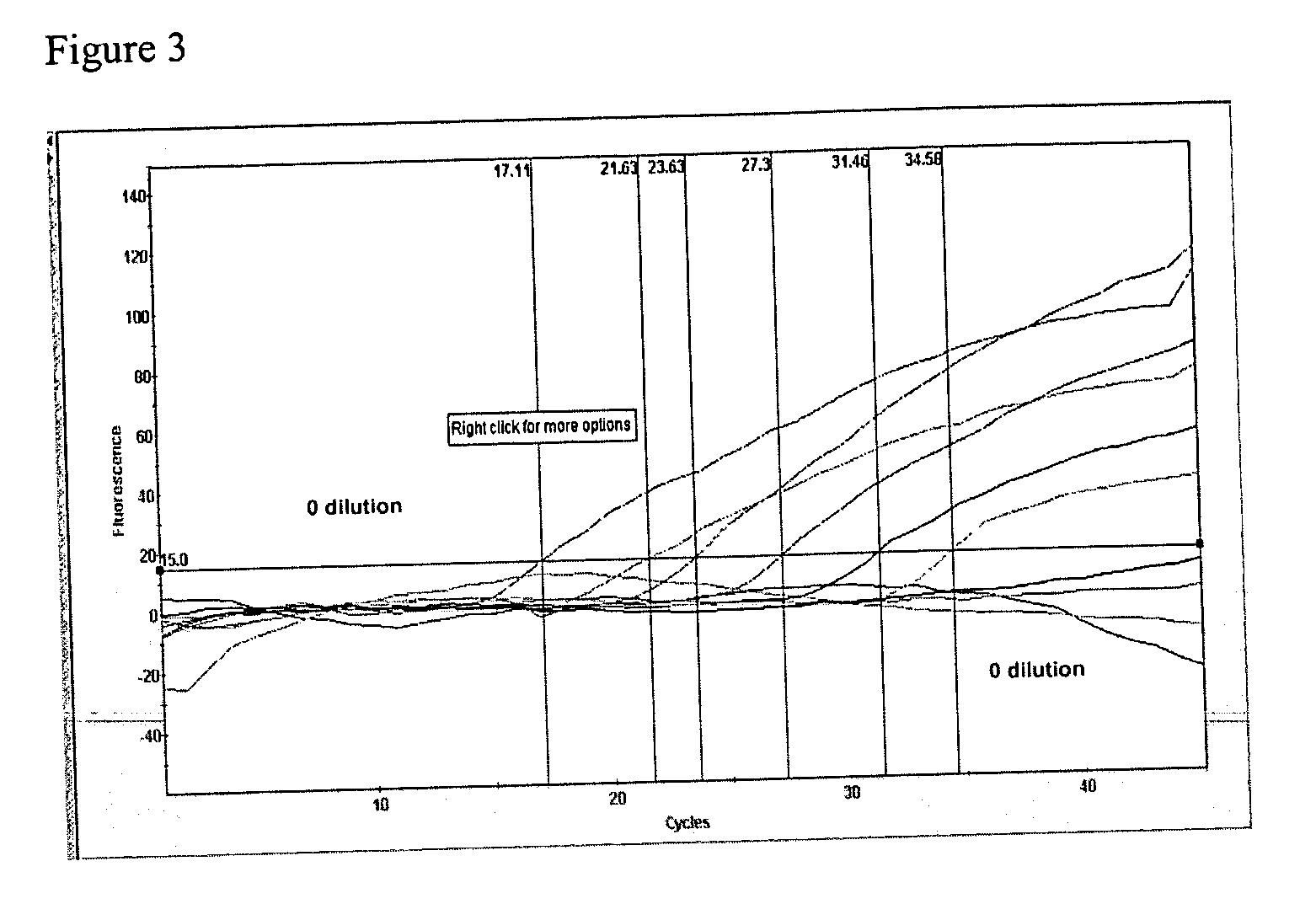
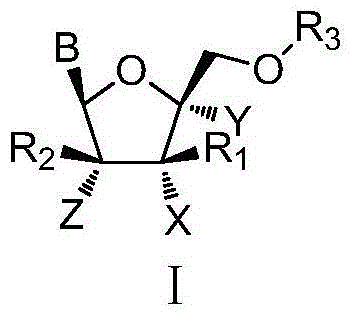
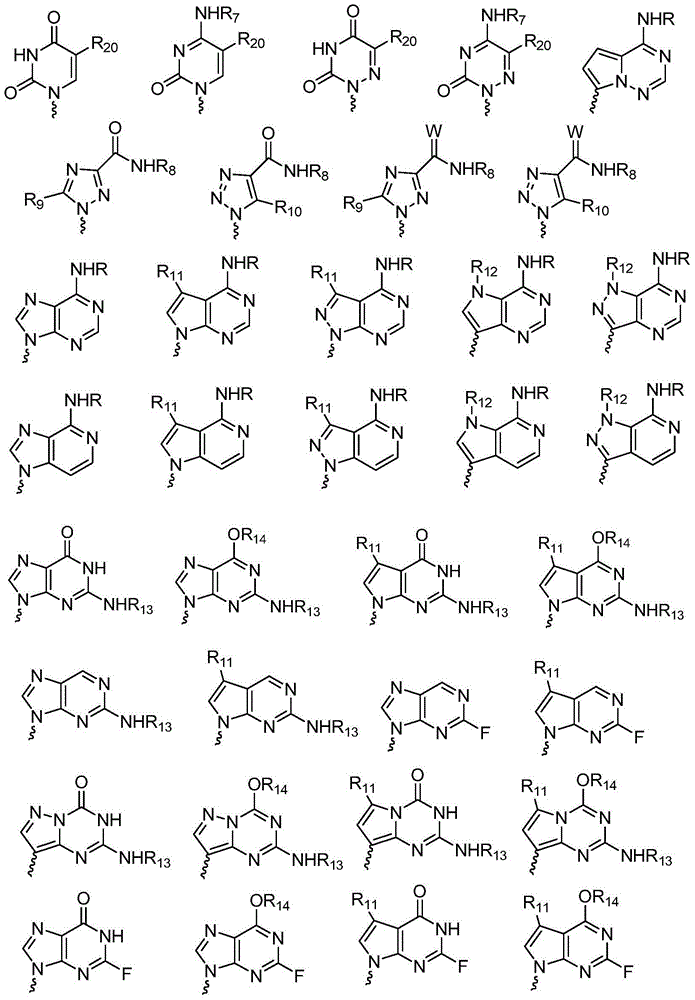
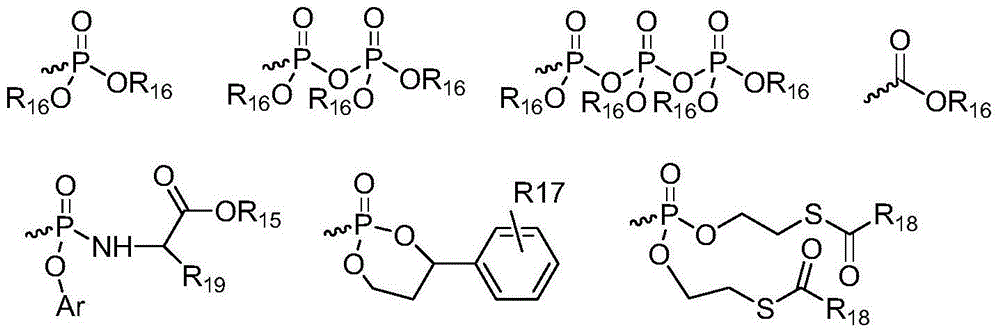
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117 results about "Enterovirus RNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
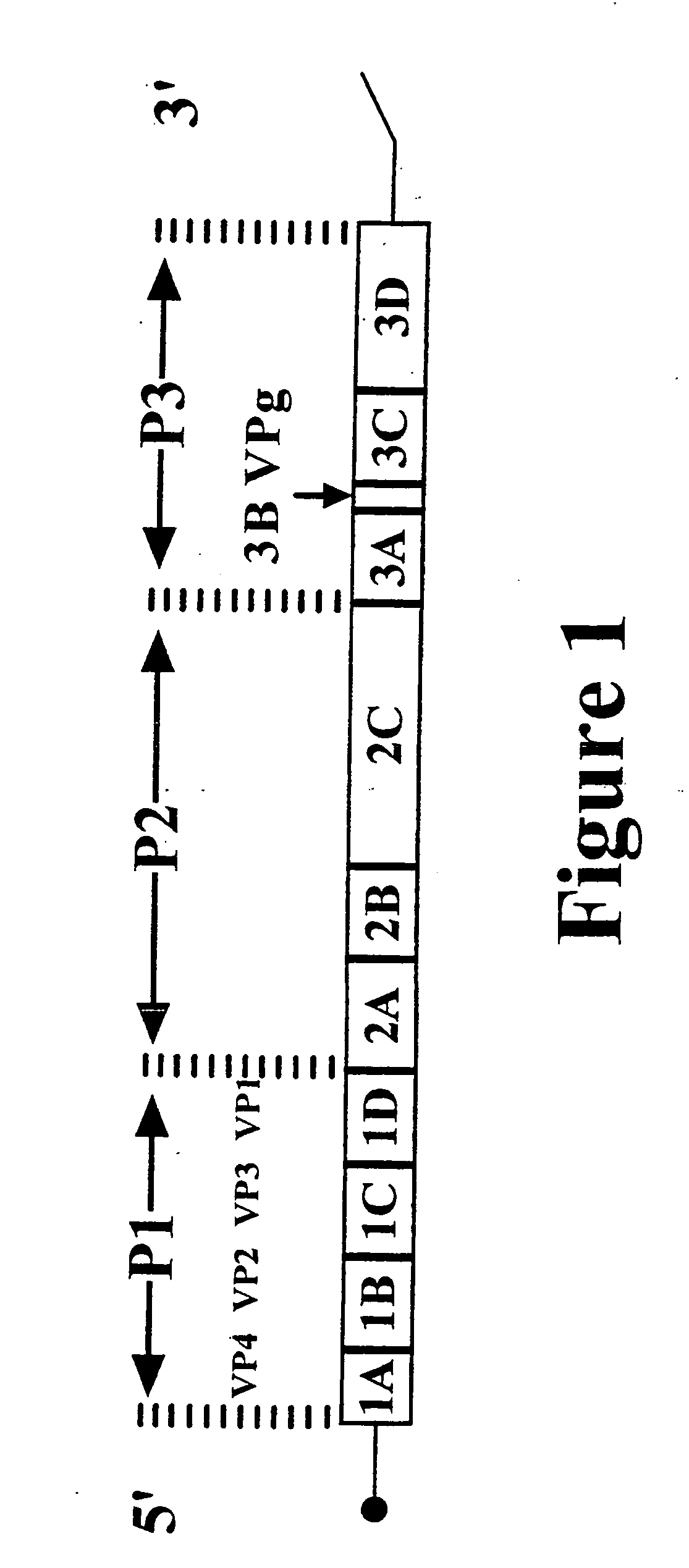
Enteroviruses are a genus of positive-sense single-stranded RNA viruses associated with several human and mammalian diseases. Enteroviruses are named by their transmission-route through the intestine (enteric meaning intestinal).
Antisense antiviral compound and method for treating picornavirus infection
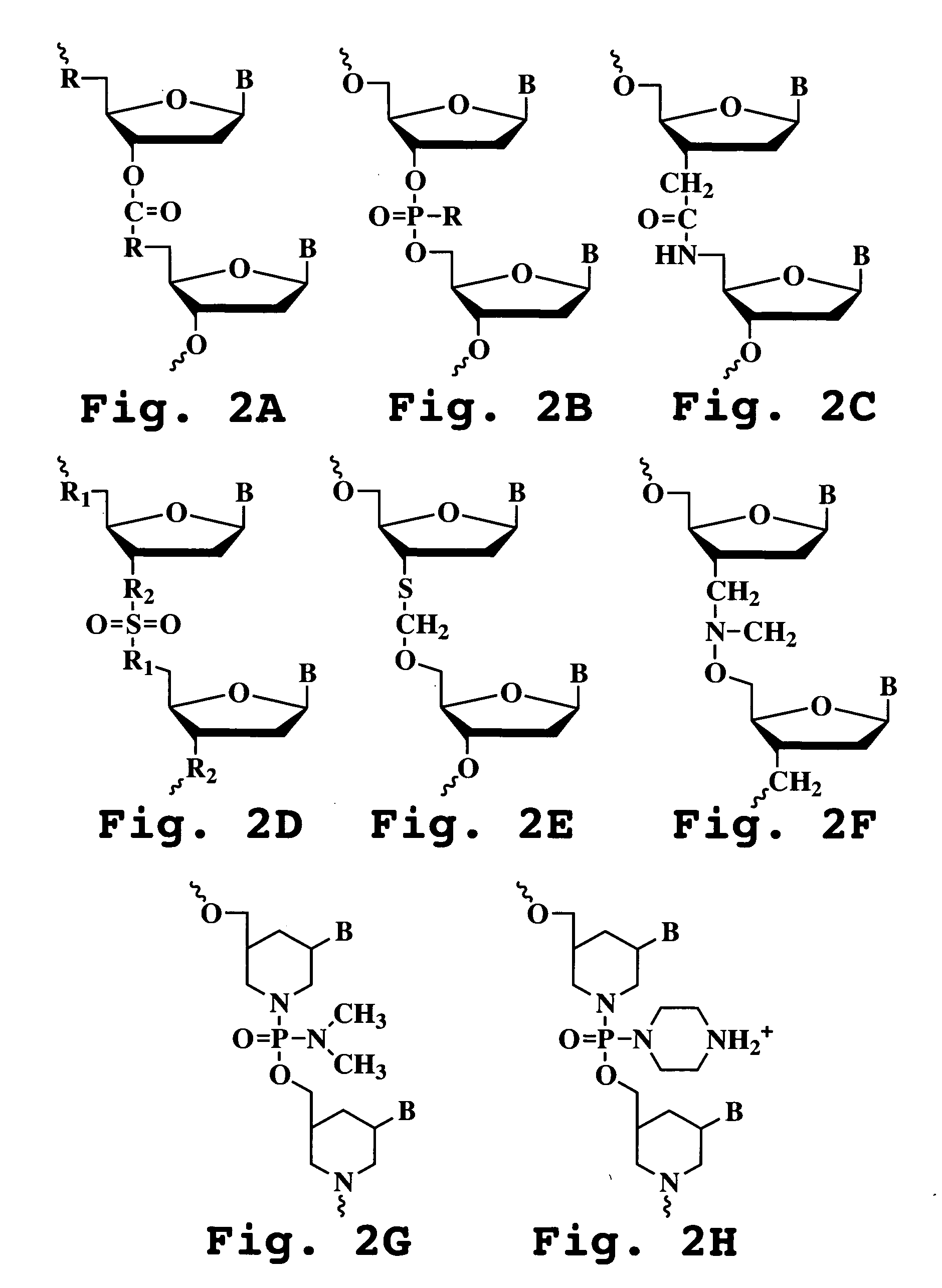
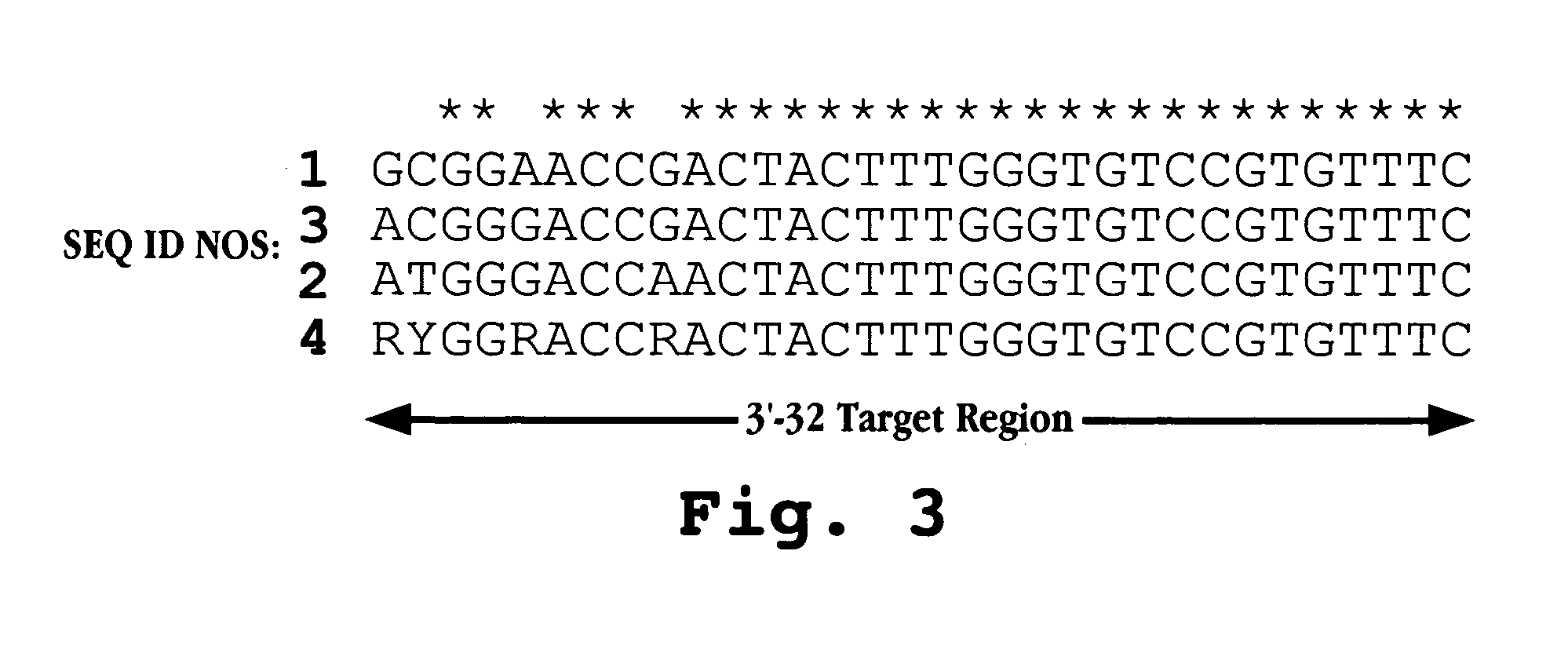
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:7.
