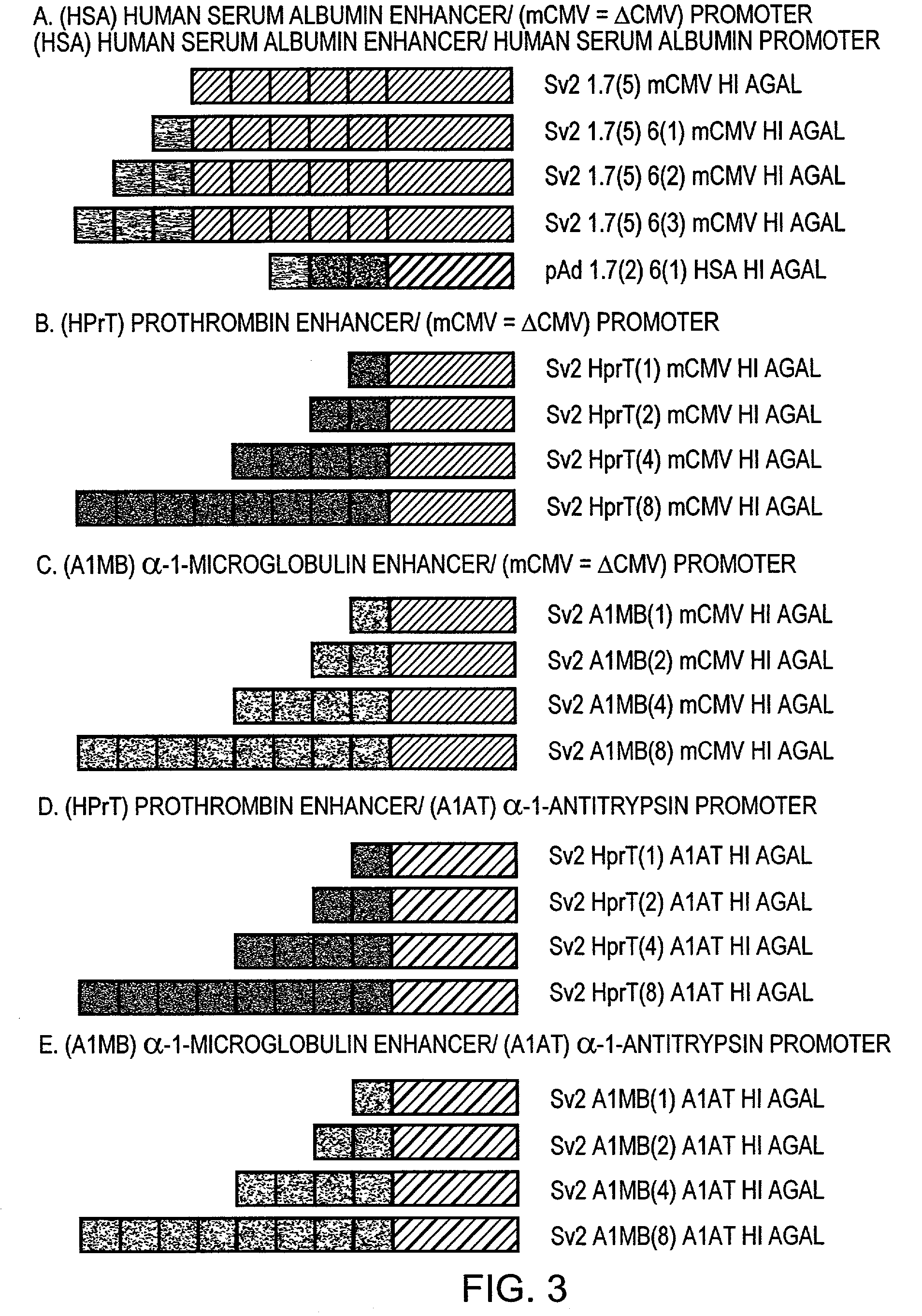
Regulatory elements for delivery to the liver
The invention is directed to novel combinations of liver specific enhancers and promoter elements for achieving persistent transgene expression in the liver. The liver specific enhancer elements may be derived from either the human serum albumin, prothrombin, α-1microglobulin or aldolase genes in single copies or in multimerized form linked to elements derived from the cytomegalovirus intermediate early (CMV), α-1-antitrypsin or albumin promoters. In a preferred embodiment of the invention, an adenoviral vector comprising a liver specific enhancer / promoter combination operably linked to a transgene is administered to recipient cells. In other embodiments of the invention, adeno-associated viral vectors, retroviral vectors, lentiviral vectors or a plasmid comprising the liver specific enhancer / promoter combination linked to a transgene is administered to recipient cells. Also within the scope of the invention are promoter elements derived from the human prothrombin gene and the β-fibrinogen gene.
