Des-gamma-carboxy-pro-thrombin microplate chemiluminescence immune analysis determination reagent kit and preparing method thereof
A chemiluminescence immunoassay and prothrombin technology, applied in the field of chemiluminescence immunoassay kits and their preparation, can solve the problems affecting the early diagnosis of small liver cancer, low detection sensitivity, large cross-reaction, etc., achieve good market value and improve linearity Range, easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of de-γcarboxyprothrombin chemical method photoimmunoassay assay kit of the present invention
[0044] 1. Preparation of standard products
[0045] Horse serum, 50% horse serum (diluted in normal saline), adult bovine serum, 50% adult bovine serum (diluted in normal saline), 3% BSA+0.6% hydrolyzed gelatin buffer (diluted in normal saline) were used for five kinds of matrices Select, preferably the one with the highest sensitivity (in RLU S1 / RLU S0 )express.
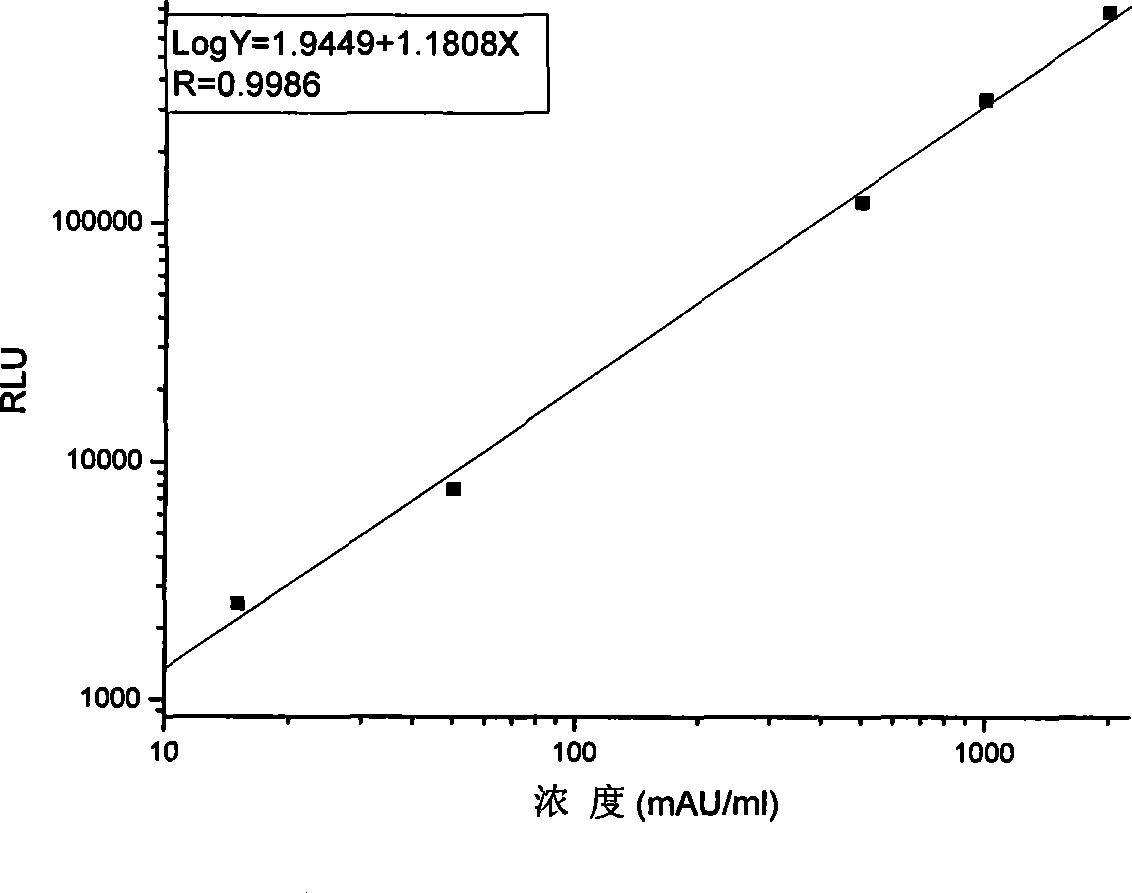
[0046] Preparation of point S1: Dissolve 10mAU into 1ml of the above five matrices, mix thoroughly, and set aside.
[0047] Then according to the operation process of the present invention, that is: 50 μl of standard substance and 50 μl of enzyme marker were respectively added to the coated microwell plate, incubated at 37°C for 40 min, washed, and 100 μl of luminescent substrate was added to detect S 0 , S 1 point RLU. The results are shown in Table 1. It can be seen from the results ...
