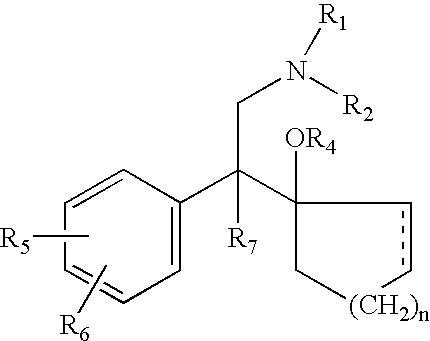
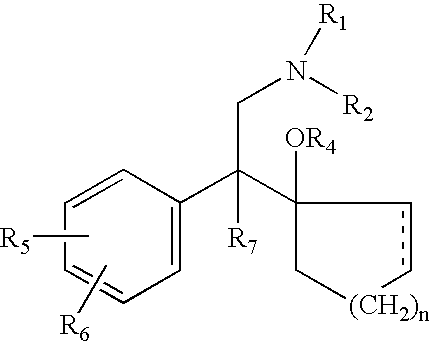
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Therapy is also rapid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
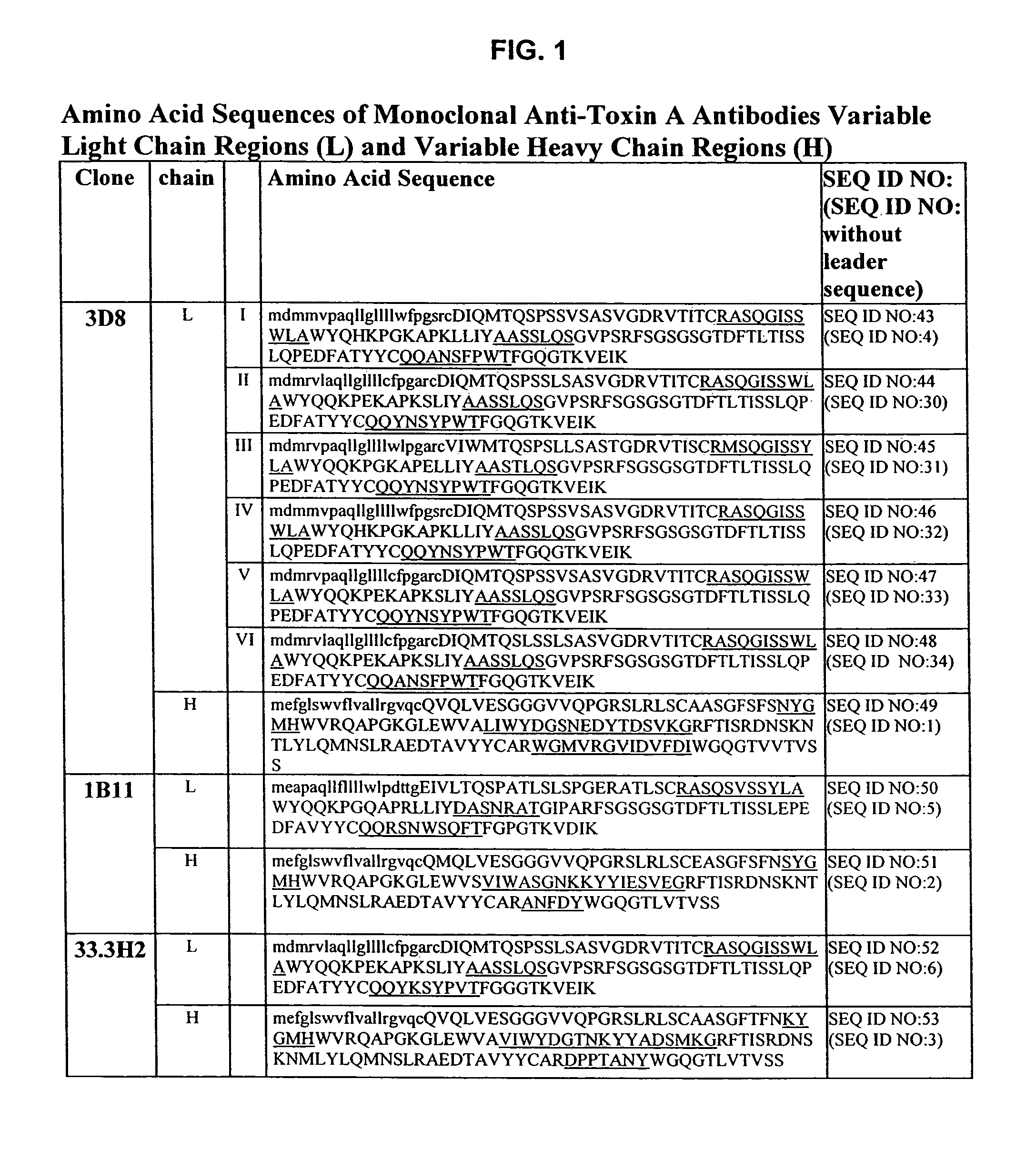
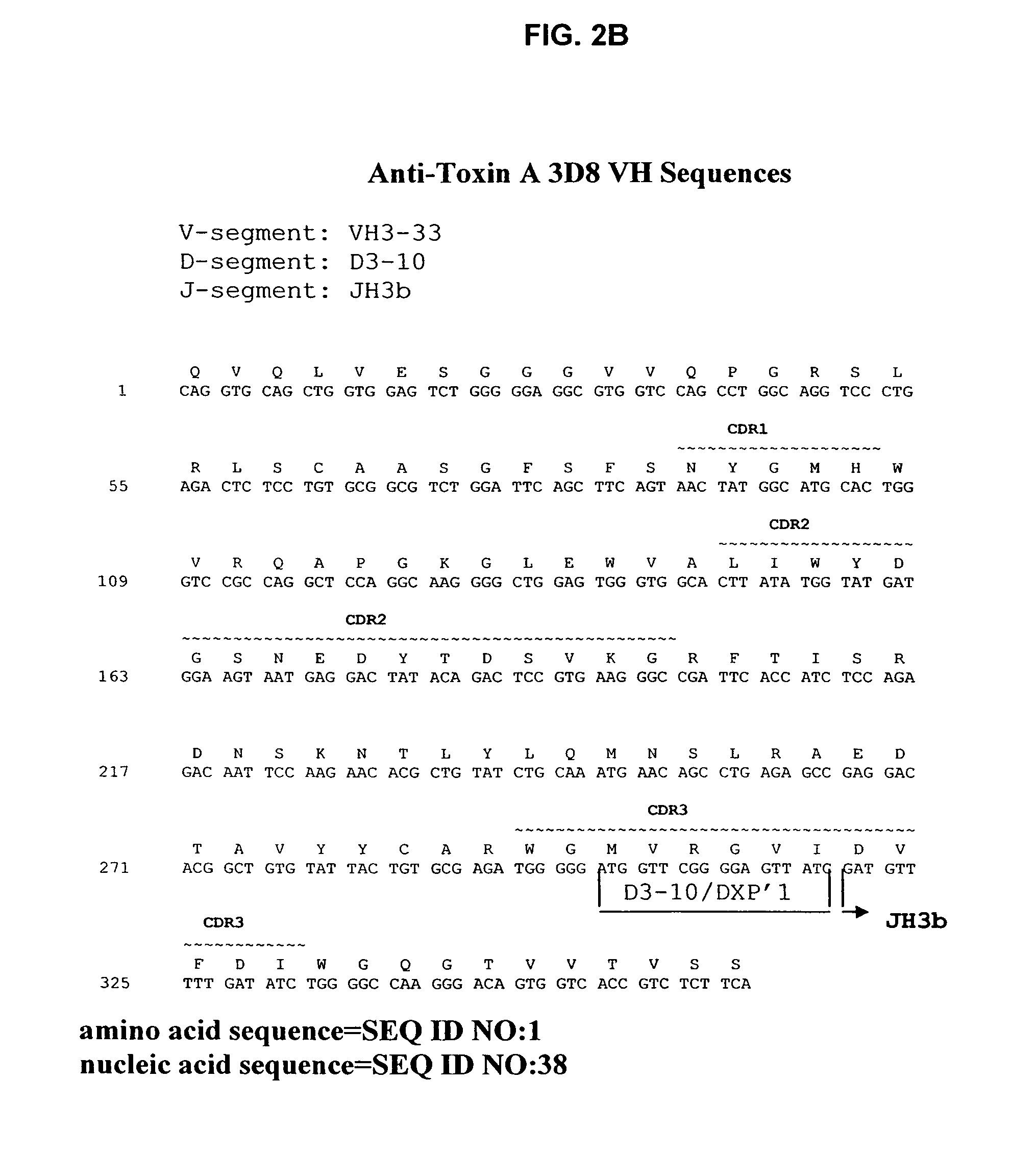
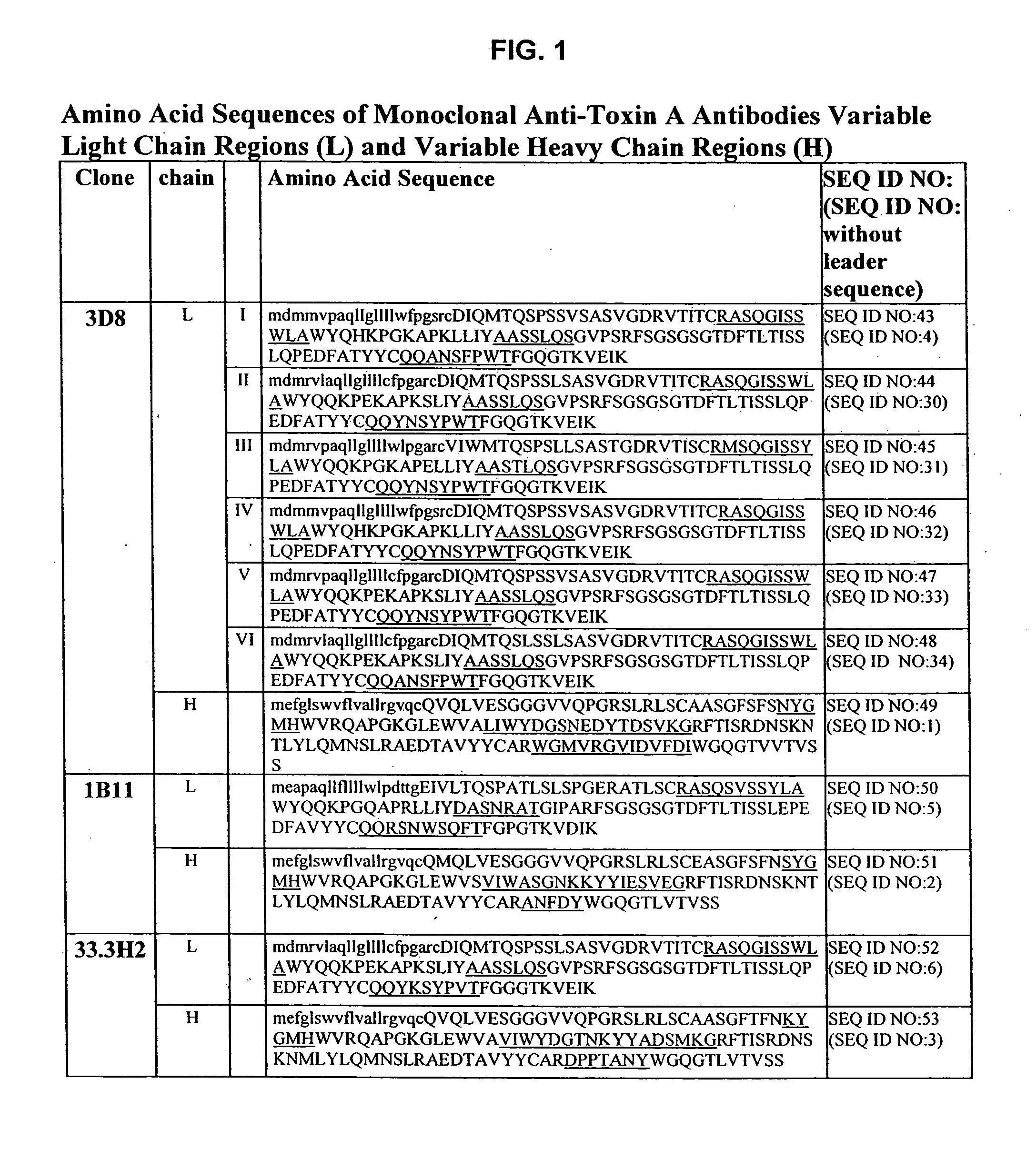
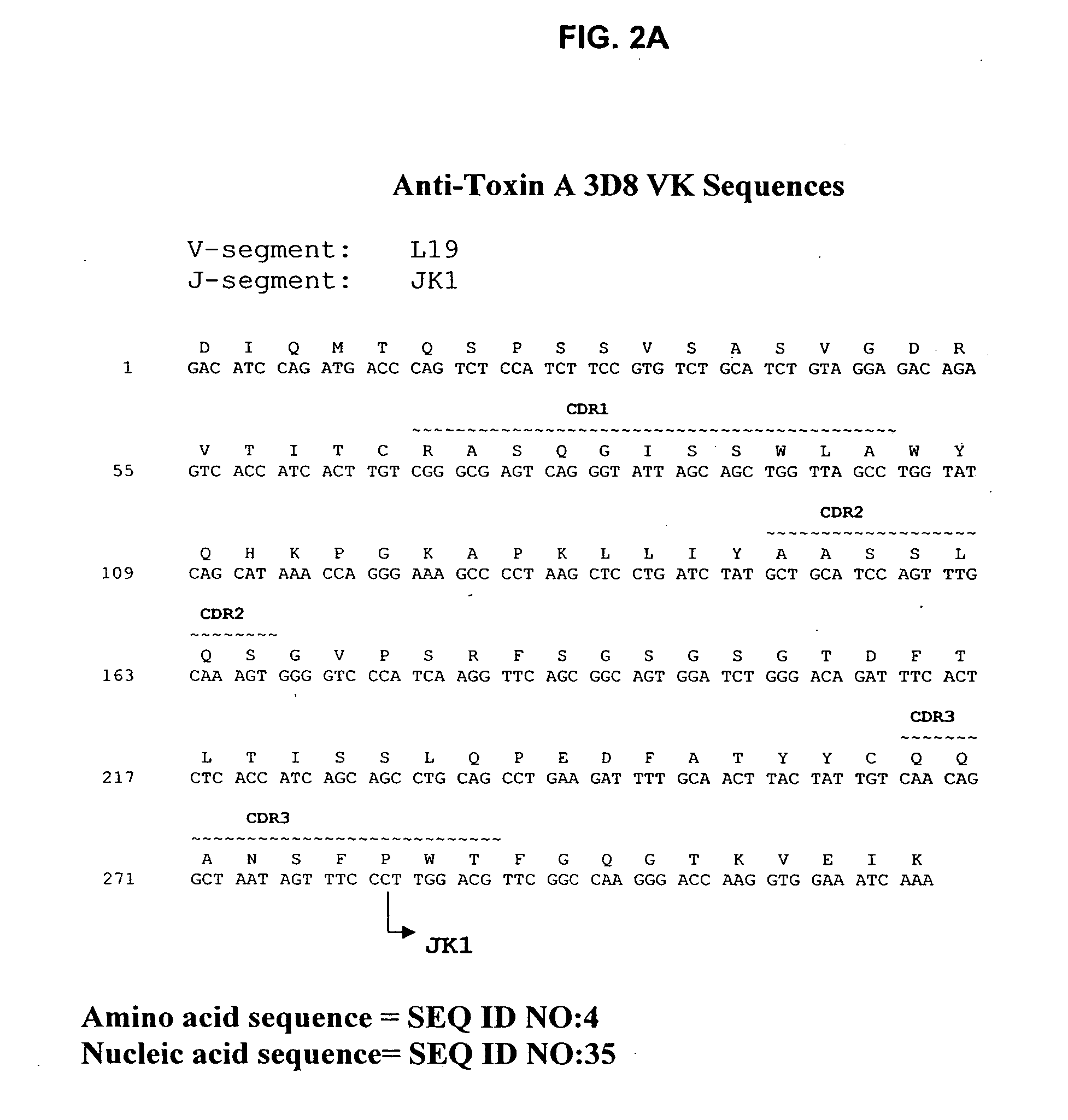
Antibodies against Clostridium difficile toxins and uses thereof
ActiveUS7625559B2High affinityLess immunogenicAntibacterial agentsGenetic material ingredientsClostridium difficile toxin AClostridium difficile (bacteria)
Owner:MASSACHUSETTS UNIV OF +2
Antibodies against Clostridium difficile toxins and uses thereof
ActiveUS20050287150A1High affinityLess immunogenicAntibacterial agentsBacteriaClostridium difficileClostridium difficile (bacteria)
Owner:MASSACHUSETTS UNIV OF +2
Active body cooling with vasodilation to reduce body temperature
ActiveUS7361186B2Improve scalabilityRapid coolingSurgical drugsEster active ingredientsActive coolingAnti-emetic
Active cooling of a person, such as to induce mild / moderate hypothermia, is accomplished by transferring heat from the persons body. Heat transfer and patient comfort are aided by administration of an anti-shivering drug and an anti-emetic drug.
Owner:MEDIVANCE
Method of administering antidepressant dosage form