Owner:SAREPTA THERAPEUTICS INC
Antisense antiviral compound and method for treating picornavirus infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Picornaviridae family and in the treatment of a viral infection. The compounds are particularly useful in the treatment of Enterovirus and / or Rhinovirus infection in a mammal. The antisense antiviral compounds are substantially uncharged, including partially positively charged, morpholino oligonucleotides have a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with viral RNA sequences within a 32 nucleotide region of the viral 5′ untranslated region identified by SEQ ID NO:4.
Owner:AVI BIOPHARMA
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
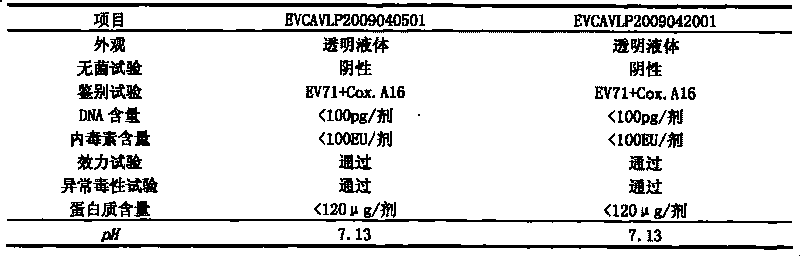
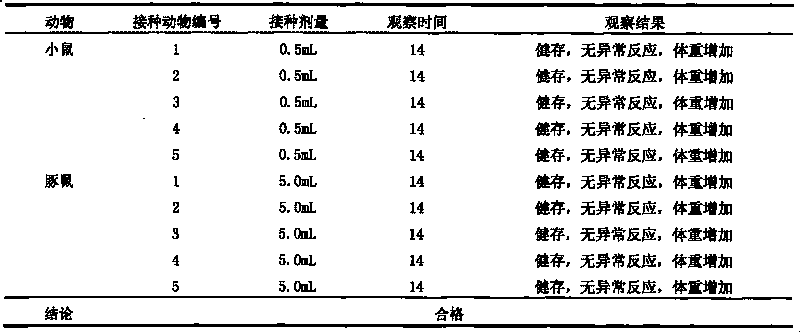
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Nucleic acid sequences for the amplification and detection of respiratory viruses
InactiveUS20100279273A1Improve the situationSsRNA viruses negative-senseSsRNA viruses positive-senseSpecific detectionHuman respiratory virus
The present invention relates to methods of detection, as well as assays, reagents and kits for the specific detection of 15 clinically important respiratory viruses including influenza A and B viruses, human respiratory syncytial viruses, human metapneumoviruses, human enteroviruses, all serotypes of rhinoviruses, 7 serotypes of adenoviruses, parainfluenza viruses types 1, 2, 3, and 4, as well as coronaviruses NL, 229E, OC43, and SARS-CoV. The present invention allows for the detection of each of these respiratory viruses in a single assay.
Owner:UNIV LAVAL
Quantitative real-time assay for noroviruses and enteroviruses with built in quality control standard
InactiveUS20060110724A1Efficient RT-PCRMicrobiological testing/measurementFermentationEnterovirusQuality control
A method is provided for reverse transcription-polymerase chain reaction (RT-PCR) comprising a) amplifying a reverse transcribed cDNA in a mixture comprising Norovirus Genogroup I and Norovirus Genogroup II primers and probes, wherein said Norovirus primers and probes distinguish between Genogroup I and Genogroup II viruses; b) quantifying virus; and c) normalizing data based on a universal internal RNA control. Optionally, the method may also include primers and probes for Enteroviruses. A reaction mixture comprising Norovirus Genogroup I and Norovirus Genogroup II primers and probes, wherein said Norovirus primers and probes distinguish between Genogroup I and Genogroup II viruses and universal internal RNA control primers and probes are also included.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE US SEC DEPT OF
L-nucleoside compounds and application thereof
InactiveCN105646629AInhibitory activityOrganic active ingredientsSugar derivativesEbola virusRNA Virus Infections
The invention discloses L-nucleoside compounds having the structure characteristic represented by the formula (I) or pharmaceutically acceptable salts thereof, and belongs to the technical field of pharmaceutical chemistry. The compounds can inhibit the activity of RNA viral polymerase, so the compounds can be used as potential drugs for prevention and treatment of infection of RNA viruses such as HCV, influenza virus, HRV (rhinovirus), RSV, Ebola virus, dengue virus, intestinal virus and the like.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Antigens and Vaccines Directed Against Human Enteroviruses
The instant invention provides materials and methods for producing immunologically active antigens derived from members of the Picornaviridae virus family. The picornavirus antigens of the invention may be in a form for use as a vaccine administered to a subject in a therapeutic treatment or for the prevention of a picornavirus infection. The picornavirus antigens of the invention may be in the form of an immunogenic composition for use in vaccines which are administered for the prevention of an Enterovirus infection. The instant invention further encompasses immunogenic compositions comprising Human enterovirus A, Human enterovirus B, Human enterovirus C, Human enterovirus D antigens and their use in vaccines for the prevention of an Enterovirus infection.
Owner:SENTINEXT THERAPEUTICS
Oligonucleotide chip capable of detecting five enteroviruses simultaneously and application thereof
InactiveCN101654713AStrong specificityHigh compliance rateNucleotide librariesMicrobiological testing/measurementFluorescenceOligonucleotide chip
The invention discloses an oligonucleotide chip capable of detecting five enteroviruses simultaneously and an application thereof. The chip comprises a substrate, probes of pathogens of five enteroviruses coated on the substrate, negative contrast, positive contrast and blank contrast, wherein the probes of pathogens are five 54-70mer of HAV-P, ROV-P, NOV-P, ASV-P and ADV-PV1 probes. The chip of the invention adopts multiple PCR to amplify a plurality of target sequences of pathogens simultaneously and uses the downstream primer Tamara labeling method to perform fluorescence labeling to PCR product, the labeled PCR product is hybridized with the chip to realize the accurate detection of pathogens, the detection sensitivity is equal to that of PCR and the specificity is high. The detectionefficiency and accuracy are obviously shortened. The technology system of the invention is applicable to fields such as sea water and marine life specimens monitoring, food hygiene surveillance, customs quarantine, related clinical detection and the like.
Owner:SHANDONG MEDICAL BIO TECH RES CENT +1
