Purification and preparation process of recombinant staphylokinase mutant
A preparation process, a technology of staphylokinase, which is applied in the directions of biochemical equipment and methods, hydrolase, microorganisms, etc., can solve the problems of complex purification steps, low yield, endotoxin pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Homogenate of Engineering Bacteria and Treatment of Homogenate
[0060] Since mSAK is a soluble protein expressed in the large intestine, resuspension, homogenization, centrifugation, etc. are required before chromatographic purification to release the target protein in the cells. Therefore, it is first necessary to compare the homogenization conditions and whether the post-homogenization treatment will affect the subsequent purification. Take 50g each of three parts of the same bacteria sludge, and perform homogenization and post-homogenization treatment according to the method in Table 1 respectively:
[0061] Table 1 Homogenization and post-homogenization treatment
[0062]
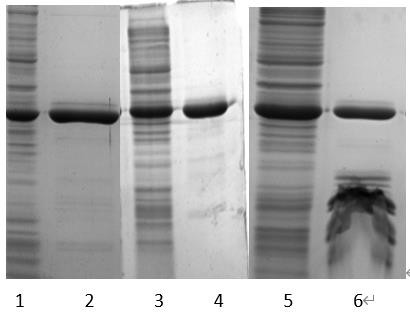
[0063] The three groups of samples were subjected to Chelating Ni2+ chromatography after homogenization and post-homogenization, and compared the influence of different processing methods on the chromatographic purity. The results are as follows figure 1 shown.
[0064] accordin...
Embodiment 2
[0065] Example 2: Determination of recombinant staphylokinase capture method and process optimization
[0066] After the homogenate treatment method is determined, the target protein needs to be extracted from the homogenate supernatant. Since the centrifuged supernatant after homogenization is relatively complicated, in addition to the released mSAK enzyme (target protein), there are also culture medium, secondary metabolites, protease released by cell rupture, etc., so a purification method that efficiently captures the target protein should be selected .
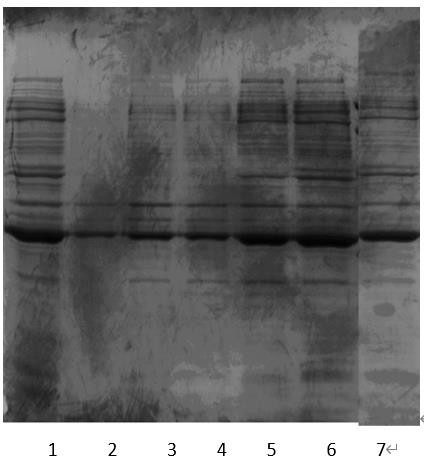
[0067] According to the common capture method of general protein products and the characteristics of mSAK being a fusion protein of Trx (the C-terminus of Trx is coupled with a His tag), the ammonium sulfate (As) fractional precipitation method and the Chelating Ni2+ metal chelation chromatography method were chosen to capture proteins for comparison. See Table 2 for details.
[0068] Table 2 Comparison of methods for r...
Embodiment 3
[0083] Embodiment 3: Chelating Ni2+ chromatographic process optimization
[0084] 3.1 Determination of elution conditions
[0085] According to the previous linear elution results, the optimal elution conditions were determined by sequential elution at 8%, 15%, 25% and 100%.
[0086] experiment procedure:
[0087] Equilibrium Buffer: 50mmol / L Tris-HCl + 0.3mol / L NaCl + 0.01mol / L imidazole pH7.5;
[0088] Elution Buffer: 50mmol / L Tris-HCl + 0.3mol / L NaCl + 0.5mol / L imidazole pH7.5;
[0089] Elution conditions: 8%, 15%, 25% and 100% elution in sequence;
[0090] Chromatographic column: XK 16 / 20 Chelating Ni2+ 20ml;
[0091] Loading amount: 10mg / ml
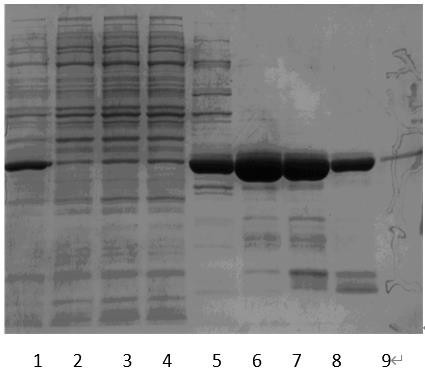
[0092] Gradient collection of each gradient elution peak, SDS-PAGE electrophoresis (results such as Figure 5 Shown), calculated yield and purity (results are shown in Table 3).
[0093] Table 3 Statistics of each elution peak yield and electrophoresis purity
[0094]
[0095] table 3, Figure 5 It shows that the electrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com