Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Angiogenesis Effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
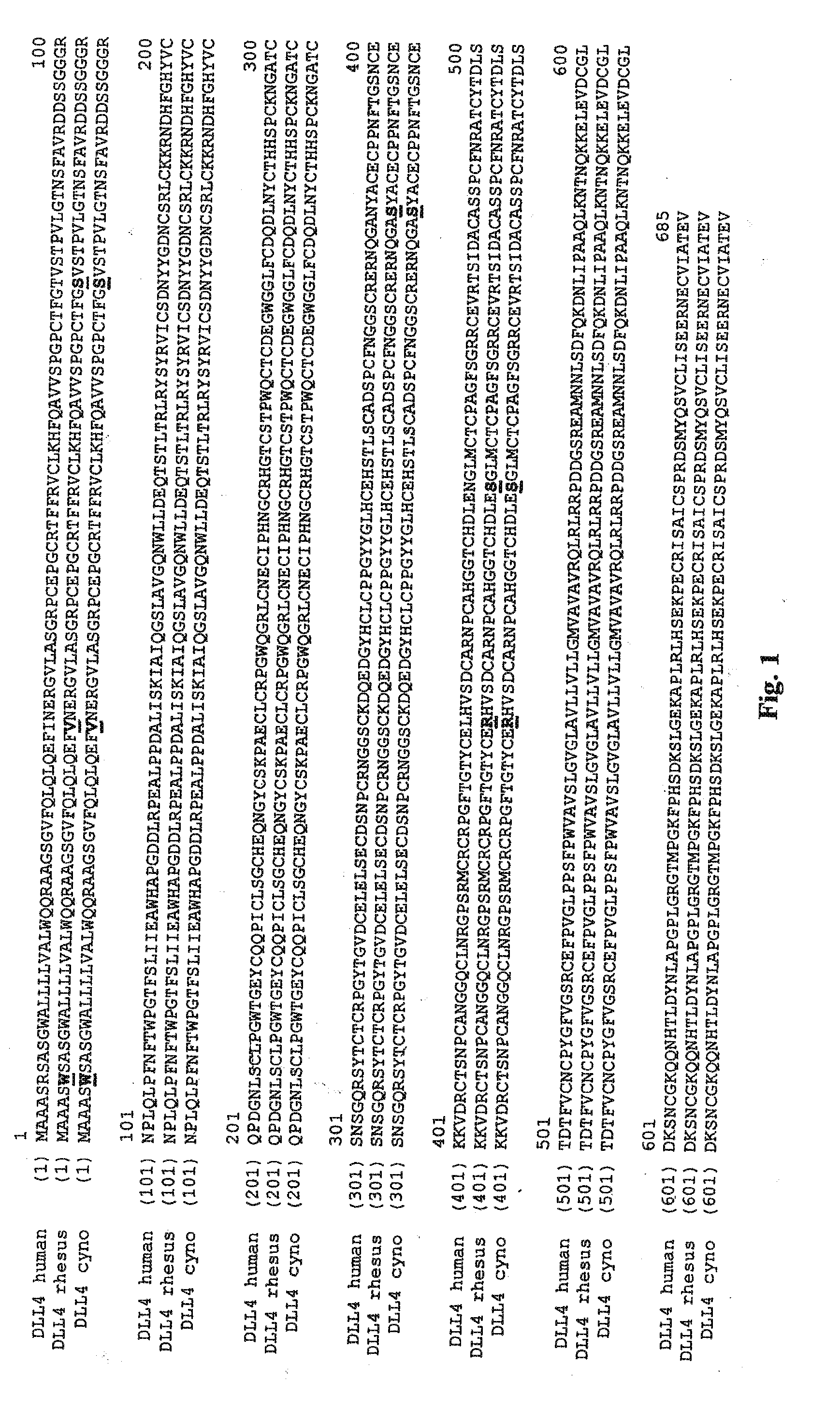
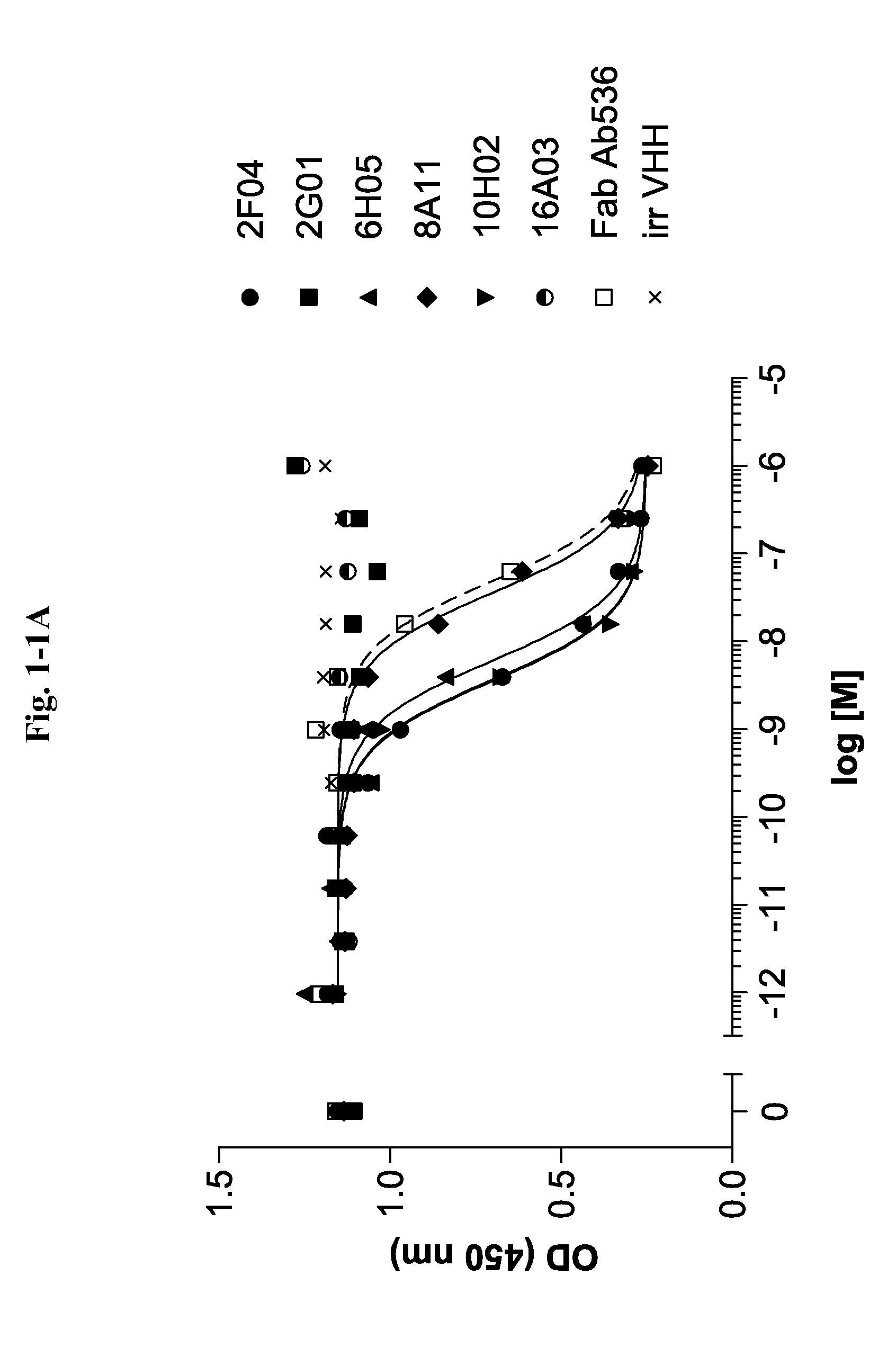
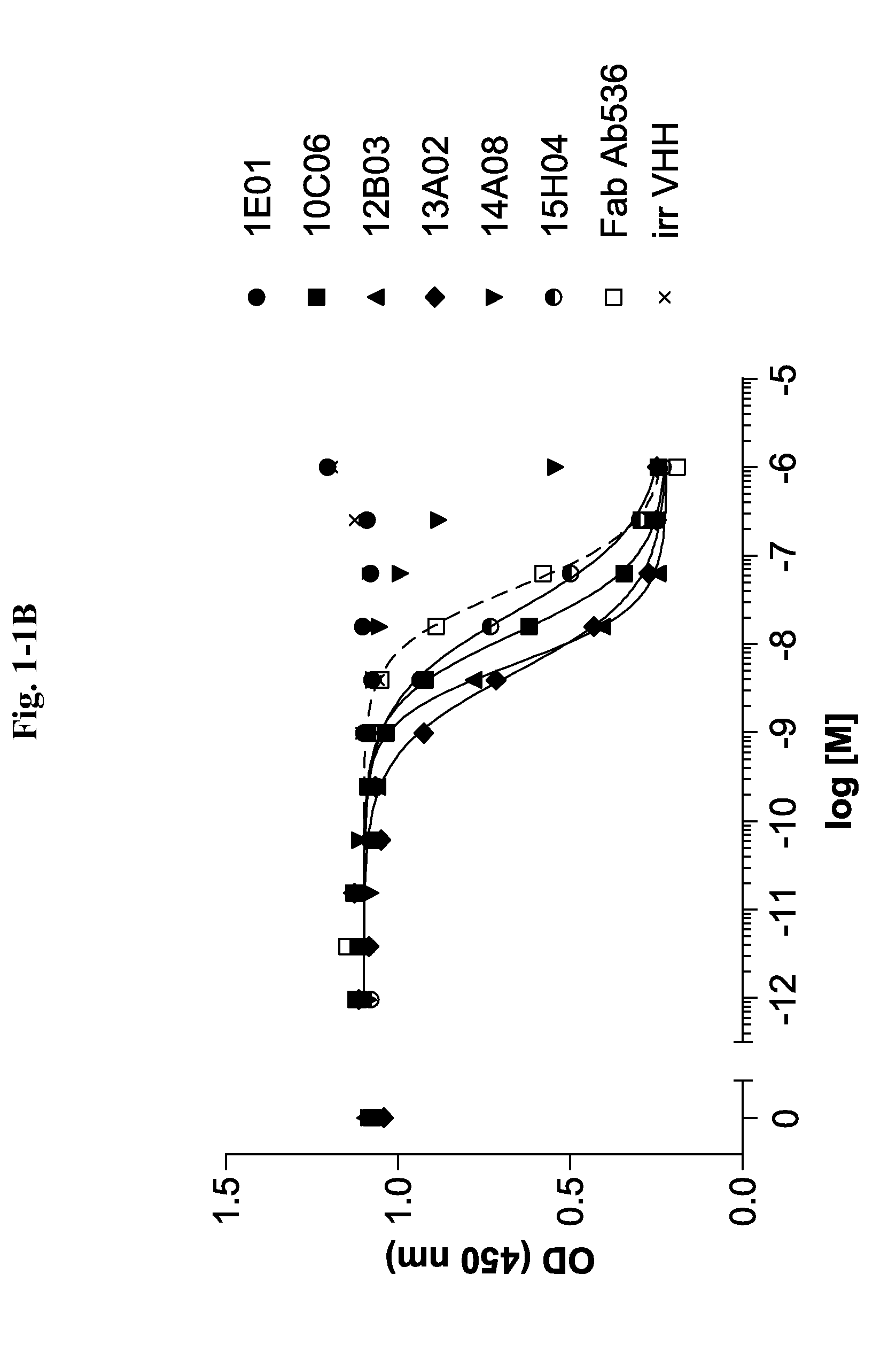
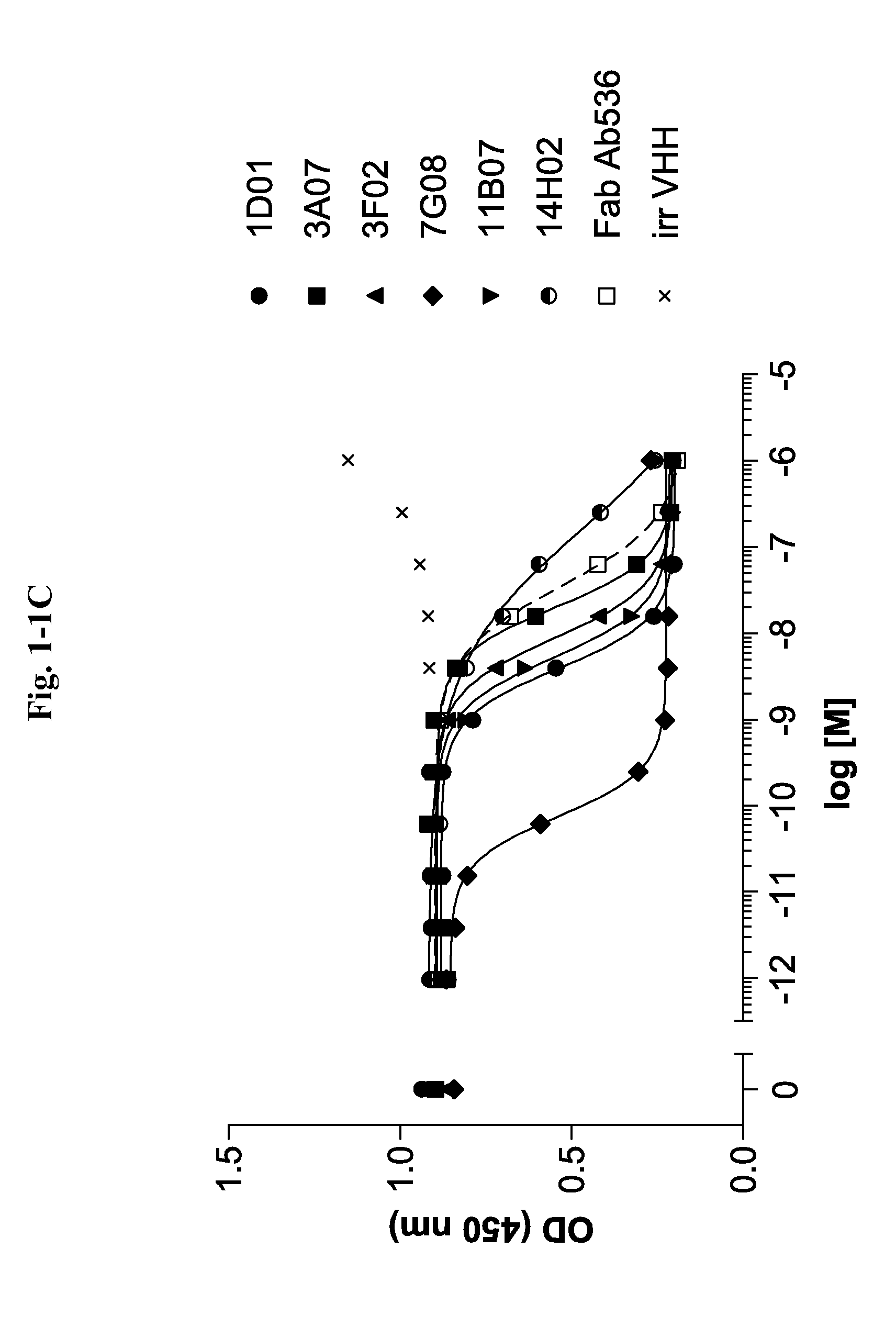
Bispecific binding molecules for Anti-angiogenesis therapy
InactiveUS20110172398A1Reduce manufacturing costImproving desired propertySenses disorderSugar derivativesAngiogenesis EffectAntiangiogenic therapy
Bispecific binding molecules, in particular immunoglobulin single variable domains such as VHHs and domain antibodies, comprising a VEGF-binding component and a Dll4-binding component in one molecule. Pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with VEGF- and Dll4-mediated effects on angiogenesis. Nucleic acids encoding the bispecific binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
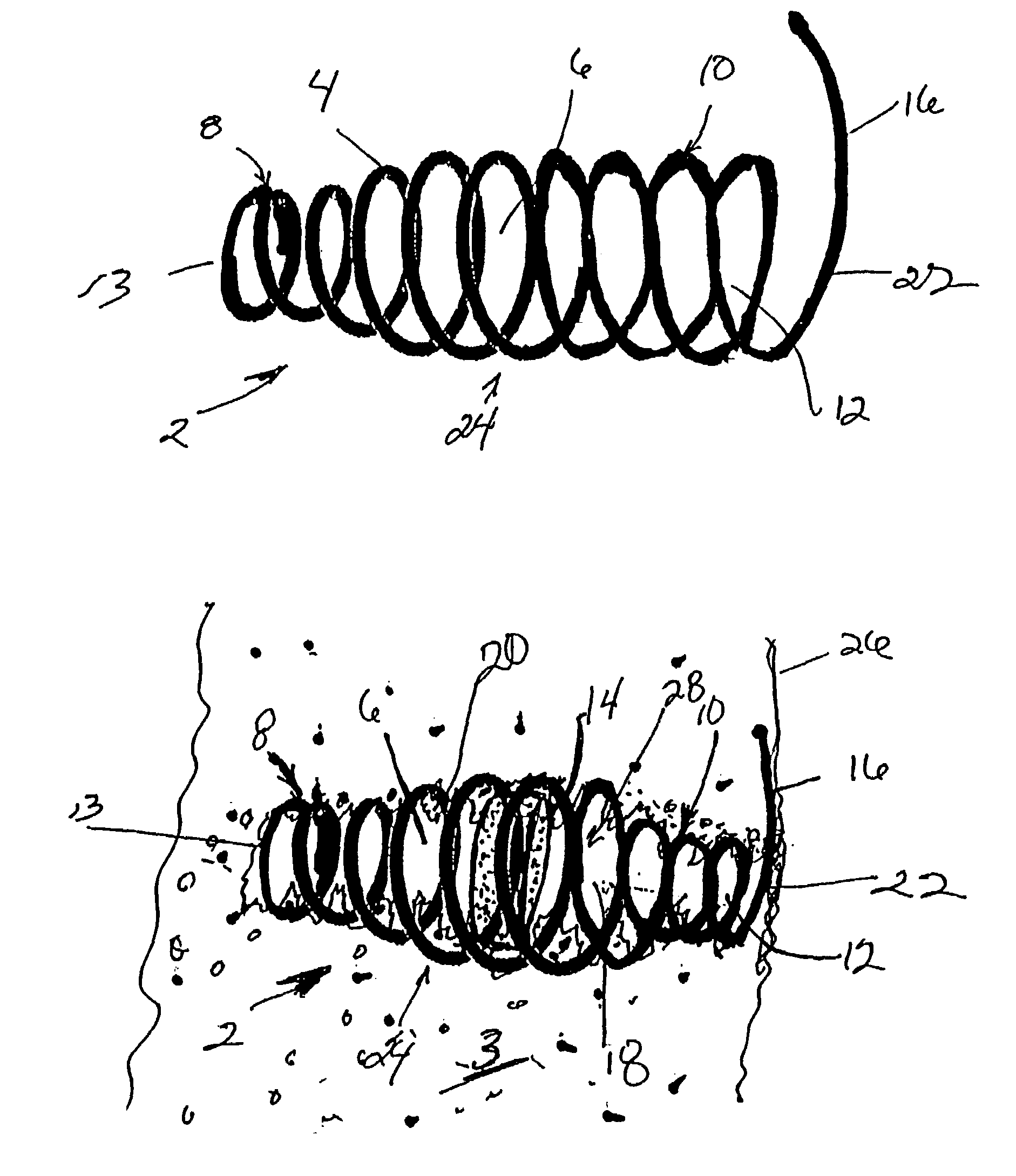
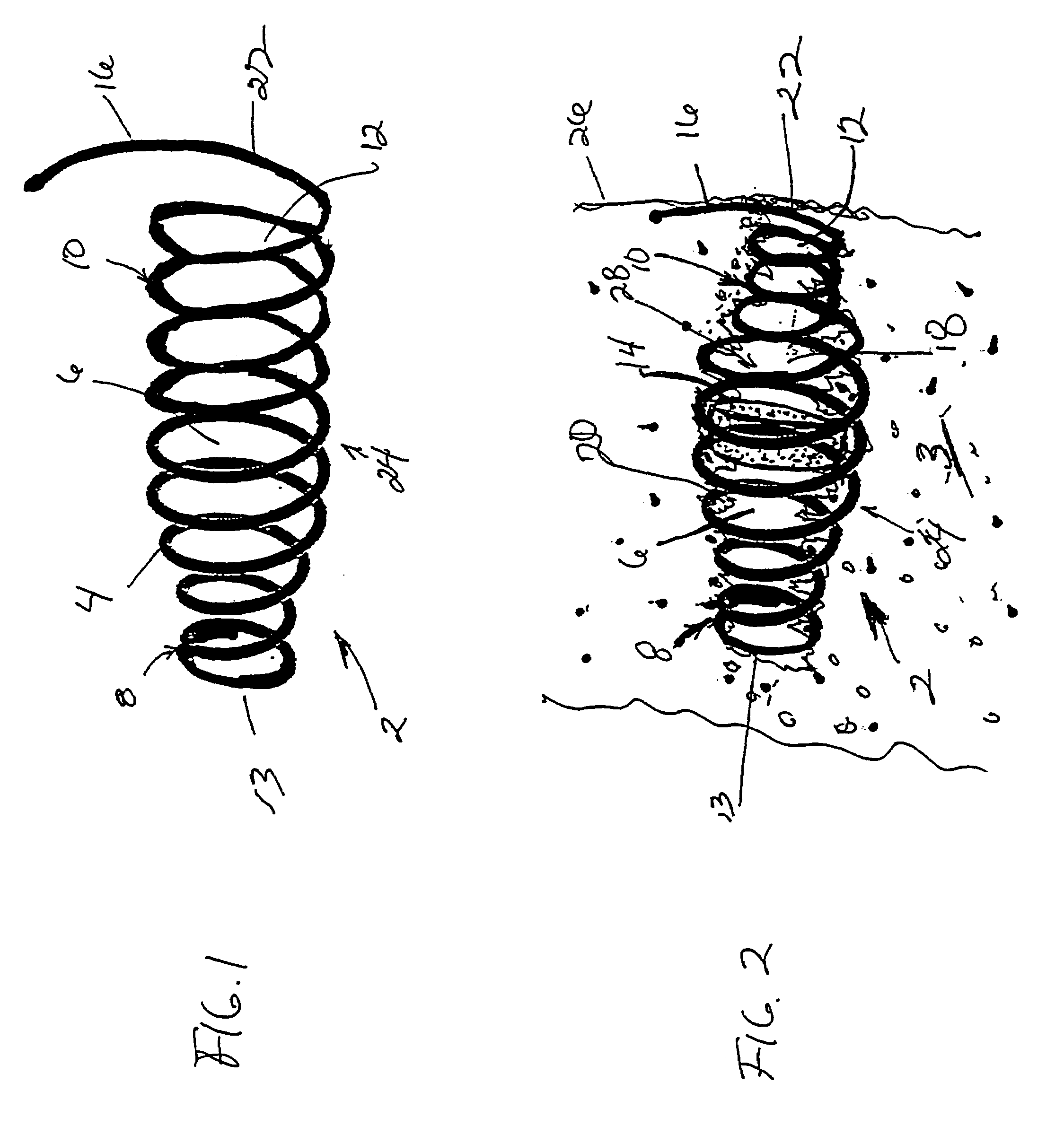
Agent delivery systems
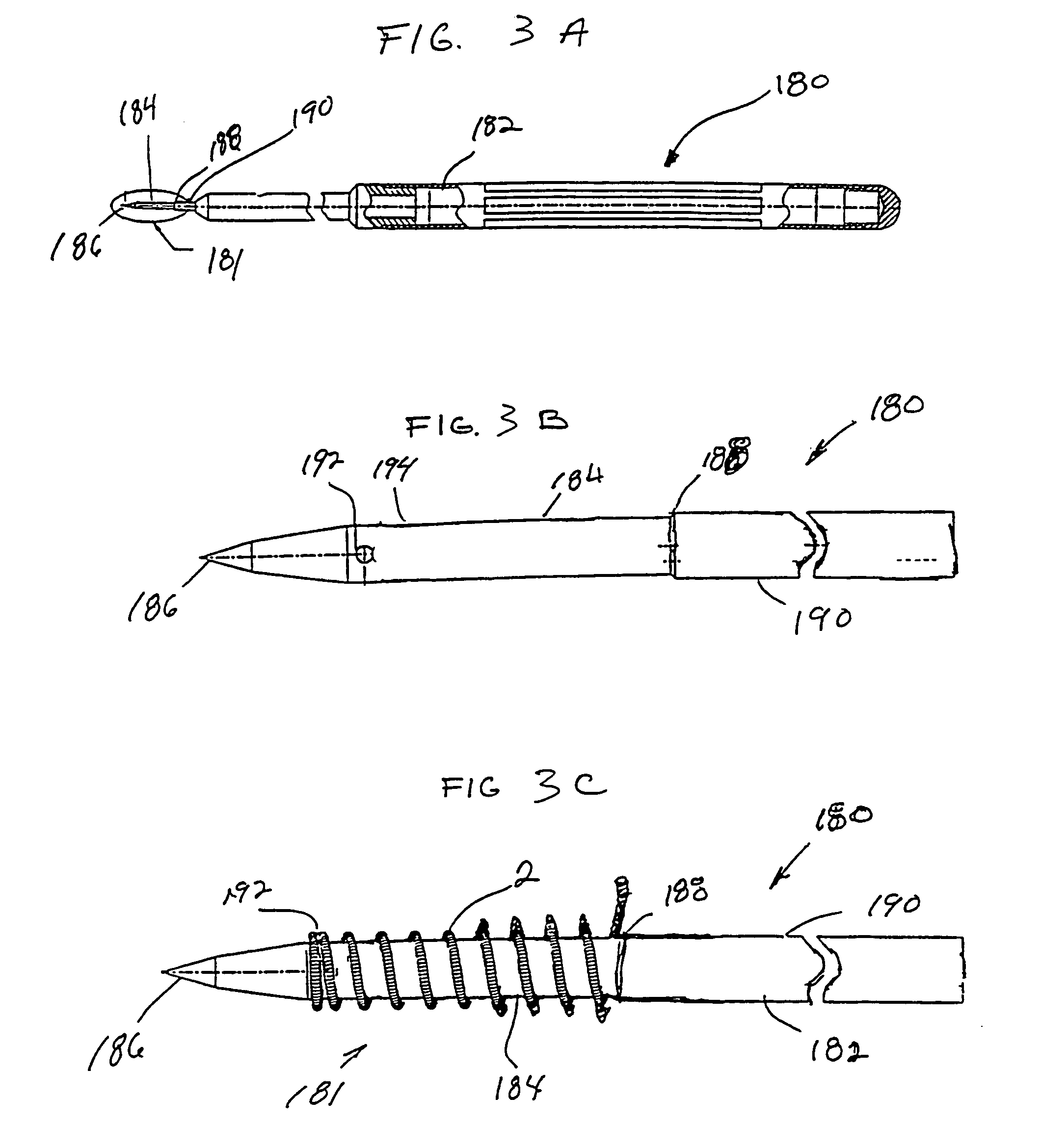
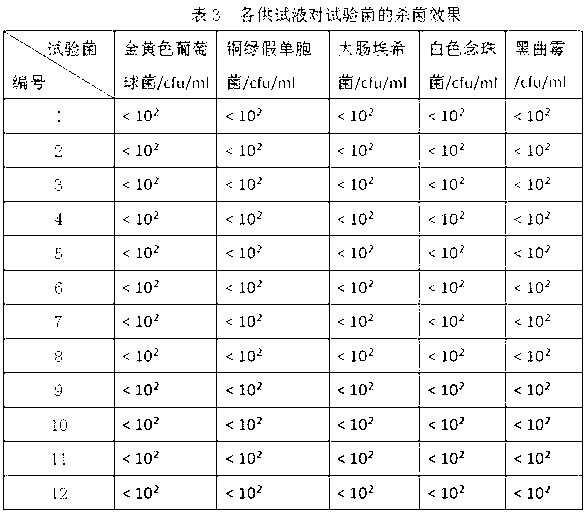
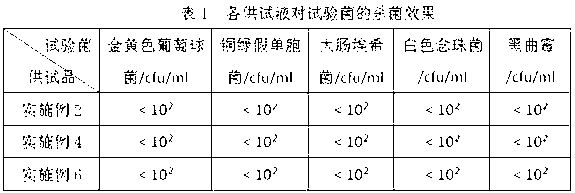
InactiveUS7232421B1The process is simple and effectiveSurgical needlesMedical devicesMechanical irritationAngiogenesis Effect
The present invention provides a system for delivering a therapeutic agent into tissue in combination with an implant device (2). Preferably the therapeutic substance is contained within a pellet form (14) that is deliverable into the interior of the device (2) after it has been implanted. In a preferred embodiment the implant device (2) comprises a flexible coiled spring body, the coils (4) of which have the proper diameter and spacing to contain the pellet (14) within the interior of the device (2) to contact the therapeutic agent. In treatment of ischemic tissue such as that of the myocardium of the heart, the