InactiveUS6440457B1Constant drug levelFacilitated releaseOrganic active ingredientsBiocideDosage formAntidepressant
The invention pertains to a dosage form 10 and to administering an antidepressant medicament 16 for an extended period of time in a rate-known dose.
Owner:ALZA CORP
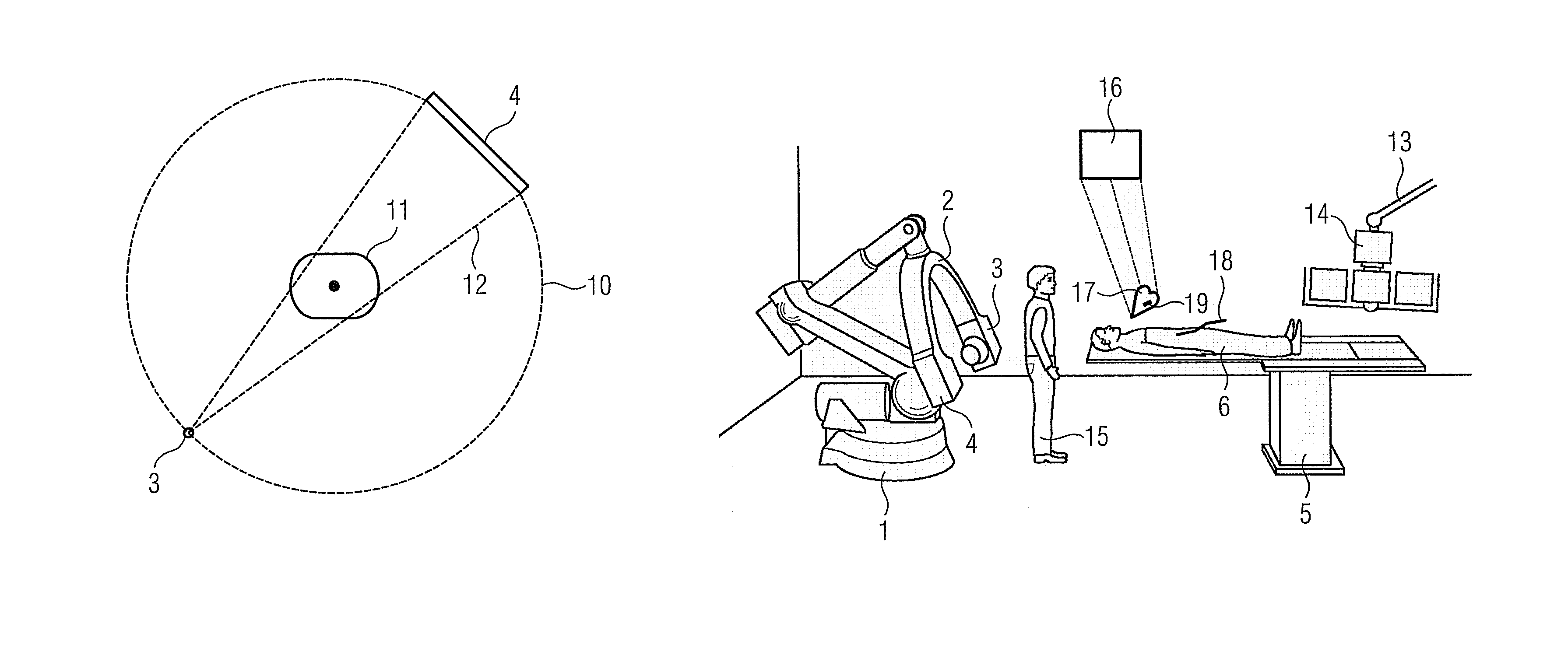
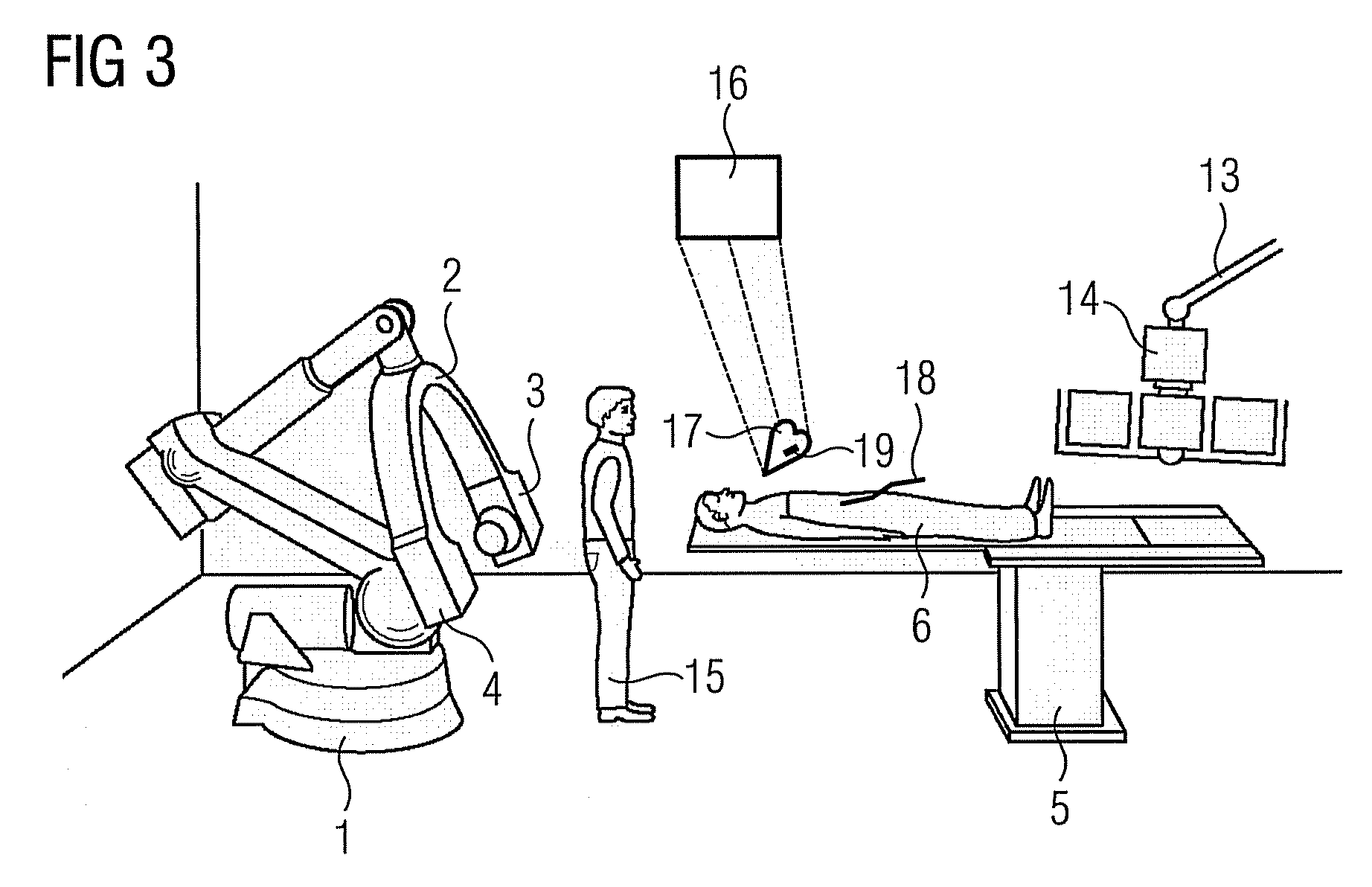
Method for representing interventional instruments in a 3D data set of an anatomy to be examined as well as a reproduction system for performing the method
ActiveUS20100020926A1Faster and safer minimally-invasive therapyAccurate locationX-ray spectral distribution measurementSurgical navigation systemsSoft x rayData set
The invention relates to a method for presenting interventional instruments in a 3D data set of an anatomy to be treated. A 3D data set of the anatomy is recorded before introduction of an interventional instrument. Once the interventional instrument has been applied, the spatial position of the instrument is determined by x-ray fluoroscopy from images created at two different angulations. A 3D model of the instrument is formed from the x-ray images. The 3D model of the instrument is fused with the 3D data set of the anatomy. A 3D hologram is reproduced from the fused 3D data set. The 3D hologram is repeatedly reproduced in real time to follow the application of the instrument in the presentation.