Owner:GENZYME CORP
Non-toxic purification and activation of prothrombin and use thereof
InactiveUS20050265989A1Reduce leakageHigh strengthSurgical adhesivesPeptide/protein ingredientsFisheryBiological activation
The present invention relates to a prothrombin activator for activating prothrombin to thrombin comprising of an extract of fish gills or a combination of fish egg and fish gills. Said activator is suitable for use in medical applications as well as in food products.
Owner:SALMON BRANDS
Liquid ready-to-use prothrombin time detection reagent
InactiveCN107356768AOvercome the difference between bottlesOvercome the defect of large batch differenceBiological testingTissue factorCholesterol
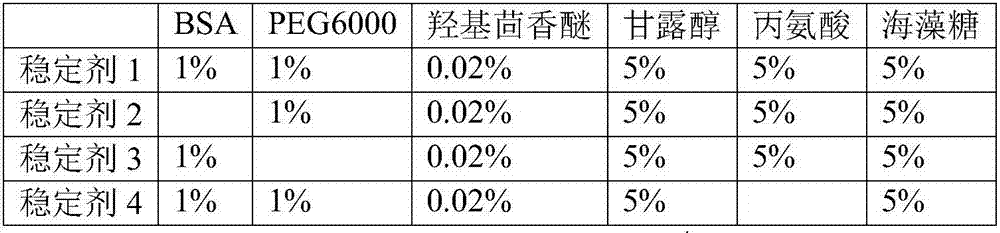
The invention discloses a liquid ready-to-use prothrombin time detection reagent, which includes a buffer, a synthetic phospholipid, a recombinant rabbit tissue factor, a surfactant and a stabilizer. The synthetic phospholipid is composed of phosphatidylserine, phosphatidylcholine and Cholesterol composition. The present invention uses rabbit recombinant factors and synthetic phospholipids to prepare prothrombin time detection reagents by selecting synthetic phospholipid components and optimizing stabilizers. It does not need to be reconstituted during use and can be used immediately after opening the bottle. The reagent overcomes the problem of difficult-to-control batch-to-batch variation of existing prothrombin time detection reagents and has high sensitivity, good stability, small batch-to-batch variation, easy quality control, and easy production.
Owner:NINGBO ACCUTECH BIOSCI LTD
Pharmaceutical preparations and medicines capable of generating, and/or containing, thrombin
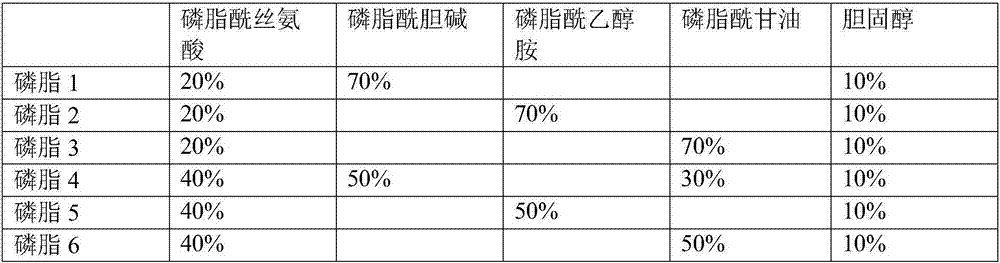
The invention relates to a pharmaceutical active ingredient preparation for producing a medicament that contains thrombin or has a thrombin-generating capacity and compositions comprising thereof. The inventive preparation contains: (A) prothrombin obtained from plasma or by means of genetic engineering (coagulation factor II), (B) coagulation factors V, VIII, IX, X obtained from plasma or by means of genetic engineering, which can be at least partially in the activated state, and coagulation factor Xla obtained from plasma or by means of genetic engineering, and (C) phospholipids which are safe from prions and contribute to the clotting process, said phospholipids being optionally contained in liposomes.