mechanical irritation of the tissue caused by the implanted device (2) can help to provide an angiogenic effect. Additionally, the cavity (18) provided by the interior of the device (2) permits blood to pool around the pellet (14) and mix with the therapeutic agent. Several delivery systems have provided for delivering the implant and pellet (14) sequentially or simultaneously.
Owner:CR BARD INC
Wound nursing dressing containing hyaluronic acid
ActiveCN103191461AHigh viscoelasticityGood moisturizing effectAbsorbent padsBandagesAngiogenesis EffectWound care
The invention discloses a wound nursing dressing containing hyaluronic acid. The dressing comprises hyaluronic acid and salt thereof, and soluble silver salt effective ingredients, wherein the hyaluronic acid comprises high molecular weight hyaluronic acid and low molecular weight hyaluronic acid or oligomeric hyaluronic acid, wherein the high molecular weight hyaluronic acid is excellent in viscoelasticity, moisture retention and hydroscopicity, and the low molecular weight has a remarkable effect of promoting angiogenesis; the hyaluronic acids with two molecular weights both have multiple effects to wound healing, and have the effect of playing quick response and long action; and the soluble silver salt is stable in quality, has a good bacteriostatic effect, and plays a synergetic effect when being compounded with hyaluronic acids with high and low molecular weights. The dressing can be used for disinfection, anti-infection and healing acceleration of various skin wounds, ulcer and the like, and has a remarkable effect.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Ang2-binding molecules
InactiveUS20130259859A1Reduce manufacturing costReduced activityAnimal cellsSenses disorderDiseaseAngiogenesis Effect
