Mutant for improving thermal stability of hexotensin I by increasing disulfide bond and preparation method thereof
A technology of thermal stability and mutant, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as being unable to adapt to industrial application well, poor thermal stability of heparinase I, etc., and achieves reduced screening workload and excellent thermal stability performance. , the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] The present invention will be described in further detail below in conjunction with specific examples. The following examples are only descriptive, not restrictive, and cannot limit the protection scope of the present invention.
[0040] The raw materials used in the present invention, unless otherwise specified, are conventional commercially available products; the methods used in the present invention, unless otherwise specified, are conventional methods in the art.
[0041] Unmarked test methods in the implementation of the present invention, such as the preparation of competent state, heat shock transformation plasmid and Escherichia coli Rosetta (DE3) medium, etc., all belong to routine operation methods, and can refer to the third edition of "Molecular Cloning Experiment Guide" in the method.
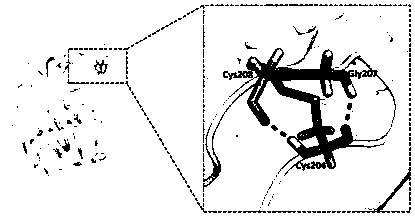
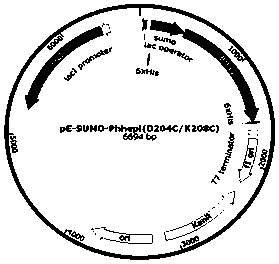
[0042] A mutant BtHepI that improves the thermostability of heparanase I by increasing disulfide bonds D204C / K208C , the amino acid sequence of the mutant is: SEQ ID NO.1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com