Human embryo lung fibroblast strain and method for using human embryo lung fibroblast strain for producing hand-foot-mouth viral vaccine
InactiveCN102911910AAvoid Residual EffectsReduced purification stepsAntiviralsEmbryonic cellsEmbryoViral Vaccine
The invention provides a novel human embryo lung diploid fibroblast strain Walvax-2, CCTCCC201055. The cell strain is sensitive to main epidemic disease viral strains-coxsackievirus 16-type COX.A16 in a group A and enterovirus 71-type EV71 second-strain virus of a hand-foot-mouth disease, and virus output is high. The invention further provides a method for preparing a divalent hand-foot-mouth virus inactivated vaccine and application of the human embryo lung diploid fibroblast strain in preparation of the hand-foot-mouth virus inactivated vaccine. The divalent inactivated vaccine produced by the human embryo lung diploid fibroblast strain can effectively prevent hand-foot-mouth disease.
Owner:云南沃森生物技术股份有限公司
Methods for producing virus for vaccine production
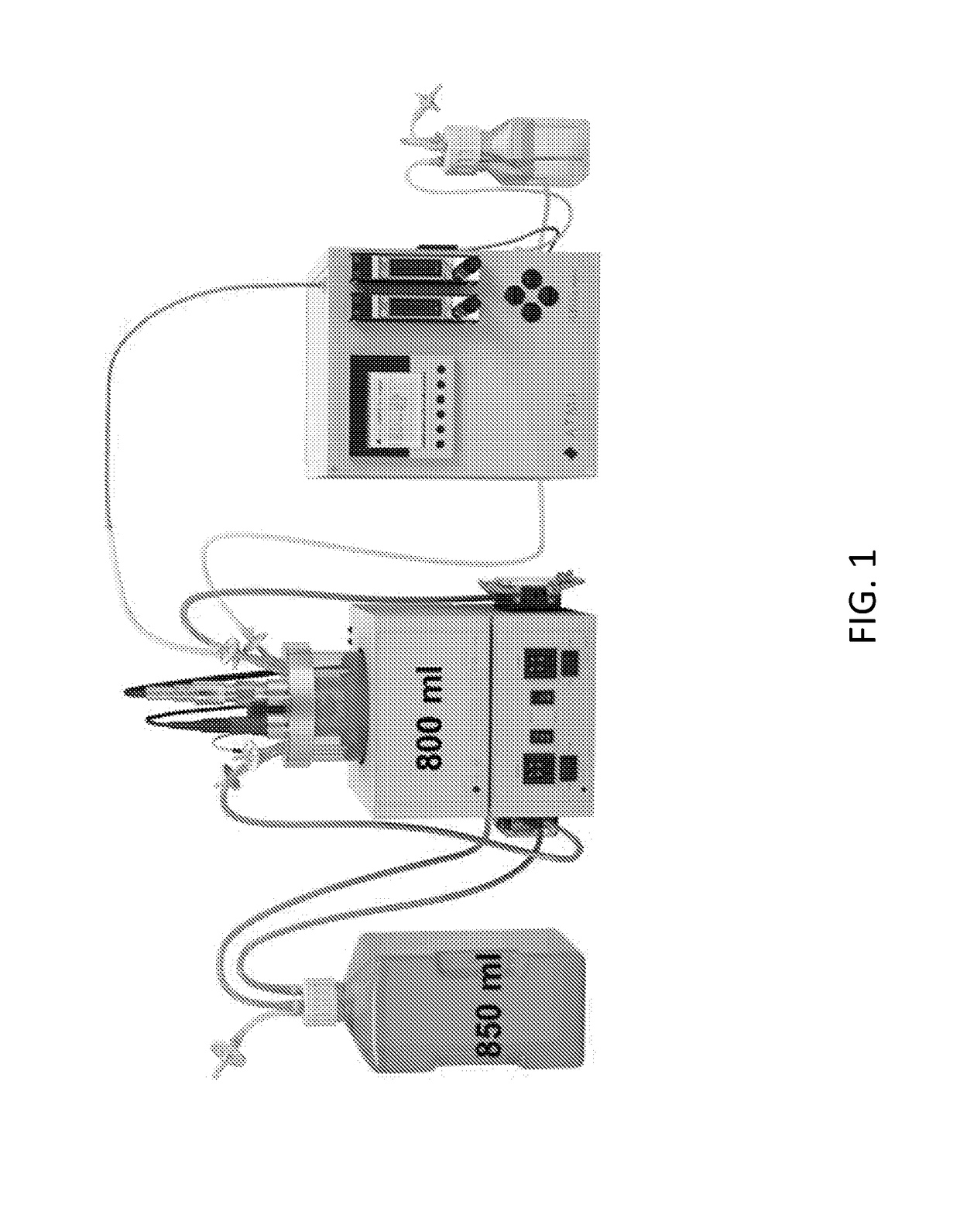
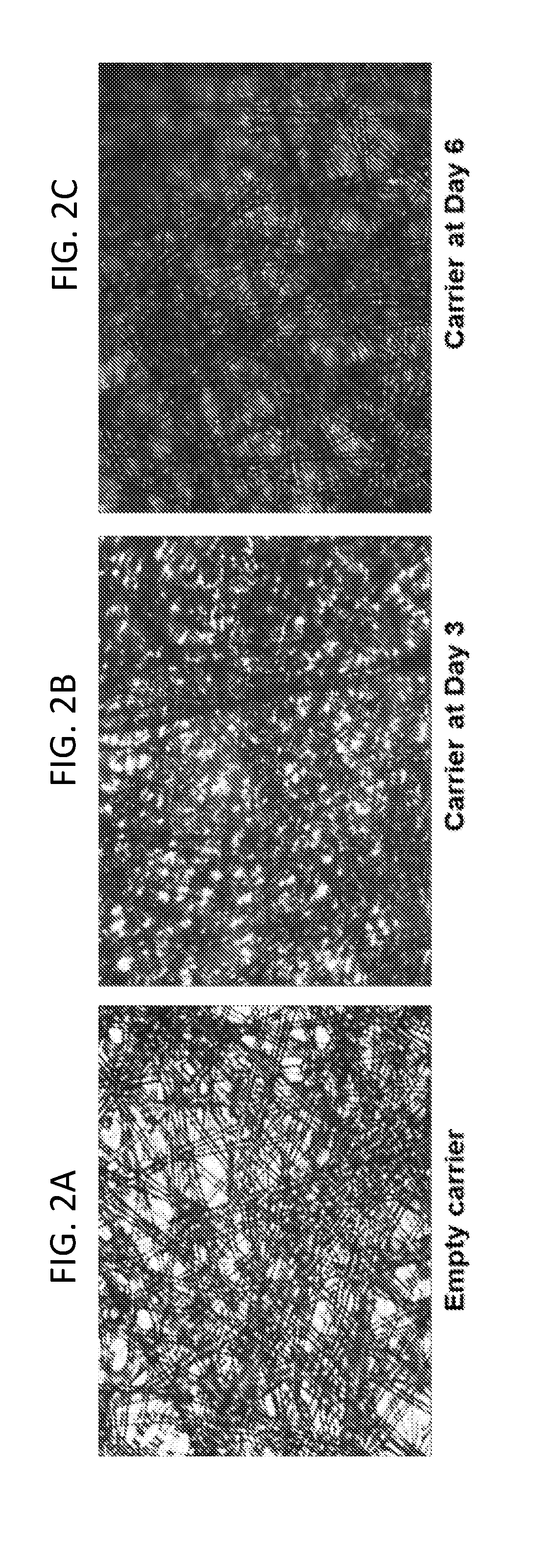
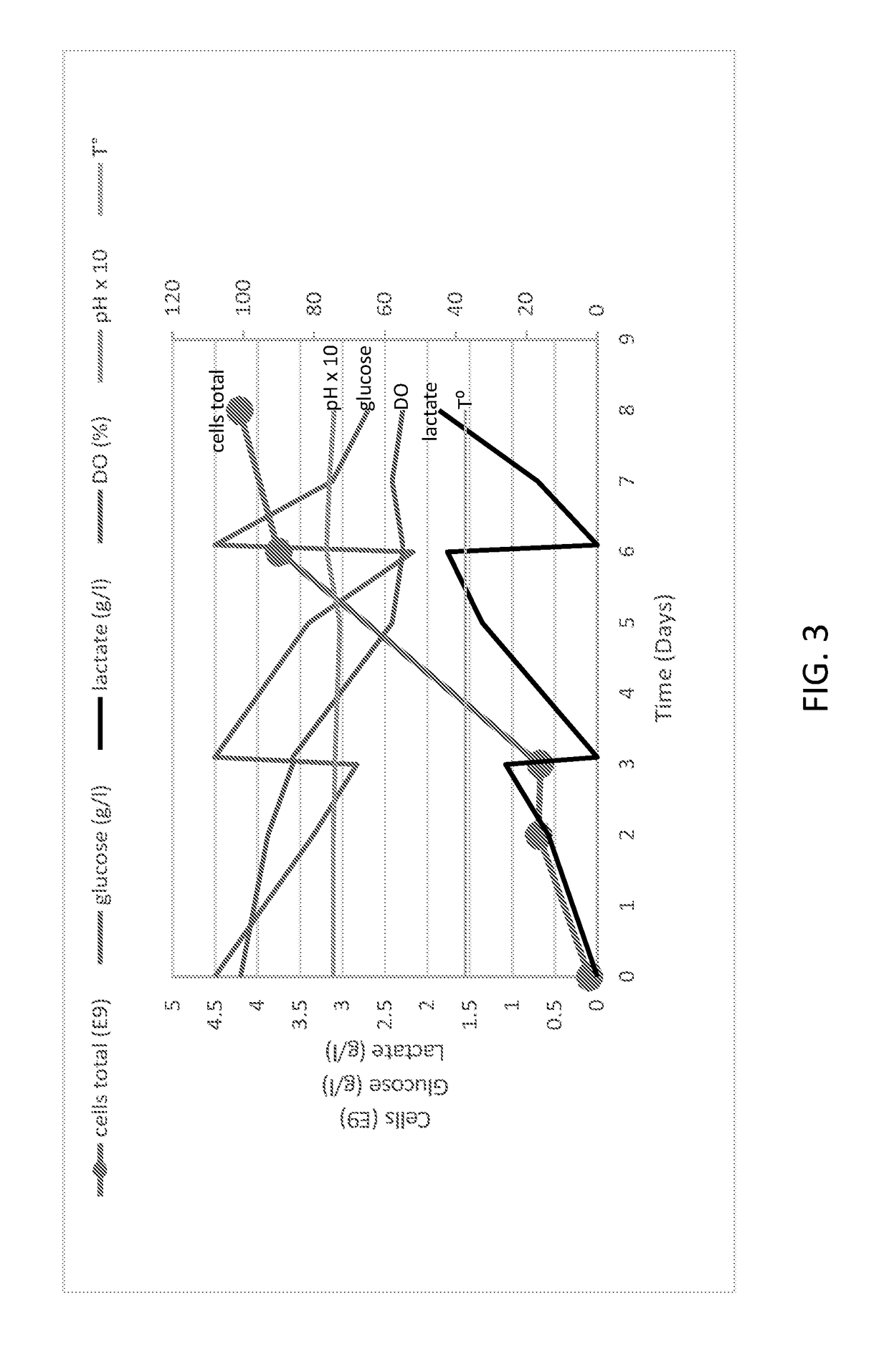
InactiveUS20180195048A1Enhanced viral productionImprove cost efficiencySsRNA viruses positive-senseViral antigen ingredientsCulture cellVaccine Production
The present disclosure relates to methods of producing Enterovirus A, e.g., for vaccine production, that include culturing cells in a fixed bed bioreactor. Further provided herein is an Enterovirus A produced by the methods of production disclosed herein, as well as compositions, immunogenic compositions, and vaccines related thereto.
Owner:TAKEDA VACCINES INC
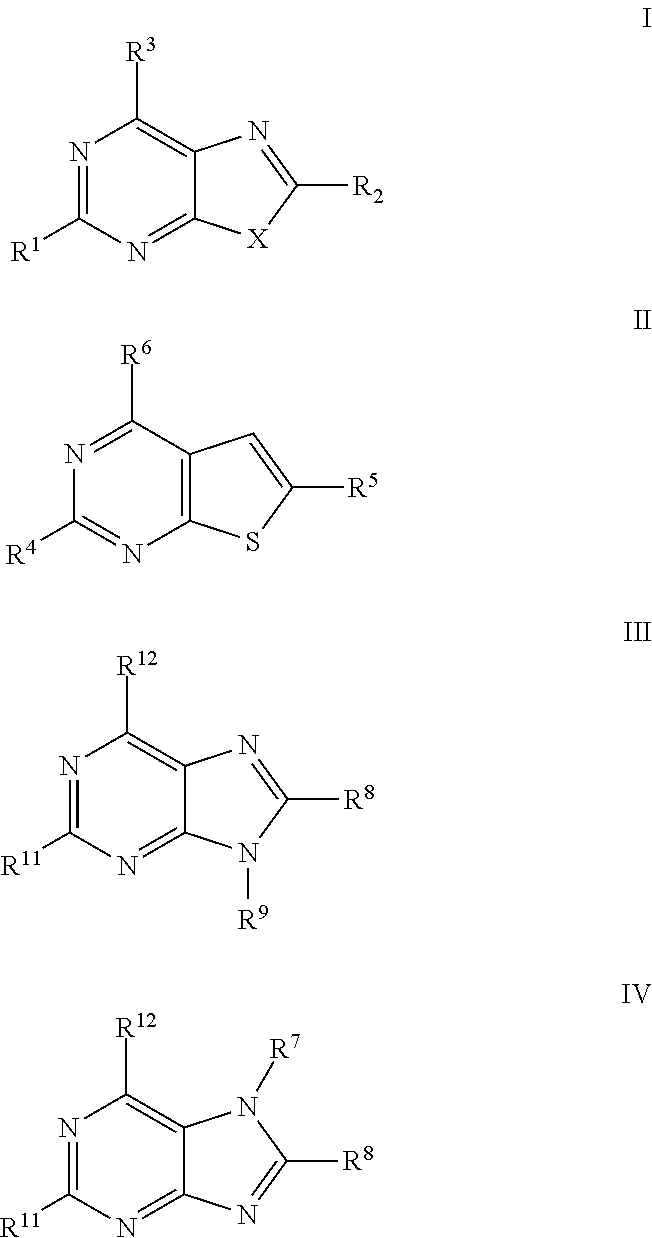
Antiviral Activity of Novel Bicyclic Heterocycles
The present invention relates to compound of Formula I, II, III, or IV, and / or a pharmaceutical acceptable addition salt thereof and / or a stereoisomer thereof and / or a solvate thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R11, and R12 are as defined in the claim 1 or as described in detail in the description of the invention, and to the use of said compounds to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, particularly infections with RNA-viruses belonging to the family of the Retroviridae, the family of the Flaviviridae and the family of the Picornaviridae and more preferably infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus. The present invention also relates to pharmaceutical compositions of said compounds and the use of said pharmaceutical compositions to treat or prevent viral infections. The present invention further relates to the use of said compounds as biologically active ingredients, more specifically as medicaments for the treatment of viral disorders and pathologic conditions such as, but not limited to, viral infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus.
Owner:KATHOLIEKE UNIV LEUVEN
Composition of starwort sulphonic acid or vitriolic acid polyoses ester total phenolic glycoside and method of preparing the same and antiviral application
The invention relates to a kind of natural medicine of broad spectrum antibiotic. At present, the broad spectrum antibiotic medicine with high effect and safety is at shortage all round the world. The invention is intended to extract laminarinsulphate or sulphonic acid sugar ester or sulphosalts from plant chickweed or other chickweed plant with two resin adsorption methods or a water extraction and alcohol precipition method. The spectrum antibiotic in the invention is distributed under 50,000 in the formula weight formed by carbon glycosidic bond and / or oxide glycosidic bond with phenol, especially the total flavones comprising apigenin. However, the invention mainly acts as total phenolic glycoside with the formula weight under 4,000. Besides, the invention can form brownish compound with the total flavones comprising apigenin and the glycosidic ingredients without sulfur element, so as to be applied as broad spectrum antibiotic drug. Therefore, the compound in the invention can be applied to cure ADIS virus, hepatitis virus, influenza virus and parainfluenza virus comprising SARS, adenovirus, verruca acuminate virus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus and varicella. No toxic effect has been found in the application. What is more, the invention can be made into 10 sorts of formulation, disinfector and health-improving products.
Owner:朱耕新
Hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof
InactiveCN102190725ASignificant effectImprove survival rateImmunoglobulins against virusesAntiviralsTotal proteinPasteurization
The invention relates to a hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof in pharmacy. The preparation method comprises the following steps of: separating component I+II+III, I+III and II precipitates in turn by using a low-temperature methanol protein separation method; performing pasteurization on a component II precipitate; refining and purifying; performing dealcholization; preparing; and sterilizing, packaging and performing low-pH incubated inactivation. The product has antibody titer of not less than 1:640 for enteroviruses (including one or more of coxsackie virus, Echo and EV71), the immunoglobulin content is not less than 95.0 percent of the total protein content, and the sum of IgG monomer and dimer is not less than 95 percent; and the using method is that a specific antibody with titer of 160,000-320,000 is intravenously infused. The invention is suitable for industrial production; and the product has high-titer enterovirus resistant specificity, is safe and reliable, and can become an effective medicine for treating the hand-foot-and-mouth disease.
