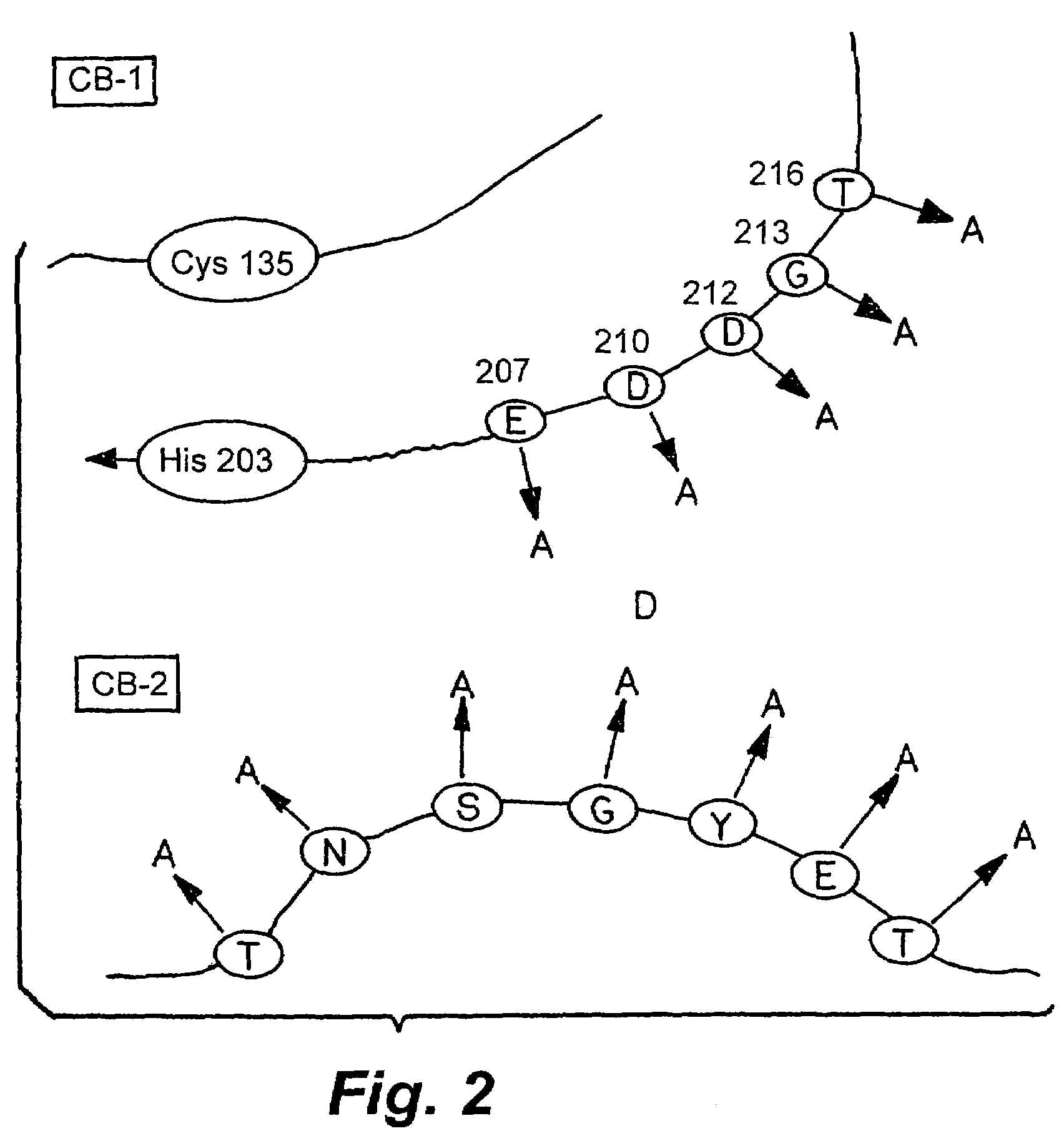
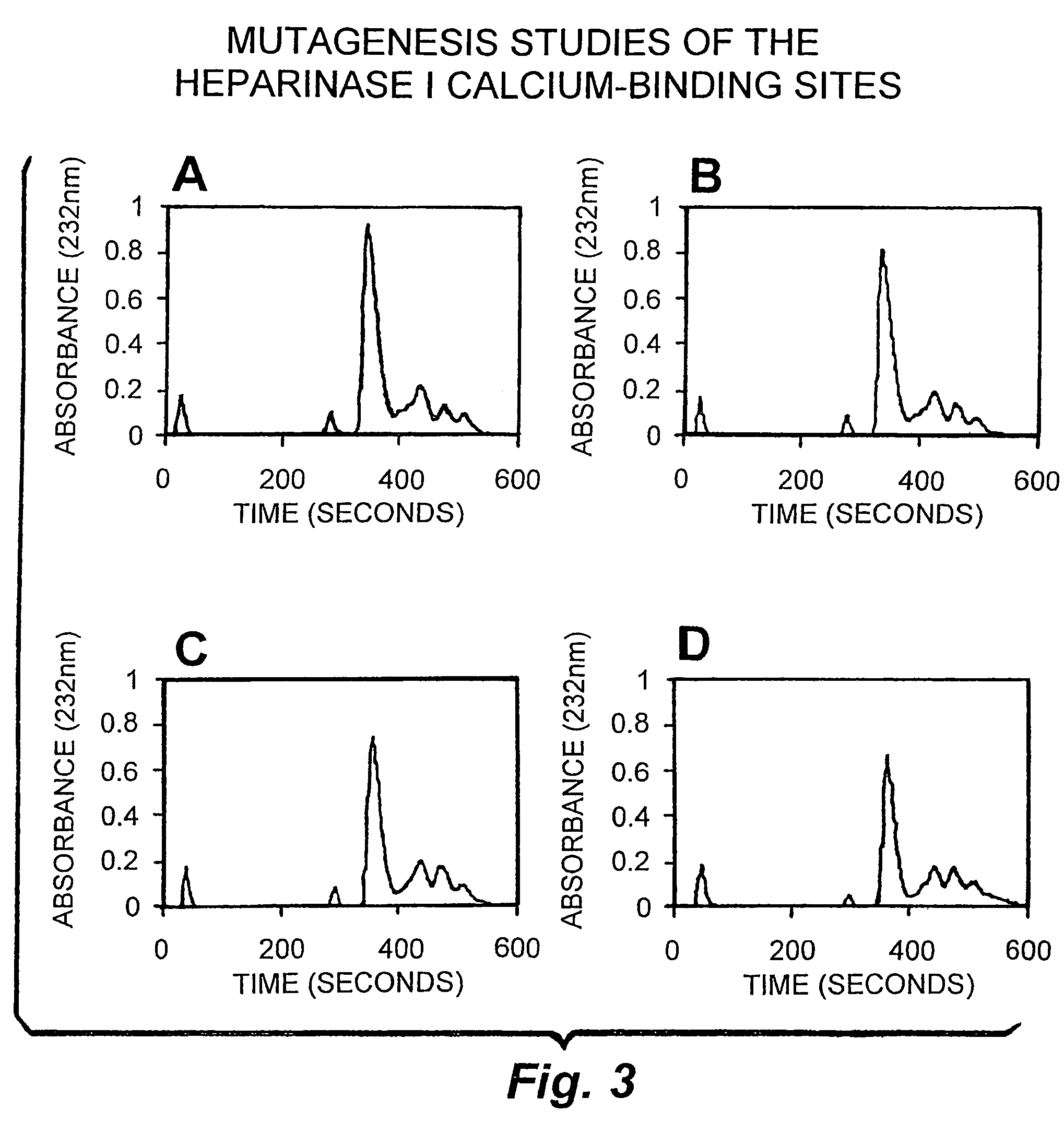
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Heparinase I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rationally designed heparinases derived from heparinase I and II
Owner:MEDI GLOBE VERTRIEBS +1
Rationally designed heparinases derived from heparinase I and II and methods of sequencing therewith
InactiveUS20060105430A1Increase freedomImprove stabilityOrganic active ingredientsFungiHeparinase INucleic acid
Owner:MASSACHUSETTS INST OF TECH
Rationally designed heparinases derived from heparinase I and II and methods of sequencing therewith
Owner:MASSACHUSETTS INST OF TECH
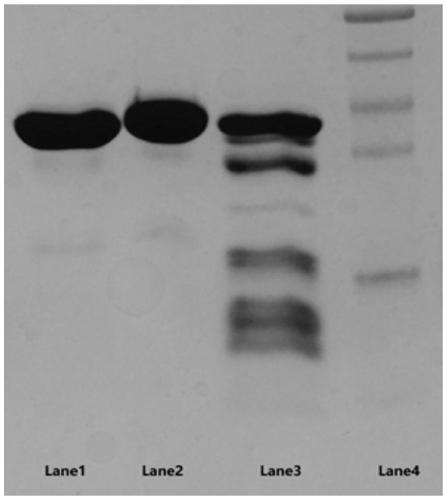
A kind of preparation method of Flavobacterium heparinase heparanase I, ii, iii
ActiveCN102286448AHigh purification process activity yieldSimple processMicroorganism based processesEnzymesChromatographic separationFlavobacterium heparinum
The invention provides a preparation method of flavobacterium heparinum heparinases I, II and III, which comprises the following steps: inoculating flavobacterium heparinum used as the raw material into a seed culture medium for culture, then inoculating into a fermentation culture medium, centrifuging, collecting the precipitate, carrying out ultrasonication on the precipitate, and centrifuging to obtain a crude enzyme liquid of flavobacterium heparinum heparinases I, II and III; carrying out SP-Sepharose FF on the crude enzyme liquid to obtain an enzyme II and enzymes I and III through chromatographic separation; carrying out SP-Sepharose FF on the enzymes I and III to obtain a heparinase I and a heparinase III through separation; respectively carrying out SP-Sepharose FF on the obtained enzyme I and enzyme III twice to obtain high-purity heparinases I and III through purification; and respectively carrying out HEP-Sepharose 4B, HA-Ultrogel and SP-Sepharose FF on the obtained enzymeII to obtain a high-purity heparinase II.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
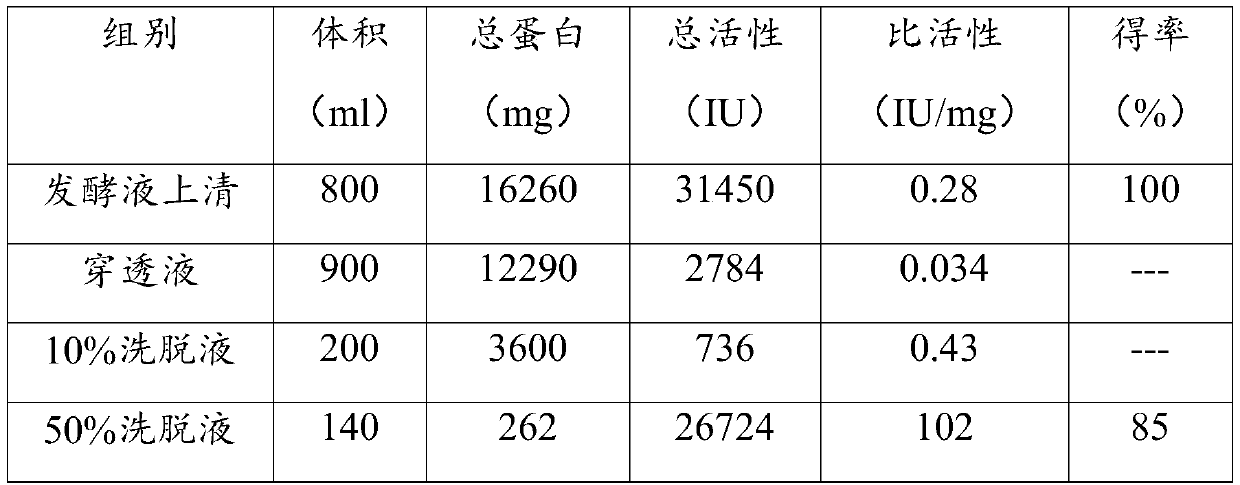
Method for preparing flavobacterium heparinum heparinase I
ActiveCN101886067ATaller than aliveEnsure stabilityMicroorganism based processesLyasesCentrifugationFlavobacterium heparinum