Owner:SIEMENS HEALTHCARE GMBH
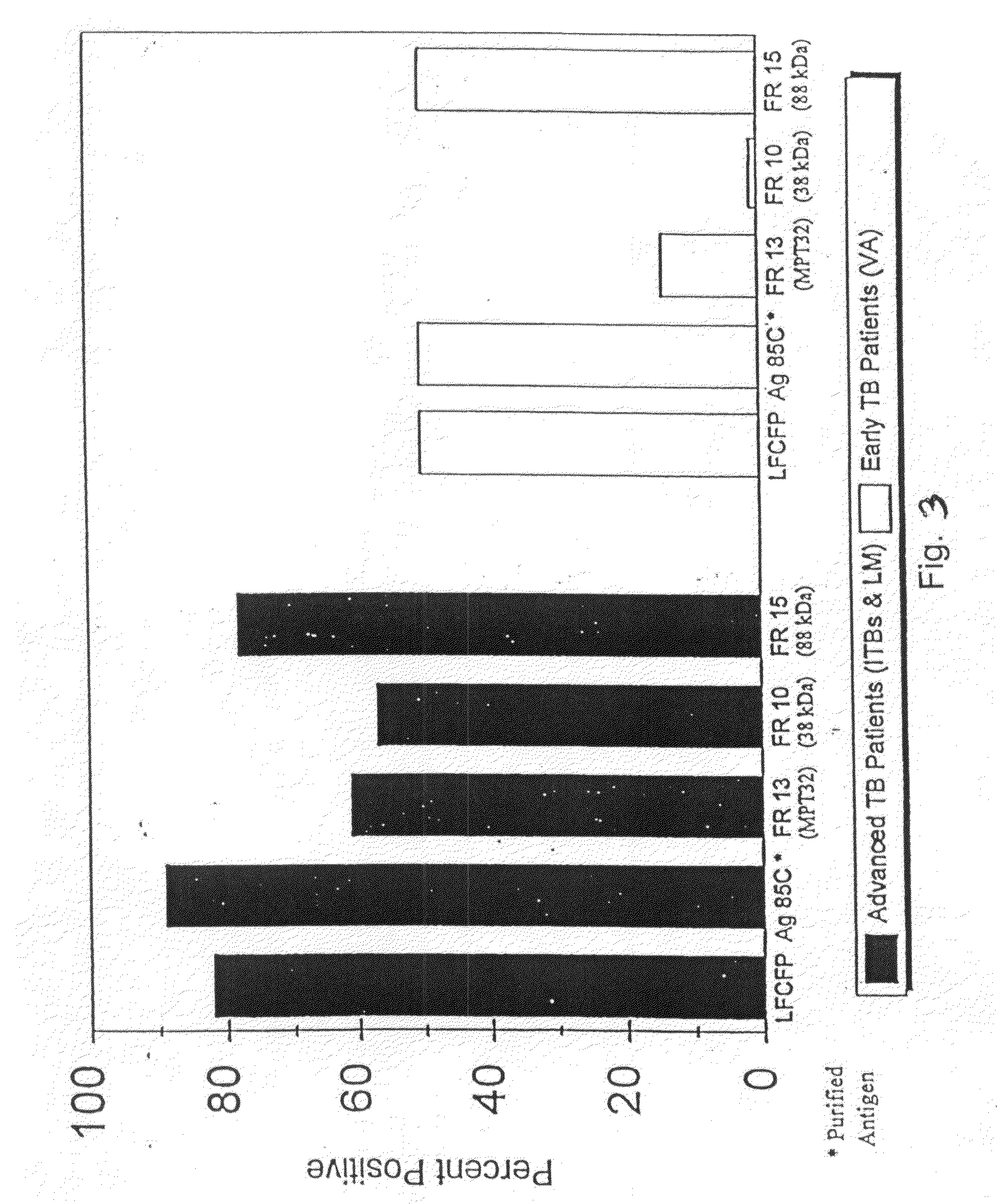
Early detection of mycobacterial disease using peptides
InactiveUS20090280140A1Reduce distractionsUse toolAntibacterial agentsPeptide/protein ingredientsMycobacteriumProtein C
A number of protein and glycoprotein antigens secreted by Mycobacterium tuberculosis (Mtb) have been identified as “early” Mtb antigens on the basis early antibodies present in subjects infected with Mtb prior to the development of detectable clinical disease. Epitope-bearing peptide fragments of these early Mtb antigens, in particular of an 88 kDa secreted protein, GlcB (SEQ ID NO:106) and of Mtb antigen MPT51 (SEQ ID NO:107) have been identified. These peptides, variants thereof, peptide multimers thereof that include two or more repeats of one or more of the peptides, and fusion polypeptides that include early Mtb antigenic proteins, peptides or both, are useful in immunoassay methods for early, rapid detection of TB in a subject. Preferred immunoassays detect the antibodies in the subject's urine. Also provided are antigenic compositions, kits and methods to useful for detecting an early Mtb antibodies. The antigenic proteins and peptides are also used in vaccine compositions.
Owner:NEW YORK UNIV +1
Antibodies against clostridium difficile toxins and uses thereof
ActiveUS20100233181A1High affinityLess immunogenicAntibacterial agentsGenetic material ingredientsClostridium difficile toxin AClostridium difficile (bacteria)
Owner:MASSACHUSETTS UNIV OF +1
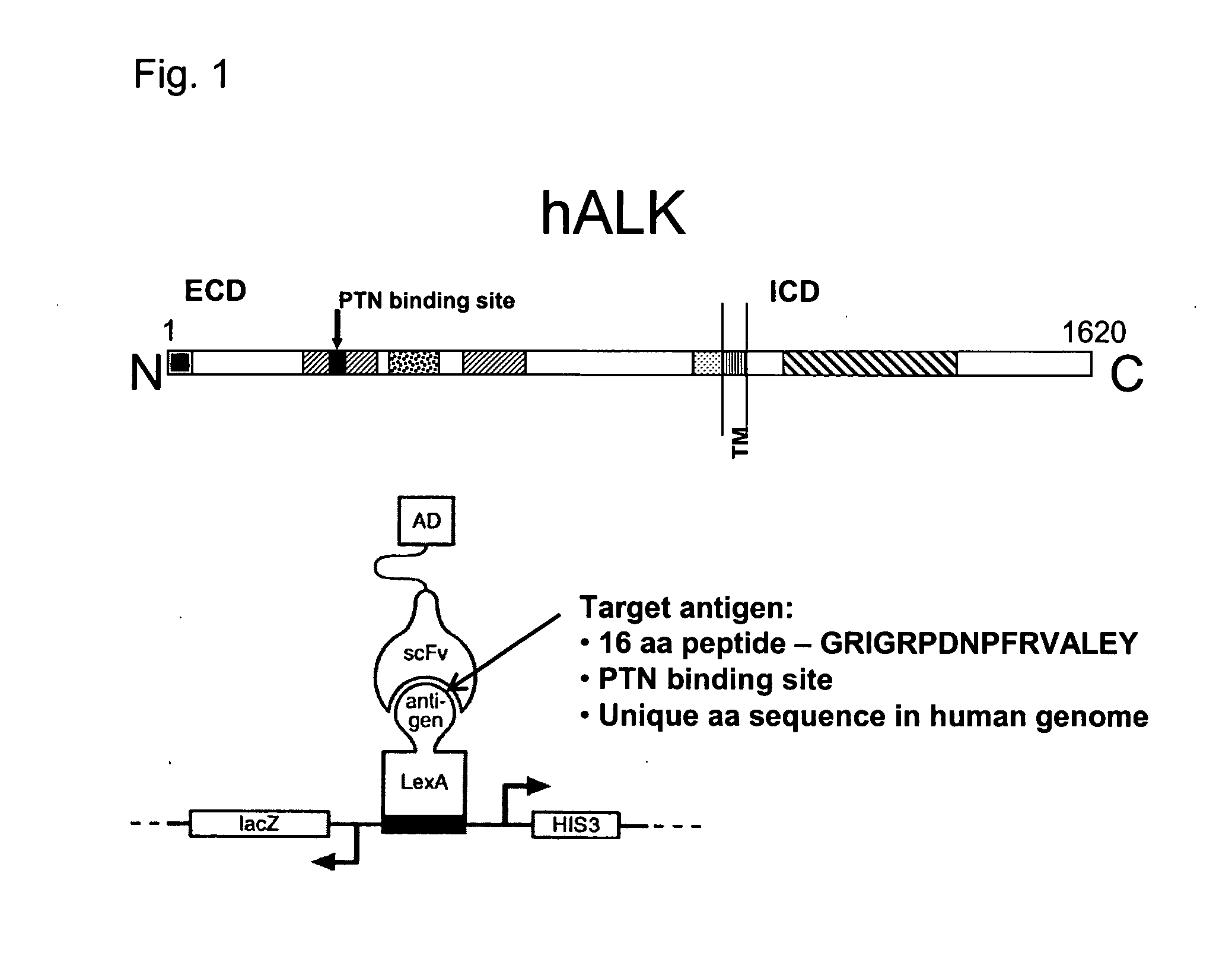
Antibodies binding to the extracellular domain of the receptor tyrosine kinase ALK
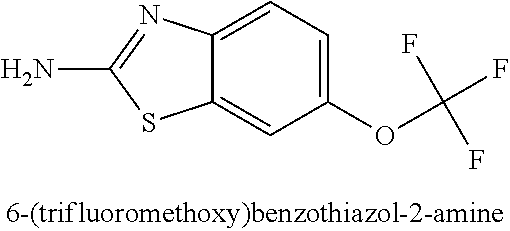
InactiveUS20080118512A1Less immunogenicTherapy is also rapidBacteriaSugar derivativesGlioblastomaTopical treatment
The present invention concerns an antibody specific for human ALK (Anaplastic Lymphoma Kinase), in particular a scFv, a nucleic acid sequence encoding it, its production and its use as a pharmaceutical or for diagnostic purposes. Said antibody is suitable for the local treatment of tumors, in particular glioblastoma.
Owner:CELL MEDICA SWITZERLAND AG
Methods for evaluating lung cancer status
InactiveUS20150088430A1Readily availableUseful predictionSugar derivativesNucleotide librariesOncologyLung cancer
The invention in some aspects provides methods of determining the likelihood that a subject has lung cancer based on the expression of informative-genes. In other aspects, the invention provides methods for determining an appropriate diagnostic intervention plan for a subject based on the expression of informative-genes. Related compositions and kits are provided in other aspects of the invention.