Ang2-binding molecules, preferably Ang2-binding immunoglobulin single variable domains like VHHs and domain antibodies, pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with Ang2-mediated effects on angiogenesis. Nucleic acids encoding Ang2-binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Dll4-binging molecules
InactiveUS20110195494A1Reduce manufacturing costImproving desired propertySenses disorderAntipyreticMedicineAngiogenesis Effect
DII4-binding molecules, preferably DII4-binding immunoglobulin single variable domains like VHHs and VHs, pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with DII4-mediated effects on angiogenesis. Bispecific DII4-binding molecules that also bind to VEGF-A. Nucleic acids encoding DII4-binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Katsutoxin extract, preparation method and application thereof
InactiveCN101366733ASuppress generationInhibition of productionAnthropod material medical ingredientsDigestive systemFreeze-dryingAngiogenesis Effect
The invention discloses a scorpion venom extract extracted from East Asia scorpions. A preparation method for the scorpion venom extract comprises the following steps: scorpion venom freeze-dried powder is dissolved, centrifuged, filtered by use of 0.45 mu m and 0.22 mu m microporous membranes and freeze-dried; sephadex is added in; III-IV protein peaks of scorpion venom are collected; cation exchange chromatography is carried out; detection is carried out by use of an ultraviolet spectrophotometer; and the protein peaks are collected, so as to obtain the scorpion venom extract. Experiments prove that the scorpion venom extract has definite functions of inhibiting angiogenesis and hepatoma cell reincrease value. The invention successfully extracts effective components inhibiting angiogenesis and hepatoma cell reincrease value from the East Asia scorpions, which provides a foundation for further research and application in the future.