The invention provides a method for preparing heparinase I. The method for preparing the heparinase I comprises the following steps of: inoculating flavobacterium heparinum serving as a raw material to a seed culture medium for culture; then inoculating the flavobacterium heparinum to a fermentation culture medium; centrifugally collecting precipitate; performing ultrasonication on the precipitate; performing centrifugation again to obtain crude enzyme liquid of the flavobacterium heparinum heparinase I; and performing SP-sepharose FF chromatographic purification on the crude enzyme liquid for three times to obtain the high-purity flavobacterium heparinum heparinase I, wherein the SP-sepharose FF chromatographic purification for three times is protected by calcium chloride in the whole course, so that the yield of pure enzymic activity is greatly increased. The method for preparing the heparinase I has the characteristics of simple process, easy amplification, large preparation amount of products at a time, low cost of reagents and the like. The specific activity of the prepared heparinase I reaches 223 IU / mg and the yield of the pure enzymic activity reaches 30 percent. Compared with the conventional newest method, the method for preparing the flavobacterium heparinum heparinase I has the advantages of increasing the specific activity by more than two times, and the purification yield by about one time.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Engineering strain construction method for improving activity of heparinase I
InactiveCN106497897AImprove the efficiency of enzymatic hydrolysis preparationLow costNucleic acid vectorFermentationProtein targetGenetic engineering
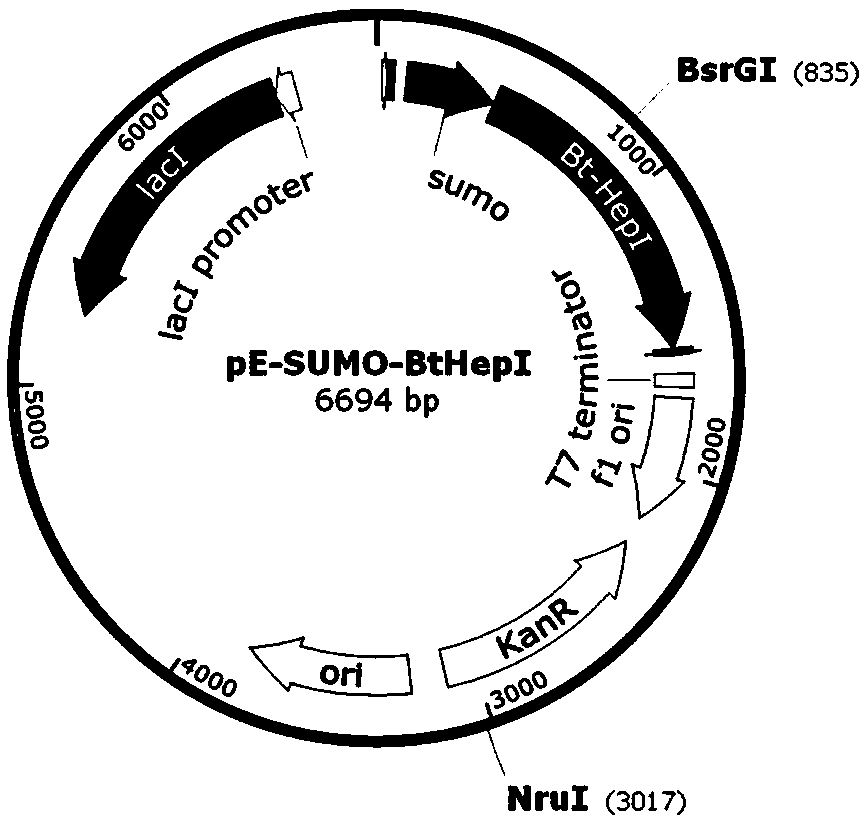
The invention relates to an engineering strain construction method for improving the activity of heparinase I. An amino acid sequence is optimized according to spatial structure analysis on the heparinase I to obtain Hep169 with the activity improved by 48% compared with the heparinase; a Hep169 gene is optimized according to codon preference, a gene DNA is obtained through artificial synthesis and is cloned into an expression vector to perform fusion expression with SUMO and other labels, host cell conversion and screening are performed to