Embodiment 2
[0103] Example 2 Method of using the de-gamma carboxyprothrombin chemical method photoimmunoassay assay kit of the present invention
[0104] 1. Sample pretreatment
[0105] Take human fasting serum, centrifuge at 2000-4000rpm for 10min, and take the supernatant for analysis without any other special treatment.
[0106] 2. Detection steps
[0107] Before using this kit for detection, it is necessary to take out the packaged medicines in the kit first: the coated plate, enzyme markers, and standard products are allowed to stand at room temperature, and then used after equilibrating to room temperature; after that, adjust the incubator or water bath. to 37°C; then, prepare a suitable micro-injector and corresponding tips and check whether the chemiluminescence instrument is working normally.
[0108] The specific operation steps of using this kit to carry out the experiment according to the method of embodiment 1 are as follows:
[0109] 1) Take out the kit and equilibrate to r...
Embodiment 3
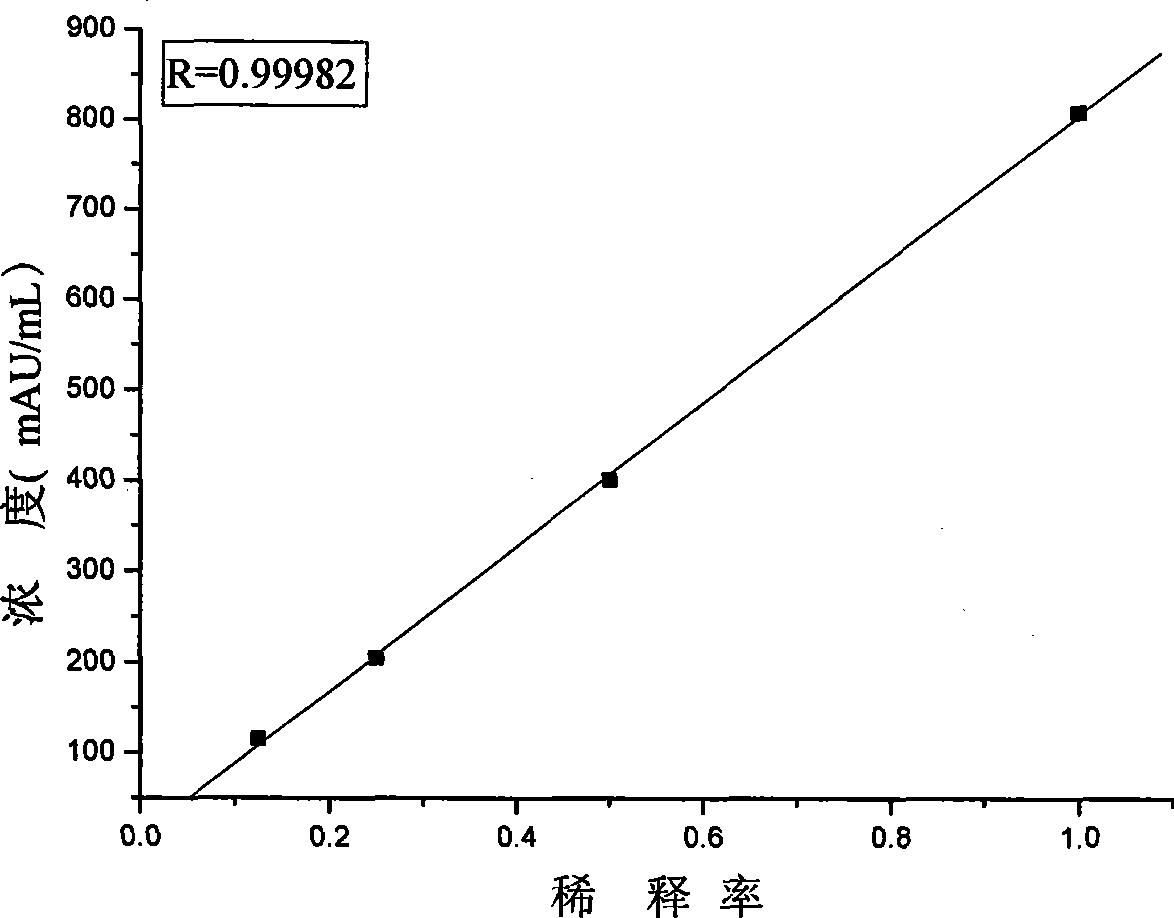
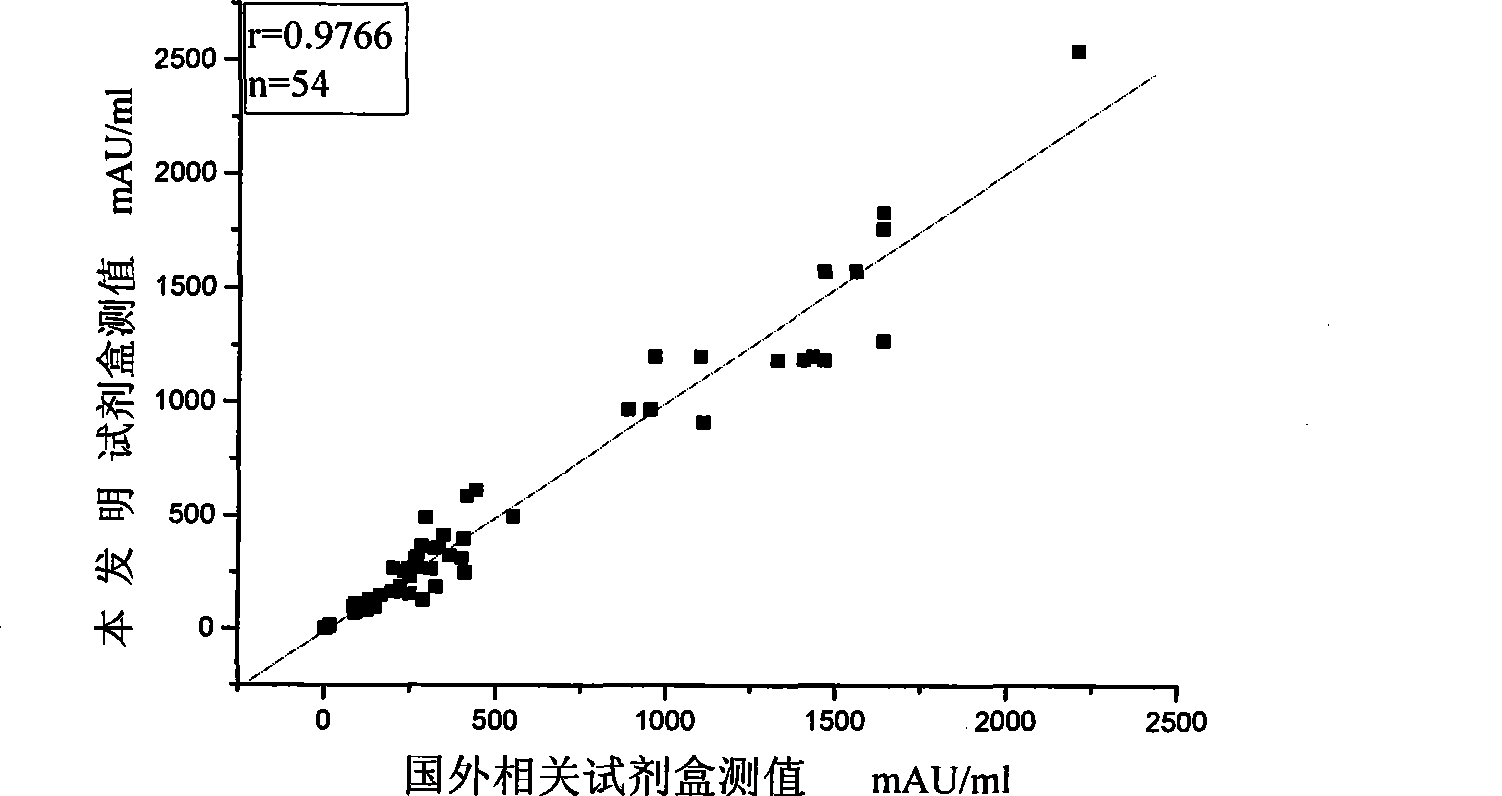
[0120] Embodiment 3 The methodological index of the kit of the present invention
[0121] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0122] 1. Kit sensitivity
[0123] Sensitivity is the concentration that is distinguishable from the zero dose point. Measure 10 zero standard points at the same time to obtain the average value and standard deviation of RLU. Calculate the difference between the average value of RLU and twice the standard deviation, and obtain the corresponding concentration value from the working curve by interpolation. The sensitivity determined in the experiment was 4.37mAU / ml.
[0124] 2. Kit precision
[0125] Precision refers to the reproducibility of the analysis results. Prepare three samples with 8 high / medium / low values, and test each sample at the same time in the same analysis. The ratio of the coefficient of variation to the standard...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com