Owner:BIO PRODS & BIO ENG AKTIENGES
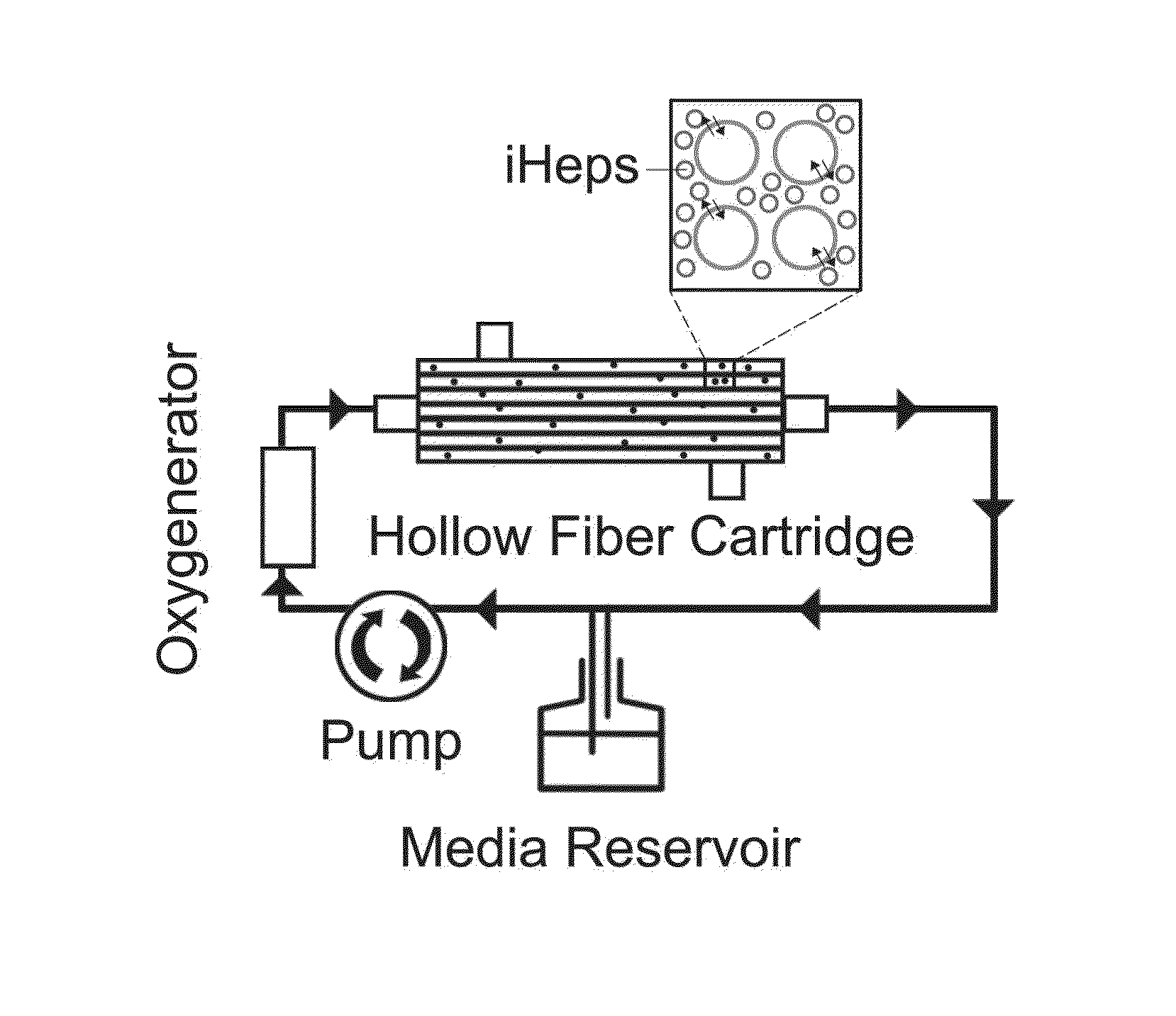
Induced pluripotent stem cell-derived hepatocyte based bioartificial liver device
InactiveUS20160256672A1Reduce toxic buildupQuality andCulture processDialysis systemsBioartificial liver deviceHuman albumin
Human induced pluripotent stem cell (iPSC) technology combined with a hollow fiber based bioartificial liver (BAL) device can benefit patients with liver failure. Defined iPSC lines can provide unlimited supply of functional hepatocytes by developing iPSC derived hepatocytes (iHeps). Disclosed herein is a protocol for deriving metabolically active hepatocytes from iPSCs. In some embodiments, iHeps were cultured on microcarrier beads in spinner flasks. Subsequently, the iHep-microcarrier complexes were loaded into the extracapillary space of a hollow fiber bioreactor cartridge and cultured using closed circuit continuous flow system. The iHeps secreted human albumin, prothrombin and apolipoprotein B into the hollow fiber intracapillary space media which indicated the maintenance of plasma protein secretory function. In addition, the continuous flow system improved the maturation of iHeps. Thus, the iPSC hepatocytes in the bioartificial liver device maintained the secretory function and exhibited cell maturation.