establish a soluble genetic engineering production system of the Hep169, analysis results show that a target protein obtains an efficient soluble expression, has very good biological activity and can efficiently crack heparin to generate low-molecular-weight heparin. By the adoption of the method, the highly active HepI is provided, a new method is provided for efficient soluble expression gene engineering production of the heparinase I, the production cost of the low-molecular-weight heparin and other drugs can be effectively reduced, and the engineering strain construction method has wide application prospect.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of oligosaccharide containing N-acetylated structure heparin
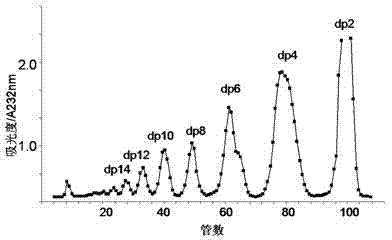
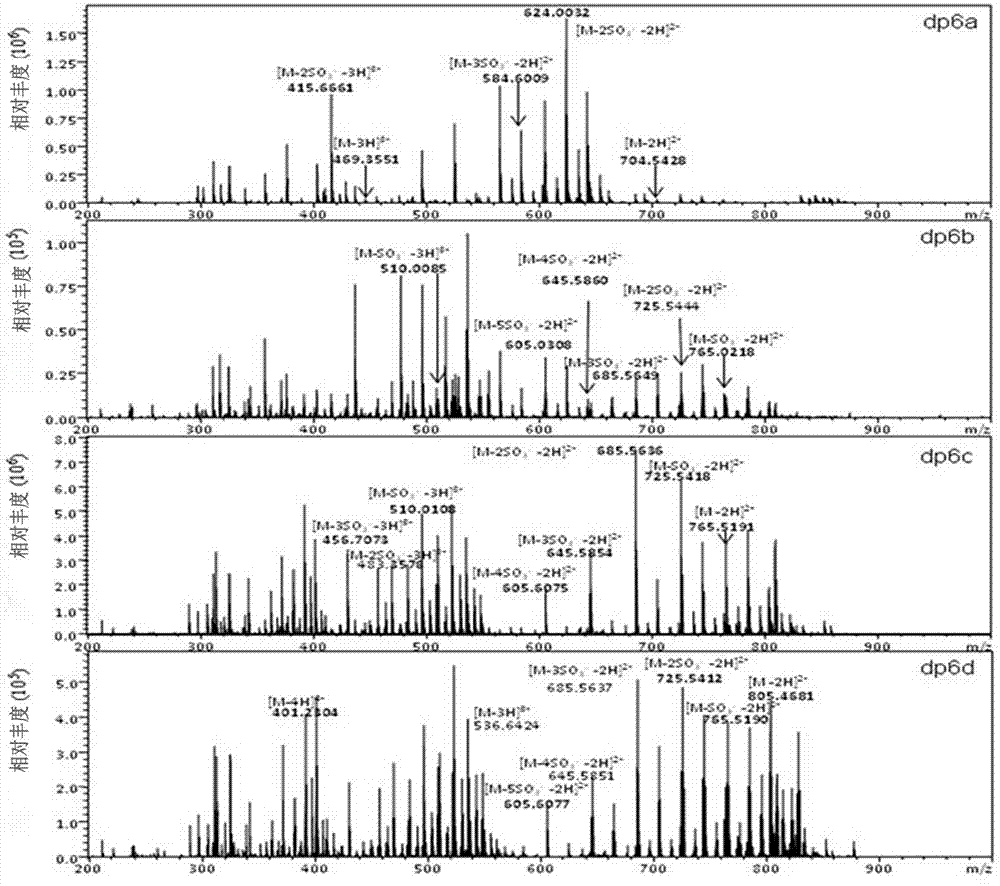
The invention relates to a preparation and purification method of a carbohydrate library containing N-acetylated heparin. The method comprises the following steps: enriching oligosaccharide containing an N-acetylated structure through deep enzymolysis of low molecular weight heparin with heparinase I, then, preparing a series of heparin oligosaccharide crude samples ranging from disaccharide to tetradecasaccharide by Bio-Gel P10 gel chromatography, further separating the crude samples by means of strong anion high performance liquid chromatography and other methods, and respectively purifying the crude samples to obtain four hexasaccharide segments and three octasaccharide fragments; analyzing the disaccharide constituent of each purified oligosaccharide by compound enzymolysis with heparinase I, heparinase II and heparinase III and strong anion chromatography, and primarily deducing sequence structures of the four hexasaccharide and the three octasaccharide in combination with heparinase I substrate specificity; and finally, identifying the structure by electrospray ionization-ion trap-time of flight mass spectrometry (ESI-IT-TOF-MS). The preparation method provided by the invention can be used for solving the problem of difficulty in preparation and structure determination of oligosaccharide containing N-acetylated heparin, which makes research on a relationship between a special structure and functions of heparin / heparan sulfate developed further.