Owner:VERACYTE INC
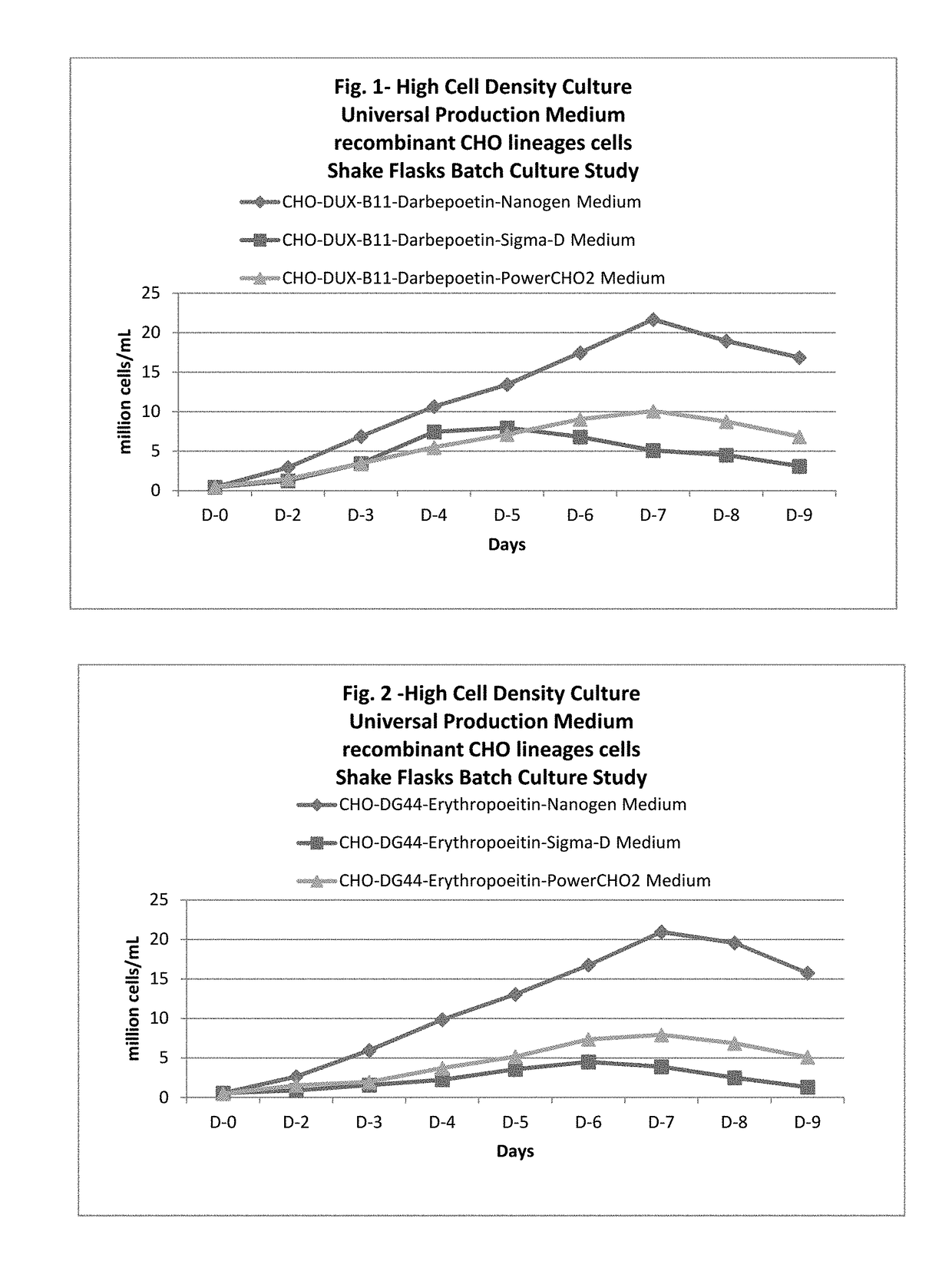
Universal, glycosylation enhancer, completely chemically defined medium formulation
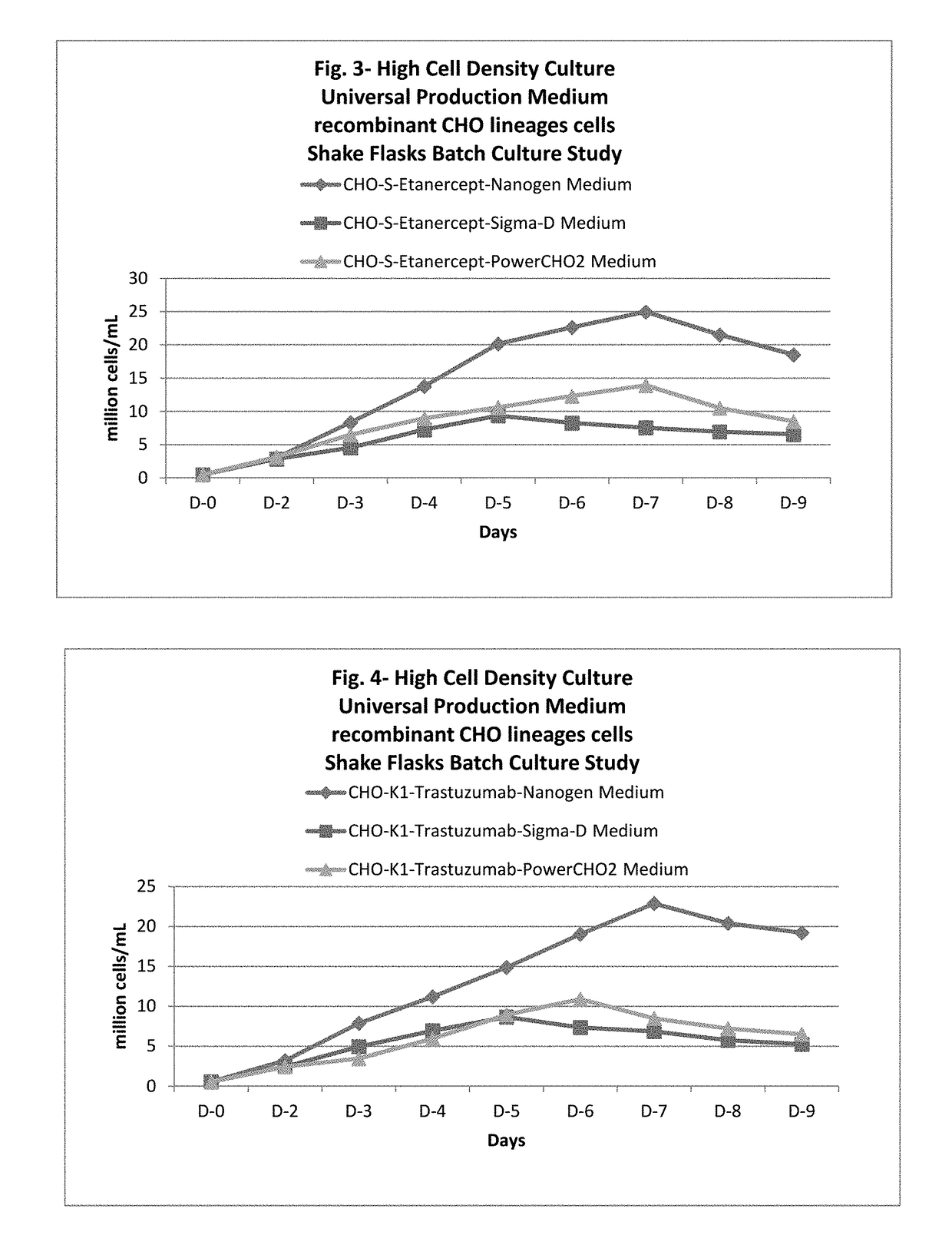
ActiveUS20170218328A1Therapy is also rapidCulture processCell culture mediaSodium bicarbonateHydrolysate
In one embodiment, the present application discloses a cell culture medium for culturing cell lines suitable for producing a therapeutic protein, comprising an amino acid selected from a group consisting of L-arginine, L-asparagine, L-proline, L leucine and L hydroxyproline and a mixture thereof; a vitamin selected from a group consisting of ascorbic acid Mg2+ salt, biotin, pyridoxine HCL, folic acid, riboflavin and D-calcium pantothenate, and a mixture thereof; an element selected from a group consisting of ammonium meta vanadate, sodium meta vanadate, germanium dioxide, barium acetate, aluminum chloride, rubidium chloride, cadmium chloride, ammonium molybedate, stannous chloride, cobalt chloride, chromium sulfate, silver nitrate, sodium metasilicate, zinc sulfate, manganese sulfate H2O, manganous chloride, ferric nitrate 9H2O, ferrous sulfate 7H2O, ferric ammonium citrate, magnesium chloride anhydrous, and magnesium sulfate anhydrous, and a mixture thereof; a nucleoside selected from a group consisting of uridine and cystidine; a sugar selected from a group consisting of galactose, mannose and N-Acetyl-D-Mannosamine; and a triple buffering system comprising sodium carbonate, sodium bicarbonate and HEPES; wherein the cell culture medium is animal component-free, plant component-free, serum-free, growth factors-free, recombinant protein-free, lipid-free, steroid-free, and free of plant or animal hydrolysates and / or extracts.
Owner:NANOGEN PHARMA BIOTECH CO LTD
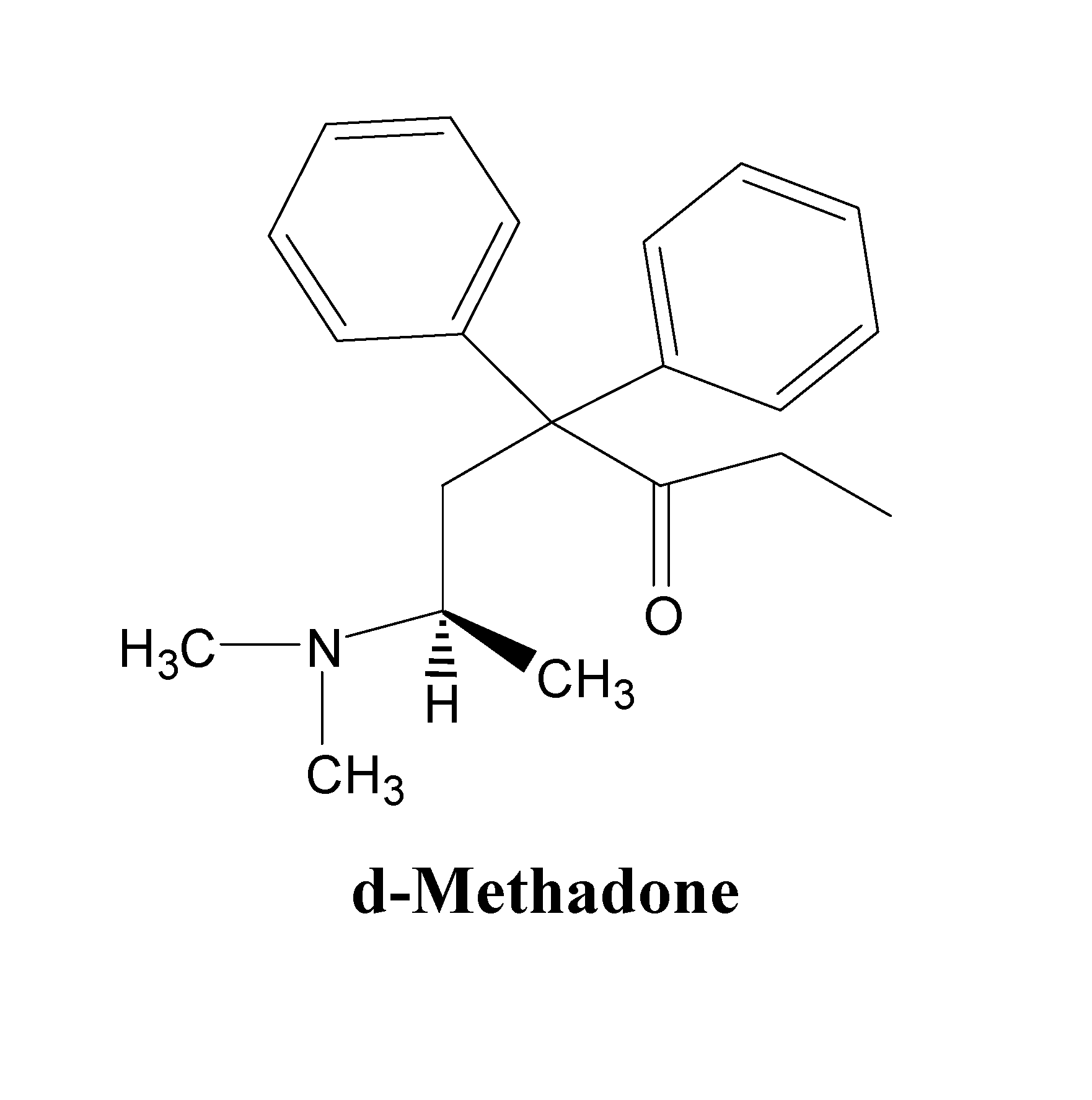
d-Methadone for the treatment of psychiatric symptoms
ActiveUS9468611B2Rapid onsetEffective for patientOrganic active ingredientsNervous disorderNR1 NMDA receptorMethadone treatments
The present invention relates to a method of treating psychiatric symptoms in a subject having a NMDA receptor and a NE receptor which includes administering d-methadone, d-methadol, d-alpha-acetylmethadol, l-alpha-acetylmethadol, d-alpha-normethadol, l-alpha-normethadol, pharmaceutically acceptable salts thereof, or mixtures thereof, to the subject under conditions effective for the substance to bind to the NMDA receptor and NE receptor of the subject.