Owner:INST OF BASIC MEDICINE OF SAMS
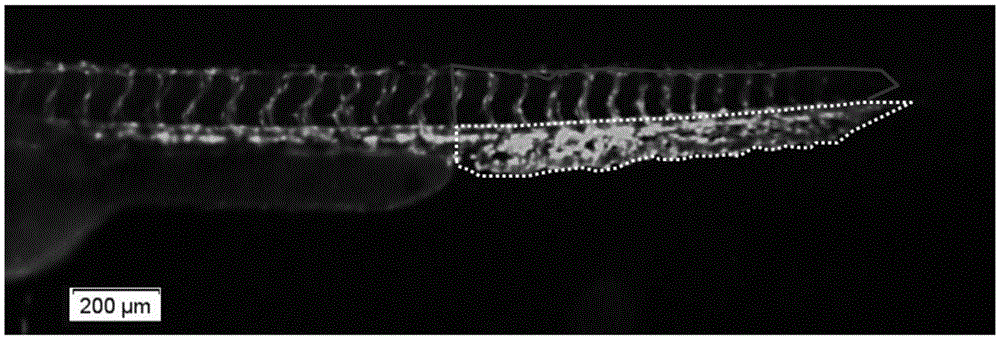
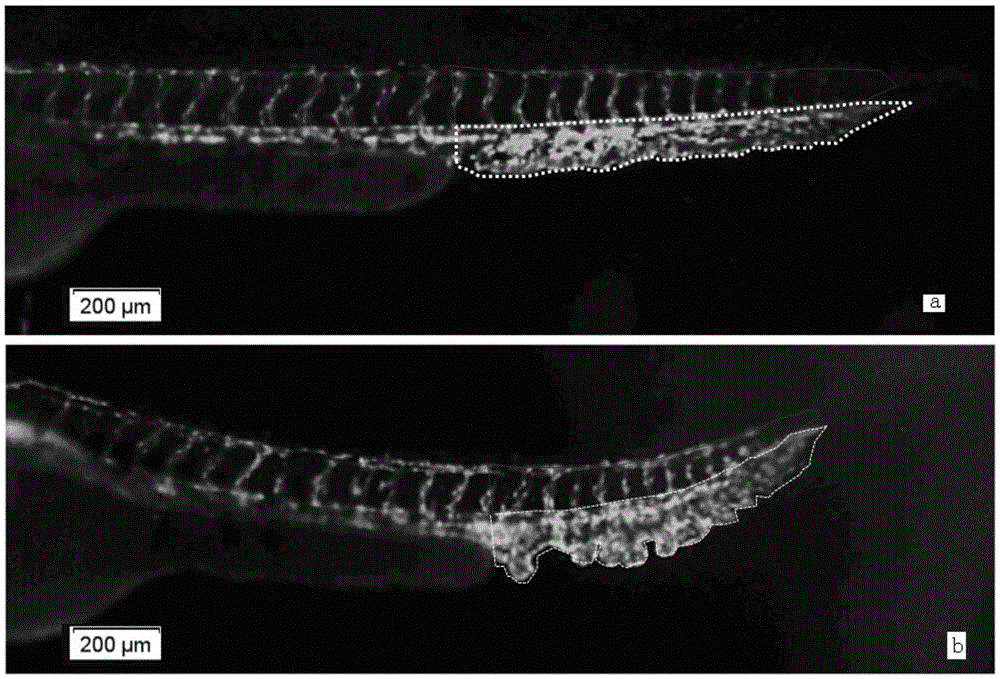
Method for quickly and quantitatively evaluating blood vessel generation promotion function of chemical compounds on zebra fish
ActiveCN105424666AImprove accuracyEasy to operateUsing optical meansFluorescence/phosphorescenceChemical compoundAngiogenesis Effect
The invention relates to a method for quickly and quantitatively evaluating a blood vessel generation promotion function of chemical compounds on zebra fish. According to the method, transgenic zebra fish with blood vessels specially expressing green fluorescent protein are adopted, a to-be-detected medicine is directly added to water for culturing the zebra fish, the relative area of tail venous rete of the zebra fish is detected, and the method for quickly and quantitatively evaluating the blood vessel generation promotion function of the chemical compounds on the zebra fish is established and used for medicine screening. In the experiments of the zebra fish, the relative area of the tail venous rete is initially taken as a new evaluation index about the blood vessel generation function, whether the to-be-detected medicine has a blood vessel generation promotion function is analyzed more scientifically and more objectively, and the accuracy of results is improved.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
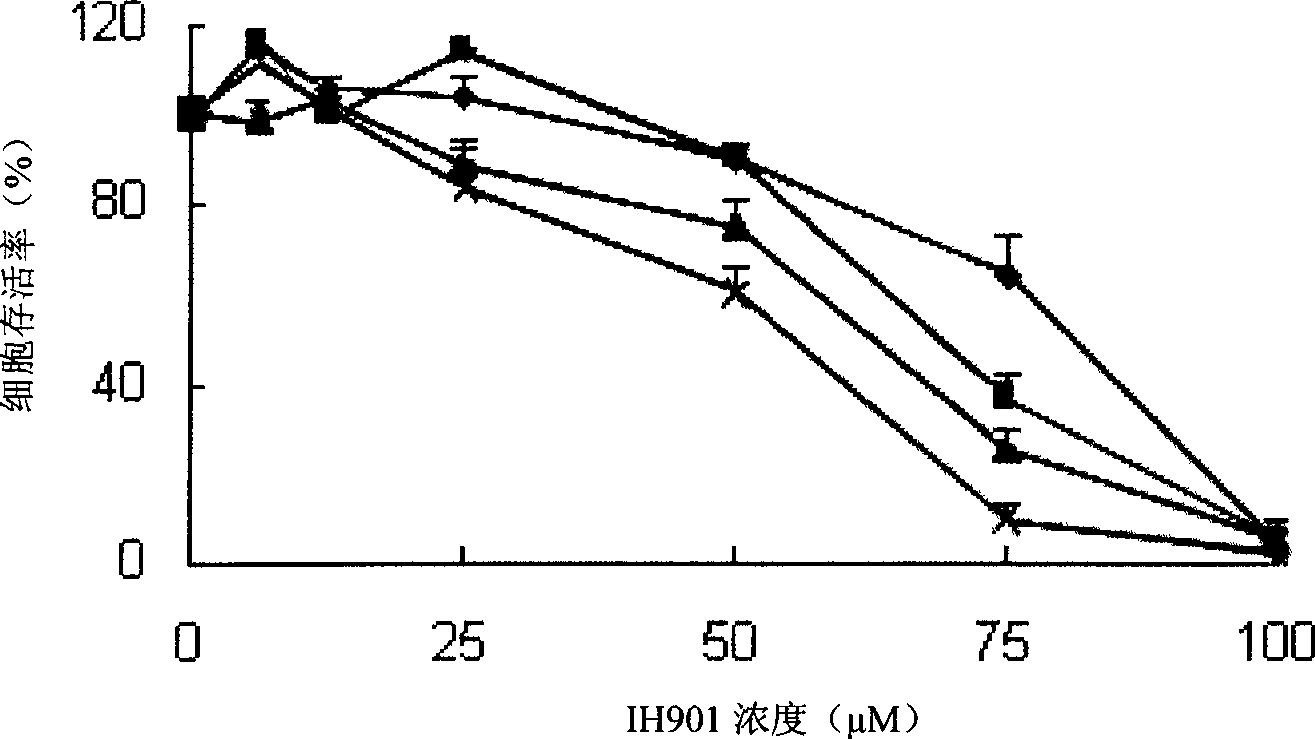
Application of ginseng saponin IH901 in preparing angiogenesis inhibitor
InactiveCN101474193AResistant to invasion and metastasisOrganic active ingredientsAntipyreticDiseaseLymphatic Spread
Application of ginsenoside IH901 in preparing angiogenesis inhibitors relates to the application of the ginsenoside IH901 in the medicament field. The invention provides application of ginsenoside IH901 and a precursor thereof in preparing angiogenesis inhibitors. The ginsenoside IH901 is 20-O-beta-D-glucopyranoside-20(S)-protopanoxadiol which can be used for preparing the angiogenesis inhibitors, and the precursor of the ginsenoside IH901 can be used for preparing the angiogenesis inhibitors. The extracted ginsenoside IH901 and the precursor have inhibitory effect on infiltration metastasis and angiogenesis and are used for preparing medicaments for treating diseases related to tumor angiogenesis and other angiogenesis. The ginsenoside IH901 and precursor are prepared into anti-tumor drugs and other drugs for angiogenesis, thus providing a research base for pharmacodynamics and action mechanism and having values of developing the ginsenoside IH901 and the precursor into drugs for tumor chemotherapy and / or auxiliary chemotherapy.
Owner:厦门华侨亚热带植物引种园
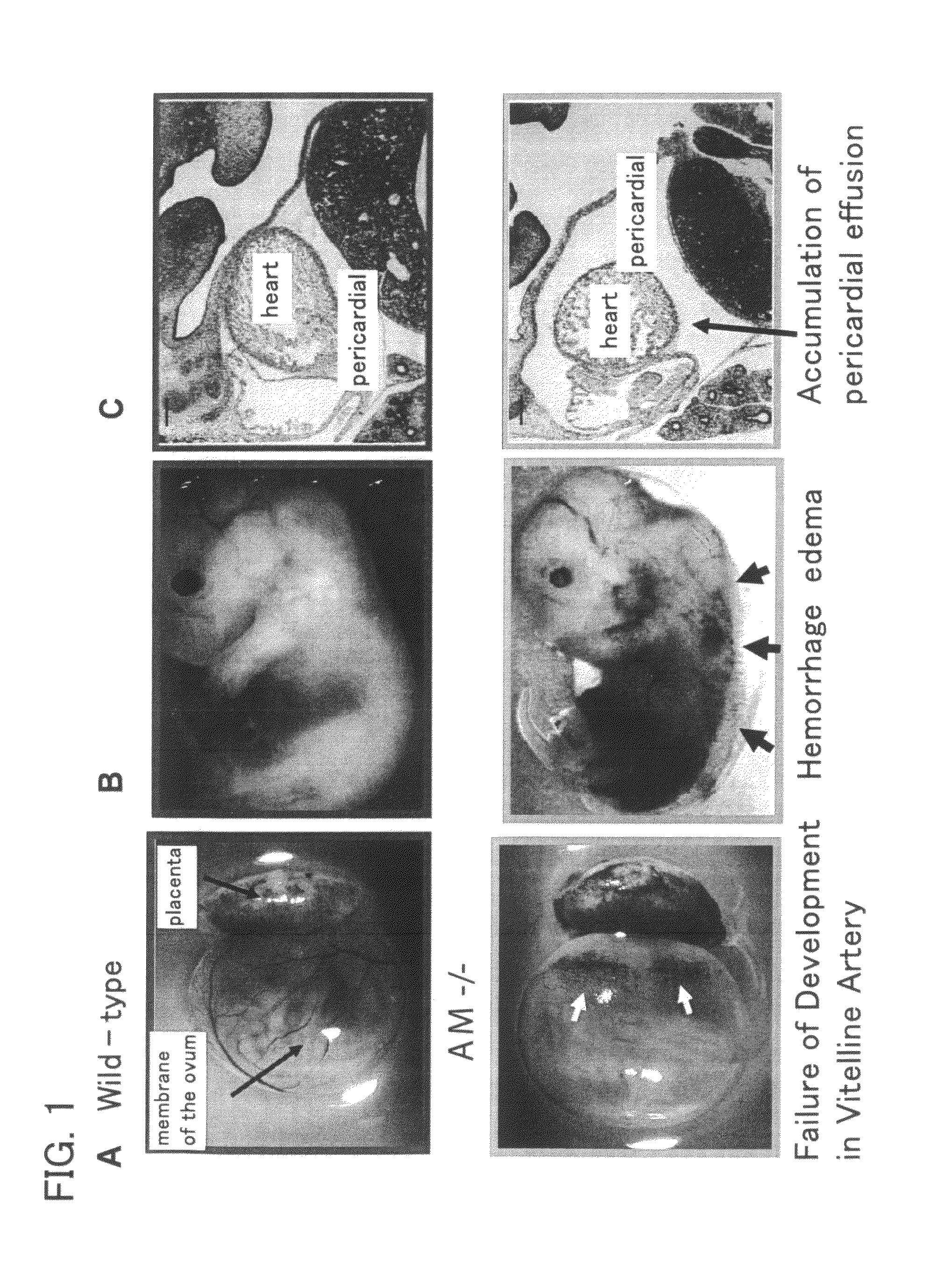
Angiogenically effective unit dose of FGF-2 and method of use
InactiveUS20050143298A1Reduce needTherapy is also rapidOrganic active ingredientsPeptide/protein ingredientsCoronary artery diseaseArterial disease
The present invention has multiple aspects. In particular, in one aspect, the present invention is directed to a unit dose composition comprising 0.2 μg / kg to 48 μg / kg of an FGF-2 of SEQ ID NO: 2, or an angiogenically active fragment or mutein thereof in a pharmaceutically acceptable carrier. In another aspect, the present invention is directed to a method for treating a human patient for coronary artery disease, comprising administering into one or more coronary vessels or a peripheral vein of a human patient in need of treatment for coronary artery disease a safe and angiogenically effective dose of a recombinant FGF-2, or an angiogenically active fragment or mutein thereof. The single unit dose composition of the present invention provides an angiogenic effect in a human CAD patient that lasts 2 months before re-treatment is required. In another aspect, the present invention is directed to a method of administration which optimizes patient's safety. In this embodiment, fluids, heparin and / or rate of infusion all play a role. In another aspect, the present invention is directed to a pharmaceutical composition comprising a therapeutically effective amount of FGF-2, alone or in combination with heparin, in a therapeutically effective carrier. The magnitude and duration of benefit were unexpected; in addition benefit with the IV route was unexpected.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
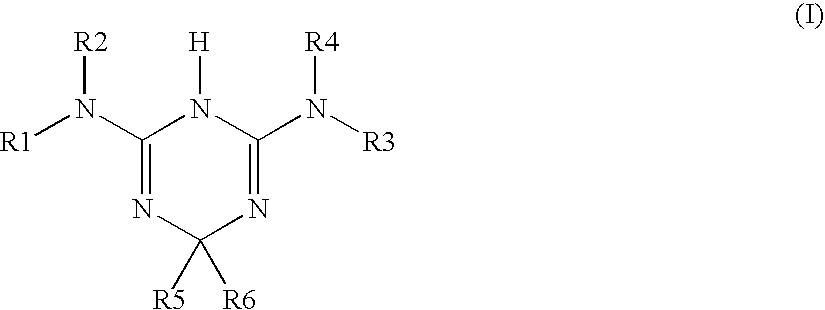
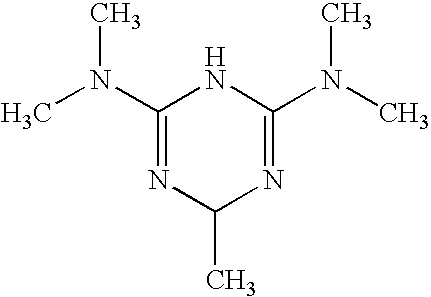
Use of triazine derivatives for the manufacture of a medicament having a cicatrising or angiogenic effect
The present patent application relates to the use of triazine derivatives as cicatrising or angiogenic agents.