Owner:FUZHOU UNIV
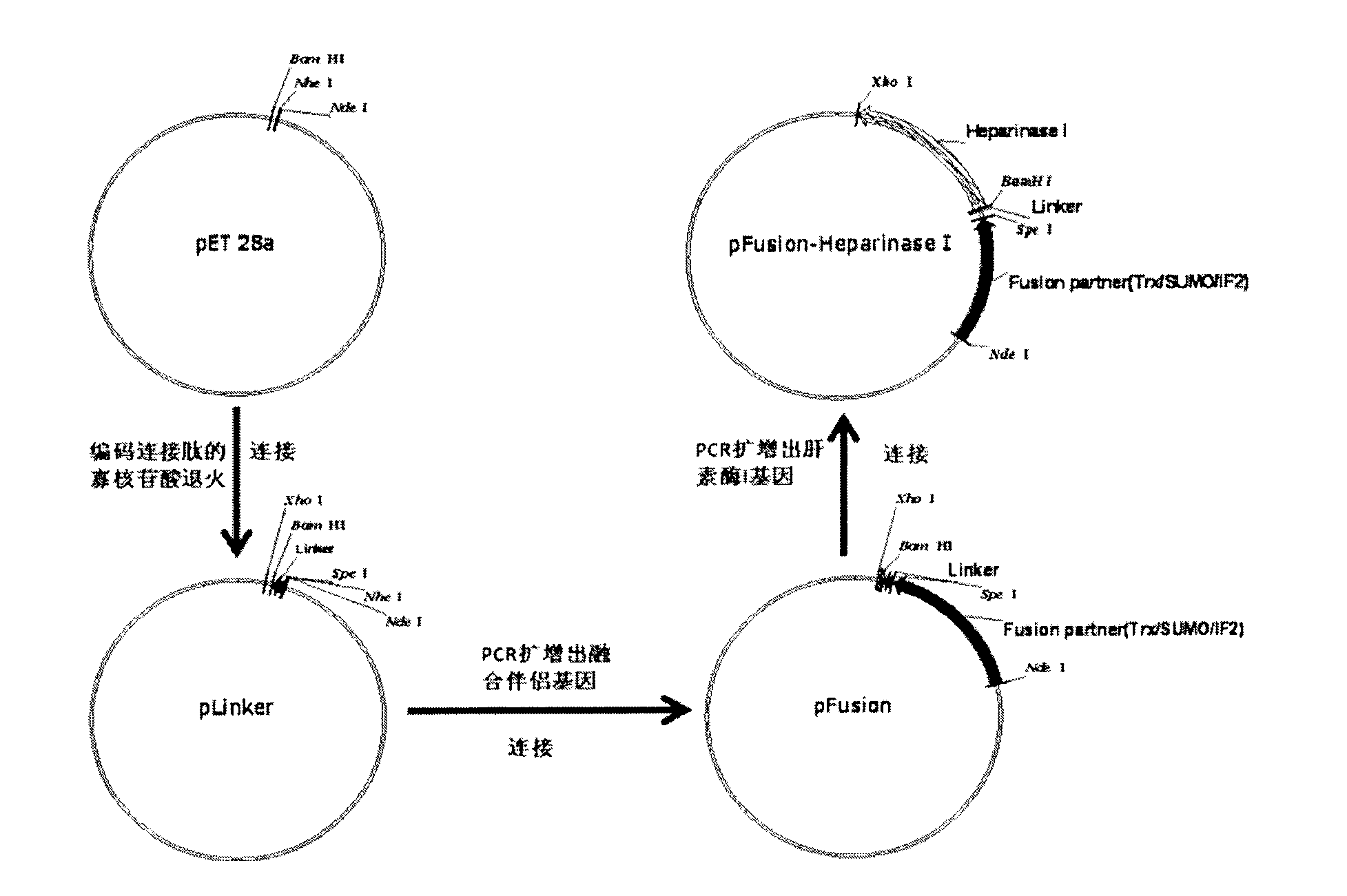
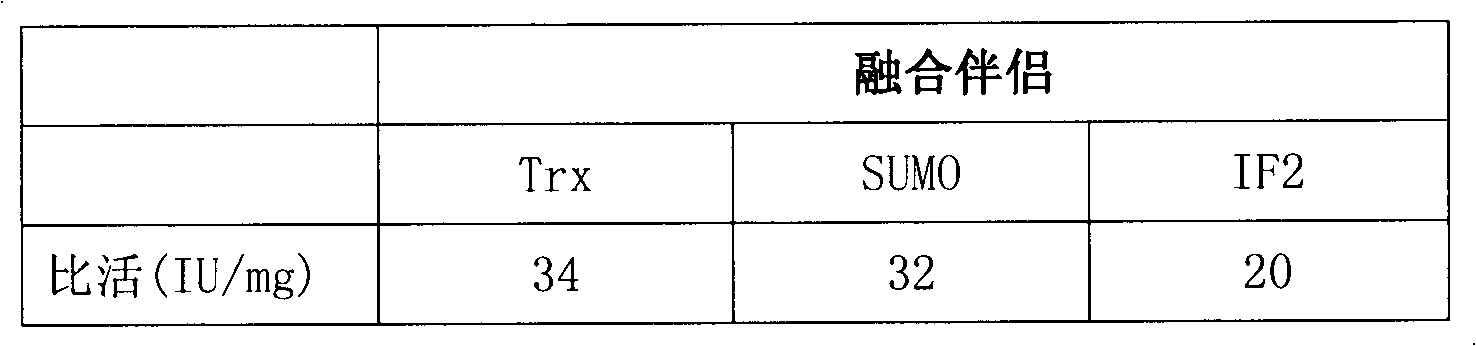
Heparinase I fusion protein
The invention provides one or several heparinase I fusion proteins and coding genes thereof. The heparinase I fusion protein contains three structural domains: a fusion structural domain, a connection structural domain and a heparinase I structural domain. Specifically, the fusion structural domain is selected from thioredoxin Trx, small ubiquitin-like modifier SUMO or translation initiation factor 2IF2, and the heparinase I structural domain includes Flavobacterium heparinum heparinase I. The invention also provides expression and purification methods of the heparinase I fusion protein.
Owner:曹林 +2
Method for controlling the production of low molecular weight heparin
This article discloses a production method of low molecular weight heparin or ultra-low molecular weight heparin. This method utilizes two or more heparinase enzymes selected from heparinase I, II and III to degrade heparin to produce low molecular weight heparin or ultra-low molecular weight heparin.