Owner:HUALAN BIOLOGICAL ENG INC
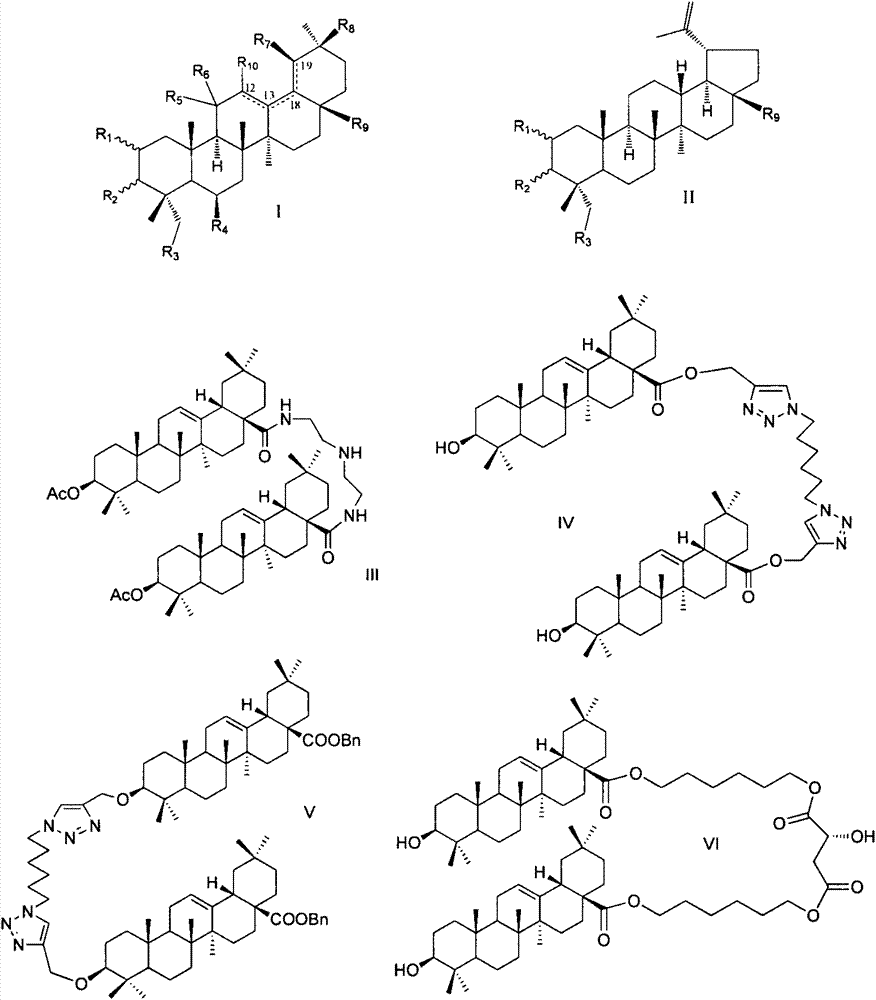
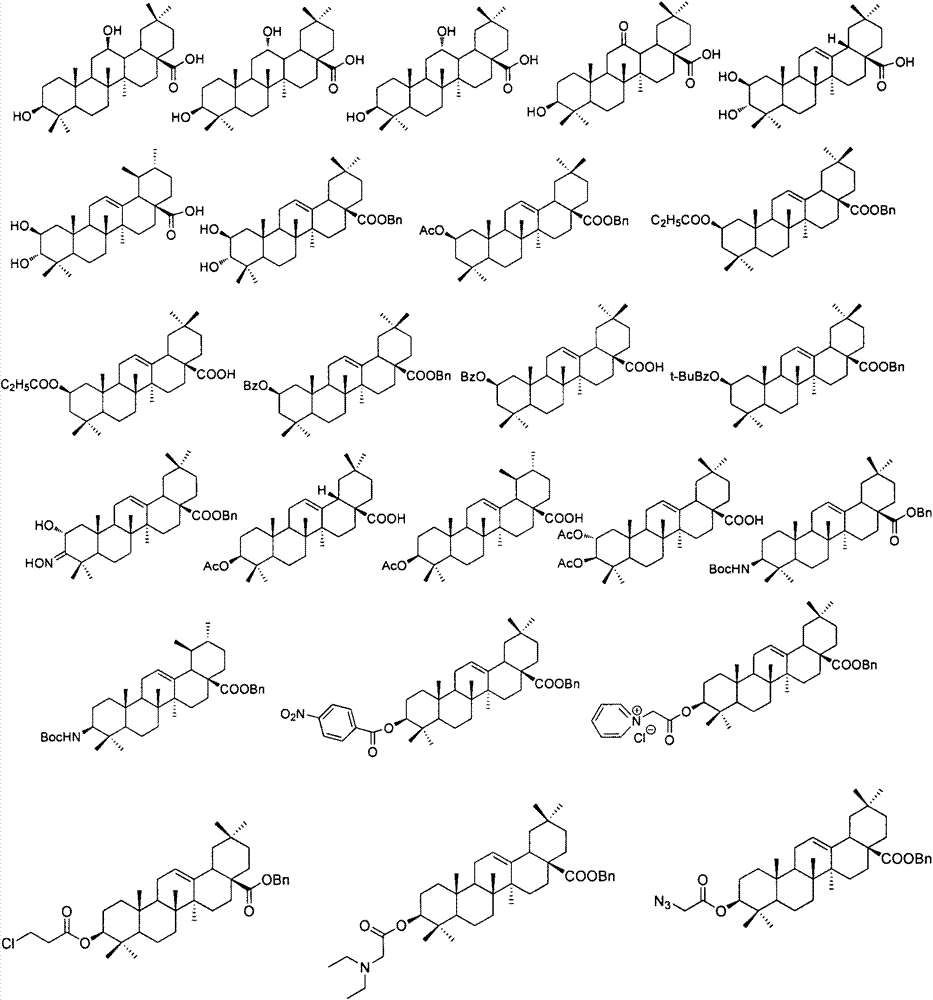
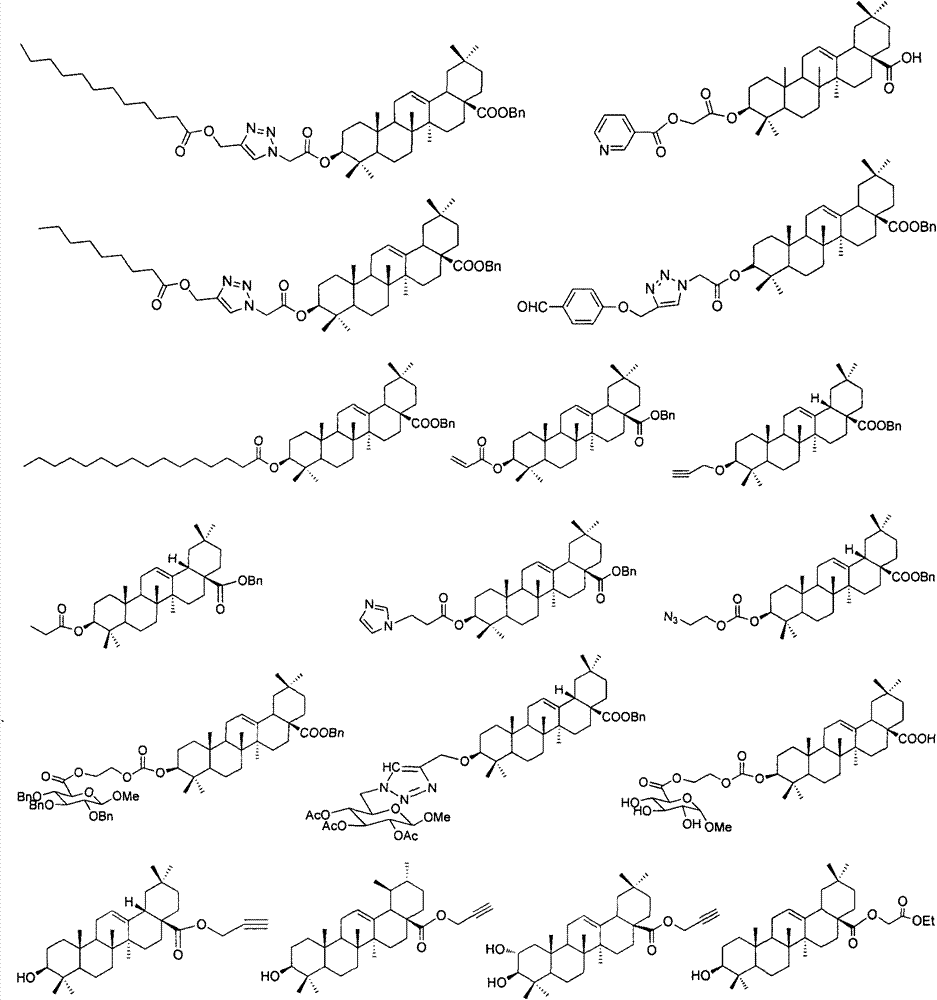
Pentacyclic triterpene enterovirus EV71 inhibitors, and medicinal compositions and medicinal use thereof
InactiveCN104840467AOrganic active ingredientsNervous disorderAcute hyperglycaemiaHand-foot-and-mouth disease
The invention relates to the pharmacy field, concretely relates to a use of a series of pentacyclic triterpene compounds as enterovirus EV71 inhibitors, and especially relates to an application in the preparation of medicines for preventing and treating EV71 infection induced hand-foot-and-mouth diseases and complications thereof, such as synanche, myocarditis, pulmonary edema, encephalitis, herpes, septicemia, hypertension, hyperglycemia, cognitive function disorder, poliomyelitis-like paralysis and many nerve system associated diseases. The invention also discloses medicinal compositions of the series of the pentacyclic triterpene compounds as enterovirus EV71 inhibitors.
Owner:CHINA PHARM UNIV
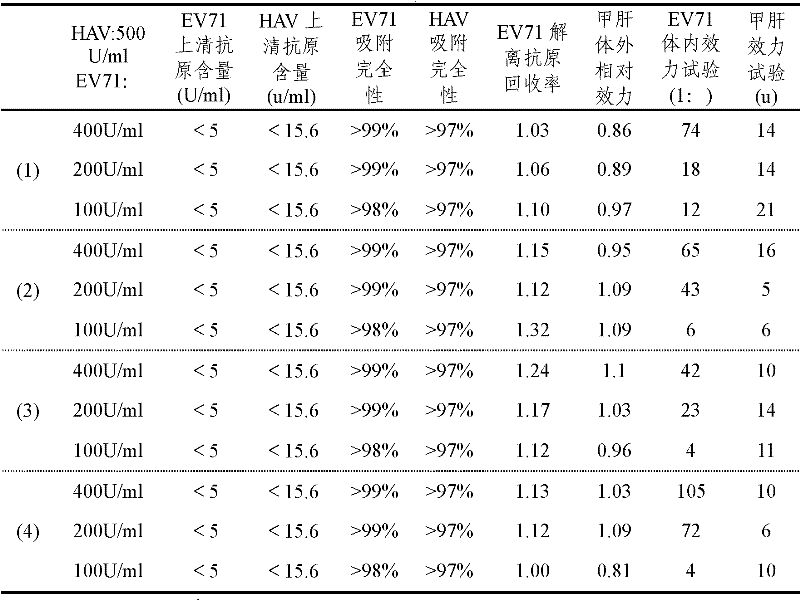
Kits for extracting enterovirus RNA and corresponding method for extracting and purifying enterovirus RNA
ActiveCN102839169AMicrobiological testing/measurementMicroorganism based processesMagnetic beadSodium sulfate
The invention firstly provides a kit for extracting enterovirus RNA. The kit comprises the following components: 1 RNA extraction solution I containing lauryl sodium sulfate, triton, guanidinium isothiocyanate and 100-400mu g / ml magnetic bead; 2, RNA extraction solution II containing 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid and sodium chloride; 3, RNA extraction solution III containing triton and sodium chloride; and RNA extraction solution IV: silicone oil. The kit for extracting enterovirus RNA can work together with an automatic instrument to quantitatively detect enterovirus RNA, has high RNA extraction yield and high detection sensitivity, is capable of quickly and accurately detecting RNA concentration in various samples and provides reliable experiment basis for early diagnosis of various pathogene infections. The invention also provides a magnetic bead RNA extraction kit containing RNA eluant and a kit for extracting RNA by one-step method. The three kits provided by the invention all can effectively extract enterovirus RNA of different types of samples containing various repressors, such as anal swab, excrement, throat swab.
Owner:SANSURE BIOTECH INC
Typing of human enteroviruses
The present invention discloses a method for detecting the presence of an enterovirus in a clinical sample. The invention additionally discloses a method for typing an enterovirus in a clinical sample. Both methods employ a set of primer oligonucleotides for reverse transcription and amplification that hybridize to conserved regions of the enterovirus genome, and that provide amplicons that include significant portions of the VP1 region that are characteristic of the various serotypes. In the typing method, the invention further provides a database consisting of nucleotide sequences from prototypical enteroviral serotypes, which is used to type the clinical sample by comparing the sequence of its amplicon with each prototypical sequence in the database. The invention additionally provides mixtures of primer oligonucleotides, and a kit for use in conducting the typing method that includes a mixture of the primer oligonucleotides,