Owner:POXEL
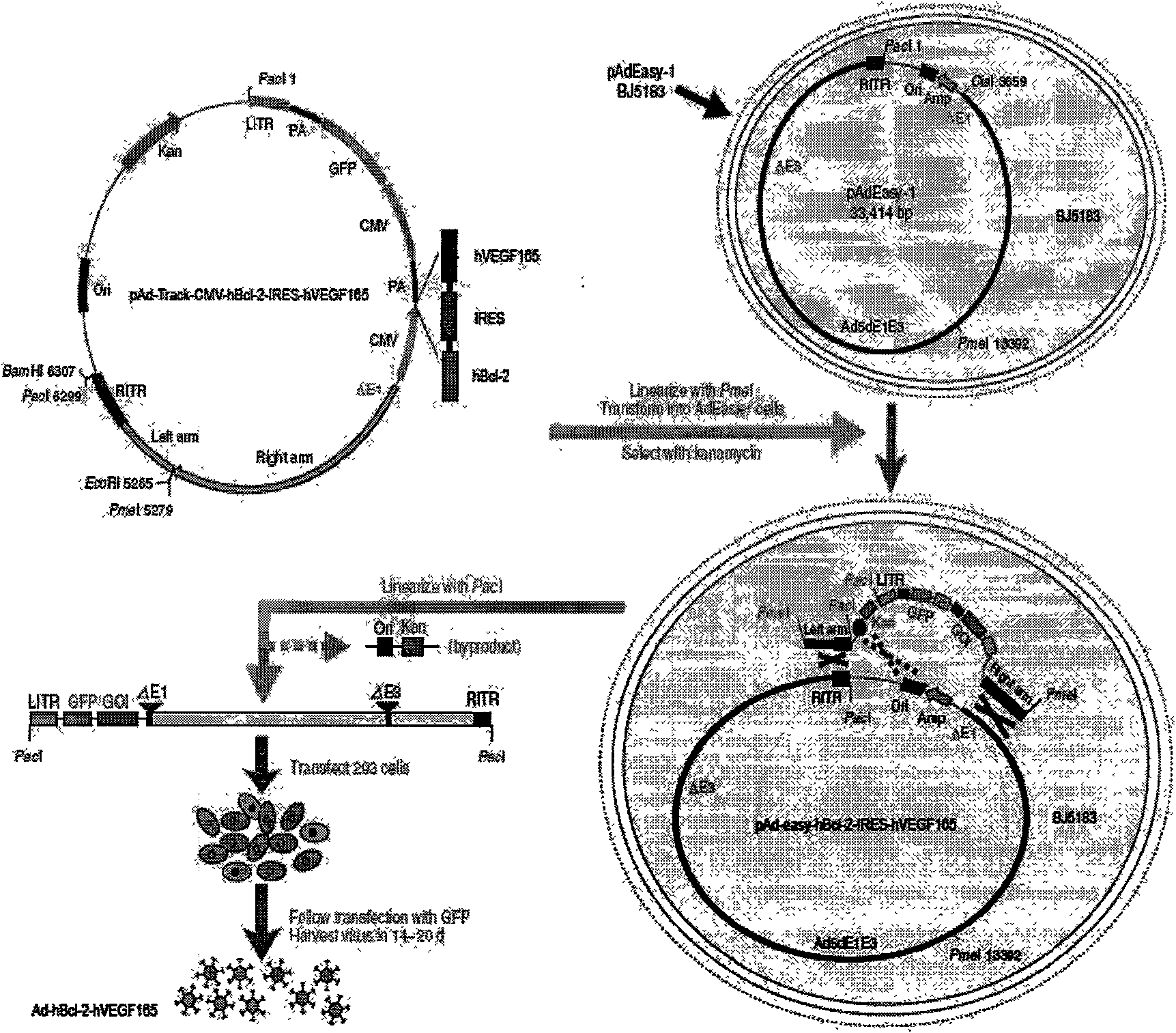
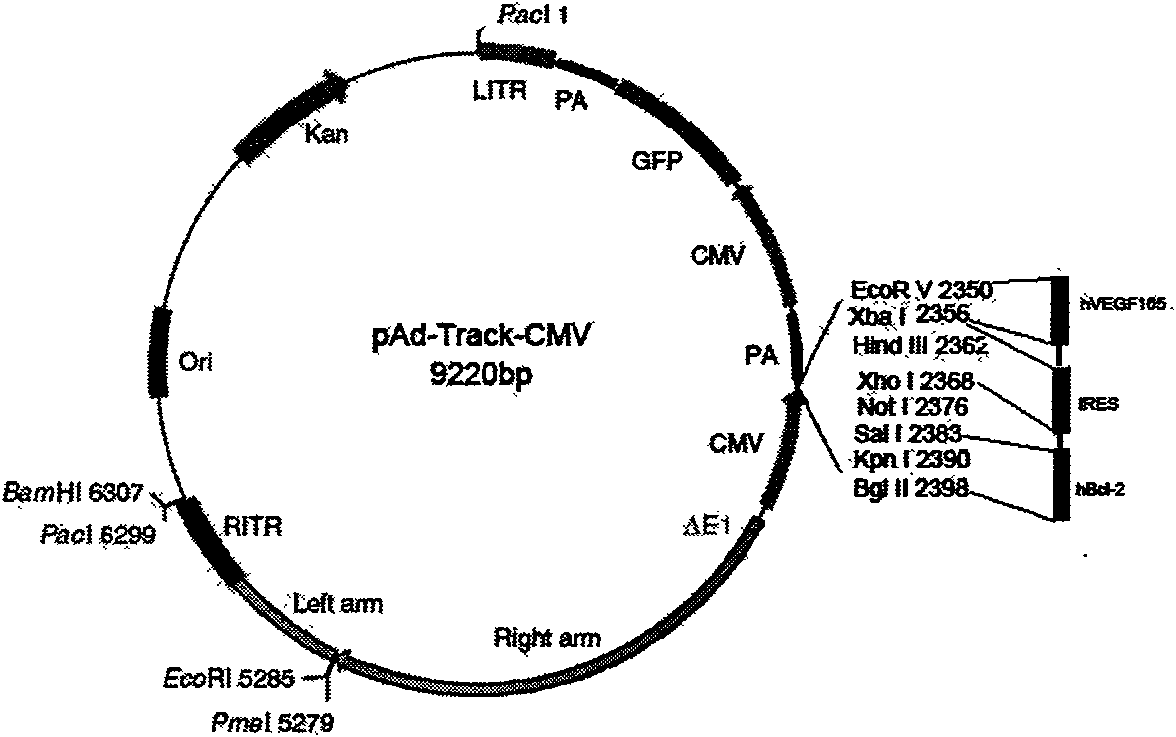
Human Bcl-2 and human VEGF165 double-gene co-expression recombinant vector and building method thereof
InactiveCN101654685APromote regenerationSolve the problem of low survival rateFermentationVector-based foreign material introductionAngiogenesis EffectAngiogenesis growth factor
The invention relates to a human Bcl-2 and human VEGF165 double-gene co-expression recombinant vector and a building method thereof, and is characterized in that IRES segment is utilized to connect an hBc1-2 gene and an hVEGF165 gene, and homologous recombination mechanisms in bacterium are utilized to recombine and build the human Bcl-2 and human VEGF165 double-gene co-expression recombinant vector according to an AdEasy system. The invention leads the Bcl-2 gene with anti-apoptosis capacity and the VEGF165 gene which currently promotes angiogenesis cytokine with strongest function to recombine on a same vector to be co-expressed, and solves the problem of low survival rate of transplanted cells under the condition of hypoxia and inflammation, simultaneously plays the function of promoting angiogenesis of VEGF165, plays an important part in the gene therapy of myocardial infarction and the cell transplantation research field, and has wide application prospect.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
In vitro assay for evaluation of angiogenic effects
InactiveUS20050064521A1Quick and easy and economical methodDiagnosticsPreparing sample for investigationAngiogenesis EffectCollagen fiber
The present invention provides an in vitro method of assaying angiogenic effects, using the morphology of an in vitro model prepared by culturing endothelial cells in a three-dimensional collagen fiber gel as an index. The invention also provides a method for screening of prospective pro- and anti-angiogenic compounds.
Owner:TUNGHAI UNIVERSITY
Application of carnosic acid in preparing medicament for suppressing angiogenesis
InactiveCN102058570AHas anti-angiogenic activityPrevent proliferationOrganic active ingredientsSenses disorderDiseaseArthritis
The invention relates to application of carnosic acid in preparing a medicament for suppressing angiogenesis. The invention has the advantages that: the novel application of the carnosic acid discovers the effect of the carnosic acid in resisting angiogenesis for the first time, and the carnosic acid can effectively suppress proliferation, migration and canaliculization of vascular endothelial cells for the first time, which proves that the carnosic acid has the activity of resisting the angiogenesis, can be used as an angiogenesis suppressant, and can be applied to preparation of angiogenesis medicaments as the angiogenesis suppressant to treat neovascularization dependent and neovascularization related diseases, such as tumors, arthritis, psoriasis, ocular diseases, atherosclerosis, and the like.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
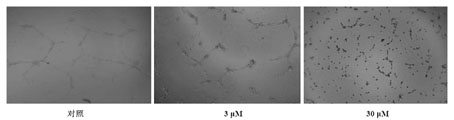
Application of two halogen-phenol compounds to effect of promoting angiogenesis
InactiveCN105193795AGood pro-angiogenic effectLittle side effectsKetone active ingredientsCardiovascular disorderHalogenAngiogenesis Effect
The invention relates to two halogen-phenol compounds, namely 2, 4', 5'-trihydroxy-5, 2'-dibromo benzophenone and 2',5-dibromo-2-(2-phenyl imidazole-1-group) methyl-4',5'-dihydroxy benaophenonel, and novel pharmacological application of medicine compositions of the two compounds in the effect of promoting angiogenesis. According to the application of the two halogen-phenol compounds, the angiogenesis promotion effect of the two halogen-phenol compounds is defined, and the compounds have the specific effects that the length of a damaged internode blood vessel of a zebra fish can be remarkably increased, and the zebra fish suffering from vascular injury can be protected. The compounds can be used independently or in combination with other pharmaceutic adjuvant ingredients acceptable to pharmacy for preparing medicine having the angiogenesis promotion function, and potential application prospects in prevention and treatment of ischemic heart disease, apoplexy, muscle atrophy, far-end limb necrosis and the like are achieved.