Owner:TSINGHUA UNIV
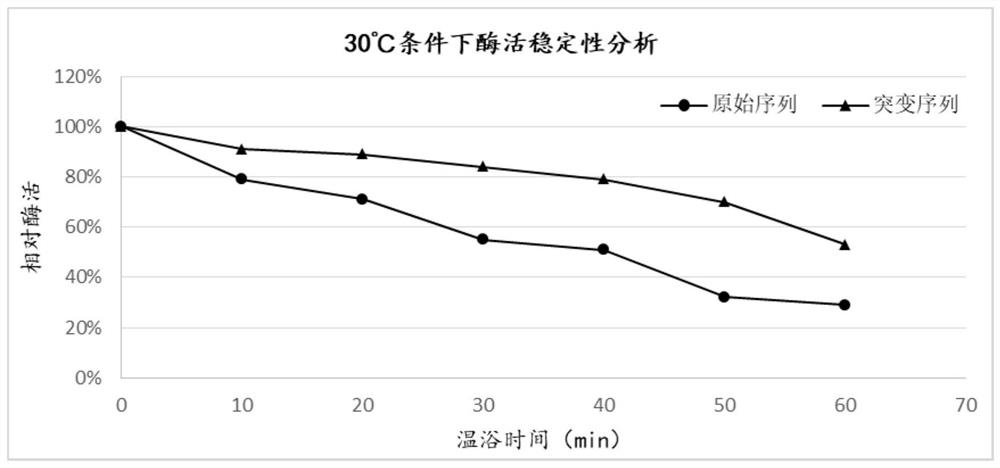
Mutant for improving thermal stability of heparinase I by enzyme flexibility analysis based on molecular dynamics and preparation method thereof
ActiveCN109666666ALong shelf lifeImprove operational stabilityBacteriaMicrobiological testing/measurementThermal stabilityOperational stability
The invention relates to a mutant for improving the thermal stability of heparinase I by enzyme flexibility analysis based on molecular dynamics, wherein the mutant is a mutant BtHepIQ198R and / or a mutant BtHepIK247W, and the amino acid sequence of the mutant BtHepIQ198R is SEQIDNO. 1, and the amino acid sequence of the mutant BtHepIK247W is SEQIDNO. 2. The mutant of the invention is a heparin enzyme I mutant strain with excellent thermal stability, has larger industrial application potential and economic value, and has important significance for prolonging the shelf period of the heparin enzyme I improveing the operation stability of the heparin enzyme I in a catalytic process and the reusing batches, reducing production cost and the like.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
High-expression water-soluble heparinase I fusion protein and coding gene thereof
ActiveCN103992995AAdd nucleotide sequenceAchieve one-step purificationFungiBacteriaNucleotideFermentation
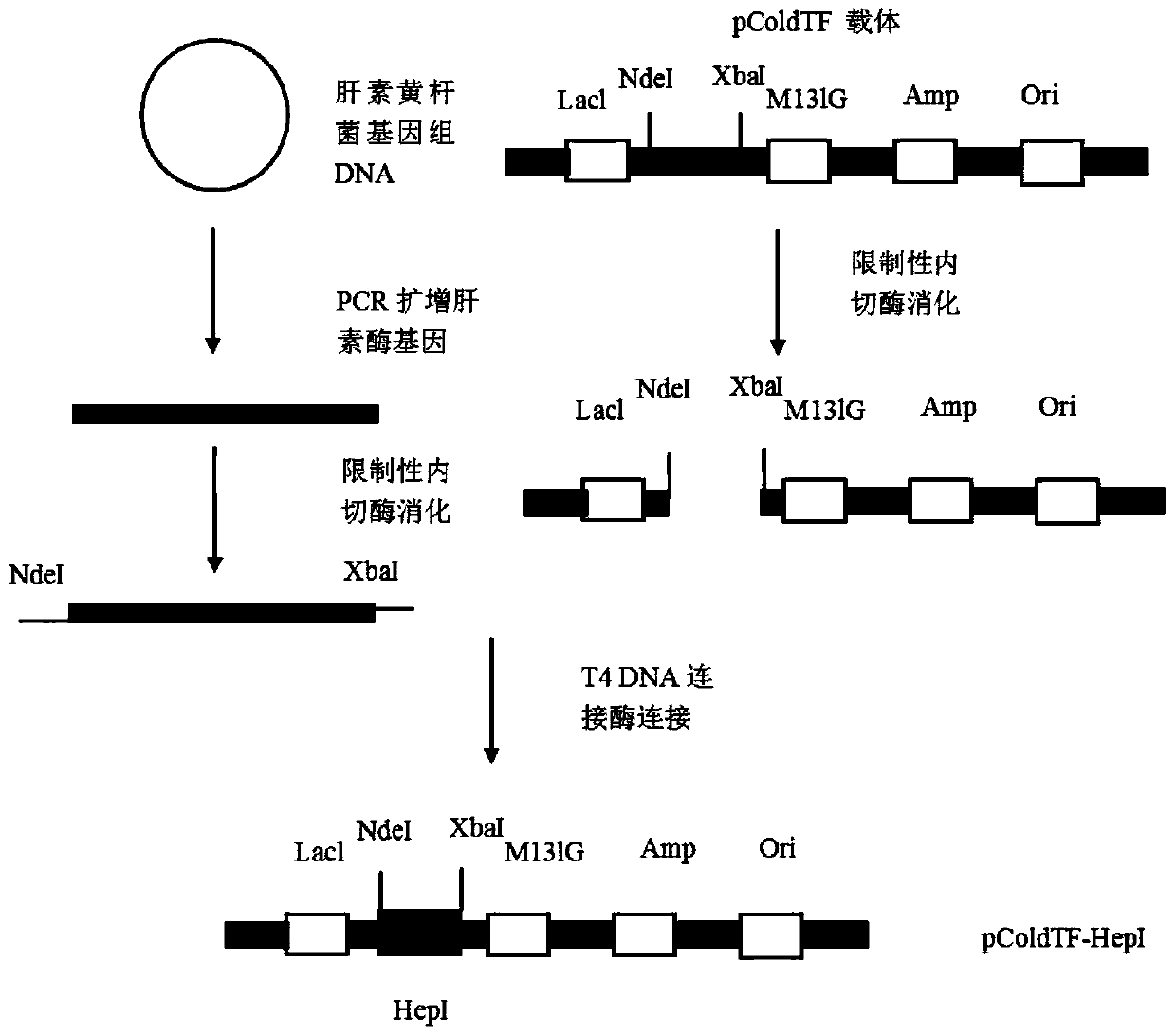
The invention relates to a high-expression water-soluble heparinase I fusion protein and a coding gene thereof. The amino acid sequence of the heparinase I fusion protein is as shown in SEQ ID NO. 2; the nucleotide sequence of the coding gene of the heparinase I fusion protein is as shown in SEQ ID NO. 1. According to the invention, a pColdTF vector is utilized to transform an expression gene of heparinase I, a section of nucleotide sequence expressing a pColdTF protein is added and the heparinase I fusion protein is obtained; the enzyme activity of the heparinase I fusion protein can reach 64000U / L of fermentation solution, the expression level can reach 320mg / L of fermentation solution and the specific enzyme activity can reach 200U / mg. Furthermore, the enzyme can further realize one-step purification of the fusion protein by nickel column separation.
