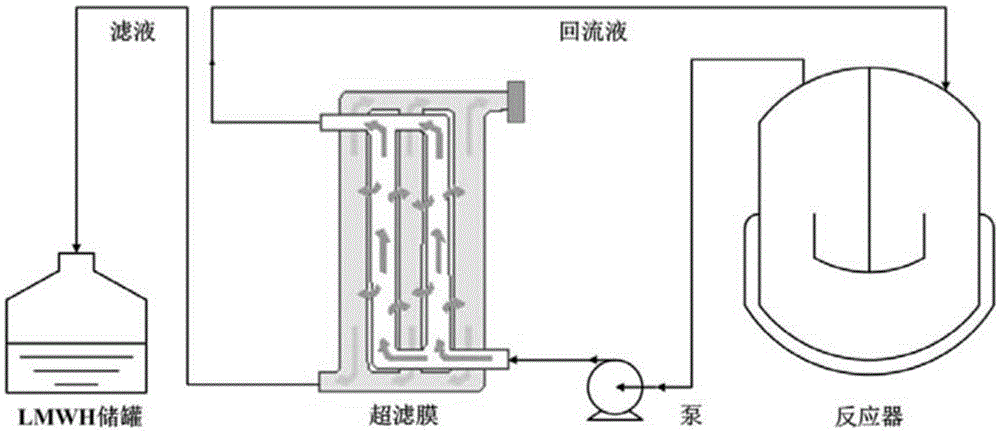
Method for controlling the production of low molecular weight heparin
A low molecular weight heparin, molecular weight technology, applied in the field of controlled production of low molecular weight heparin or ultra-low molecular weight heparin, can solve problems such as limiting the application of heparin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] The (ultra) low molecular weight heparin product obtained in Example 1 adopts high performance exclusion chromatography to measure its average molecular weight (including the weight average molecular weight M w and number average molecular weight M n ) and distribution. The gel chromatography column used is TSKGelG3000SW, 30mm×750mm, Tosoh Co., Japan. The HPLC system used includes computer control system, peristaltic pump (LC-10ATvp), autosampler (SIL-10ADvp), ultraviolet detector (SPD-M10Avp), differential detector (RID-10A). Wherein the ultraviolet detector and the differential detector are connected in series, and the differential detector is connected behind the ultraviolet detector. The mobile phase used is pH5.0, containing 2.84% Na by mass percentage 2 SO 4 buffer. The column temperature is 35°C, the flow rate is 0.5mL / min, and the detection wavelength of the ultraviolet detector is 235nm.
[0113] (Ultra) low molecular weight heparin molecular weight stand...
Embodiment 2
[0141] Example 2 Comparison of heparinase I and heparinase III degrading heparin
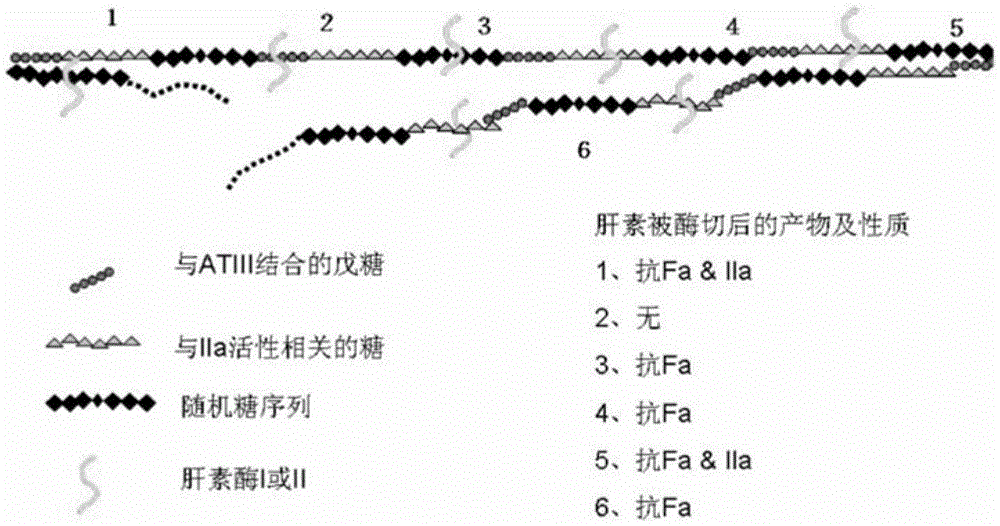
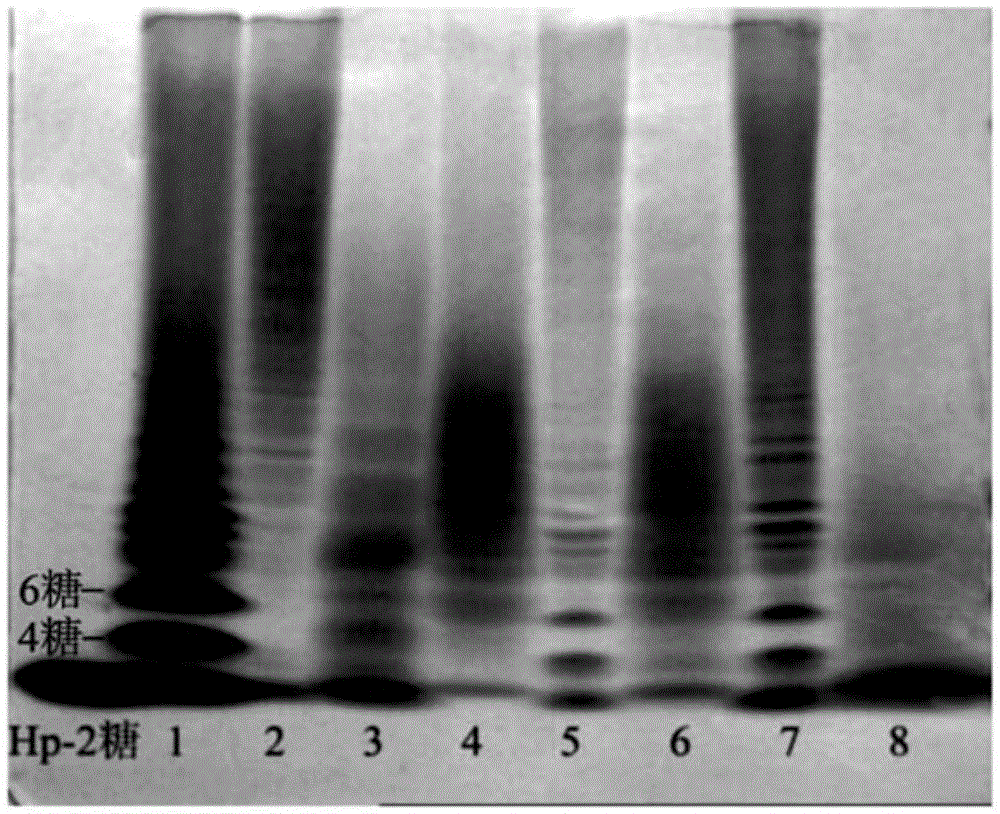