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Recombinant human adenovirus 3, and preparation method and application thereof
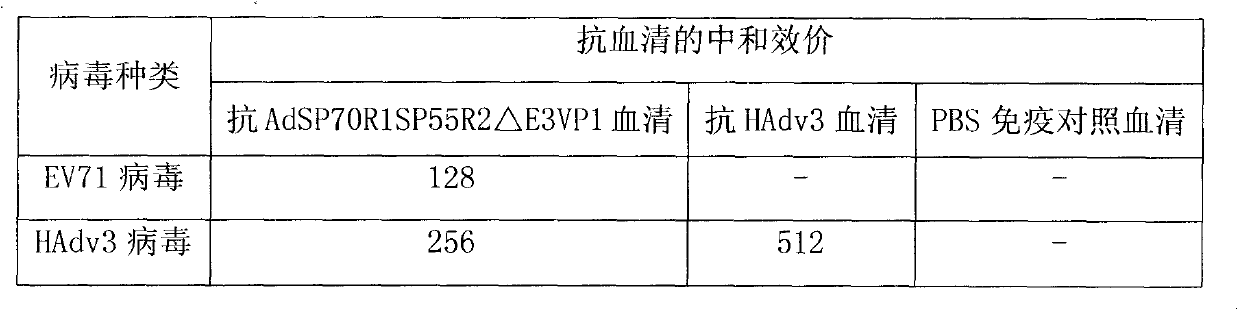
InactiveCN103966263ARetain major antigenic activityAvoid infectionMicroorganism based processesFermentationEnterovirus 71Genome
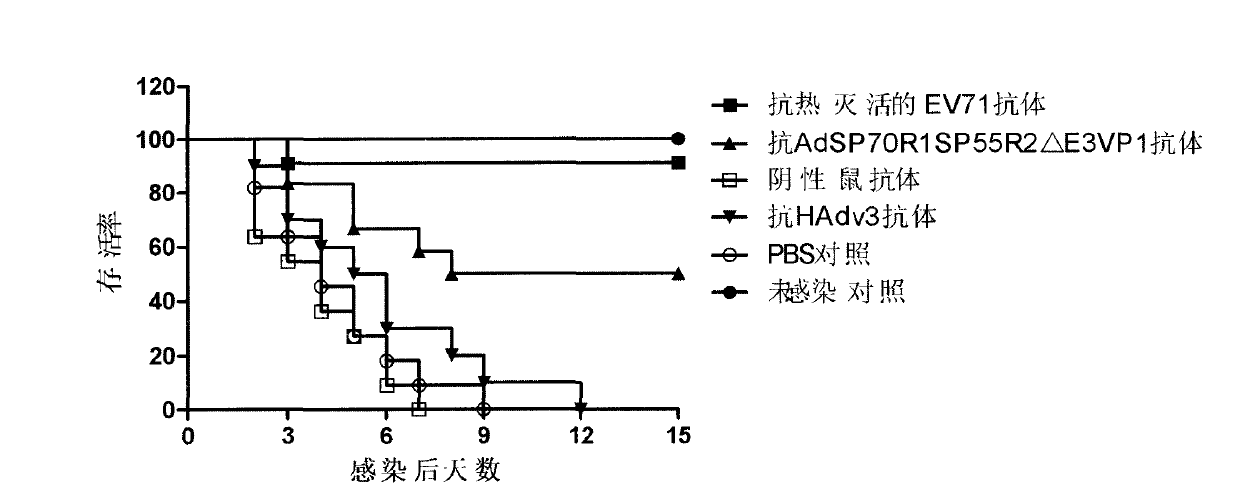
The invention discloses a novel enterovirus 71-recombinant human adenovirus 3 vaccine candidate strain with human adenovirus 3 (HAdv3) as a carrier, and a preparation method thereof. Two EV71 neutralizing epitopes are embedded to the hexon of the human adenovirus 3, and the VP1 protein cassette of EV71 is inserted to the genome E3 region of the human adenovirus 3. The vaccine candidate strain can induce a strong anti-EV71 infection and anti-HAdv3 infection immunization reaction, and can be used for making bivalent vaccines for preventing the EV71 infection and the HAdv3 infection.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
Quantitative determination process for enterovirus in environment water body
InactiveCN101333568AQuantitatively accurateHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceConserved sequenceFluorescence
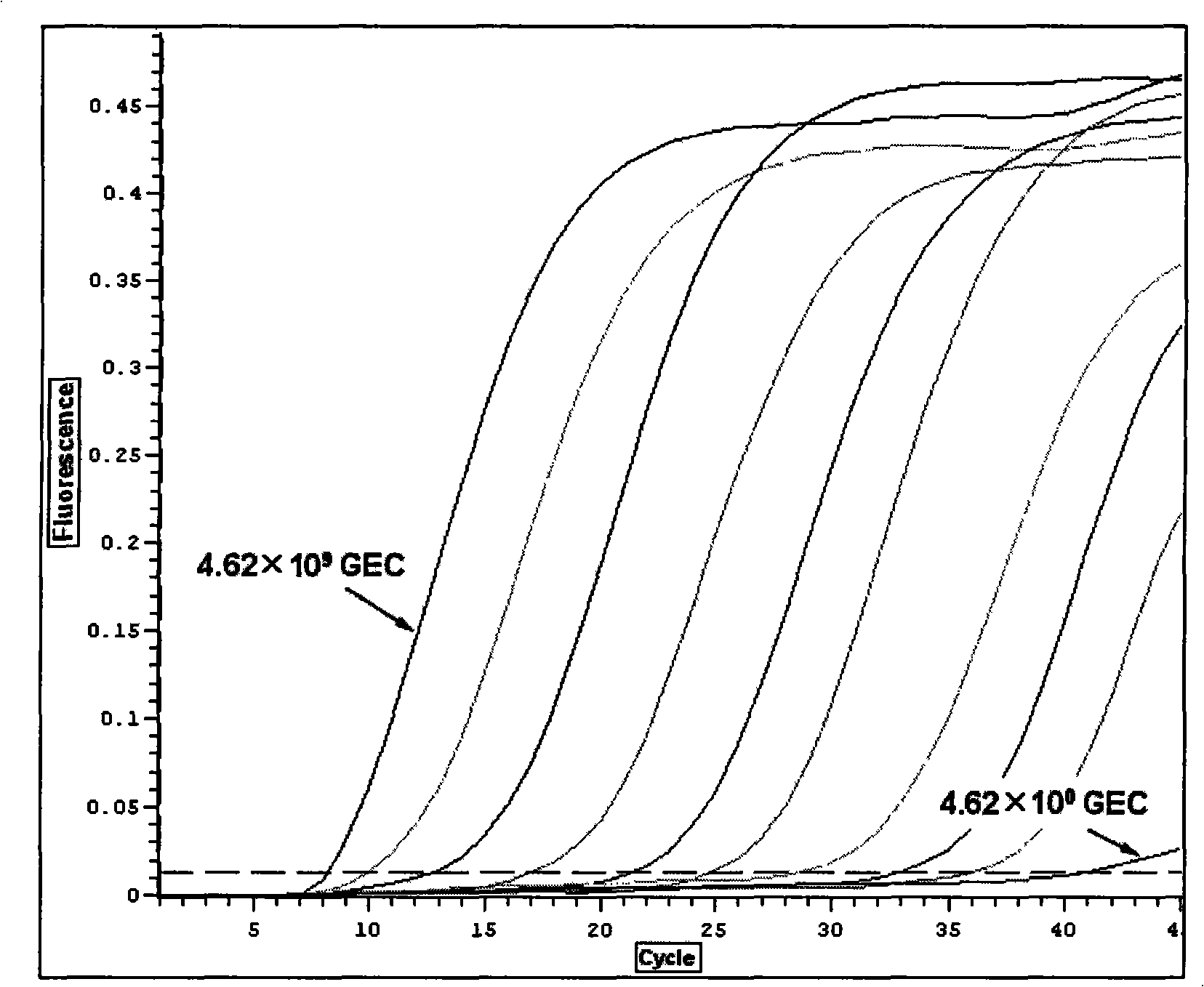
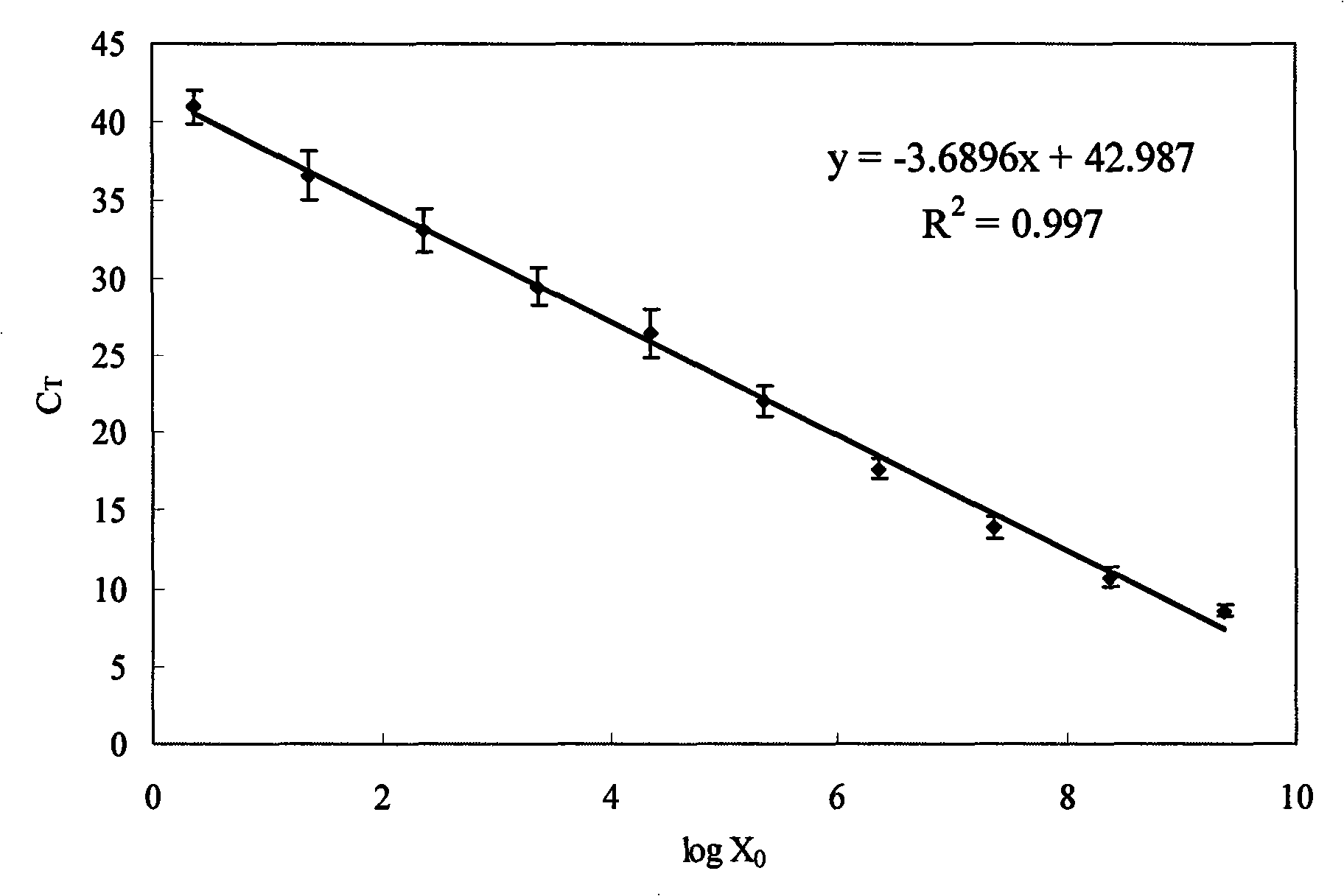
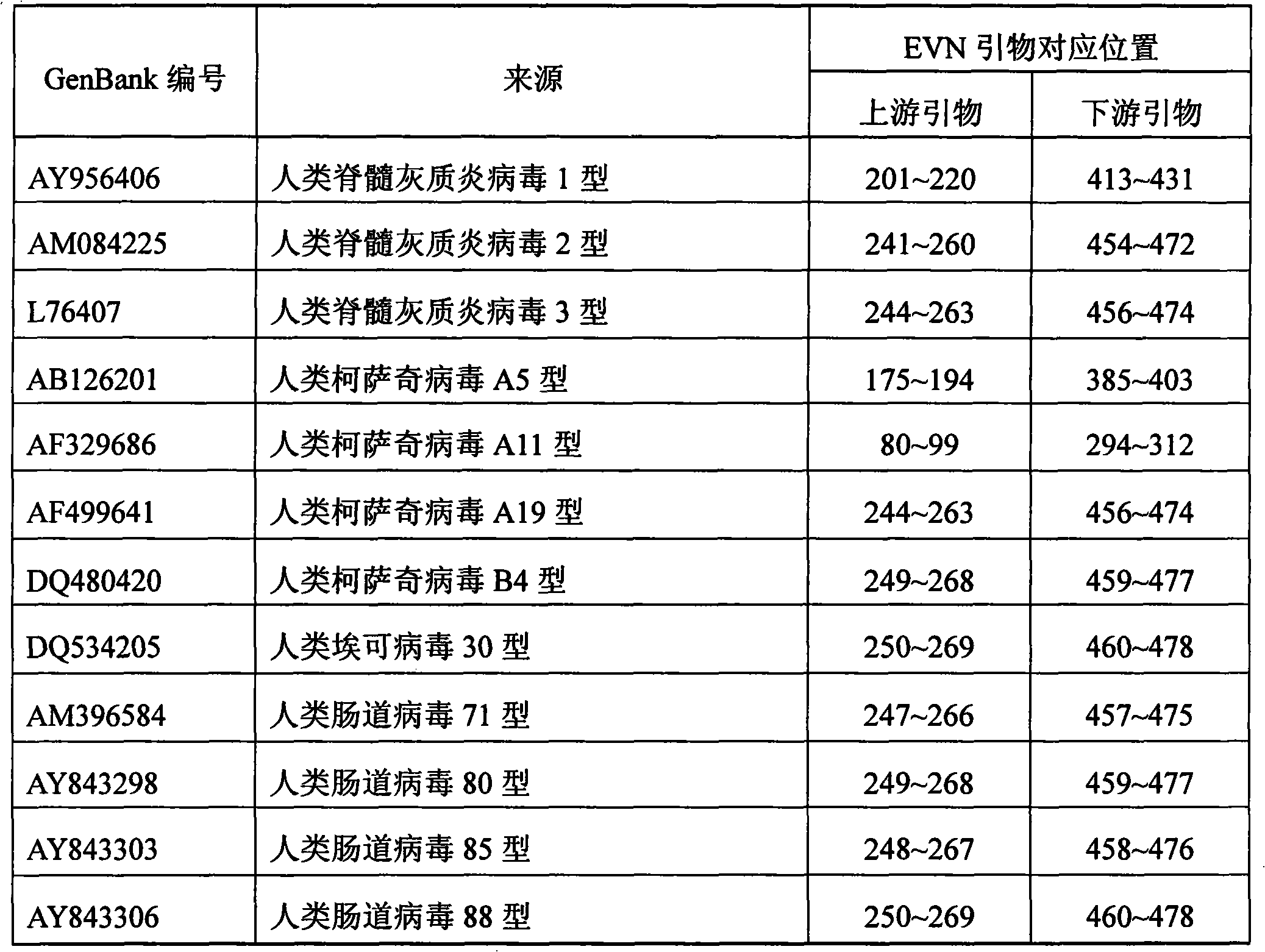
The invention discloses a quantitative detection method of enteroviruses in environmental water, according to the homology of a conserved sequence at an RNA5` non-coding region of enteroviruses and on the basis of the existing primers of EV1 and EV2, the method further designs a pair of enterovirus universal primers EVN, prepares a series of recombinant plasmids adopting gradient dilution as standard substances, and builds a real-time fluorescence quantitative PCR reaction system applying the enterovirus universal primers EVN, so as to quantitatively detect the enterovirus content in the environmental water. The method has advantages of accurate quantification, greatly improving the sensitivity of the enterovirus qualitative RT-PCR detection technique in the current environmental water, broad linearity range, high precision, good specificity, and having no cross reaction with other common pathogenic microorganisms in the environment.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
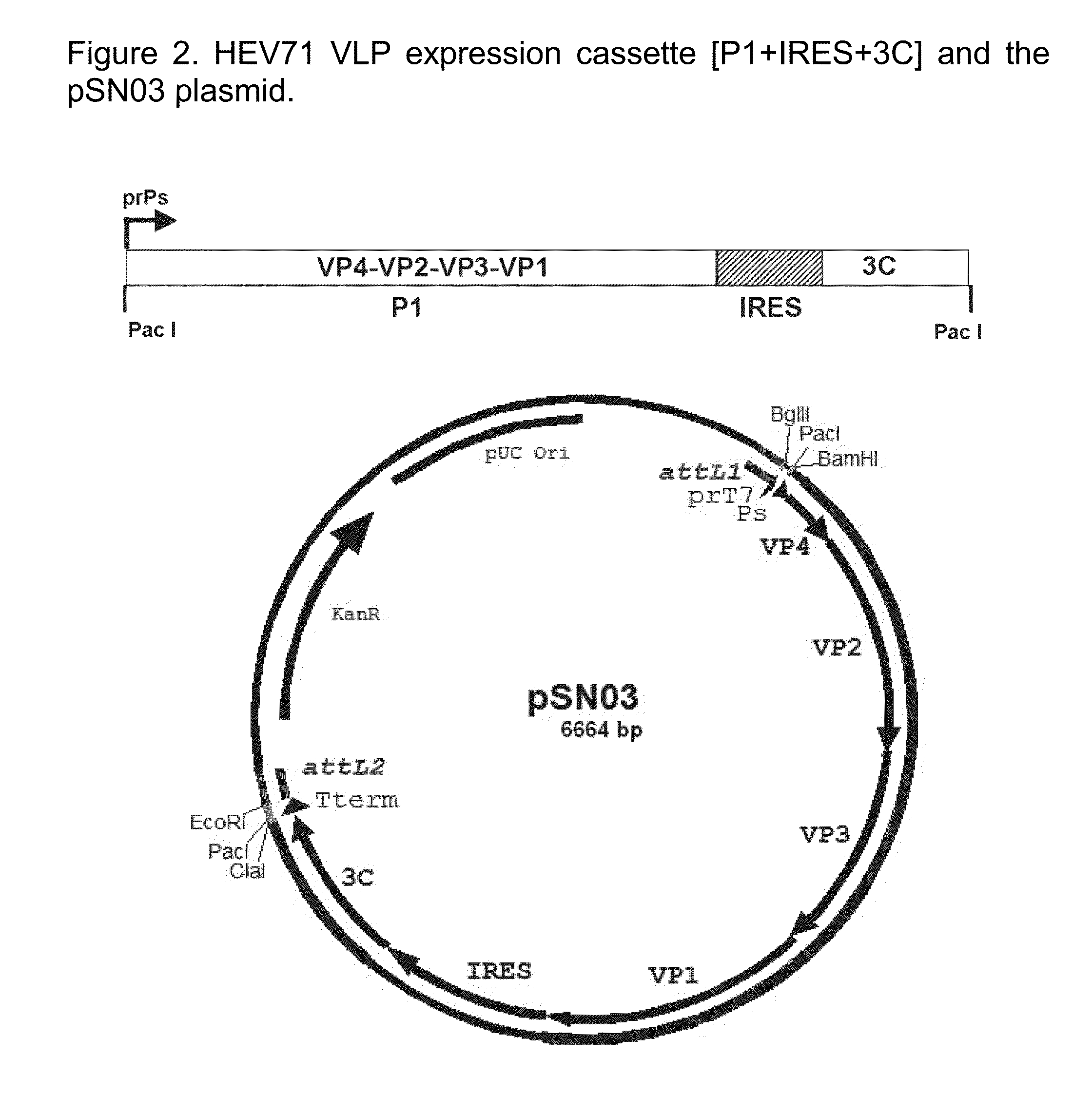
EV71 virus-like particles and hand-foot-and-mouth disease vaccine prepared from EV71 virus-like particles
InactiveCN102154229ASuitable for industrial productionEasy to purifyInactivation/attenuationAntiviralsPichia pastorisSaccharum
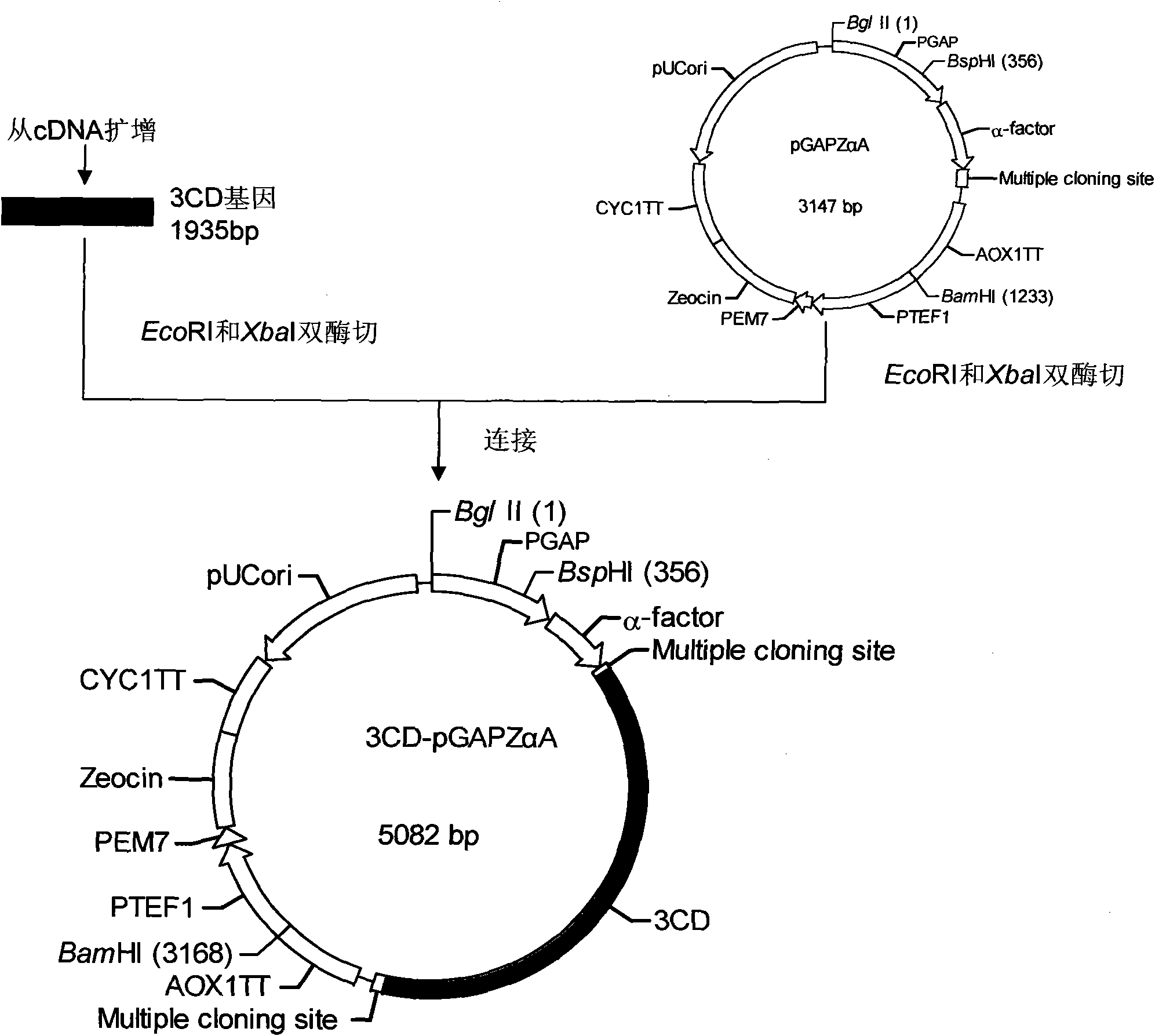
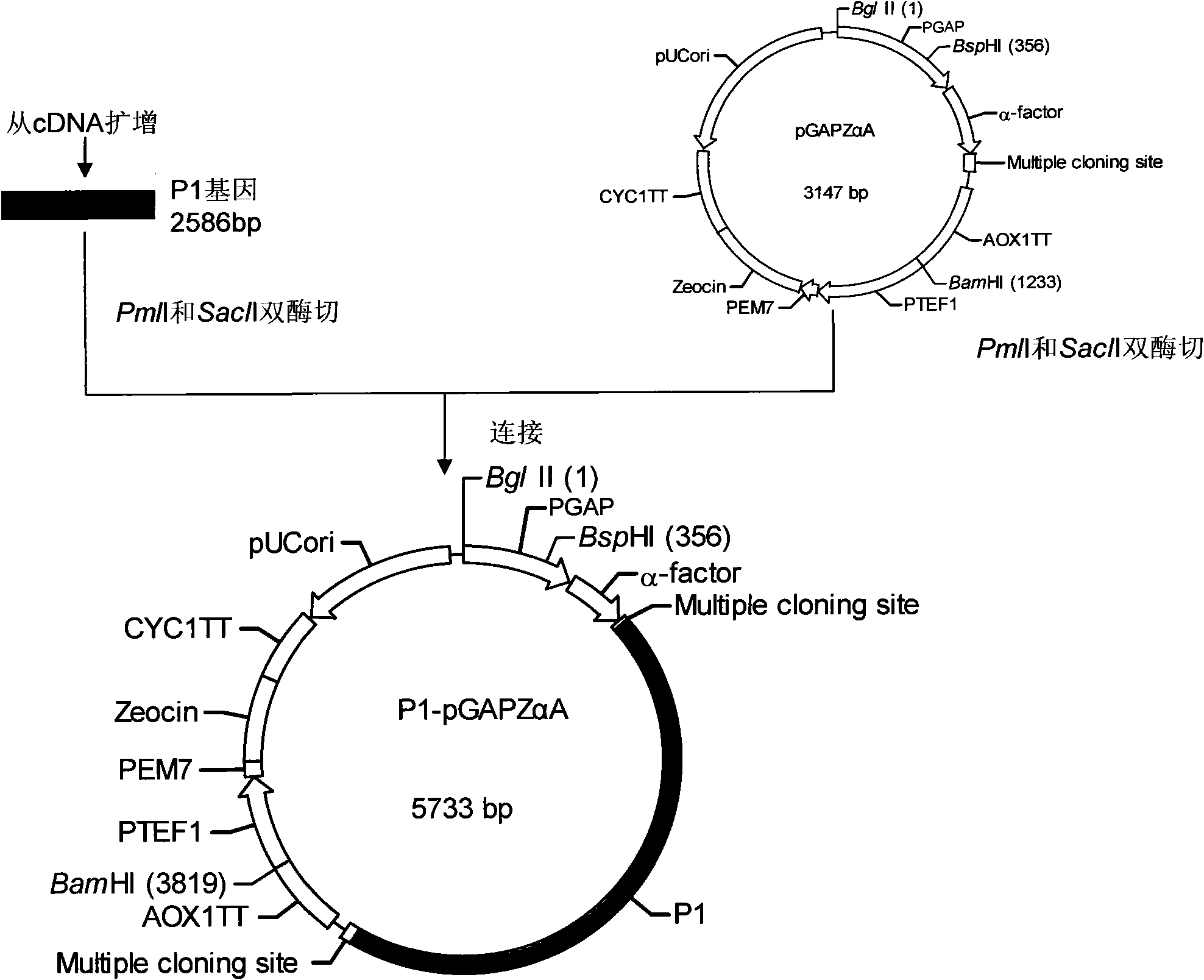
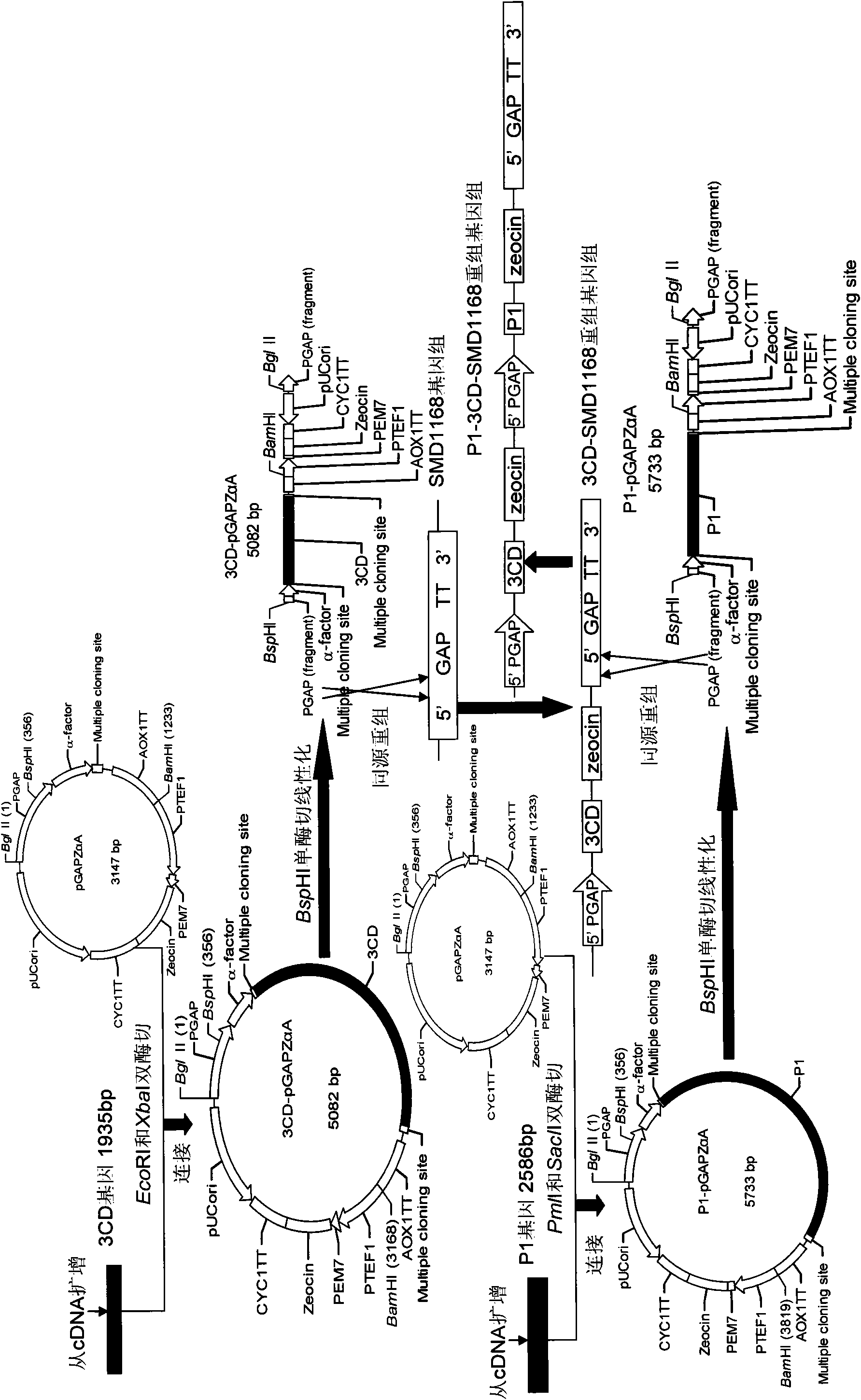