Owner:INTURRISI CHARLES E +1
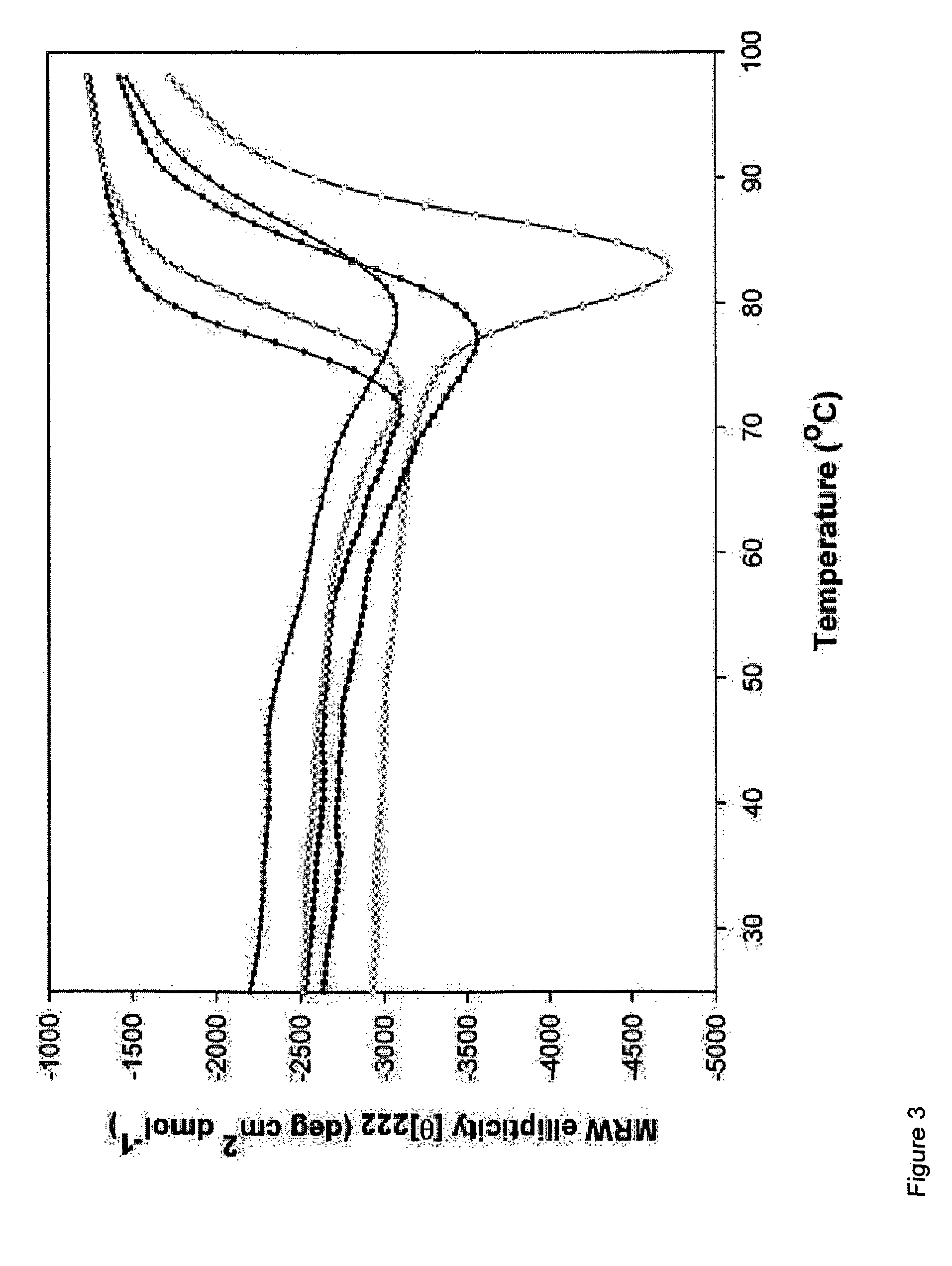
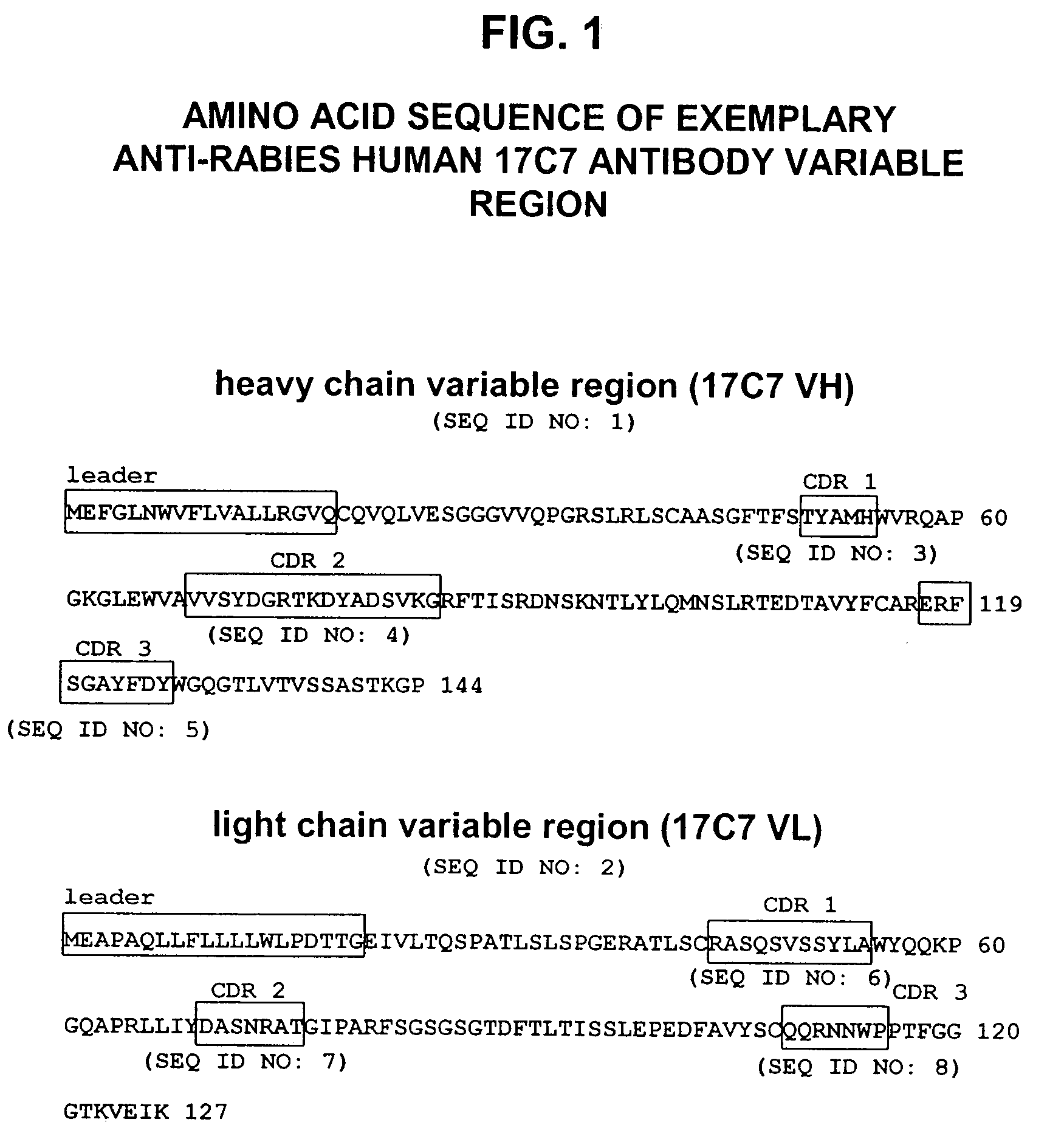
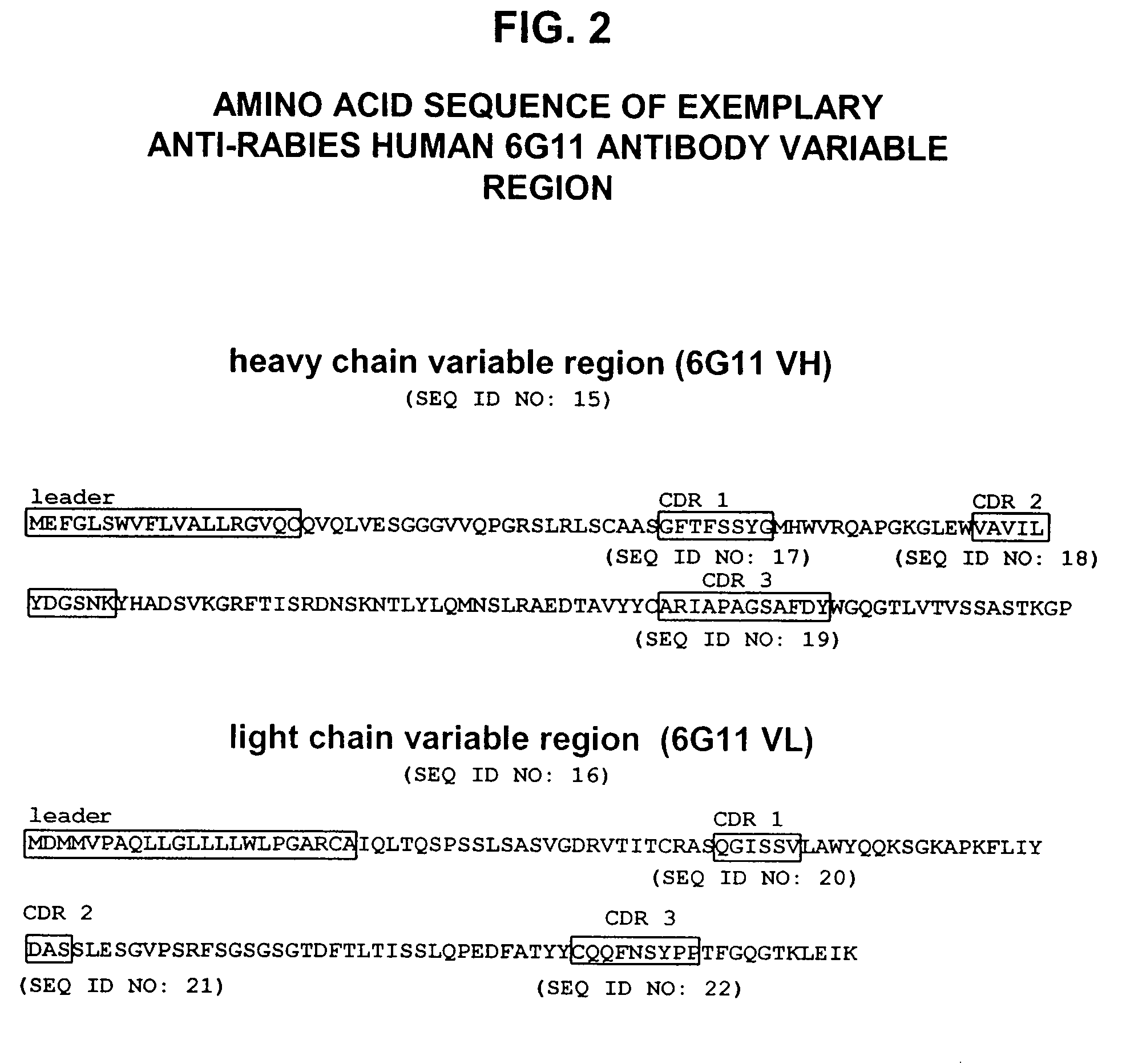
Human antibodies against rabies and uses thereof
ActiveUS7727532B2Improve survival rateIncrease exposureSsRNA viruses negative-senseVirus peptidesAntigen bindingRabies virus
Owner:MASSACHUSETTS UNIV OF +1
Cytokine design
ActiveUS7994281B2Effective therapyTherapy is also rapidTumor necrosis factorNGF/TNF-superfamilyCytokineBiology
Owner:EURO LAB FUER MOLEKULARBIOLOGIE EMBL +2
Transfection system
InactiveUS20040043007A1Improve availabilityAttain therapeutic effectBiocideNervous disorderWound healingTissue defect
The present invention relates to a method of preparing a composition for wound healing, and for repairing and regenerating human and animal tissue, said method comprising the following steps: a. providing a plasmid DNA in substantially pure form, which encodes a gene that has a positive effect on the progression of the regeneration of the tissue, b. providing a component / components of a self-hardening bio-polymer, and c. providing a cell suspension with cells which promote regeneration, characterized in that components (a), (b) and (c) are incubated with each other simultaneously or successively so that the plasmid and the cell suspension are obtained homogenously distributed in one of the biopolymer components. Furthermore, transfection systems containing a plasmid DNA, a component of a self-hardening biopolymer and a cell suspension with cells promoting regeneration are disclosed. This transfection system does not contain any further transfection-promoting or transfection-mediating substances. Moreover, therapeutical kits, pharmaceutical compositions and their use for the treatment of tissue defects, in particular burn wounds, and for wound healing in the skin are described. In particular, the present invention relates to a transfection system containing a plasmid DNA, a component of the fibrin adhesive and a cell suspension.
Owner:UNIVERSITATSKLINIKUM FREIBURG
d-Methadone for the Treatment of Psychiatric Symptoms
ActiveUS20140088155A1Fast onset of treatmentAvoiding and reducing increased risk of suicideBiocideNervous disorderMethadone treatmentsL-alpha-acetylmethadol
The present invention relates to a method of treating psychiatric symptoms in a subject having a NMDA receptor and a NE receptor which includes administering d-methadone, d-methadol, d-alpha-acetylmethadol, l-alpha-acetylmethadol, d-alpha-normethadol, l-alpha-normethadol, pharmaceutically acceptable salts thereof, or mixtures thereof, to the subject under conditions effective for the substance to bind to the NMDA receptor and NE receptor of the subject.
Owner:INTURRISI CHARLES E +1
Cool vest
InactiveUS20120151951A1Limit traumaTherapy is also rapidLighting and heating apparatusOvergarmentsAdhesiveEngineering
A method and apparatus for adjusting the body temperature of a patient comprises a vest receiving at least a portion of the body and head. The vest comprises a frontal opening with adjustable Velcro adhesive for accommodating different patients and two openings for the arms with rubber-tubing coiled between two layers of fabric, one being thermal insulated. The rubber-tubing from the vest will be connected to a pump then connected to a copper-tubing coil inside a cooler to form a closed circuit loop between the rubber-tubing, copper-tubing coil and pump. Water or other liquids will be circulated via pump into vest from copper-tubing coil inside a cooler filled with ice. The vest, pump and copper coil will be portable and there will be two union connectors, two tee connectors, and a manual diverter to enable unit to also be powered via a stationary cooling motor if so desired.
Owner:TERRY DENNIS T
Early detection of mycobacterial disease using peptides
InactiveUS7807182B2Reduce distractionsUse toolAntibacterial agentsPeptide/protein ingredientsSecretory proteinTGE VACCINE
A number of protein and glycoprotein antigens secreted by Mycobacterium tuberculosis (Mtb) have been identified as “early” Mtb antigens on the basis early antibodies present in subjects infected with Mtb prior to the development of detectable clinical disease. Epitope-bearing peptide fragments of these early Mtb antigens, in particular of an 88 kDa secreted protein, GlcB (SEQ ID NO:106) and of Mtb antigen MPT51 (SEQ ID NO:107) have been identified. These peptides, variants thereof, peptide multimers thereof that include two or more repeats of one or more of the peptides, and fusion polypeptides that include early Mtb antigenic proteins, peptides or both, are useful in immunoassay methods for early, rapid detection of TB in a subject. Preferred immunoassays detect the antibodies in the subject's urine. Also provided are antigenic compositions, kits and methods to useful for detecting an early Mtb antibodies. The antigenic proteins and peptides are also used in vaccine compositions.