Owner:SHANXI MEDICAL UNIV
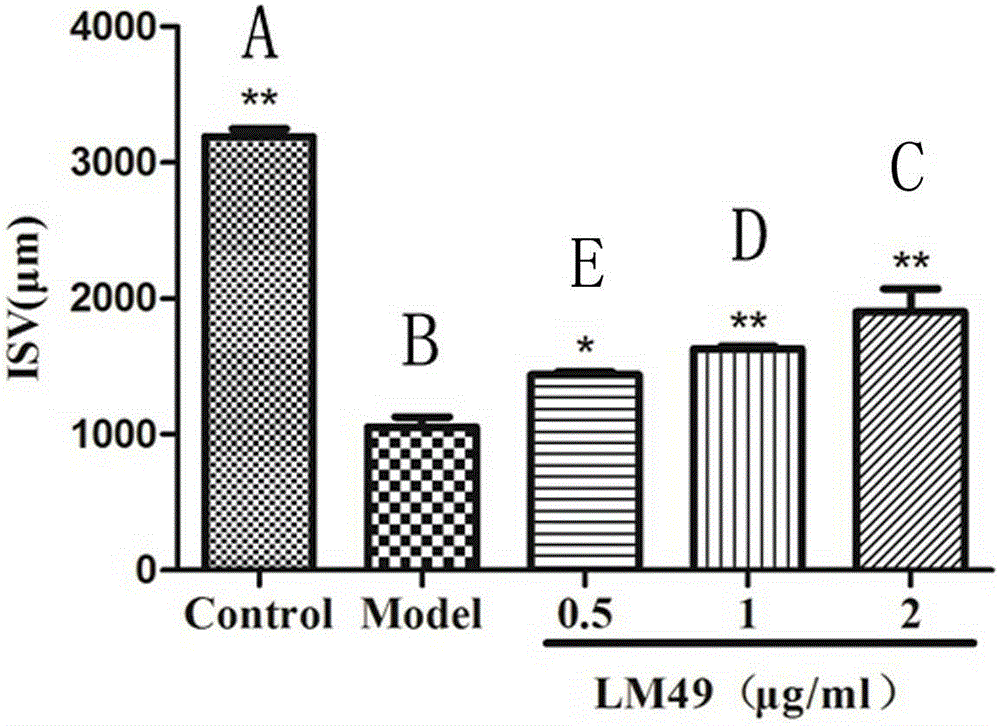
Method for screening substance having proangiogenic effect
ActiveUS9072777B2Stable structurePeptide/protein ingredientsAntipyreticAngiogenesis EffectAdrenomedullin receptor activity
Owner:SHINDO DENSHI KOGYO KK
Preparation method of nerve repair drug-carrying system with electrical stimulation and angiogenesis promotion effects
ActiveCN110975008AMeet mechanical performance requirementsPrevent collapseElectro-spinningTissue regenerationCell-Extracellular MatrixNerve repair
The invention relates to a nerve repair drug-carrying system with bioelectrical stimulation and angiogenesis promoting effects and a preparation method thereof. Gelatin, chitosan, polylactic acid andother natural polymer components in the nerve repair drug-carrying system ensure good biocompatibility and no toxicity, polylactic acid can also meet the good mechanical property requirement of a catheter, catheter collapse in the nerve growth process is avoided, and excellent flexibility enables the nerve repair drug-carrying system not to hinder nerve regeneration. Due to the introduction of a conductive copolymer GO, the catheter has weak conductivity, meets the requirement of physiological current of a human body, and is beneficial to accelerating nerve repair; and by introducing a VEGF growth factor, an extracellular matrix microenvironment in a human body can be simulated to the maximum extent, nerve repair is promoted from the microvascular regeneration acceleration direction, and finally the nerve injury repair quality is improved.
Owner:深圳南泥湾科技有限公司
Stem cell combined growth factor injection for promoting angiogenesis in ischemic tissue and its preparation method and use method
InactiveCN102274491ALimitations of benefit in overcoming ischemiaGuaranteed to get enoughPeptide/protein ingredientsPharmaceutical delivery mechanismProgenitor Cell EngraftmentBasic fibroblast growth factor
The invention relates to a stem cell combined growth factor injection for promoting angiogenesis in ischemic tissue, a preparation method and a use method thereof. The body’s protective response to peripheral tissue ischemia is relatively complex. Although a single growth factor intervention is effective, it cannot complete this process; although stem and progenitor cell transplantation can improve the vascular density and blood perfusion of ischemic tissue, the main source of cells is bone marrow. There are serious limitations in the acquisition of umbilical cord blood, umbilical cord blood, etc., and the in vivo viability of cells is extremely limited, which seriously affects its therapeutic effect. The invention utilizes human basic fibroblast growth factor to intervene in adipose mesenchymal stromal cells, and cooperates with vascular endothelial cell growth factor to promote angiogenesis in ischemic tissue. The present invention overcomes the benefit limitation of single factor or cell therapy, ensures sufficient stem cell acquisition, and improves the survival ability and angiogenesis effect of transplanted cells, thereby further improving blood perfusion of ischemic lower limbs and improving limb survival Rate.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
DLL4-binding molecules
InactiveCN102648210AAntibacterial agentsSenses disorderAngiogenesis EffectAngiogenesis growth factor
DII4-binding molecules, preferably DII4-binding immunoglobulin single variable domains like VHHs and VHs, pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with DII4-mediated effects on angiogenesis. Bispecific DII4-binding molecules that also bind to VEGF-A. Nucleic acids encoding DII4-binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Sugar transferase gnt-v having angiogenic effect
InactiveUS20050148516A1Increase neovascularizationPromote growthAntibacterial agentsSenses disorderNeovascularization inhibitorsAngiogenesis Effect
The present invention provides a peptide or protein having a neovascularization action and containing a basic amino acid cluster region of β1,6-N-acetylglucosaminyltransferase, a neovascularization accelerator containing the above-mentioned peptide or protein, a method of screening an inhibition substance for the above-mentioned peptide or protein, and a neovascularization inhibitor containing this inhibition substance.
Owner:SUNTORY HLDG LTD
Application of total triterpenes in preparing drugs inhibiting angiogenesis
InactiveCN102657660AGood inhibitory effectHas anti-angiogenic activityOrganic active ingredientsSenses disorderDiseaseArthritis
The invention relates to application of total triterpenes in preparing drugs inhibiting angiogenesis. The application has the advantages that novel application of the total triterpenes in extractive of Actinidia chinensis roots is provided. An experiment proves that the total triterpenes 1,2,3,4 have remarkable inhibiting effect on multiplication of human umbilical vein endothelial cells (HUVEC) and canaliculization of HUVEC, have anti-angiogenic activity, and can serve as angiogenesis inhibitors. By means of the application of the total triterpenes in preparing the drugs inhibiting the angiogenesis, the anti-angiogenesis effect of the total triterpenes in the extractive of Actinidia chinensis roots is firstly found out, and the total triterpenes can serve as the angiogenesis inhibitors to be applied to treat new blood vessel dependence and new vessel related diseases including tumors, arthritis, skin and eye diseases, atherosclerosis and the like.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE +1
Oxidase-loaded microneedle for promoting wound healing of diabetic patient and preparation method of oxidase-loaded microneedle
ActiveCN114376993AEfficient removal of biological activityReduces levels of oxidative stressAntibacterial agentsHeavy metal active ingredientsUltraviolet lightsOxidative enzyme
The invention belongs to the technical field of biological materials and biological medicines, and discloses an oxidase-loaded microneedle for promoting wound healing of diabetics and a preparation method thereof.The preparation method comprises the steps that SilMA is dissolved in deionized water, and a photoinitiator HMPP is added under the dark condition to obtain a mixed solution A; adding PBZs and VEGF solution into the mixed solution A to obtain a mixed solution B; adding the mixed solution A into a polymyxin solution to obtain a mixed solution C; and respectively adding the mixed solution B and the mixed solution C into a microneedle mold, carrying out crosslinking under ultraviolet light, and separating the mold to obtain the composite microneedle. The PBZs and the VEGF are creatively loaded into the SilMA composite hydrogel microneedle to treat diabetic skin wounds, the oxidative stress level of diabetic skin wound tissues can be effectively relieved, a proper oxidation microenvironment is provided for the VEGF to play a role in promoting angiogenesis, and healing of the diabetic skin wounds can be better promoted.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Bispecific binding molecules for Anti-angiogenesis therapy
InactiveUS20140120095A1Improving desired propertyExtended half-lifeSenses disorderSugar derivativesAngiogenesis EffectAntiangiogenic therapy
Bispecific binding molecules, in particular immunoglobulin single variable domains such as VHHs and domain antibodies, comprising a VEGF-binding component and a DII4-binding component in one molecule. Pharmaceutical compositions containing same and their use in the treatment of diseases that are associated with VEGF- and DII4-mediated effects on angiogenesis. Nucleic acids encoding the bispecific binding molecules, host cells and methods for preparing same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Angiogenesis drugs
InactiveUS7091175B2Simple designEasy to synthesizeBiocidePeptide/protein ingredientsAngiogenesis EffectAngiogenesis growth factor
A novel angiogenesis drug which can be clinically used for therapies of human or the like is disclosed. The angiogenesis drug according to the present invention comprises a peptide as an effective component, which has a prescribed amino acid sequence and has an angiogenesis action.
Owner:NOKIHARA KIYOSHI
Fusion protein comprising Anti-c-met antibody and vegf-binding fragment
ActiveUS20140294837A1Improved anticancerImproved anti-angiogenesis effectSenses disorderHybrid immunoglobulinsAntiendomysial antibodiesAngiogenesis Effect
There are provided a fusion protein formed by coupling of anti-c-Met antibody and VEGF-binding fragment, a bispecific antibody comprising the fusion protein, a polynucleotide encoding the fusion protein, a transformant comprising the polynucleotide, a pharmaceutical composition comprising the bispecific antibody as an active ingredient, and a method for preparing the bispecific antibody which is targeted at c-Met and VEGF at the same time, with improved anticancer and anti-angiogenesis effects, comprising coupling an anti-c-Met antibody with a VEGF-binding fragment.
