Mutants of Staphylokinase Carrying Amino and Carboxy-Terminal Extensions for Polyethylene Glycol Conjugation
a technology of polyethylene glycol and amino and carboxyterminal extensions, which is applied in the field of cysteine variants of staphylokinase, can solve the problems of hammering its use, affecting the allergic response of patients, and relatively short plasma half-life, and achieves the effect of higher temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
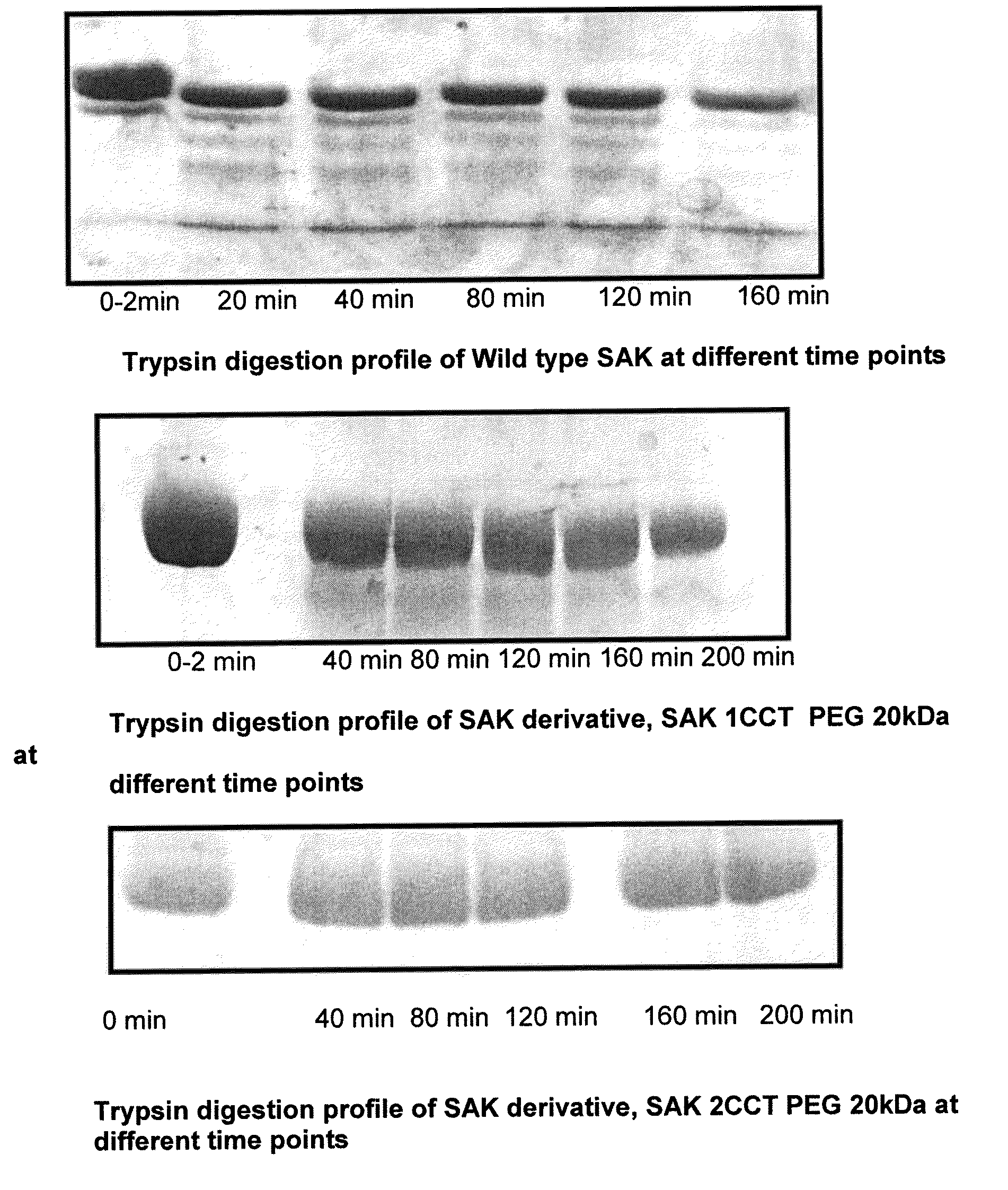
[0073]Construction of SAK mutants carrying extended amino and carboxy terminal regions: SAK is the smallest known protein that has an efficient plasminogen activator activity. Major portion of SAK polypeptide is important for either forming a bimolecular complex with plasminogen or interaction with a substrate molecule. Therefore, addition or a deletion within the core region of SAK often results [Schlott, B., Gurhs, H. K., Hartmann, M., Rocker, A., Collen, D. (1997) Staphylokinase requires NH2-terminal proteolysis for plasminogen activation. J. Biol. Chem. 273; 22346-22350; Rajamohan, G. and Dikshit, K. L.(2000) Role of the N-terminal region of Staphylokinase (SAK) evidence for the participation of the N-terminal region of SAK in the enzyme-substrate complex formation. FEBS Lett. 474; 151-158] in a non-functional form of protein. Therefore, to generate derivatives of SAK, extension of one or more amino acid residues carrying one or multiple cysteine residues at terminal positions, ...