Owner:NEW YORK UNIV +1
Human antibodies against rabies and uses thereof
ActiveUS20090041777A1Therapy be also rapidLess immunogenicSsRNA viruses negative-senseOrganic active ingredientsAntigen bindingMonoclonal
Owner:MASSACHUSETTS UNIV OF +1
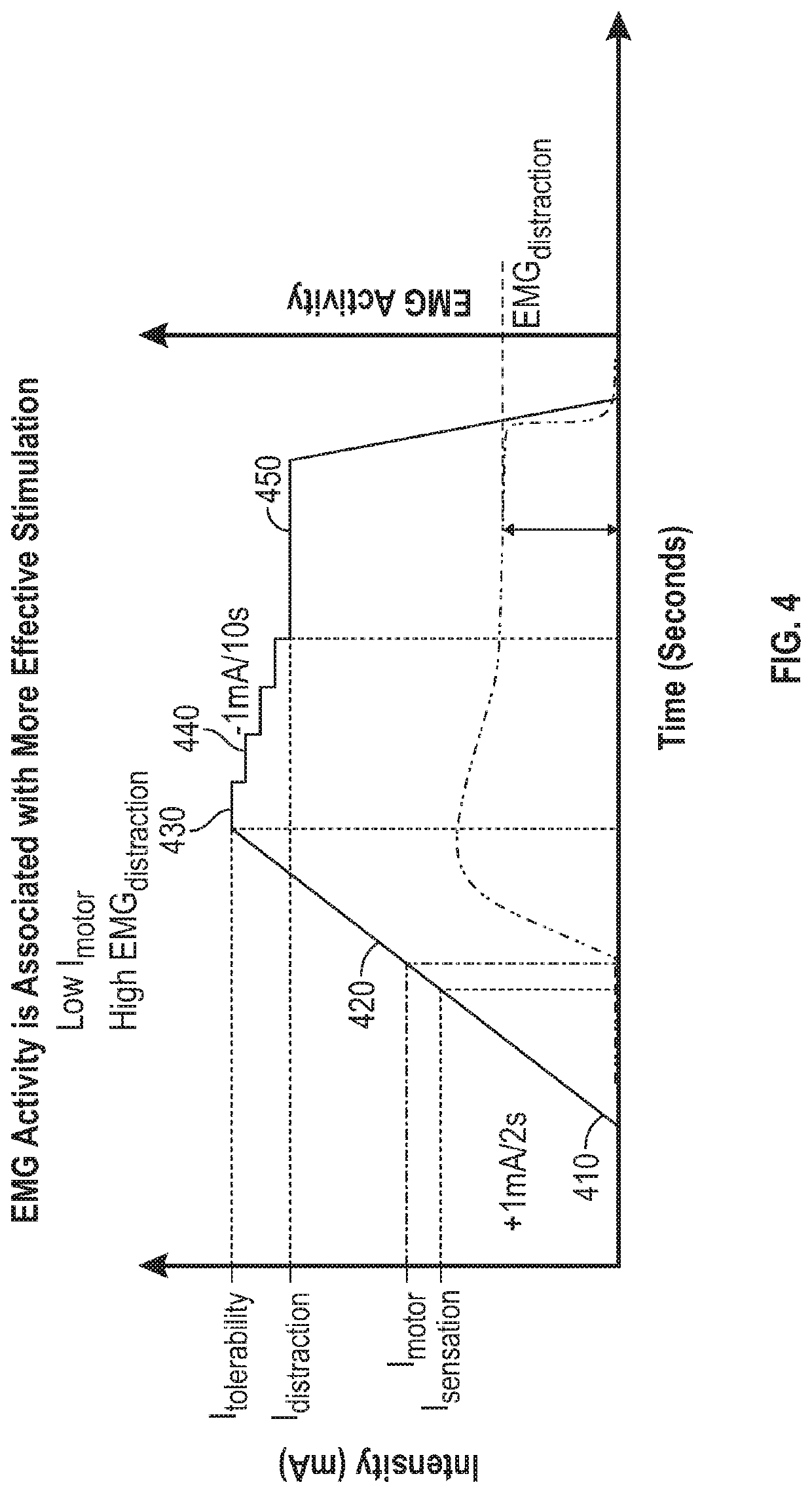
Peripheral nerve stimulation for restless legs syndrome
ActiveUS20210100998A1Therapy is also rapidImprove individual patient outcomeDiagnostic recording/measuringSensorsPhysical medicine and rehabilitationHigh frequency stimulation
Systems and methods for treating a patient having symptoms of restless legs syndrome (RLS) or Periodic Limb Movement Disorder (PLMD) using high-frequency stimulation by applying a high-frequency pulsed electrostimulation therapy signal to a peroneal nerve or a branch thereof, where the therapy signal is above a motor threshold of a muscle innervated by the peroneal nerve or branch thereof. Surface EMG (sEMG) response to neurostimulation can be used to evaluate patient responsivity to neurostimulation, or to evaluate neurostimulation efficacy, such as to compare various neurostimulation parameter settings and to select between such settings to meet or balance between one or more goals. The sEMG response can be obtained with the muscle at rest, or during muscle activation.
Owner:NOCTRIX HEALTH INC
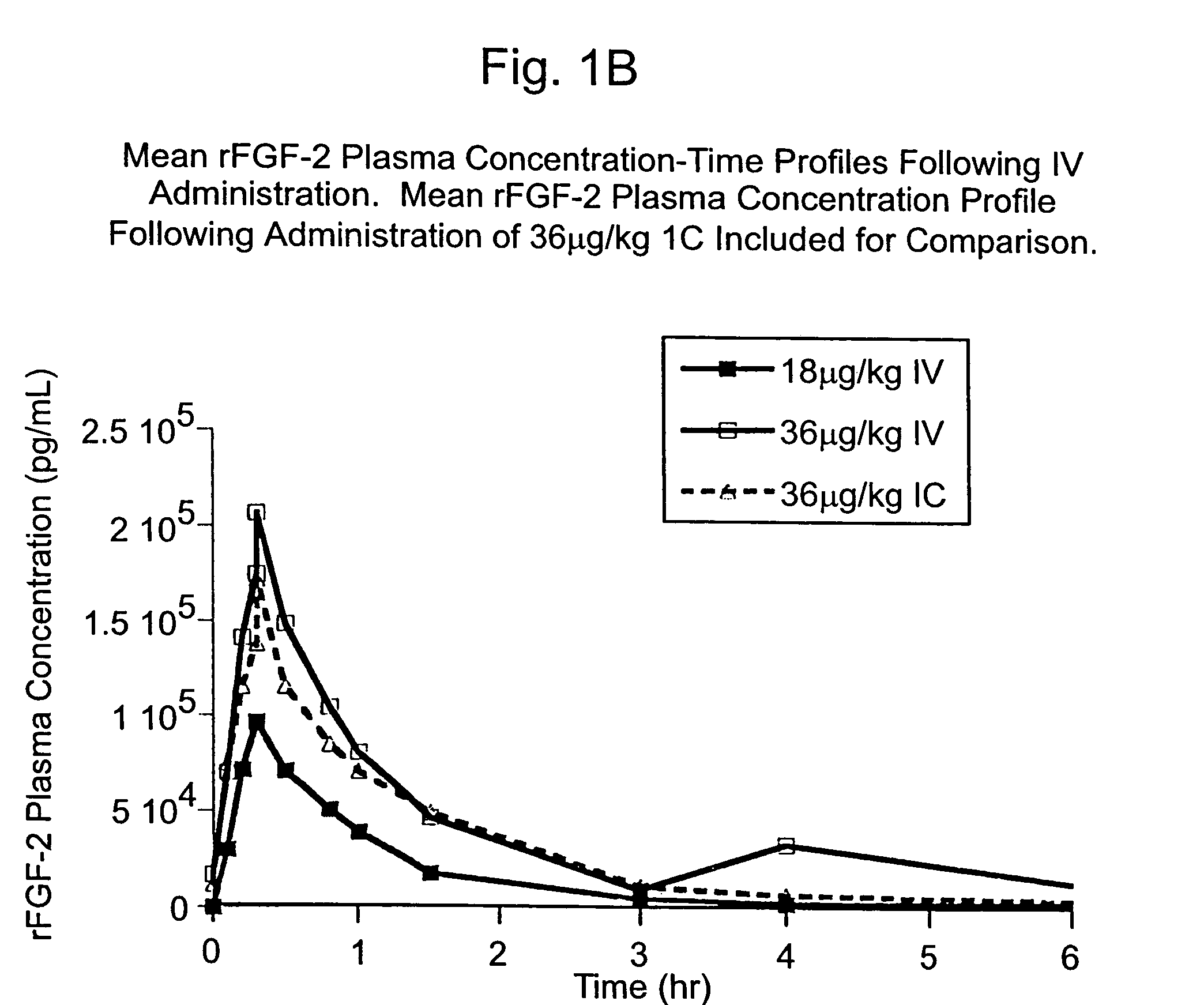
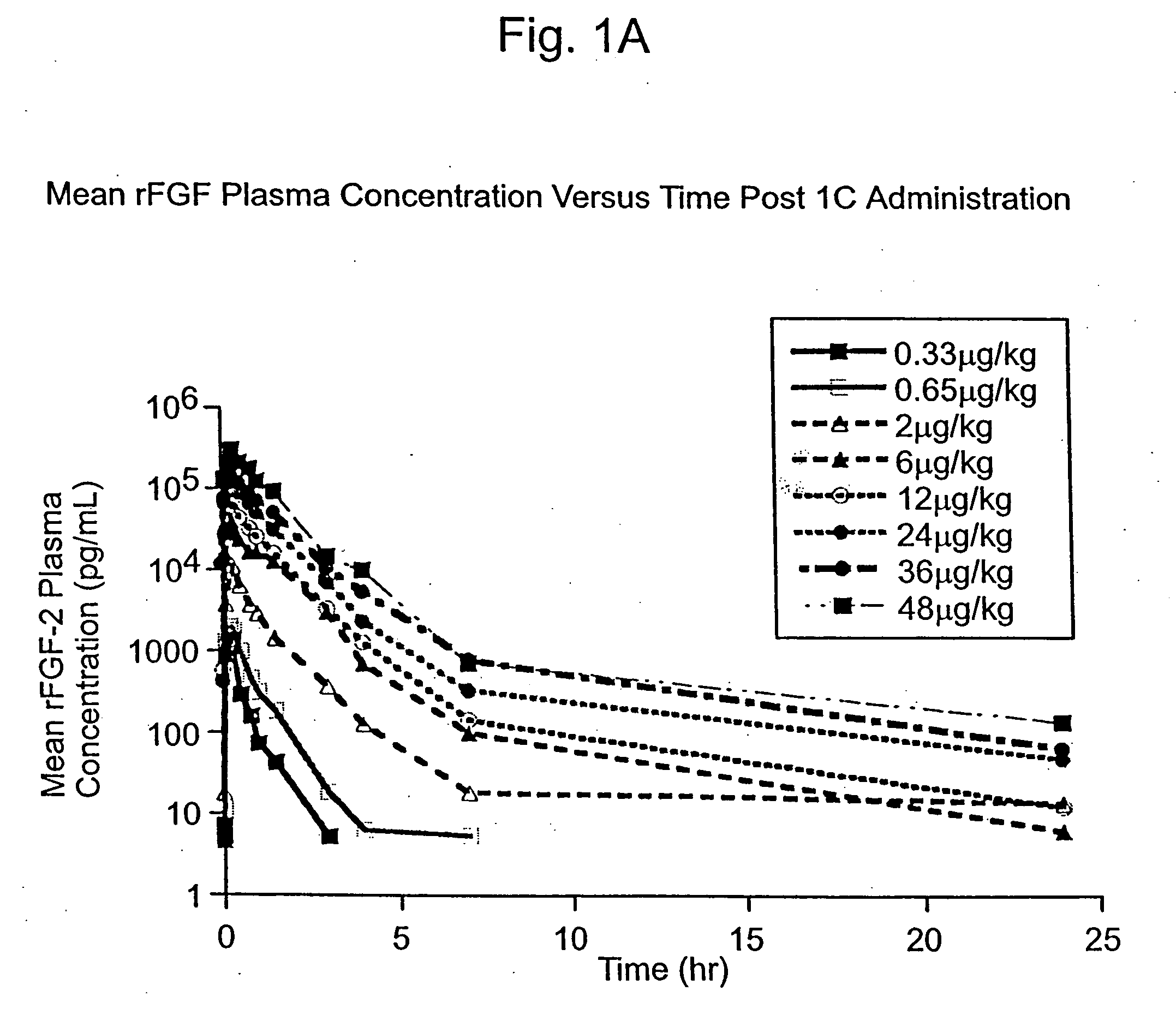
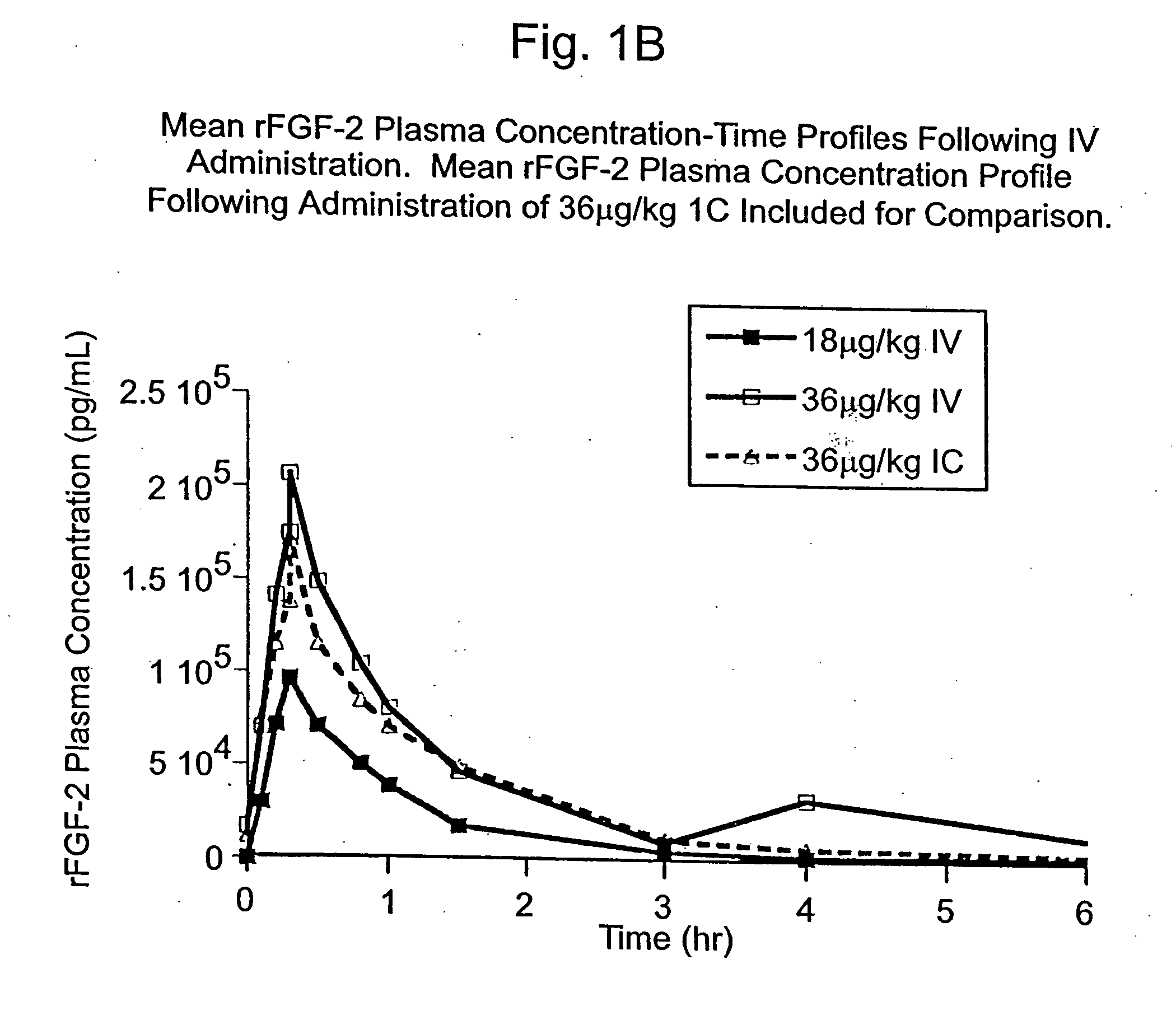
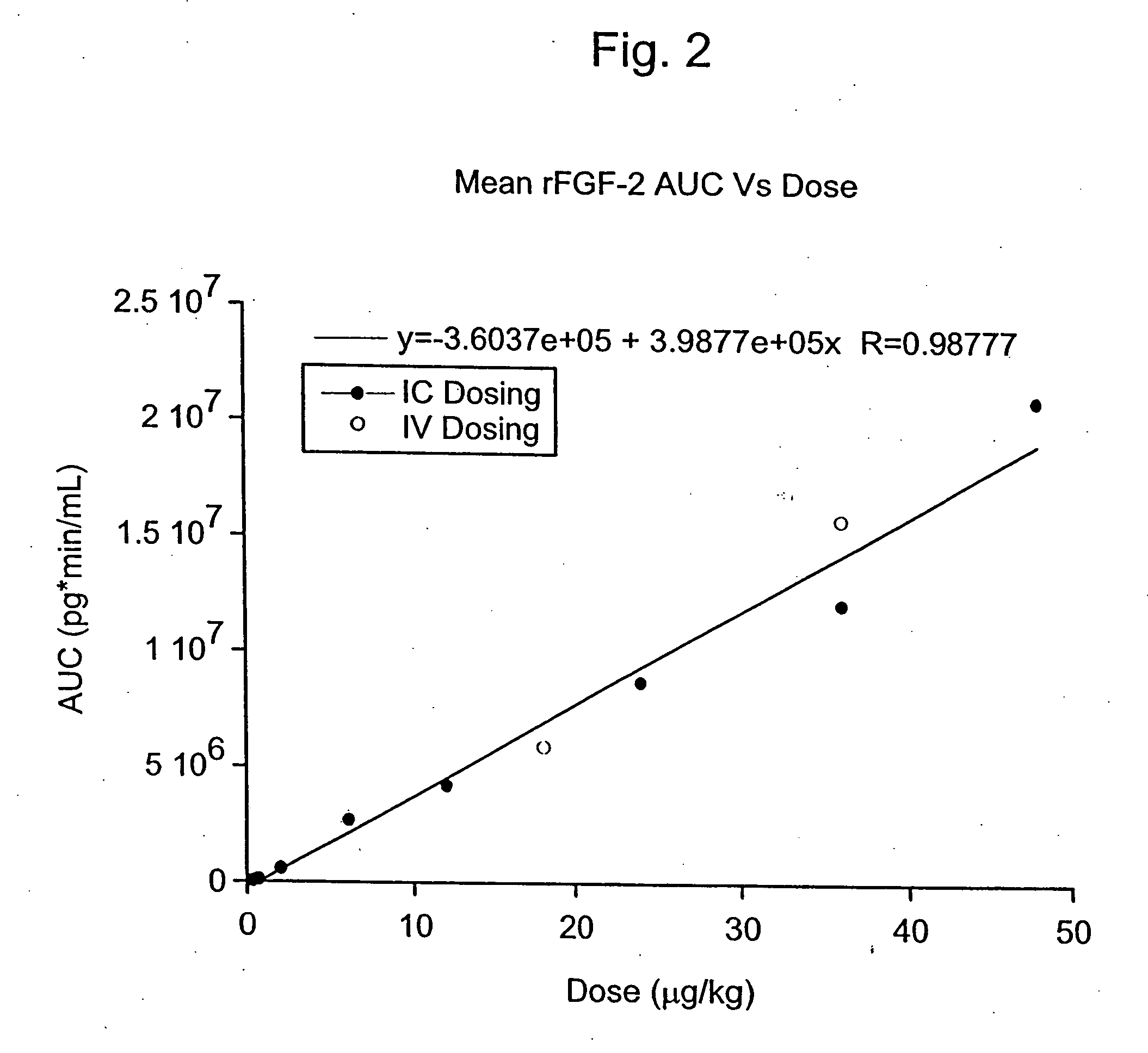
Angiogenically effective unit dose of FGF-2 and method of use
InactiveUS7112560B2Reduce needTherapy is also rapidOrganic active ingredientsPeptide/protein ingredientsCoronary artery diseaseArterial disease
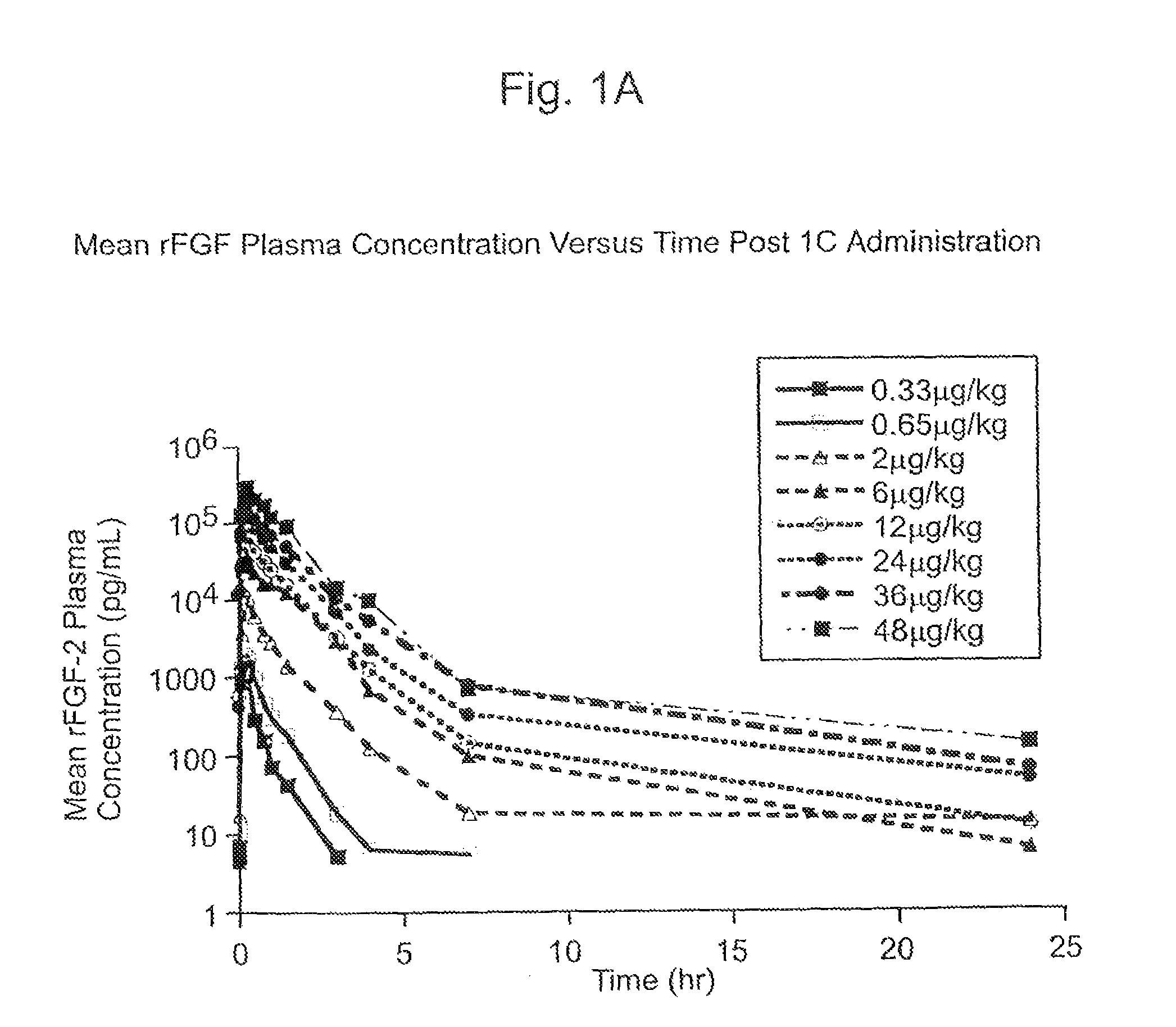