Owner:SAMSUNG ELECTRONICS CO LTD
Application of dehydrocostuslactone to preparing drug for inhibiting angiogenesis
InactiveCN102058579AHas anti-angiogenic activityPrevent proliferationOrganic active ingredientsSenses disorderAngiogenesis EffectPharmaceutical drug
The invention relates to application of dehydrocostuslactone to preparing a drug for inhibiting angiogenesis. The invention has the advantages of providing the novel application of the dehydrocostuslactone, finding the anti-angiogenesis of the dehydrocostuslactone for the first time, finding that the dehydrocostuslactone can effectively inhibit the multiplication, the migration and the canaliculization of vascular endothelial cells for the first time, showing that the dehydrocostuslactone has the activity of the anti-angiogenesis and can be used as an angiogenesis inhibitor as well as can be used as the angiogenesis inhibitor used for preparing the angiogenesis drug and treating vascularization dependent and vascularization relevant diseases of tumors, arthritis, parapsoriasis guttata, eye disorders, atherosclerosis and the like.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Modified blood vessel endothelium growth factor polypeptide segment and application thereof
InactiveCN101260147AHigh affinityImprove stabilityPeptide/protein ingredientsRadioactive preparation carriersVEGF receptorsFactor ii
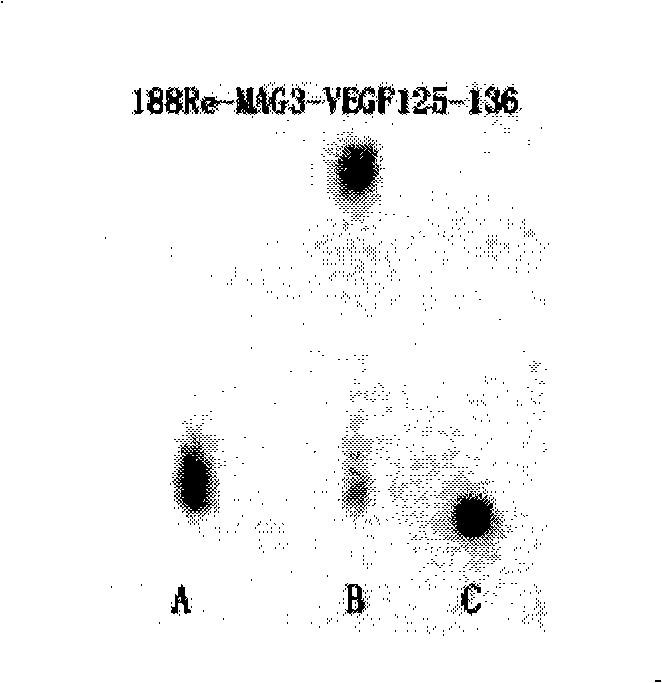
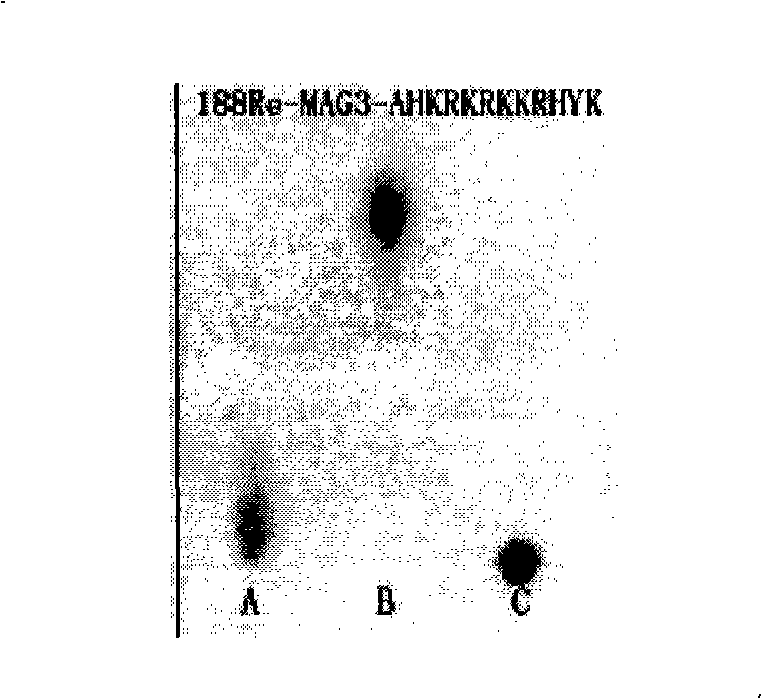
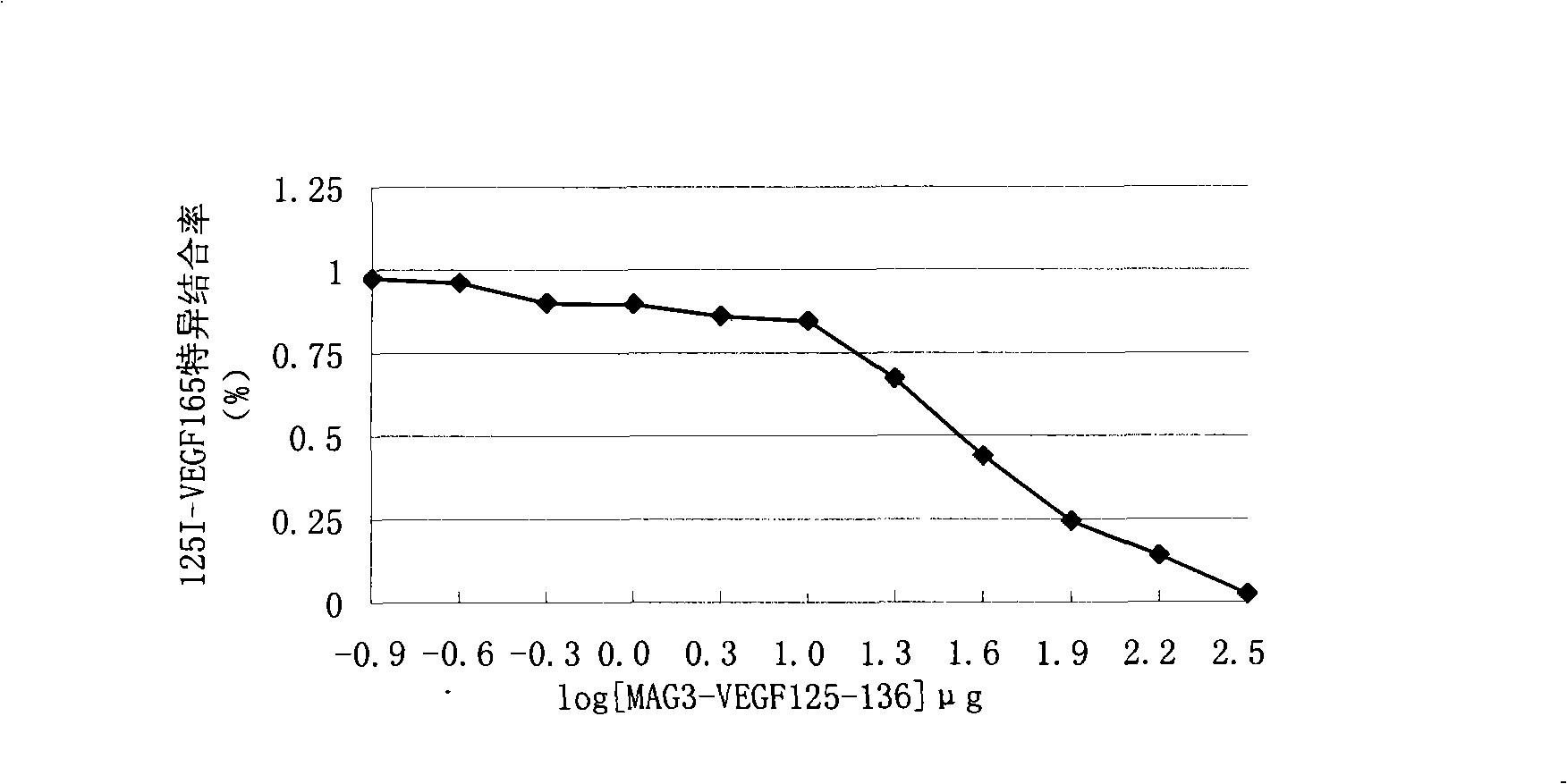
The invention discloses an improved endothelium growth factor polypeptide fragment of blood vessel and an application thereof. A polypeptide amino acid, the sequence of which is AHKRKRKKRHYK, compete with and antagonize the promotion effect of angiogenesis of VEGF by the distinguished combination with a VEGF acceptor and are used to prepare anti-tumor angiogenesis drugs; moreover, radionuclide marks used to prepare tumor nuclide developing drugs and internal irradiation of radioactive nuclide drugs can be further made; the appetency between the polypeptide of the invention and the VEGF acceptor is obviously higher than VEGF125-136; S-acetyl-MAG3 is used as a double function chelating agent to make the radionuclide marks for the polypeptide, and the mark rate is more than 95 percent, moreover, the marks are good in stability, do not need to be purified and can be directly used.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Pharmaceutical Compositions for Angiogenic Therapy
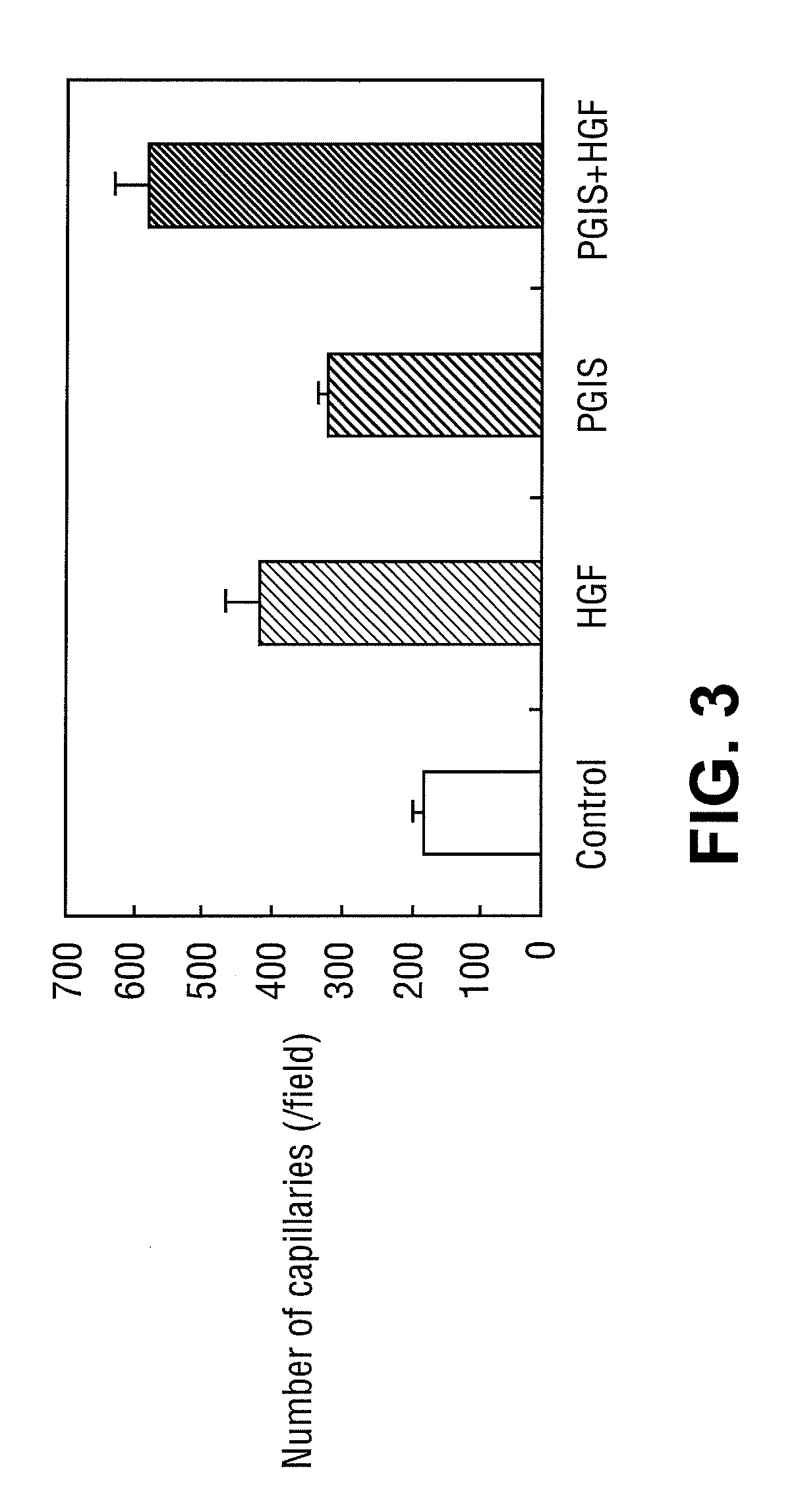
InactiveUS20100240735A1Enhancing angiogenic effectGood effectBiocideOrganic active ingredientsProstacyclin synthaseBULK ACTIVE INGREDIENT
The present invention provides: (1) pharmaceutical compositions for angiogenic therapy which contain, as the active ingredients, at least one substance selected from substances having vasodilating effect and / or platelet aggregation inhibitory effect, and substances producing them; and a gene encoding an angiogenesis factor; (2) agents for potentiating the angiogenic effect of a gene encoding an angiogenesis factor that contain, as the active ingredient, at least one substance selected from substances having vasodilating effect and / or platelet aggregation inhibitory effect and substances producing them; (3) an angiogenic agent which contains a prostacyclin synthase gene as the active ingredient; (4) pharmaceutical compositions for angiogenic therapy which contain ets-1 gene and another gene encoding an angiogenesis factor as the active ingredients; (4) an agent which contain ets 1 gene as the active ingredient for potentiating the angiogenic effect of another gene encoding an angiogenesis factor; and (5) an angiogenic agent which contains ets-1 gene as the active ingredient.
Owner:ANGES MG INC
Neovascularization promoters
InactiveCN1278177AImprove generation effectElcosanoid active ingredientsPeptide/protein ingredientsDiseaseHydrogen atom
An angiogenesis promoter containing a compound of the following formula (I) <CHEM> wherein R<1> is acyl, R<2> is alkyl, R<3> and R<4> are the same or different and each is hydrogen atom or hydroxy protecting group and R<5> is alkyl, as an active ingredient. This compound itself not only has an angiogenesis promoting effect but also potentiates the angiogenic effect by a drug (e.g., b-FGF) having such effect. Therefore, it can express the angiogenesis promoting effect more effectively in the ischemic tissues and the site under different disease state, where b-FGF has locally increased.