Owner:SHANDONG UNIV
Freeze-drying preservation method for heparin flavobacterium heparinum heparinase I, II and III
The invention relates to a freeze-drying preservation method for heparin flavobacterium heparinum heparinase I, II and III, in particular to a protective agent optionally comprising calcium chloride and trehalose, and the protective agent is sued for protecting activity of the heparinase I, II and III during freeze-drying. The enzyme freeze-drying powder prepared according to the method provided by the invention is convenient to store, transport and use, and the application value is improved.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Specific enzyme activity improved directionally modified enzyme of heparinase I as well as molecular modification method and expression engineered bacterium
ActiveCN109321549AIncreased specific enzyme activityGreat application potentialBacteriaFermentationSpecific enzymeNucleotide
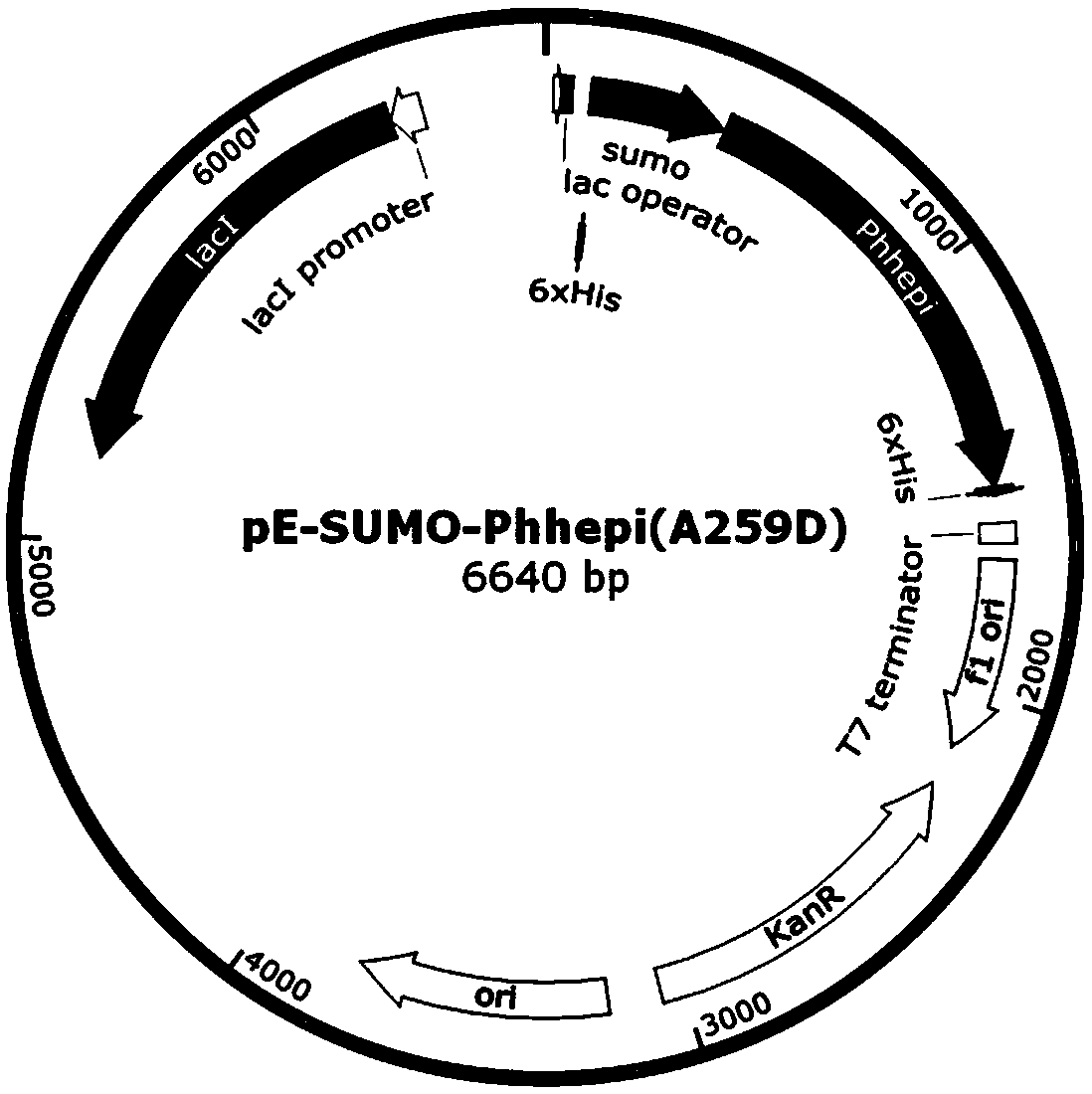
The invention relates to a specific enzyme activity improved directionally modified enzyme PhHepI<A259D> of heparinase I. The nucleotide sequence of the enzyme is SEQ ID NO: 1. The invention providesa design and construction method of two mutant enzymes PhHepI<A259D> and PhHepI<S169D / A259D>, and a method for the construction of a novel mutant-enzyme engineered bacterium and the high-efficiency expression of the mutant enzymes. Compared with a wild type, the mutant enzymes provided by the invention have higher specific enzyme activity and greater application potential, and the theoretical basis is also laid for the research of HepI.
Owner:TIANJIN UNIV OF SCI & TECH +1
Preparation method of heparin oligosaccharides
A preparation method of heparin oligosaccharides comprises the following steps: 1, carrying out enzymic degradation on heparin: dissolving 20 mg of heparin in 1 ml of a reaction solution Digestion Buffer, adding 500 [mu]l of heparinase I, 500 [mu]l of heparinase II and 500 [mu]l of heparinase III, carrying out an oscillatory reaction for 72 h at a constant temperature of 37 DEG C with heparinase supplement every 12 h, heating in a water bath with the temperature of 100 DEG C for 15 min after ending the reaction, carrying out high speed centrifugation for 10 min with a speed of 12000 rpm, taking out a supernatant and setting aside; and 2, carrying out stage purification on heparin oligosaccharides: carrying out stage purification through passing oligosaccharide fragments obtained in step 1 through a Bio-gel P2 column, collecting and lyophilizing. So the finished products are obtained.
Owner:GUANGYUAN HAIPENG BIOLOGICAL TECH
Strain of bacillus subtilis engineering bacteria and application thereof in producing heparinase I
ActiveCN102533628AHigh activityIncrease productionBacteriaMicroorganism based processesMicroorganismMicrobiology
The invention discloses bacillus subtilis engineering bacteria expressing recombinant flavobacteriun heparinum heparinase I, wherein the class name is bacillus subtilis which is preserved in the CGMCC (China General Microbiological Culture Collection Center) of CCCCM (China Committee for Culture Collection of Microorganisms) on 15th February, 2012, with preservation number CGMCC No.5757. A construction method comprises the following steps: transferring the coding gene of the flavobacteriun heparinum heparinase I into the bacillus subtilis WB600 to obtain the engineering bacteria capable of secreting and expressing heparinase. The bacillus subtilis engineering bacterial disclosed by the invention can stably and efficiently express the recombinant heparinase I; and the heparinase I can specifically degrade heparin and heparan glucosidic bond, and can be applied to the industrial production of low-molecular heparin, research of heparin and clinical medicine.
Owner:SHANDONG UNIV
Method for preparing high-activity and low-molecular-weight heparin by enzymic method
InactiveCN102660610AIncrease the number ofHigh anticoagulant activityFermentationHeparin biosynthesisSulfate radicals
The invention discloses a method for preparing high-activity and low-molecular-weight heparin by an enzymic method. The method includes using heparin as substrate; degrading the heparin by HepI (histidine-tagged heparinase I) in a controlled manner; then utilizing a PAPS (3'-phosphoadenosine-5'-phosphosulfate) regeneration system; selectively decorating the heparin by heparin biological synthetase 3-O-sulfotransferase; and increasing the quantity of anticoagulation activity centers to obtain the low-molecular-weight heparin with high anticoagulation activity. The HepI, the 3-O-sulfotransferase and AST-IV which are adopted in the method can be prepared by means of high-density fermentation in high yield; the PAPS regeneration system uses PNPS (P-nitrophenol sulfonic acid potassium salt) as sulfate radical for enzyme modification reaction, and production cost is greatly reduced. The method provides a new way for enzymic method industrialization of the high-activity and low-molecular-weight heparin.
Owner:JIANGNAN UNIV
Heparin detection kit and application thereof
PendingCN109709292AStable storageNeutralizes anticoagulant activityTesting medicinal preparationsGlycerolBlood coagulations
The invention, which relates to the technical field of anticoagulant effect detection, discloses a heparin detection kit and application thereof. To be specific, the heparin detection kit comprises aheparin detection reagent including heparinase I being a liquid enzyme preparation; for long-time preservation, the heparinase I needs to be frozen and dried and then the processed heparinase I becomes solid one for preservation; however, after a certain amount of anhydrous calcium chloride, glycerol and D-trehalose that are used as protective agents are added into the heparinase I, the detectionreagent exists stably for long time, so that the long-time preservation requirement is met. With the heparin detection kit, the anticoagulant activity of heparin can be neutralized rapidly and specifically without affecting other blood components of blood coagulation, so that the residual situation of the heparin sodium salt and the anticoagulant effect of heparin sodium salt can be evaluated rapidly and precisely and thus the basis is provided for clinical medication.