[0142] Add equal amounts of heparinase I and heparinase III to the heparin solution, degrade completely at 30°C, and react until A 235 No change occurs. The product was then passed through a membrane, precipitated and evaporated to dryness under reduced pressure. The products after evaporation to dryness were subjected to molecular weight identification and anti-Xa, IIa factor activity detection, and the results are shown in Table 2. Among them, the 1# sample is the product obtained by the degradation of heparinase I, with low molecular weight and low anti-Xa and anti-IIa activities, indicating that heparanase I can cut the anticoagulant active center structure of heparin. With the prolongation of cutting time, Most of the active centers are destroyed; 2# sample is the degradation product of heparanase III, which has a larger molecular weight than 1# sample, and the important thing is that t...
Embodiment 3
[0146] Example 3 Comparison of heparinase III and heparanase III, I combined use to degrade heparin
[0147] In order to test the differences in the action sites of heparanase I and heparanase III, the following experiments were designed. First use heparanase III to degrade heparin, degrade it completely at 30°C, and react until A 235 No change occurs, then take out half of the solution, then add heparanase I to it for cleavage, keep the other half of the solution as a control, and finally evaporate the two samples to dryness under reduced pressure. The products after evaporation to dryness were subjected to molecular weight identification and anti-Xa, IIa factor activity detection, and the results are shown in Table 3. Among them, the 2# sample is the product obtained by the degradation of heparanase III (see Example 2), with a molecular weight of 8299; the 3# sample is firstly degraded completely by using heparanase III under the same conditions as in Example 5 at 30°C ,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com