Owner:LTT BIO PHARMA +1
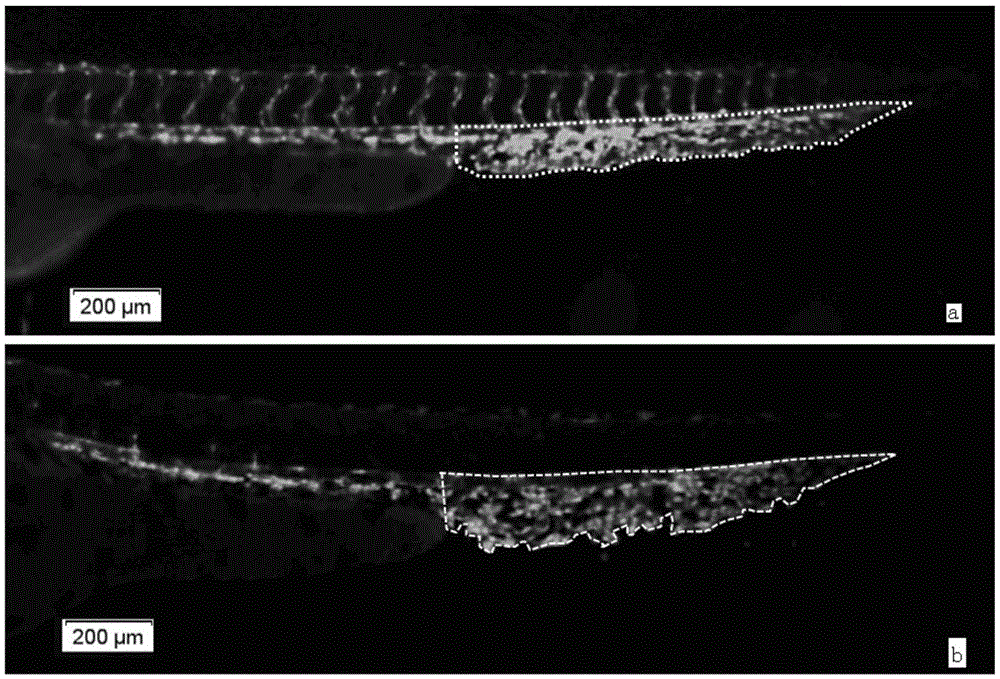
Method for evaluating effect of compound on angiogenesis in pathological state
ActiveCN106370833AEasy to operateGood repeatabilityBiological testingAngiogenesis EffectAngiogenesis growth factor
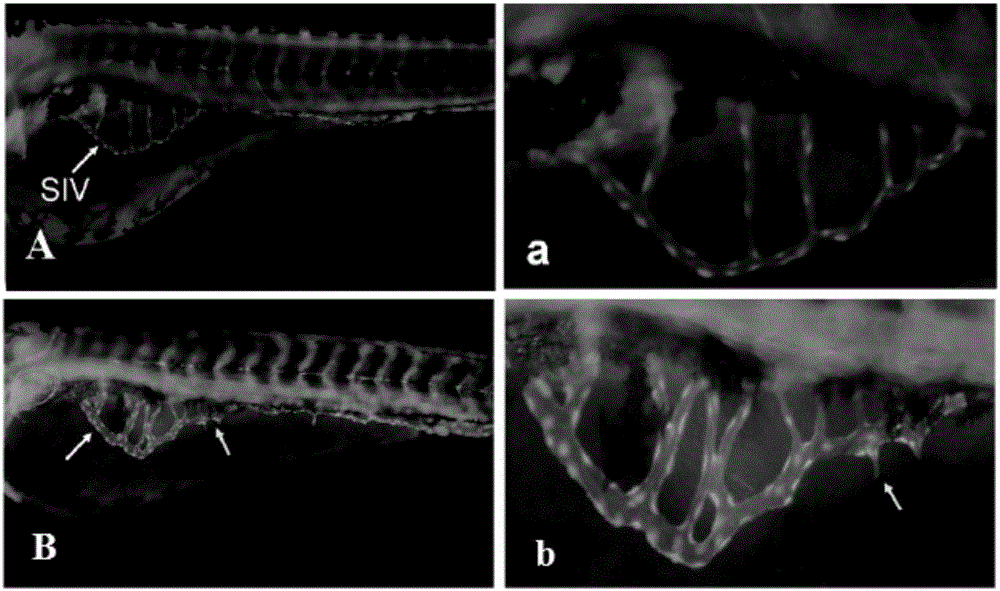
The invention relates to a method for evaluating the effect of a compound on angiogenesis in a pathological state. According to the invention, a vascular endothelial growth factor (VEGF) is used for treating young zebra fish in a specific period to simulate a pathological state, then complete subintestinal peripheral vein length is used as an evaluation index, so the evaluation method for the effect of a drug on angiogenesis in the pathological state is successively established. The method has the advantages of simple operation, rapidness, stability, reliability and good repeatability, is more scientific and objective, presents the generation condition of spinous process and improves accuracy of results.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Bone marrow mononuclear cell infected by recombinant co-gene adeno-associated virus and use thereof
InactiveCN1594556APromote generationEnsure stabilityMammal material medical ingredientsVector-based foreign material introductionVascular endotheliumLate gene
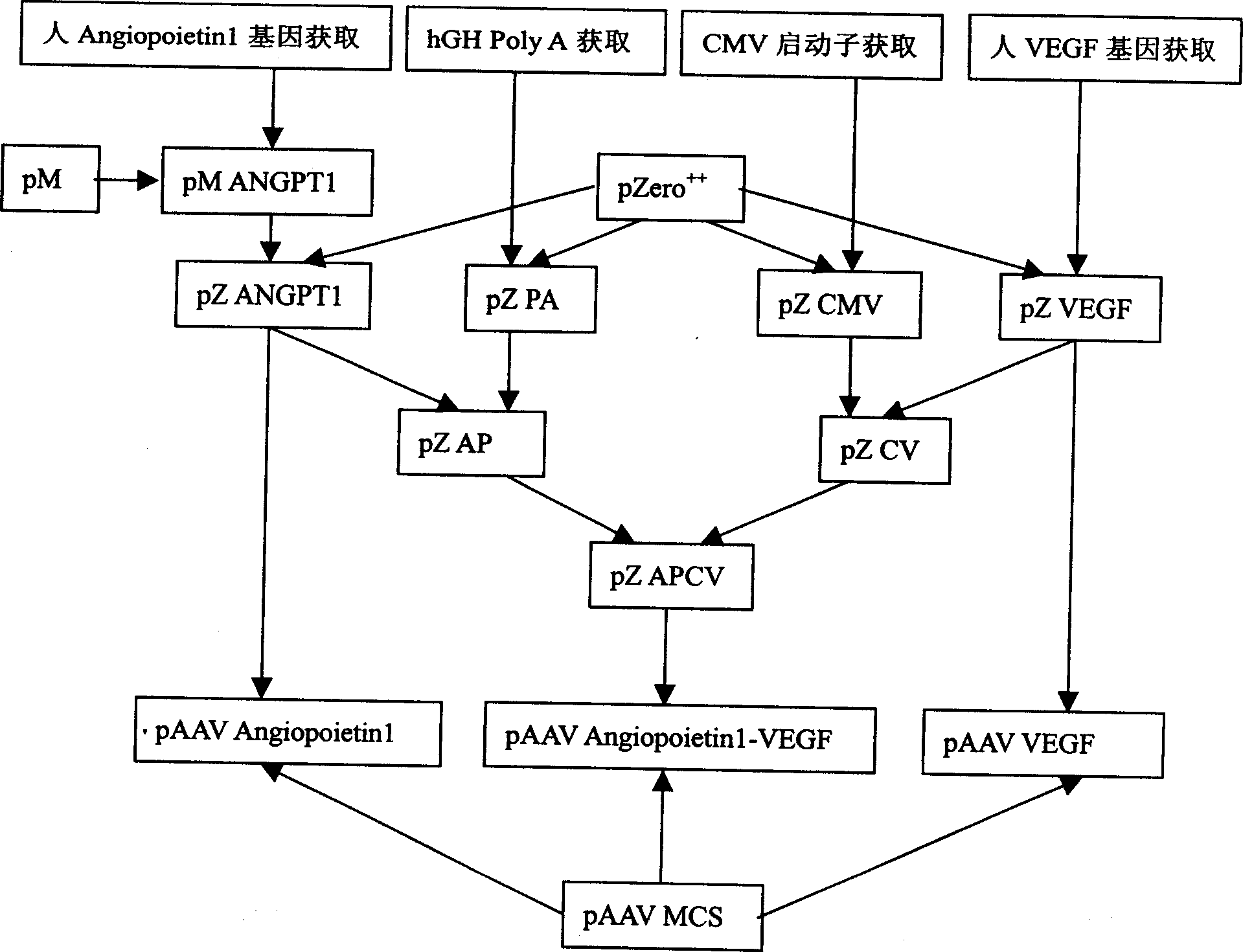
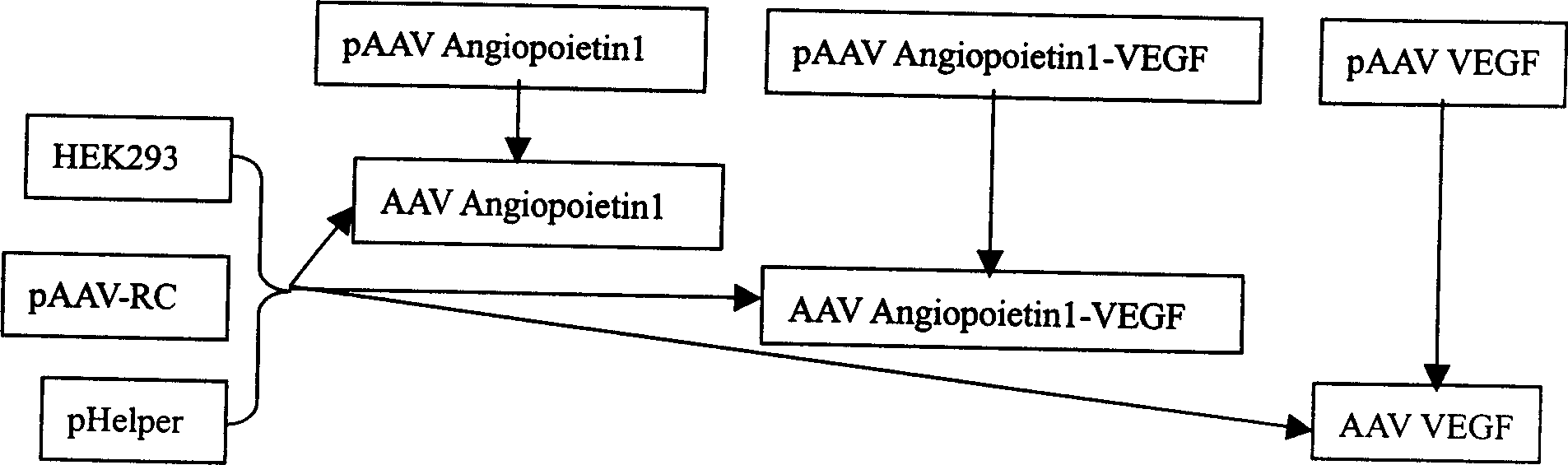
The invention relates to marrow monocyte infected with recombinant cogene AAV and its uses. Marrow monocyte infected with AAV Angiopoietinl-VEGFíóAAV Angiopoietinl and AAV VEGF is used in treating ischemia disease. The preparation steps include: Forming pAAV plasmid system containing single or united gene of blood vessel regeneration early gene and late gene, each plsmid containing a set of CMV promotor and Poly A teminator, recombining pAAV plamid system in AAV, forming three kinds of recombinant cogenen AAV, infecting blood vessel endothelial cell differentiated from marrow monocyte with AAV, injecting infected blood vessel endothelial cell ischemia tissue. The invention is safe and effective, has function of promoting blood vessel genesis.
Owner:谭最
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com