The invention relates to EV71 virus-like particles and hand-foot-and-mouth disease vaccine prepared from the EV71 virus-like particles. The EV71 virus-like particles are prepared according to the following steps: (1) constructing recombinant plasmid: respectively connecting pGAPZ alpha A plasmid with P1 protein gene of intestinal EV71 virus and 3CD protease gene to construct P1-pGAPZ alpha A and 3CD-pGAPZ alpha A recombinant plasmid; (2) transferring the recombinant plasmid to expression bacterial strains: sequentially transferring the recombinant plasmid to Pichia pastoris SMD1168 expression bacterial strains to obtain P1-pGAPZ alpha A-3CD-pGAPZ alpha A-SMD1168 recombinant expression strains; and (3) culturing thallus and purifying the EV71 virus-like particles: culturing the Pichia pastoris recombinant expression bacterial strains, centrifugalizing to separate supernate, and carrying out sucrose density gradient centrifugation on precipitation of the supernate after ultracentrifugation, thus obtaining the EV71 virus-like particles. In the invention, the EV71 virus-like particles are easy to obtain through thallus culture, have good stability, are easy to purify and suitable for preparing vaccine, and are convenient for industrial production.
Owner:张定梅 +3
Application of lycorine in preparing medicament for treating diseases caused by human enterovirus 71 type infection
ActiveCN102178678AReduce mortalityRelieve symptomsOrganic active ingredientsNervous disorderViral MyocarditisPulmonary edema
The invention belongs to the field of medicaments, discloses application of lycorine, lycorine salt, lycorine hydrate, lycorine optical isomer or lycorine prodrug in preparing medicaments for treating diseases caused by human enterovirus 71 type infection, preferably treating hand-foot-and-mouth disease, herpangina, viral meningitis, viral encephalitis, flaccid paralysis, pulmonary edema and vital myocarditis. In vitro and in vivo tests prove that the lycorine can inhibit replication and lesion of enterovirus (EV) 71 in cells, has excellent function of inhibiting the EV 71 virus, and has clinical application prospect.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Composition, kit and method used for detecting enterovirus causing hand foot and mouth disease
ActiveCN101956021AEasy to detectReduce testing costsMicrobiological testing/measurementFluorescence/phosphorescenceHand-foot-and-mouth diseaseMaterial resources
The invention discloses a composition used for detecting enterovirus causing hand foot and mouth disease, which comprises an upstream primer P1:5'-CCCTGAATGCGGCTAATCC-3' and a downstream primer P2:5'-GTCGGTTCCGCTGCAGAGT-3'. Genetic fragments of 5'UTR regions of EV71 and Cox A16 type enteroviruses in a sample can be specifically detected by the composition matched with a proper probe and other reagents through a real time Polymerase Chain Reaction (PCR) method. The invention also discloses a kit containing the composition, and a method for detecting the EV71 and / or Cox A16 type enterovirus by utilizing the composition or the kit. When the composition or the kit and / or the method is utilized for detecting the sample, whether the EV71 and / or Cox A16 type enterovirus exist in the sample can be sensitively and rapidly detected in a reaction; therefore, the efficiency and the sensitivity of the detection are improved, the detection process is simplified, and the manpower, the material resources and the detection cost are saved.
