Low-immunogenicity staphylokinase mutant and preparation method and use thereof
An immunogenicity and mutant technology, applied in the biological field, can solve the problems of decreased binding capacity, loss of staphylokinase activity, energy reduction, etc., and achieve the effect of reduced immunogenicity, decreased antibody level, and concise methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
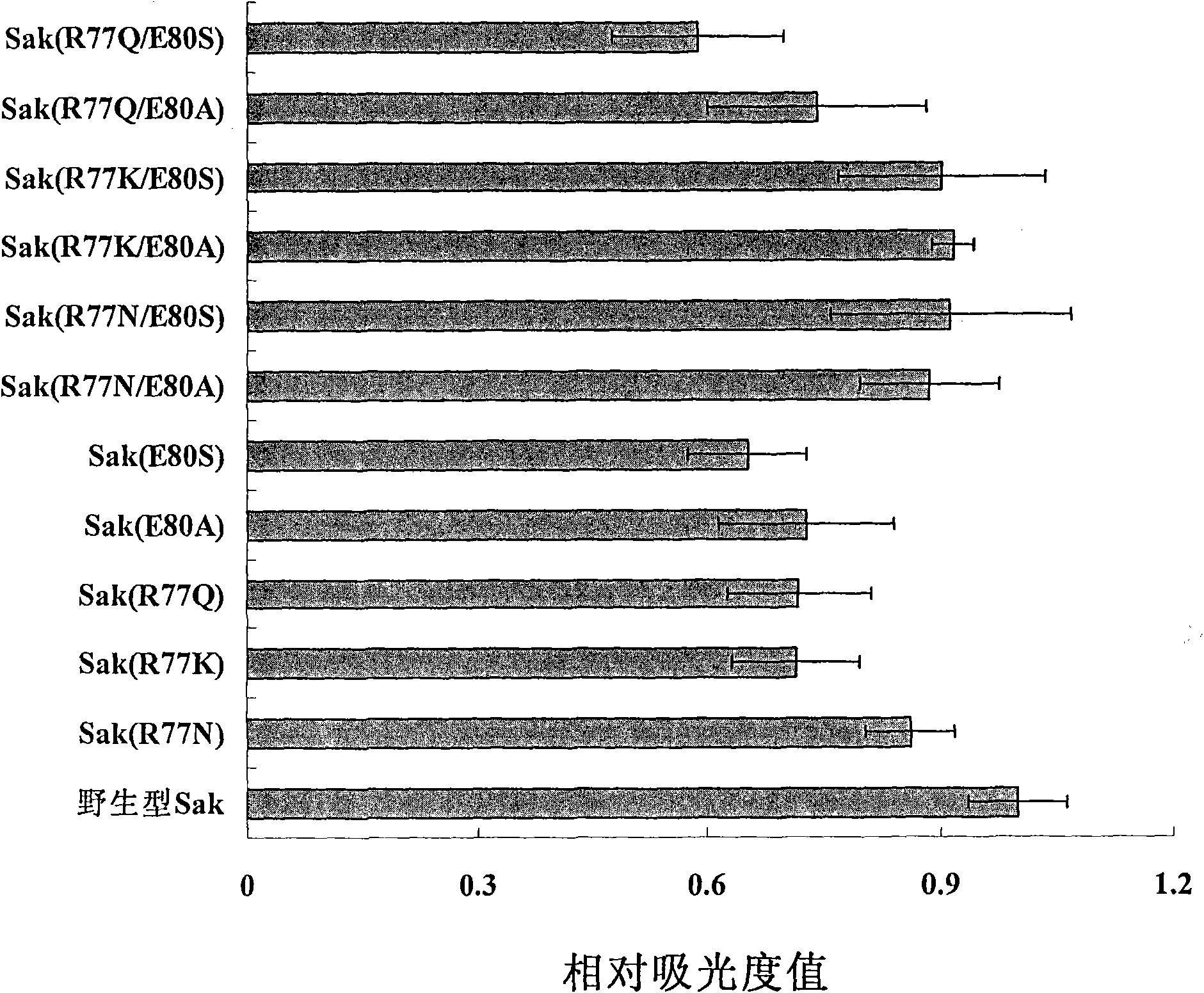
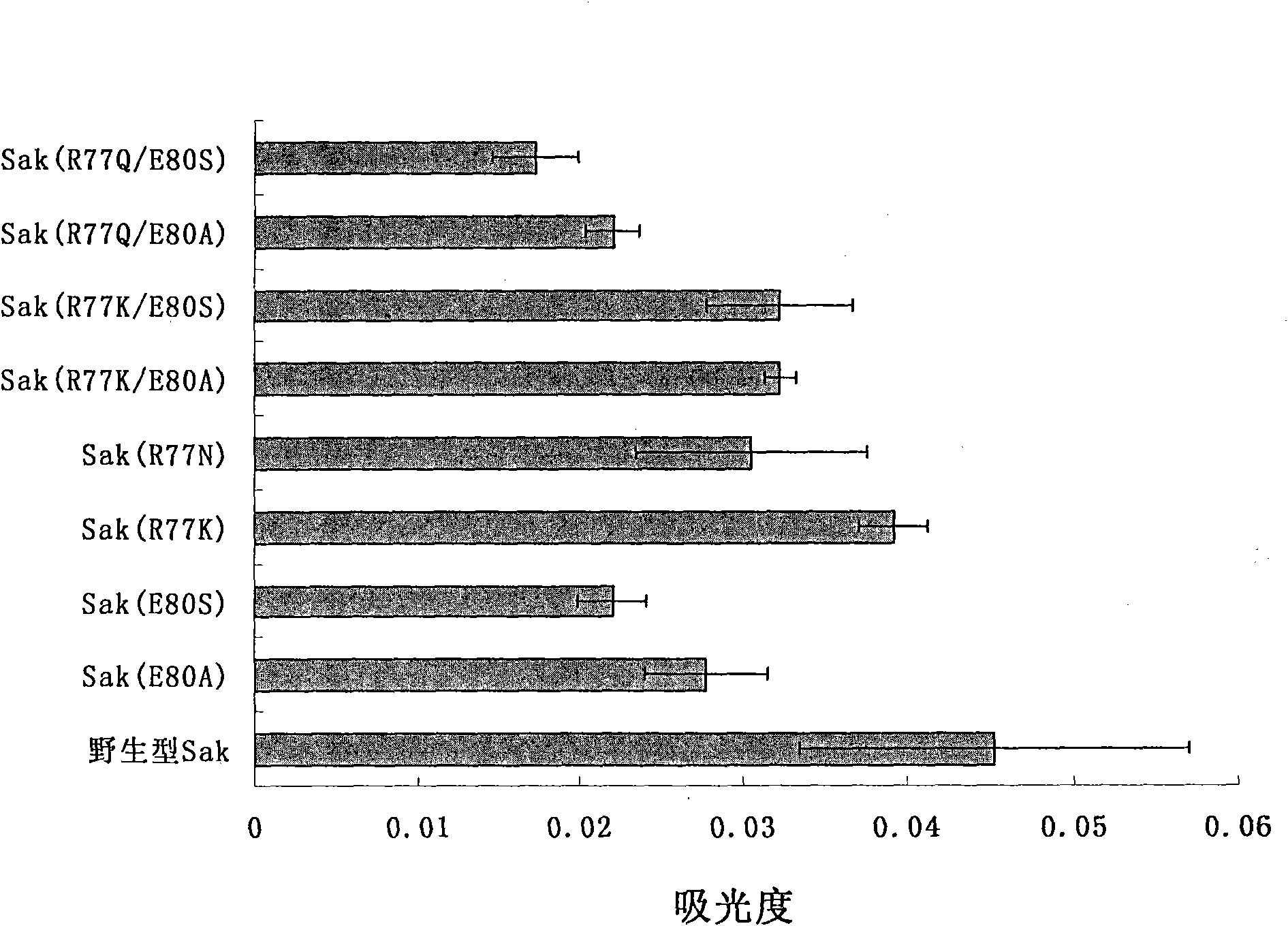
[0020] 1. Mutant design
[0021] The present invention selects R77 and E80 as mutation sites, and replaces them with other suitable amino acids, hoping to greatly reduce the immunogenicity of the constructed mutants while retaining the fibrinolytic activity of staphylokinase. The mutant Sak (R77N) designed by the present invention replaces R77 of wild-type Sak with N, or Sak (R77K) replaces R77 of wild-type Sak with K, and Sak (R77Q) replaces R77 of wild-type Sak with Q; Sak(E80A) replaces E80 of wild-type Sak with A, and Sak(E80S) replaces E80 of wild-type Sak with S; Sak(R77K / E80A) replaces R77 and E80 of wild-type Sak with K and A, Sak(R77K / E80S) is to replace R77 and E80 of wild-type Sak with K and S respectively, and Sak(R77N / E80A) is to replace R77 and E80 of wild-type Sak with N and A respectively, Sak( R77N / E80S) is to replace R77 and E80 of wild-type Sak with N and S respectively, and Sak (R77Q / E80A) is to replace R77 and E80 of wild-type Sak with Q and A respectivel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com