Recombinant human adenovirus 3, and preparation method and application thereof
An adenovirus and genome technology, applied in the field of novel enterovirus type 71-recombinant human type 3 adenovirus vaccine candidate strains and their preparation, can solve problems such as application limitations and achieve the effect of preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Bioinformatics analysis predicts the surface exposure region of human adenovirus type 3 hexon
[0021] The template structure of HAd3 hexon homology modeling was searched in Protein Data Bank (PDB) using BLAST-P program, and Modeller9.9 was used for homology modeling to construct a 3D model of HAd3 hexon protein. Experimental Pymol processing of image files. Select the hypervariable region sequence exposed on the surface of the capsid for the next experiment.
[0022] By analyzing the 3D model, the potential neutralizing epitope on the human adenovirus type 3 hexon can be located, and the sequence exposed on the capsid surface is most likely to be replaced without affecting the stability of the hexon structure . HVR1, HVR2, HVR4, HVR5, and HVR7 are potential neutralizing epitope regions, in which some amino acids may be substituted without affecting the stability of the hexon structure. The sequences of the two hypervariable regions are shown below, where t...
Embodiment 2
[0025] Embodiment 2: Construction of recombinant virus vector
[0026]The E3 region deletion shuttle vector was constructed using the HAdv3-gz01 virus strain genome (Genbank accession no. DQ099432) as a template to PCR amplify the 26,782 to 27,736, 973bp E3L fragment (KpnI+ClaI) and the 30 , 900 to 31, 901, the E3R fragment (SpeI+NotI) with a length of 1020bp, the CMV-eGFP-SV40 expression cassette (ClaI+SpeI) was amplified by PCR using the pEGFP C2 vector as a template, and ligated to pBluescript II SK (+ ) vector, the vector pSKE3LCMV-eGFP-SV40E3R (deleting the 3203bp sequence of the E3 region) including the left and right arm sequences of the E3 region was obtained.
[0027] Construction of human type 3 adenovirus backbone plasmid: AdLU1 (5'-GAATTCGCGATCGCTATCTATATAATATACCTTATAGATGG-3', introducing EcoRI and AsisI restriction sites) and AdLD1 (5'-CTGCTGTGGATAAGCTTGAG-3', including HindIII restriction site) were amplified Increase the fragment A3L of 1391bp at the left end o...
Embodiment 3
[0035] Embodiment 3: Recombinant viral vector immunogenicity test
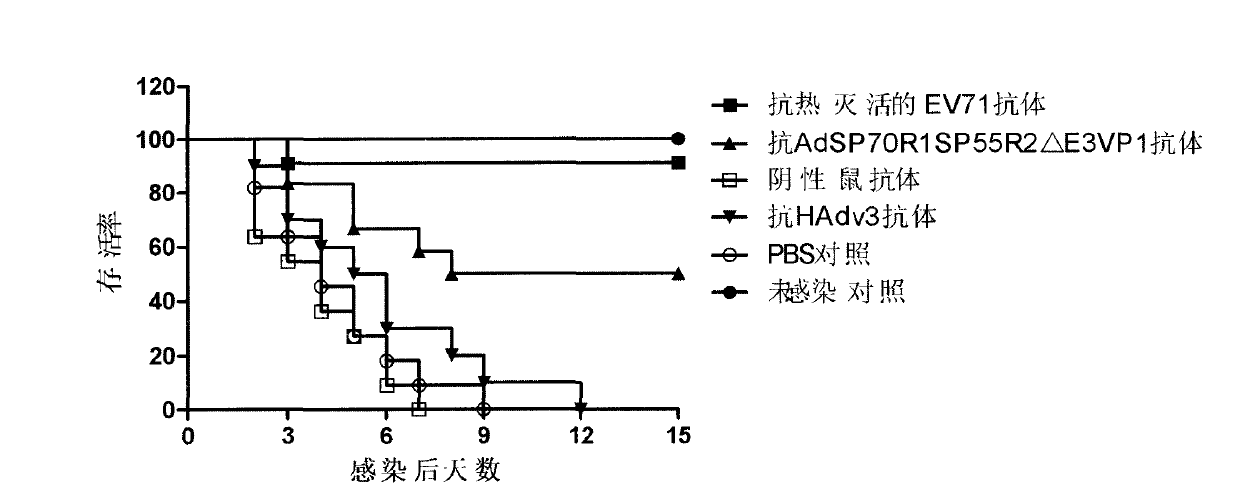
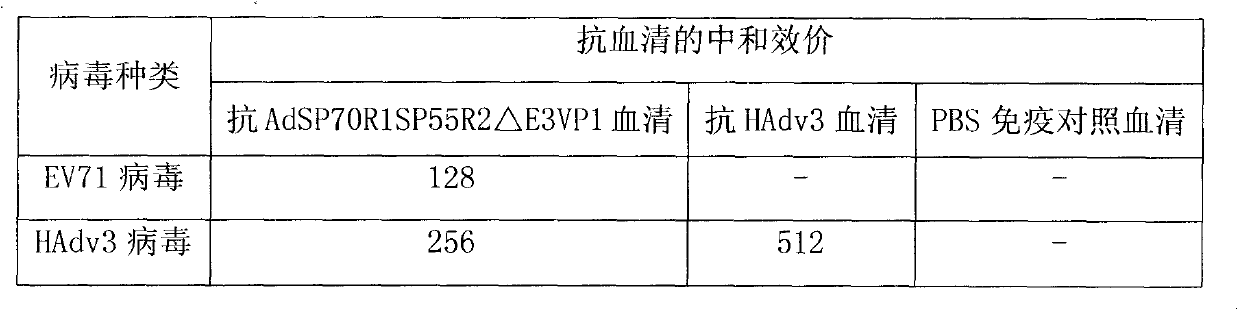
[0036] Antiserum was prepared and serum neutralization test was performed to verify the immunogenicity of the recombinant virus vector. The purified recombinant virus AdSP70R1SP55R2△E3VP1 was injected intramuscularly to immunize mice to obtain polyclonal serum, and then a microneutralization test was performed to detect whether the antibodies produced by the recombinant virus had the ability to neutralize EV71 virus and HAdv3 virus in vitro.
[0037] The experimental results of the neutralization reaction of mouse serum collected 21 days after a single immunization are shown in Table 2. A single immunization can induce high-titer neutralizing antibodies against EV71 virus and anti-HAdv3 virus; The experimental results of the neutralization reaction of the mouse serum collected on the 14th day are shown in Table 3. Table 3 is the neutralization titer of the mouse serum for multiple immunizations. be strengthen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com