RGD-recombinatn staphylokinase-human alpha microglobulin fusion protein, and preparation method and application thereof
A technology of fusion protein and microglobulin, which is applied in the field of genetic engineering, can solve the problem of RGD-recombinant staphylokinase activity decline, and achieve the effect of maintaining thrombolytic activity, high-efficiency expression, and good anticoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Expression and purification of embodiment 1 recombinant staphylokinase
[0065] For the expression and purification method of recombinant staphylokinase (rSAK), please refer to "Expression, Purification and Identification of Fibrinolytic Activity of Recombinant Staphylokinase", Journal of Chongqing Medical University, Vol. Cold, Zhou Jianzhong.
[0066] That is, the main construction methods are as follows:
[0067] Using gene synthesis technology, optimize the synthetic rSAK full-length gene sequence, while keeping the protein sequence unchanged, optimize part of the codon base sequence that may affect the translation and expression process, making it the preferred codon or the best codon for Escherichia coli Codon, using pET-28a as a vector, induced the optimized gene sequence to be expressed in competent E.coli BL21 and purified by Ni-NAT column.
[0068] The rSAK full-length gene sequence is shown in SEQ ID NO.1, specifically:
[0069] TCATTCTCCTCCATTACCAACGAAGTC...
Embodiment 2
[0072] Example 2 Expression and purification of human α-microglobulin fusion protein
[0073] For the expression and purification method of human α-microglobulin (α1M), see Zhang Y, Gao Z, Zhang Z, et al. Cloning, purification, crystallization and preliminary X-ray sstudies of human α1-microglobulin[J]. Acta Crystallogr Sect F Stryc Biol Cryst Commun, 2012, 68(pt 6):692-694.
[0074] The gene sequence of the human α-microglobulin (α1M) is shown in SEQ ID NO.3, specifically:
[0075]CAAGTGCAGGAAAACTTCAATATCTCTCGGATCTATGGGAAGTGGTACAACCTGGCCATCGGTTCCACCTGCCCCTGGCTGAAGAAGATCATGGACAGGATGACAGTGAGCACGCTGGTGCTGGGAGAGGGCGCTACAGAGGCGGAGATCAGCATGACCAGCACTCGTTGGCGGAAAGGTGTCTGTGAGGAGACGTCTGGAGCTTATGAGAAAACAGATACTGATGGGAAGTTTCTCTATCACAAATCCAAATGGAACATAACCATGGAGTCCTATGTGGTCCACACCAACTATGATGAGTATGCCATTTTCCTGACCAAGAAATTCAGCCGCCATCATGGACCCACCATTACTGCCAAGCTCTACGGGCGGGCGCCGCAGCTGAGGGAAACTCTCCTGCAGGACTTCAGAGTGGTTGCCCAGGGTGTGGGCATCCCTGAGGACTCCATCTTCACCATGGCTGACCGAGGTGAATGTGTCCCTGGGGAGCAGGAA。
[007...
Embodiment 3
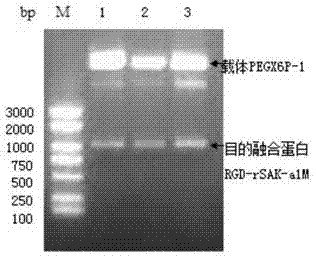
[0078] Example 3 Plasmid construction of RGD-recombinant staphylokinase-human α-microglobulin fusion protein
[0079] 1.1 Materials
[0080] Recombinant staphylokinase (rSAK) was constructed by Example 1, human α-microglobulin truncation (α1M) was constructed by Example 2, and the plasmid vector PEGX6P-1 was purchased from Novagen. Escherichia coli DH5α, BL21, 3C protease, restriction endonuclease NotI, SalI, T4 DNA ligase, high-fidelity rTaq enzyme, dNTP, plasmid purification kit, DNA Marker, molecular weight protein marker, etc. were purchased from Takara Company. Plasmid extraction kits were purchased from Promega. Thrombin, plasminogen, fibrinogen, and urokinase standard products were all purchased from China Food and Drug Control Institute. The rest of the reagents were of domestic analytical grade.
[0081] 1.2 Instruments
[0082] Water bath box, incubator, metal bath connector, PCR instrument, gel imager, ultrasonic breaker, gradient cup, protein concentration dete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com