Owner:成都新基因格生物科技有限公司
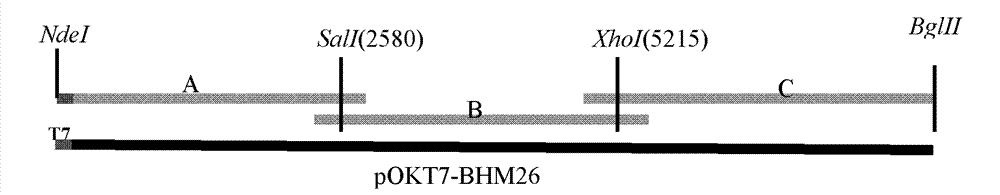
Chinese isolated strain of bovine enterovirus, and construction and application of infectious cDNA clone thereof
ActiveCN102776155ATypical small RNA virus particle morphologyStrong toleranceMicrobiological testing/measurementGenetic material ingredientsBovine enterovirusMicroorganism
The invention relates to a Chinese isolated strain of bovine enterovirus, and constructions and applications of infectious cDNA clone thereof. Isolated strain of bovine enterovirus capable of stably generating CPE on MA104 cells can be separated from bovine diarreal stool samples, with a microbial preservation number of CGMCC No. 4782. Whole-length infectious cDNA clone is constructed from the isolated strain. B family gene type 2 bovine enterovirus rescue virus is obtained by in-vitro transcribing the cDNA and transfectioning cells. The isolated strain of bovine enterovirus, the constructed whole-length infectious cDNA clone and the rescue virus thereof can be used for researches of gene structures and functions of bovine enterovirus, can be used as vaccine vectors of bovine enterovirus, or can be prepared into vaccines or reagents capable of preventing or treating bovine enterovirus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
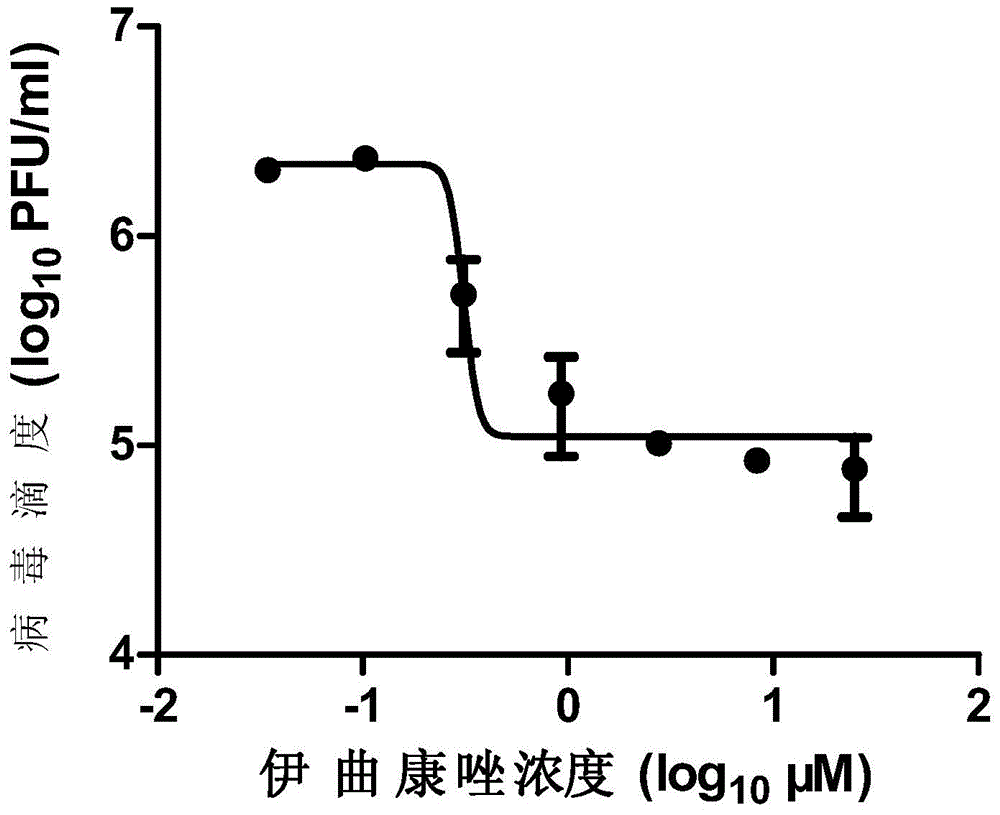
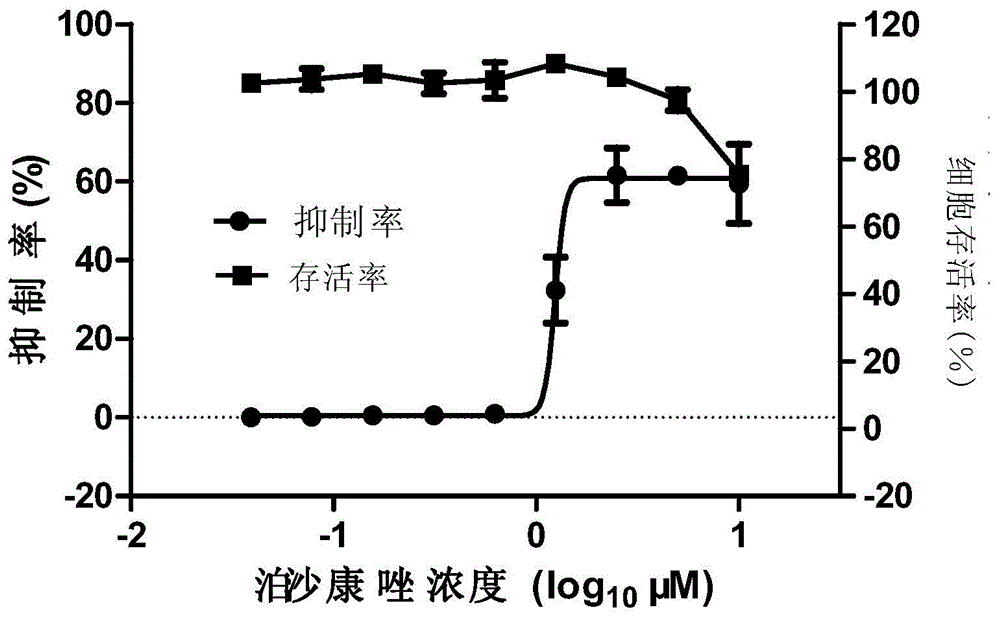
Micromolecule compound inhibitor for enterovirus and application of inhibitor
The invention provides a micromolecule compound inhibitor for enterovirus and an application of the inhibitor. Specifically, the invention provides an application of itraconazole, an itraconazole analogue, or pharmaceutically acceptable salt of itraconazole or the itraconazole analogue to preparation of a reagent, which is used for inhibiting growth or reproduction of enterovirus and / or for inhibiting synthesis of enterovirus RNA. The invention also provides an inhibitor and medicine composition containing itraconazole or the itraconazole analogue for enterovirus, and provides a method for in-vitro non-therapeutically inhibiting growth of enterovirus or killing enterovirus. Experimental results show that itraconazole and the itraconazole analogue has an excellent inhibition effect on multiple kinds of enterovirus.
Owner:中国科学院上海免疫与感染研究所
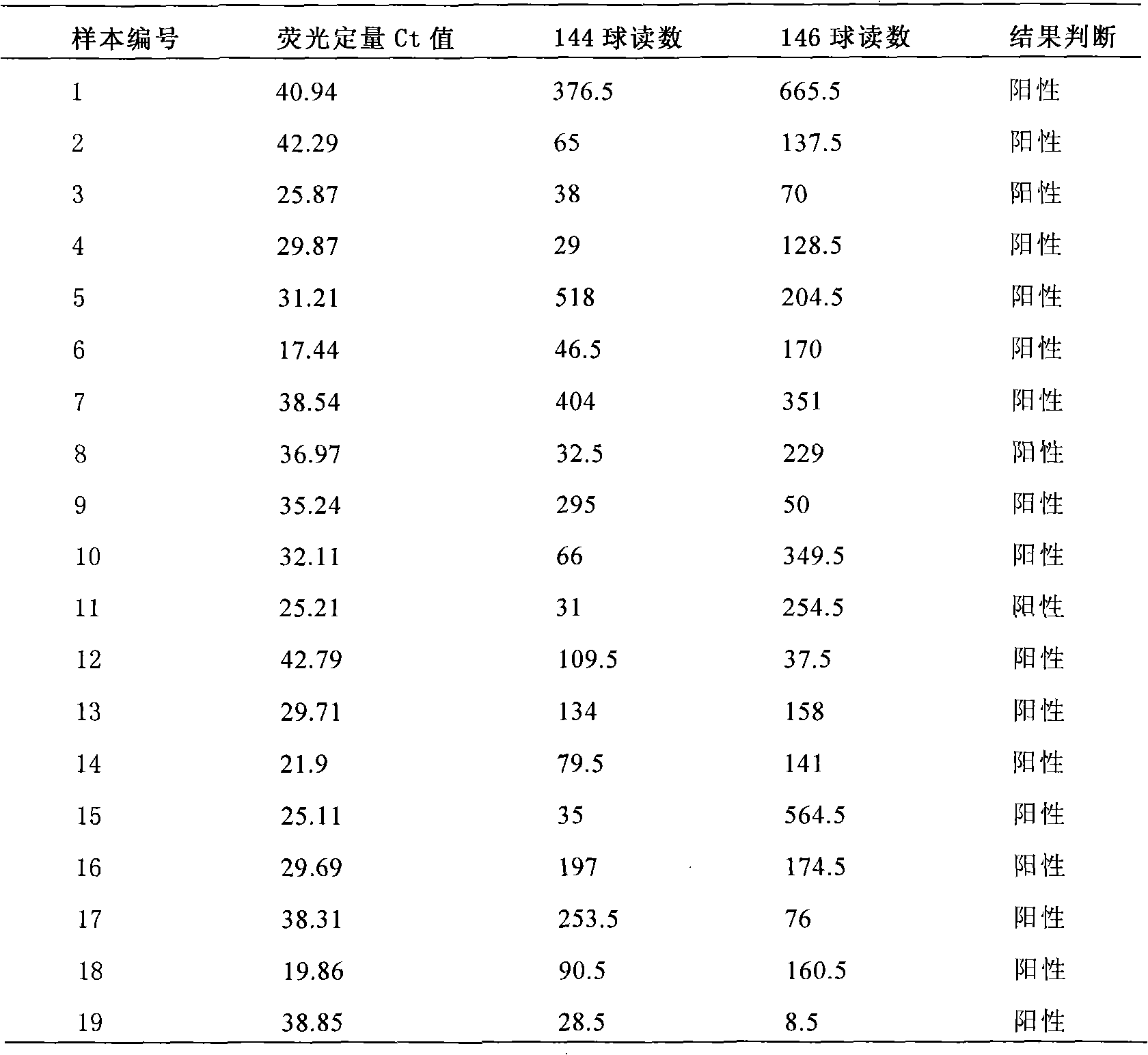
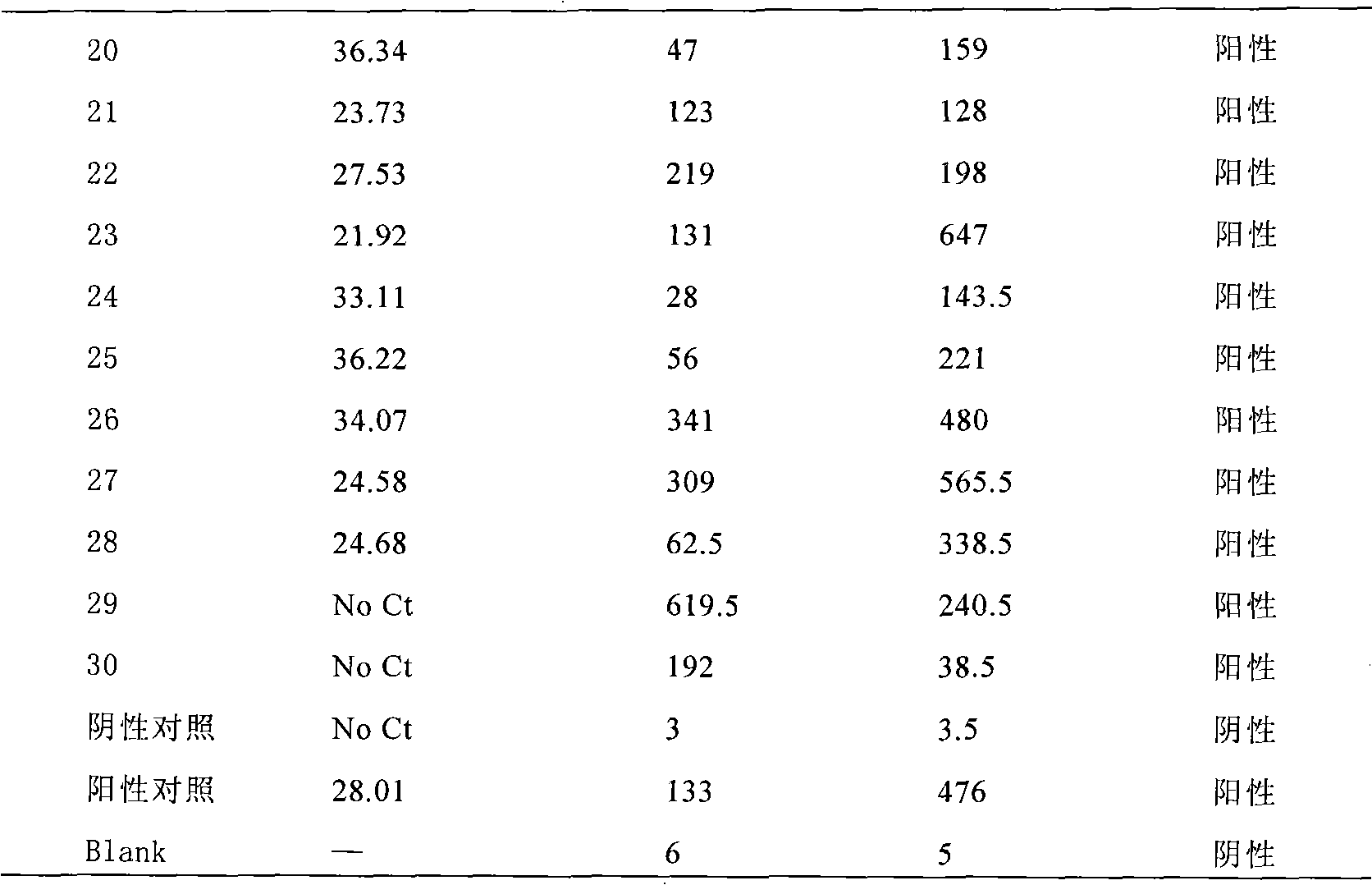
Enterovirus type-71 nucleic acid amplification fluorescent quantitative and liquid chip dual test kit
InactiveCN101665840AStrong specificityIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceCross-linkConserved sequence
The invention belongs to the field of biotechnology and particularly relates to an enterovirus type-71 test kit. The invention discloses an enterovirus type-71 nucleic acid amplification fluorescent quantitative and liquid chip dual test kit, which comprises: a pair of EV type-71 specific primers A and A' having biotin-marked 5'-terminals; a pair of EV type-71 specific primers B and B' having biotin-marked 5'-terminals; fluorescence-encoded microspheres C cross-linked with probes F matched with the sequence of amplified fragments of the primers A and A'; fluorescence-encoded microspheres D cross-linked with probes G matched with the sequence of amplified fragments of the primers B and B'; a pair of EV type-71 specific conserved sequence primers E and E'; a Taq-Man probe having a fluorescent group-marked 5'-terminal and a BHQ-marked 3'-terminal; and QRT-PCR reagent and fluorescence-marked avidin.
