Pegylated staphylokinase mutant, and preparation method and application thereof
A technology of PEGylation and polyethylene glycol, which is applied in the field of life sciences, can solve problems such as the decrease of peptide binding ability, and achieve the effects of decreased immunogenicity, reduced immunogenicity, high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
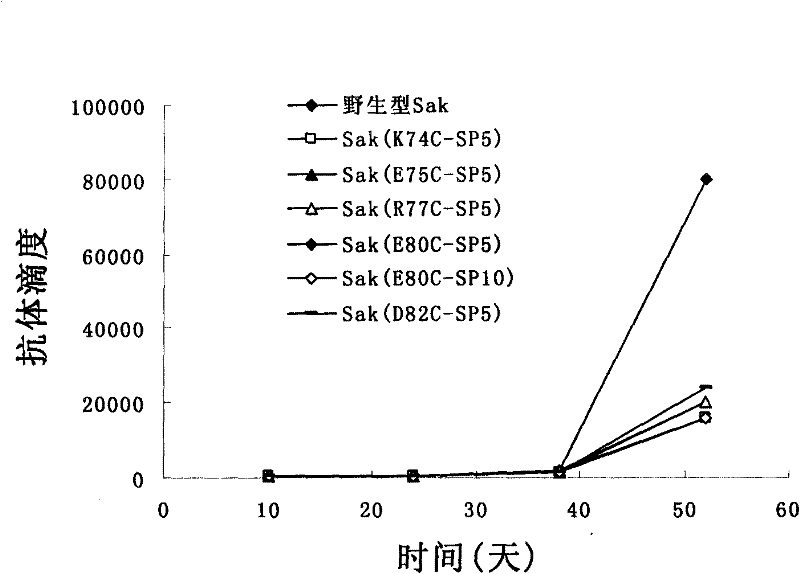
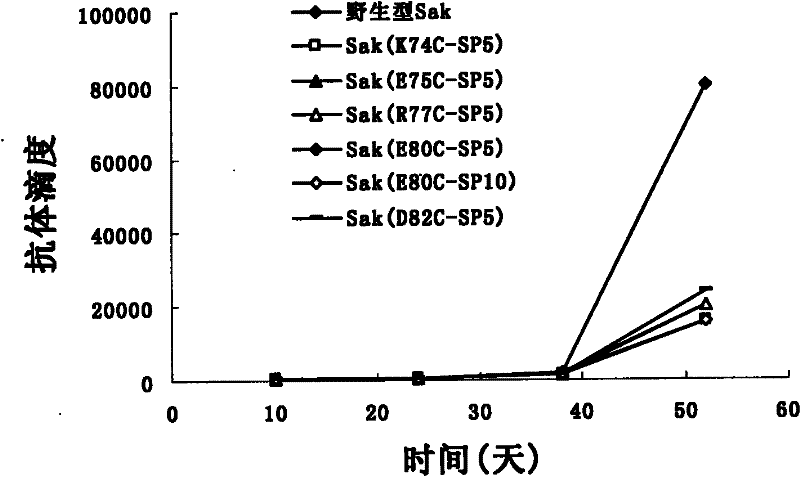
Image
Examples
Embodiment 1
[0025] 1. Construction of staphylokinase mutant Sak (K74C) gene and prokaryotic expression plasmid
[0026] Using the site-directed mutagenesis technology based on the principle of PCR reaction, the expression plasmid vector pBV-Sak containing the wild-type Sak gene was used as a template, and the expression vector was amplified with completely complementary primers containing mutant bases, and then the methyl group was digested with Dpn I. After the template DNA (the linear vector of the PCR product does not contain methylation sites, so it will not be digested by Dpn I enzyme), it is directly transformed into competent Escherichia coli DH5α (prepared by conventional molecular biology methods), and the recombinants are inoculated Culture in LB medium containing 50-100 mg / ml ampicillin, and use a plasmid extraction kit (Beijing Dingguo Biotechnology Company) to extract the plasmid DNA of positive clones. The designed mutation was confirmed by restriction enzyme digestion and n...
Embodiment 2
[0053] 1. Construction of staphylokinase mutant Sak (E75C) gene and prokaryotic expression plasmid
[0054] The construction process of the staphylokinase mutant Sak (E75C) prokaryotic expression plasmid is basically the same as that of Sak (K74C), and the only difference is the difference in PCR amplification primers. The PCR amplification primers used by Sak (E75C) are:
[0055] Sak(E75C):
[0056] Primer 1: GCGACAGCATAAATGTTTTAGAGTAGTTG
[0057] Primer 2: CAACTACTCTAAAACATTTATATGCTGTCGC
[0058] The monoclonal screening process of DH5α, JF1125, JM109 and BL21 engineered bacteria of Sak(E75C) is the same as that of Sak(K74C), and the expression products also exist in the form of intracellular soluble.
[0059] 2. Induced expression of engineered bacteria
[0060] The highly expressed bacterial strain of Sak (E75C) will be screened by shake flask as engineering bacteria, then with 2L fermentation shake flask in M 9 Low-density fermentation was carried out in CA, the cells...
Embodiment 3
[0072] 1. Construction of staphylokinase mutant Sak (R77C) gene and prokaryotic expression plasmid
[0073] The construction process of the prokaryotic expression plasmid of staphylokinase mutant Sak (R77C) is basically the same as that of Sak (K74C), the difference is only the difference of PCR amplification primers, and the PCR amplification primers used by Sak (R77C) are:
[0074] Sak(R77C):
[0075] Primer 1: GCATATAAAGAGTTTTGTGTAGTTGAATTAGATC
[0076] Primer 2: GATCTAATTCAACTACACAAAAACTCTTTATATGC
[0077] The monoclonal screening process of DH5α, JF1125, JM109 and BL21 engineered bacteria of Sak(R77C) is the same as that of Sak(K74C), and the expression products also exist in the form of intracellular soluble.
[0078] 2. Induced expression of engineered bacteria
[0079] The highly expressed bacterial strain of Sak (R77C) will be screened by shake flask as engineering bacteria, then with 2L fermentation shake flask in M 9 Low-density fermentation was carried out in C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com