The present invention has multiple aspects. In particular, in one aspect, the present invention is directed to a unit dose composition comprising 0.2 μg / kg to 48 μg / kg of an FGF-2 of SEQ ID NO: 2, or an angiogenically active fragment or mutein thereof in a pharmaceutically acceptable carrier. In another aspect, the present invention is directed to a method for treating a human patient for coronary artery disease, comprising administering into one or more coronary vessels or a peripheral vein of a human patient in need of treatment for coronary artery disease a safe and angiogenically effective dose of a recombinant FGF-2, or an angiogenically active fragment or mutein thereof. The single unit dose composition of the present invention provides an angiogenic effect in a human CAD patient that lasts 2 months before re-treatment is required. In another aspect, the present invention is directed to a method of administration which optimizes patient's safety. In this embodiment, fluids, heparin and / or rate of infusion all play a role. In another aspect, the present invention is directed to a pharmaceutical composition comprising a therapeutically effective amount of FGF-2, alone or in combination with heparin, in a therapeutically effective carrier. The magnitude and duration of benefit were unexpected; in addition benefit with the IV route was unexpected.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Sublingual administration of riluzole
InactiveUS20180153862A1Large doseDesirable effectOrganic active ingredientsNervous disorderSublingual administrationBuccal administration
Disclosed is sublingual administration of riluzole. In particular, a method for treating a neuropsychiatric disorder or symptom by administering a sublingual formulation of riluzole is provided. In addition, a method of relieving or reducing oral pain using the sublingual formulation of riluzole is disclosed.