Owner:GUIZHOU JINJIU BIOTECH
Preparation method of flavobacterium heparinum heparinases I, II and III
ActiveCN102286448BHigh purification process activity yieldSimple processMicroorganism based processesEnzymesChromatographic separationFlavobacterium heparinum
The invention provides a preparation method of flavobacterium heparinum heparinases I, II and III, which comprises the following steps: inoculating flavobacterium heparinum used as the raw material into a seed culture medium for culture, then inoculating into a fermentation culture medium, centrifuging, collecting the precipitate, carrying out ultrasonication on the precipitate, and centrifuging to obtain a crude enzyme liquid of flavobacterium heparinum heparinases I, II and III; carrying out SP-Sepharose FF on the crude enzyme liquid to obtain an enzyme II and enzymes I and III through chromatographic separation; carrying out SP-Sepharose FF on the enzymes I and III to obtain a heparinase I and a heparinase III through separation; respectively carrying out SP-Sepharose FF on the obtained enzyme I and enzyme III twice to obtain high-purity heparinases I and III through purification; and respectively carrying out HEP-Sepharose 4B, HA-Ultrogel and SP-Sepharose FF on the obtained enzymeII to obtain a high-purity heparinase II.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
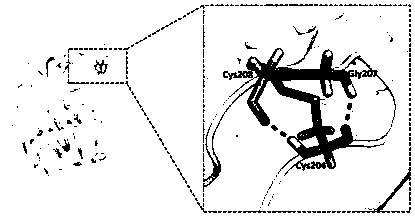
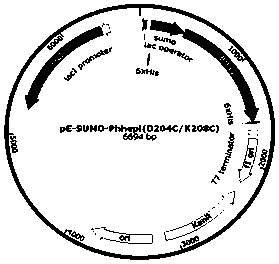
Mutant for improving thermal stability of hexotensin I by increasing disulfide bond and preparation method thereof
ActiveCN109706137ALong shelf lifeReduce screening workloadMicrobiological testing/measurementColor/spectral properties measurementsWild typeWorkload
The present invention relates to a mutant BtHepID204C / K208C for improving thermal stability of hexotensin I by increasing disulfide bond, and an amino acid sequence of the mutant is SEQ ID NO. 1. Themutant has better thermostability than wild-type heparinase I (BtHepI), and can effectively reduce the screening workload of a mutant library, at the same time, the thermostable heparinase I has greatsignificance for extending the shelf life of heparinase I, improving its operational stability and reusing batches in a catalytic process, , and reducing production costs. The industrial applicationvalue of heparinase I can be improved.
Owner:TIANJIN UNIV OF SCI & TECH +1
Mutant heparinase I, encoding nucleotide sequence thereof, recombinant carrier and host cell with nucleotide sequence and application
PendingCN110343691AImprove stabilityFungiMicroorganism based processesRestriction Enzyme Cut SiteNucleotide
The invention provides mutant heparinase I, an encoding nucleotide sequence thereof, a recombinant carrier and host cell with the nucleotide sequence and application, and relates to the technical field of molecular biology. By means of the mutant heparinase I, mutation is conducted on a protease restriction enzyme cutting site, probably influencing the stability of the heparinase I, of an amino acid sequence of existing heparinase I, and specifically, the Gln fixed point in the 2nd site of the amino acid sequence of the existing heparinase I is mutated into His, and the Glu fixed point in the78th site is mutated into Met. The mutant heparinase I obtained after mutation is higher in stability than existing heparinase I under the condition of not influencing the activity of the heparinase I.
Owner:宝锐生物科技泰州有限公司
A mutant that improves the thermal stability of heparanase I based on molecular dynamics analysis of enzyme flexibility and its preparation method
ActiveCN109666666BLong shelf lifeImprove operational stabilityBacteriaMicrobiological testing/measurementBiotechnologyEnzyme
The invention relates to a mutant that improves the thermal stability of heparanase I based on molecular dynamics analysis of enzyme flexibility, and the mutant is mutant BtHepI Q198R and / or mutant BtHepI K247W , the mutant BtHepI Q198R The amino acid sequence is SEQ ID NO.1, the mutant BtHepI K247W The amino acid sequence of is SEQ ID NO.2. The mutant of the present invention is a heparanase I mutant strain with excellent thermal stability, which has great potential for industrial application and economic value. Operational stability and batch reuse in catalytic processes, reducing production costs, etc., are of great importance.
Owner:TIANJIN UNIV OF SCI & TECH +1
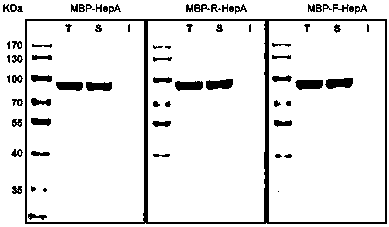
A connecting peptide and its application
The invention discloses a connecting peptide and an application thereof. The connecting peptide is mainly applied in preparation of MBP fusion polysaccharide lyases, and in particular, relates to MBP fusion heparinases I, II and III and MBP fusion chondroitinases B and AC. In an MBP fusion expression system, MBP solubilization assisting and folding assisting are improved through application of the connecting peptide, the target protein activity and thermal stability are increased, and the MBP fusion heparinases I, II and III and the MBP fusion chondroitinases B and AC having fermentation enzyme activity in escherichia coli preliminarily reaching an industrial application level and having excellent thermal stability are eventually obtained.
Owner:WUXI RES INST OF APPLIED TECH TSINGHUA UNIV
A highly expressed water-soluble heparanase I fusion protein and its coding gene
ActiveCN103992995BAdd nucleotide sequenceAchieve one-step purificationFungiBacteriaSpecific enzymeNucleotide
The invention relates to a high-expression water-soluble heparinase I fusion protein and a coding gene thereof. The amino acid sequence of the heparinase I fusion protein is as shown in SEQ ID NO. 2; the nucleotide sequence of the coding gene of the heparinase I fusion protein is as shown in SEQ ID NO. 1. According to the invention, a pColdTF vector is utilized to transform an expression gene of heparinase I, a section of nucleotide sequence expressing a pColdTF protein is added and the heparinase I fusion protein is obtained; the enzyme activity of the heparinase I fusion protein can reach 64000U / L of fermentation solution, the expression level can reach 320mg / L of fermentation solution and the specific enzyme activity can reach 200U / mg. Furthermore, the enzyme can further realize one-step purification of the fusion protein by nickel column separation.