Owner:CEDARS SINAI MEDICAL CENT
Ancylostoma caninum anticoagulant peptide and its preparation and application
InactiveCN101260150APeptide/protein ingredientsFermentationAntithrombotic AgentAnticoagulation Activity
The invention discloses a novel ancylostoma caninum anticoagulation peptide and an encoded sequence thereof and a preparation method for the anticoagulation peptide. The anticoagulation peptide acquired by the method possesses of anticoagulation activity, can markedly prolong the plasma prothrombin time (PT) and the activation part thrombozyme time(aPTT) of people and has obvious anti thrombosis effects. The invention also relates to applications of the anticoagulation peptide on aspects of anticoagulation drugs, antithrombotic drugs or anticoagulation preparations.
Owner:GUANGDONG MEDICAL UNIV
Method for preparing human thrombin from cold-removing glue plasma
InactiveCN105039295ASignificant technological progressHigh yieldPeptidasesFreeze-dryingUltrafiltration
The invention discloses a method for preparing human thrombin from cold-removing glue plasma. Firstly, anion resins are used to absorb prothrombin in the cold-removing glue plasma, then elution and filtering are conducted, and a prothrombin solution is obtained; secondly, the prothrombin solution is activated through a CaCL2 solution, and a thrombin solution is obtained; thirdly, S / D viral inactivation is conducted on the thrombin solution; fourthly, chromatography is conducted through cation columns, and a purified thrombin solution is obtained; fifthly, ultrafiltration, dialysis and concentrate are conducted on the thrombin solution, and the thrombin solution with the titer meeting the product specification requirement is obtained; sixthly, a filter element of 20 nm in size is used to conduct virus removal filtering; seventhly, sterilization filtering is conducted through a filter element of 0.22 microns in size, and then subpackage is conducted according to the required specifications; eighthly, freeze-drying is conducted, and dry heat viral inactivation is conducted on freeze-drying powder, and a human thrombin product is obtained. According to the method, the thrombin is purified through the one-step cation columns, operation is simple, thrombin specific activity is high, and safety of clinic use of the product is guaranteed through a three-step virus elimination mode.
Owner:上海洲跃生物科技有限公司
Stabilized proteins with engineered disulfide bonds
InactiveUS7205278B2High retention rateAvoidance of undesired activityOrganic active ingredientsFungiADAMTS ProteinsBlood plasma
The present invention relates to methods of introducing one or more cysteine residues into a polypeptide which permit the stabilization of the polypeptide by formation of at least one bond, preferably a disulfide bond, between different domains of the polypeptide. The invention also relates to polypeptides containing such introduced cysteine residue(s), nucleic acids encoding such polypeptides and pharmaceutical compositions comprising such polypeptides or nucleic acids. The invention also relates to vectors, viral particles and host cells containing such nucleic acids, and methods of using them to produce the polypeptides of the invention. Exemplified polypeptides include plasma proteins, including hepatocyte growth factor activator and plasma hyaluronin binding protein, as well as blood coagulation factors, such as Factor VIII, Factor V, Factor XII and prothrombin.
Owner:THE SCRIPPS RES INST
Application of peptide compound in rhizoma sparganii
ActiveCN103520160AHigh anticoagulant activityOrganic active ingredientsOrganic chemistryPartial prothrombin timeThrombus
The invention discloses application of a peptide compound in rhizoma sparganii in the preparation of medicines for resisting blood coagulation and / or thrombus. The structural formula of the peptide compound is as shown in a formula (I). The invention provides the peptide compound obtained from the rhizoma sparganii through the separation and the purification and the application thereof for the first time. The peptide compound has an elongation tendency effect on prothrombin time (PT), activated partial thromboplastin time (APPT) and thrombin time (TT) and very good anticoagulation activity and provides powerful basis for the development of antithrombus natural medicines.
Owner:GUANGDONG PHARMA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com