Owner:BIOHAVEN THERAPEUTICS LTD
Stabilized oral pharmaceutical composition
InactiveUS20050112197A1Oral administration is convenientIncrease drug concentrationBiocideNervous disorderPolyethylene glycolCyclooxygenase
An orally deliverable pharmaceutical composition is provided comprising an aminosulfonyl-comprising drug, for example a selective cyclooxygenase-2 inhibitory drug such as celecoxib, and a solvent liquid comprising a polyethylene glycol and one or more free radical-scavenging antioxidants. At least a substantial part of the drug is in dissolved form in the solvent liquid. The composition has rapid-onset properties and is useful in treatment of cyclooxygenase-2 mediated conditions and disorders.
Owner:GAO PING +8
Osteogenic Differentiation Of Bone Marrow Stem Cells And Mesenchymal Stem Cells Using A Combination Of Growth Factors
InactiveUS20100278788A1Increased quantity of usefulExpansion potential of adult stem cells,Bone marrow stroma cellsBiocideMesenchymal stem cellBlood plasma
The invention relates to methods for osteogenic differentiation of human bone marrow stem cells (BMSC) or mesenchymal stem cells (MSC), in particular using human plasma or serum and FGF and TGFB growth factors. The invention also provides the so-obtained cells and cell populations, as well as further products comprising such and uses thereof in bone therapy.
Owner:BONE THRAPEUTICS SA
Method for representing interventional instruments in a 3D data set of an anatomy to be examined as well as a reproduction system for performing the method
ActiveUS8068581B2Therapy is also rapidAccurate locationX-ray spectral distribution measurementSurgical navigation systemsData setFluorescence
The invention relates to a method for presenting interventional instruments in a 3D data set of an anatomy to be treated. A 3D data set of the anatomy is recorded before introduction of an interventional instrument. Once the interventional instrument has been applied, the spatial position of the instrument is determined by x-ray fluoroscopy from images created at two different angulations. A 3D model of the instrument is formed from the x-ray images. The 3D model of the instrument is fused with the 3D data set of the anatomy. A 3D hologram is reproduced from the fused 3D data set. The 3D hologram is repeatedly reproduced in real time to follow the application of the instrument in the presentation.
Owner:SIEMENS HEALTHCARE GMBH
Angiogenically effective unit dose of FGF-2 and method of use
InactiveUS20070142283A1Reduce needTherapy is also rapidOrganic active ingredientsPeptide/protein ingredientsCoronary artery diseaseArterial disease
The present invention has multiple aspects. In particular, in one aspect, the present invention is directed to a unit dose composition comprising 0.2 μg / kg to 48 μg / kg of an FGF-2 of SEQ ID NO: 2, or an angiogenically active fragment or mutein thereof in a pharmaceutically acceptable carrier. In another aspect, the present invention is directed to a method for treating a human patient for coronary artery disease, comprising administering into one or more coronary vessels or a peripheral vein of a human patient in need of treatment for coronary artery disease a safe and angiogenically effective dose of a recombinant FGF-2, or an angiogenically active fragment or mutein thereof. The single unit dose composition of the present invention provides an angiogenic effect in a human CAD patient that lasts 2 months before re-treatment is required. In another aspect, the present invention is directed to a method of administration which optimizes patient's safety. In this embodiment, fluids, heparin and / or rate of infusion all play a role. In another aspect, the present invention is directed to a pharmaceutical composition comprising a therapeutically effective amount of FGF-2, alone or in combination with heparin, in a therapeutically effective carrier. The magnitude and duration of benefit were unexpected; in addition benefit with the IV route was unexpected.
Owner:SCHLUMBERGER TECH CORP
Thermal performance optimization in a thermal therapy device
ActiveUS20200000628A1Minimize unnecessary thermal pollutionTherapy is also rapidTherapeutic coolingTherapeutic heatingMedicineThermal water
A rapid contrast therapy system can provide cold, heat / hot / warm (hereafter referred to as “hot”), and / or rapid contrast therapy, which involves rapidly alternating between cold therapy and hot therapy. The system can circulate cold or hot fluid, such as water, through a hose, into a therapy wrap, and then back to the fluid reservoirs of the system. The system can utilize a vapor compression system or other chiller technology to cool the cold water reservoir, and immersion heaters can be used to heat the hot water reservoir.
Owner:AVENT INC
Sublingual formation of riluzole
ActiveUS20180153794A1Large doseDesirable effectOrganic active ingredientsNervous disorderMedicineSublingual administration
Disclosed is sublingual administration of riluzole. In particular, a method for treating a neuropsychiatric disorder or symptom by administering a sublingual formulation of riluzole is provided. In addition, a method of relieving or reducing oral pain using the sublingual formulation of riluzole is disclosed.
Owner:BIOHAVEN THERAPEUTICS LTD
Method of treating coronary artery disease FGF-2
InactiveUS8796211B2Reduce needTherapy is also rapidPeptide/protein ingredientsDepsipeptidesDiseaseVein
The present invention provides a unit dose composition comprising 0.2 μg / kg to 48 μg / kg of an FGF-2 of SEQ ID NO:2, or an angiogenically active fragment or mutein thereof in a pharmaceutically acceptable carrier. Also provided is a method for treating a human patient for coronary artery disease, comprising administering into one or more coronary vessels or a peripheral vein of said patient a safe and angiogenically effective dose of a recombinant FGF-2, or an angiogenically active fragment or mutein thereof. Also provided is a pharmaceutical composition comprising a therapeutically effective amount of FGF-2, alone or in combination with heparin, in a therapeutically effective carrier.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
NSAIDs compositions containing tartaric acid
InactiveUS20050249808A1Promote absorptionIncrease drug concentrationCompounds screening/testingBiocideSodium bicarbonateAcute pain
The invention is a composition and method for treating acute pain using a composition containing one or more NSAIDs. The preferred composition includes ibuprofen, sodium bicarbonate, Gelucire, and tartaric acid
Owner:JAMALI FAHKREDDIN
B cell immunotherapy
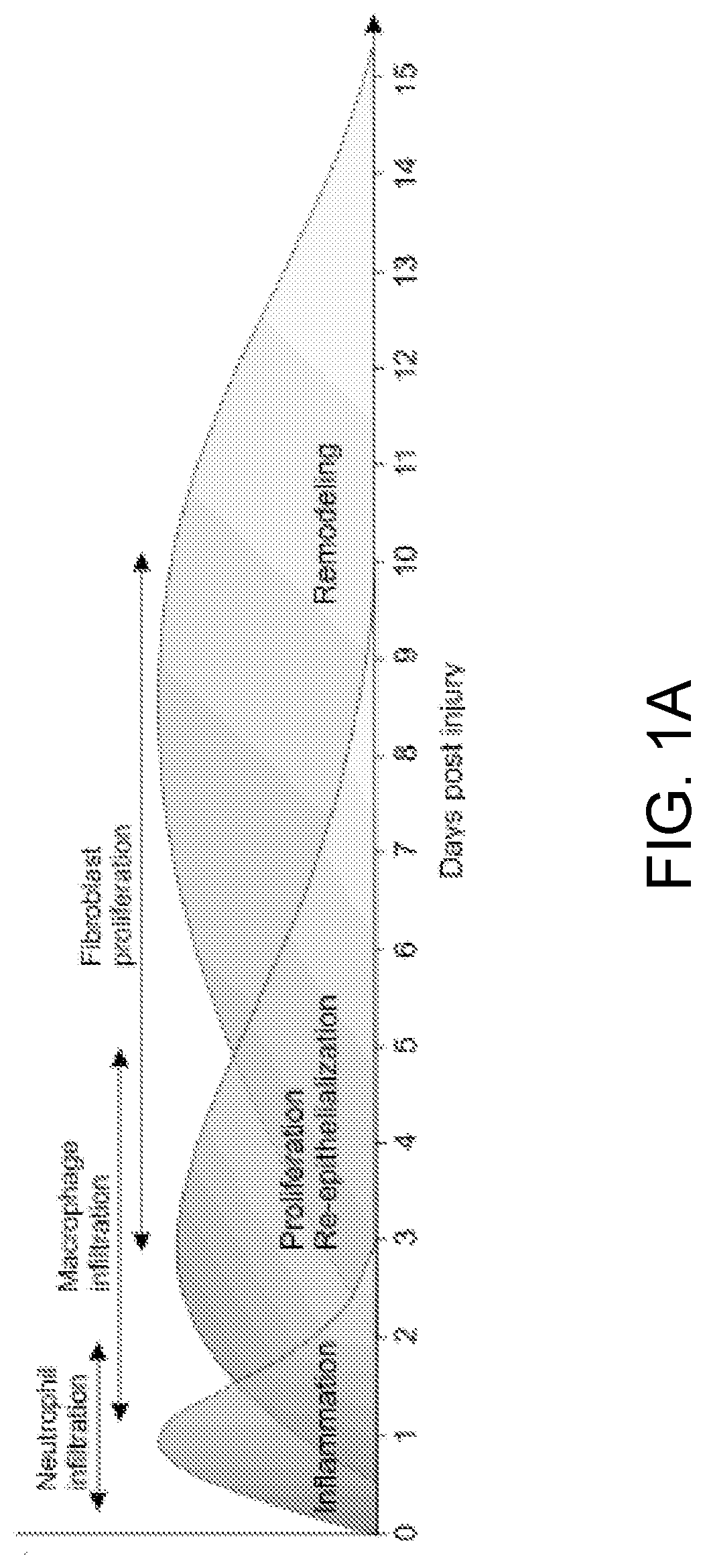
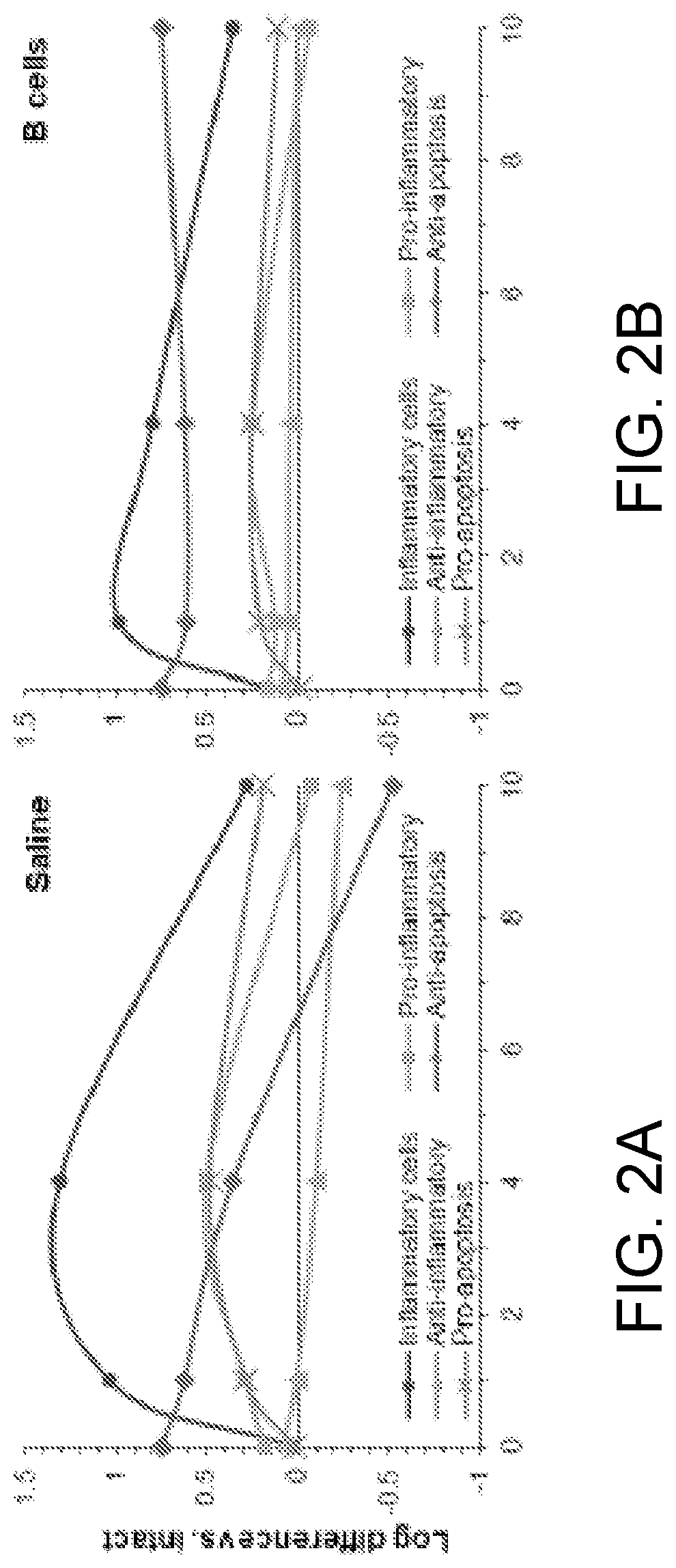
PendingUS20220079986A1Increased pro-regenerative efficiencyPromote healingOrganic active ingredientsNervous disorderAmytrophic lateral sclerosisNeuro-degenerative disease
The invention, in general, features a method of treating a neurodegenerative disease (such as amyotrophic lateral sclerosis) or a traumatic brain injury in a subject (e.g., a human) in need thereof, the method comprising administering to the subject a therapeutically effective amount of isolated B cells (such as autologous or allogeneic or xenogeneic B cells).
Owner:HOLY CROSS HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com