Owner:SHANDONG UNIV
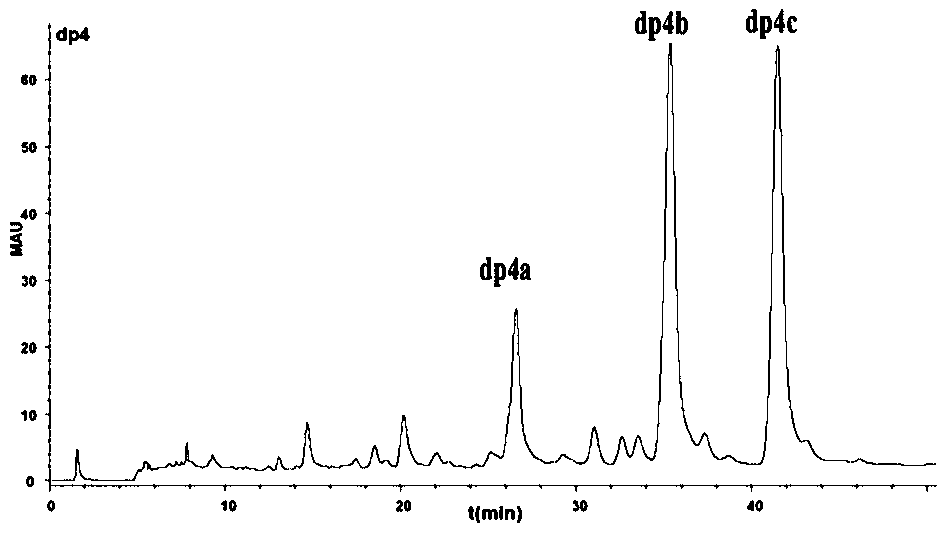
Preparation and purification method of different domain oligosaccharides of heparan sulfate/heparin
The invention relates to a preparation and purification method of different domain oligosaccharides of heparan sulfate / heparin (HS / Hp). According to the method herein, commercially available heparin is used as a raw material, a high-sulfur disaccharide with three sulfite radicals is specifically enzymatically hydrolyzed with heparinase I, enzymatic hydrolysis conditions are controlled precisely toarrive at partial enzymatic hydrolysis so as to obtain a highly sulfated domain oligosaccharide containing the high-sulfur disaccharide; lowly sulfated oligosaccharide fragments with NS and NAc structures in HS / Hp are enriched so as to prepare a series of lowly sulfated domain oligosaccharides with different structures; a series of lowly sulfated HS / Hp oligosaccharides, from disaccharide to decasaccharide, are separated and purified through gel chromatography and ion exchange high-performance liquid chromatography. The method established herein to prepare different domain oligosaccharides ofHS / Hp is fast and simple and has low cost; important carbohydrate library samples are provided for the study on structure and functionality of HS / Hp; important precursors are also provided for the development of novel heparin drugs.
Owner:FUZHOU UNIV
High-expression and high-activity bacteroides thetaiotaomicron heparinase I fusion protein, and encoding gene and application thereof
InactiveCN109385412AAdd nucleotide sequenceIncreased expression of solubleFungiBacteriaSpecific enzymeBacteroides thetaiotaomicron
The invention relates to a high-expression and high-activity bacteroides thetaiotaomicron heparinase I fusion protein. The fusion protein has the amino acid sequence shown in SEQ ID NO.1. After the fusion protein is fused with an SUMO tag, the soluble expression of a target protein is increased, the soluble expression reaches 95% or more and the specific enzyme activity is increased by 48.9%. Theenzyme activity of the bacteroides thetaiotaomicron heparinase I fusion protein in a 5L fermentation tank can reach 3.412*10<5> IU / L, the expression quantity can reach 3.4 g / L, and the foundation is laid for application development of the bacteroides thetaiotaomicron heparinase I.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
High-efficiency preparation method of heparinase I from Flavobacterium heparinum
InactiveCN109182321ALow costSimplify requirementsMicroorganism based processesCarbon-oxygen lyasesSulfateFlavobacterium heparinum
The invention relates to a high-efficiency preparation method of heparinase I of Flavobacterium heparinum, including the following steps: Flavobacterium heparinum is culture in a seed culture medium,subject to culture in fermentation medium, centrifugal sedimentation, a crude enzyme solution is obtained under osmotic stress, the crude enzyme is purified by CS (Cellufine Sulfate) column chromatography, and the specific activity of the prepared heparanase I is 135IU / mg, the pure enzyme activity yield is as high as 57%, and the purification yield is nearly doubled compared with the existing method. The invention has the advantages of simple process, easy amplification, less equipment requirement, low reagent cost, etc.
Owner:深圳市长征生物科技有限公司
Heparinase I
PendingCN114181927AImprove stabilityReduce enzyme activityFungiMicroorganism based processesNucleotideSite-directed mutagenesis
The invention discloses heparinase I, a coding nucleotide sequence thereof, a recombinant vector comprising the nucleotide sequence, a host cell comprising the nucleotide sequence and application of the heparinase I. According to the heparinase I, site-directed mutagenesis is carried out on an amino acid sequence of existing heparinase I, and particularly, glutamine (Q) at the 42nd site, the 102nd site and the 209th site of the amino acid sequence of the existing heparinase I is subjected to site-directed mutagenesis into alanine (A). Compared with the existing heparinase I, the heparinase I obtained after mutation has better stability under the condition that the activity of the heparinase I is not influenced.
Owner:刘颖
High-immobilization-tendency heparinase I coding gene and protein thereof
The invention discloses a high-immobilization-tendency heparinase I protein and a coding gene thereof. The protein can be efficiently immobilized by chitin. The amino acid sequence of the high-immobilization-tendency heparinase I protein is disclosed as SEQ ID NO:2, and the coding gene of the protein is also within the protection range. The PCR (polymerase chain reaction) technique is superposed and extended to design the heparinase I gene and chitin combination domain to fuse and express the immobilizable heparinase I.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Constructing method for prokaryotic expression vector for producing heparinase I at high yield
The invention discloses a constructing method of prokaryotic expression vector for producing heparinase I at high yield. The constructing method comprises the following steps of: taking F1:5'-TAGAATTCCAGCAAAAAAAATCCG-3' and R1:5'-GGCAAGCTTGTCTGGCAGTTTCGCTGTA-3' as primers; carrying out PCR (Polymerase Chain Reaction) to obtain a gene sequence of an encoded heparinase I, which is shown as in an SEQIDNO.1; connecting the gene sequence of the encoded heparinase I to a pET-28a vector to obtain an a recombinant expression vector pET28a-HpaI; and transferring the expression vector pET28a-HpaI plasmid into an Escherichia coli BL21 strain.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Kit for detecting functions of coagulation and platelets of heparin-added blood samples and application thereof
PendingCN107746804ATimely point-of-care coagulation assessmentAccurate point-of-care coagulation assessmentBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseThrombus
The invention provides a kit for detecting functions of coagulation and platelets of heparin-added blood samples and application thereof and relates to the technical field of medical apparatus and instruments. The detection kit comprises a reagent cup and a detection probe, wherein glass beads and a stirring rod are arranged in the reagent cup; the reagent cup also comprises a cup lid; the reagentcup is tightly bonded with the cup lid; a groove is formed in the center of the cup lid and is used for mounting the detection probe; heparinase I is arranged in the reagent cup. The detection kit takes the glass beads as activators, is capable of detecting the functions of coagulation and platelets of heparin-added blood samples through an in-vitro coagulation activating method, has great significance to early discovery of the risk of thrombosis of patients, guidance of anticoagulation treatment and component blood transfusion, and diagnosis and treatment of platelet-related diseases, and iscapable of making up the defects of detection of the functions of coagulation and platelets of the heparin-added human blood in the in-vitro study field at present.
Owner:世纪亿康(天津)医疗科技发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com