example 2
[0075]Intracellular production of SAK derivatives and their recovery from recombinant E. coli: E. coli cells transformed with recombinant plasmid carrying mutant SAK gene were streaked on a Luria Bertani (LB) agar plate containing 50 μg / ml Kanamycin and kept in an incubator set at 37° C. for overnight. Individual colonies appearing on the plate were used to raise seed culture in a 10 ml liquid LB medium supplemented with 50 μg / ml Kanamycin and grown at 37° C., 200 rpm on a gyratory shaker for 8-10 h. This primary seed culture (1% v / v) was used to inoculate 1 liter of LB medium containing 50 μg / ml Kanamycin and allowed to grow at 37° C. at 200 rpm till its optical density (OD600 nm) reaches to 0.4-0.5. The culture was then induced for SAK production by adding 0.1 mM IPTG and further grown at 37° C. for another 6-8 hours. Cells were then harvested by spinning them down by centrifugation at 6000.times.g in a GS-3 rotor (Sorvall) for 30 min at 4° C. The supernatants were discarded and t...
example 3
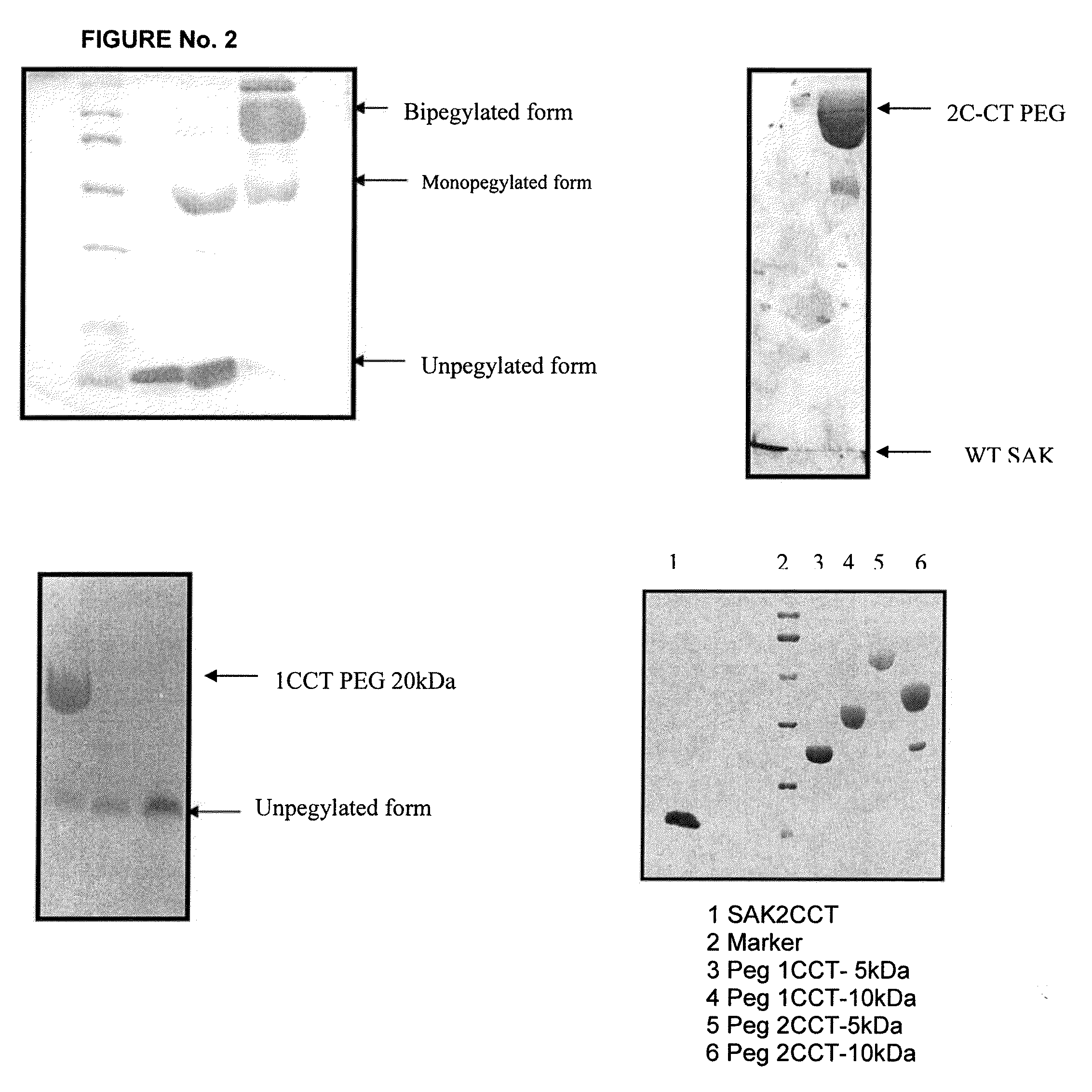
[0076]Determination of sub-unit association properties of SAK derivatives: In order to check how the extension of amino terminal regions of SAK and placement of cysteine residues within this region has affected the oligomeric state of protein, purified preparation of each SAK derivative was run on a native 10% polyacrylamide gel without adding any SDS and mercaptoethanol. Gel was stained with coomassie blue to check change in the oligomeric state of the protein. Checking the molecular mass of each SAK derivative following standard method of size exclusion chromatography on a G75 Sephadex column further substantiated change in the oligomeric state of protein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com