Owner:SHANGHAI GENEPROTECH
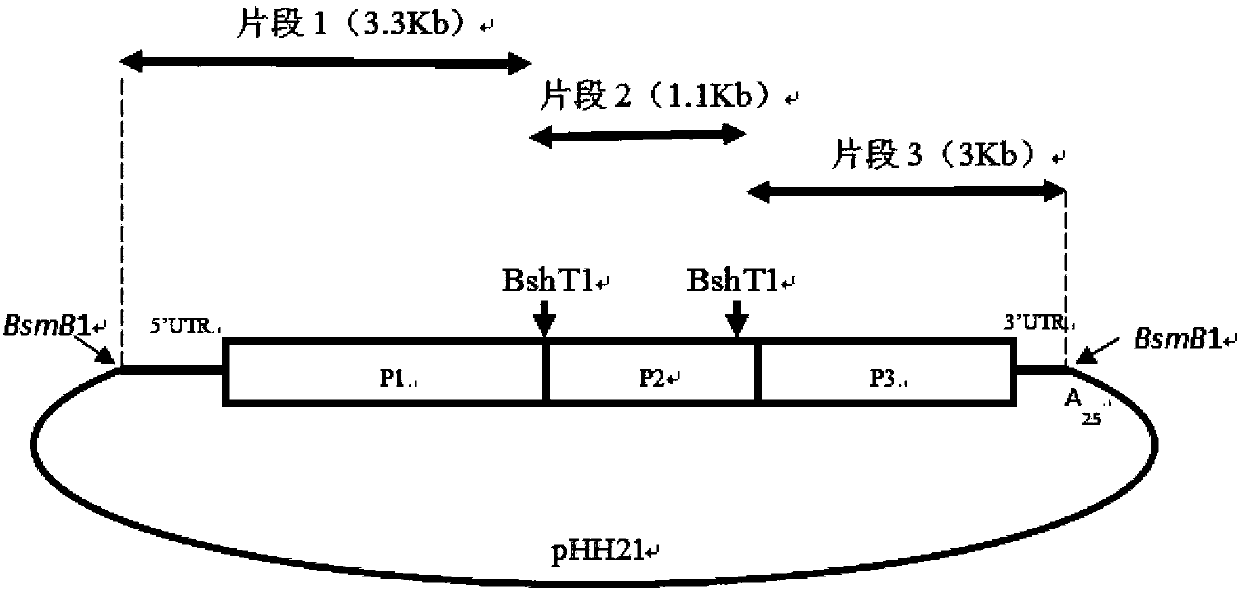
Human enterovirus D68 type infectious clone and construction method and application thereof
InactiveCN107893083AAvoid pollutionSave experimental stepsSsRNA viruses positive-senseGenetically modified cellsVirulent characteristicsRestriction site
The invention belongs to the field of biotechnology, and particularly relates to a human enterovirus D68 type infectious clone and a construction method and application thereof. The infectious clone is obtained by using a RNA polymerase I system and inserting the full-length cDNA of EVD68Fermon strain; the 1882 and 2593 BsmB1 restriction sites of the full-length cDNA are subjected to the mutationwithout changing amino acids, and the BsmB1 restriction sites are inserted at both ends. The recombinant plasmid containing the complete genomic cDNA clone of EV-D68 strain Fermon fills in the gap ofthe Chinese human enterovirus D68 type infectious clone, and the construction method is simple and highly efficient. At the same time, the application of the human enterovirus D68 type infectious clone provides a powerful tool for the search for the virulence decision sites of enterovirus 68 type and the development of drugs and vaccines resistant to enterovirus D68 type viruses.
Owner:TIANJIN UNIV
Applications of hepatocyte growth factor-regulated tyropsine kinasesubstrate in preparation of medicines preventing enterovirus 71-type infection
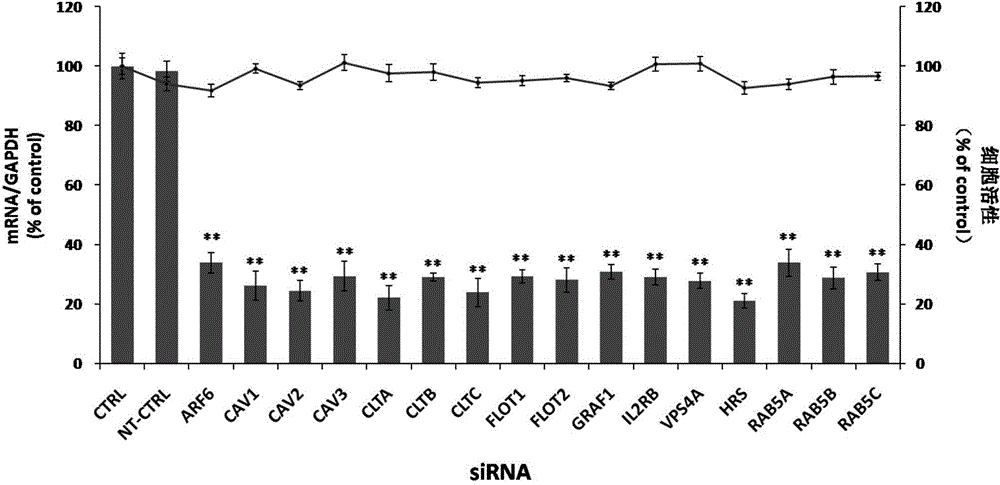
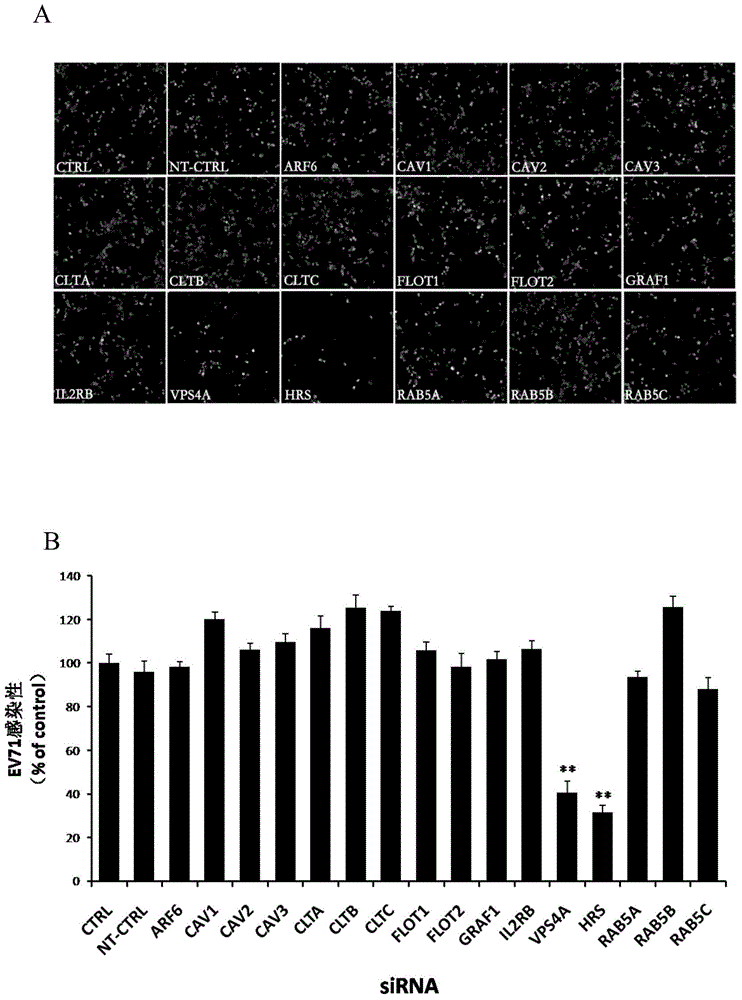
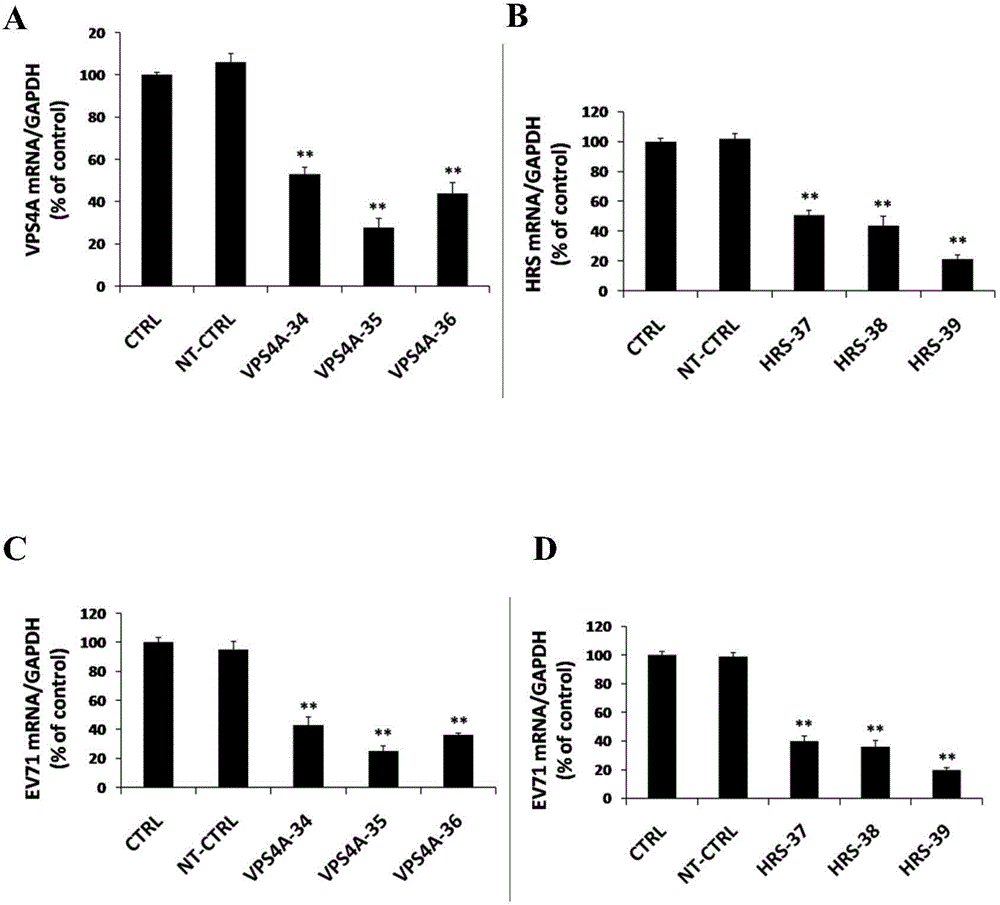
The invention relates to the biomedical technology field, and provides a new target spot for resisting enterovirus 71-type infection and applications. Human colon cancer cells (Caco-2) are employed as target cells, the RNA interference technology is employed to reduce expression of the target cell host proteins, a host factor capable of inhibiting EV71 infection of human colon cancer cells (Caco-2) effectively is sought and the purpose of cutting off EV71 infection from the source (intestinal tract) effectively is achieved. Experiments show that hepatocyte growth factor-regulated tyropsine kinasesubstrate (HRS) plays an important role in inhibiting EV71 infection of Caco-2, the HRS expression is reduced, and EV71 infection can be inhibited obviously. Applications of HRS in preparation of medicines preventing or treating enterovirus 71-type infection are provided.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
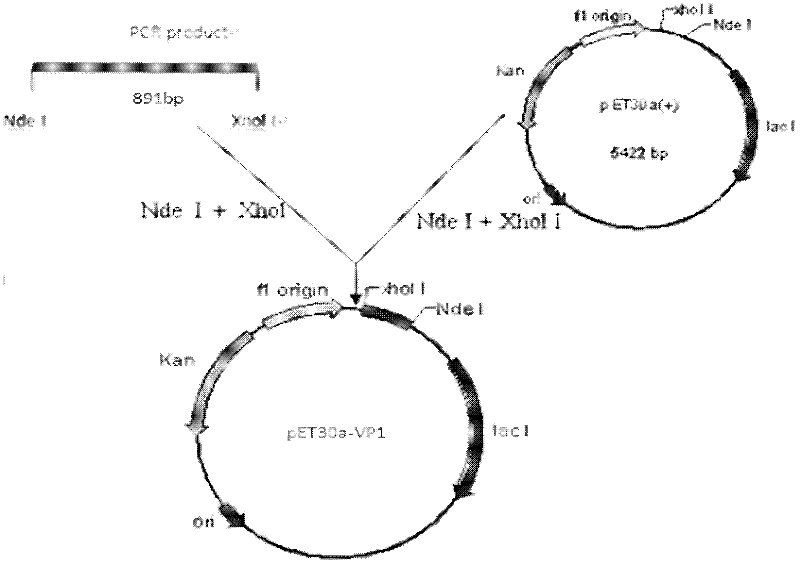
Prokaryotic expression of enterovirus 71 type VP1 (virus protein 1) and vaccine containing VP1
InactiveCN102337289AEasy to operateLow costMicroorganism based processesAntiviralsEscherichia coliVirus Protein
The invention provides a prokaryotic expression method of an enterovirus 71 type VP1 (virus protein). The method comprises the steps of: cloning an enterovirus 71 type VP1 gene sequence into a prokaryotic expression carrier, transforming into coli bacillus to be expressed in an induction way, and purifying and renaturing the expressed VP1. The obtained VP1 and an immunologic adjuvant are matched with each other to prepare a vaccine. The invention has the advantages that: a genetic engineering recombinant VP1 is firstly expressed by a coli bacillus expression system, the operation is simple, the cost is low, large-scale production is easy to achieve and the like. The prokaryotic-expressed genetic engineering recombinant VP1 is matched with a chitosan adjuvant to immunize a mouse, and an immunological effect is evaluated, so that the VP1 is primarily proved to be not only capable of inducing the generation of a humoral immune protective reaction but also capable of inducing the generation of a cellular immunity reaction, therefore, the invention lays a foundation for further developing the genetic engineering recombinant VP1 and inactivated totivirus oral vaccine for human.
Owner:中国疾病预防控制中心病毒病预防控制所
High-efficient antiviral medicament composition in chickweed as well as preparation method and use thereof
InactiveCN101371861ASafe broad-spectrum antiviral drug actionHigh-efficiency broad-spectrum antiviral drugsAntiviralsSynthetic polymeric active ingredientsCondyloma virusHighly pathogenic
The invention discloses a highly effective antiviral medicinal composition of chickweed, a preparation method and an application thereof. Presently, highly effective, safe and universal antiviral medicine is lacking all over the world. A plant, chickweed, or other stellaria plant is extracted, by two resin adsorption methods, one water-alcohol extraction and ultra-filtration, into a dark brown composition that has a molecular distribution ranging from 2,000 to 900,000 Dolton and comprises total sulfate peptidoglycan phenolic acidic components and flavonoid components; the composition is used as a safer, more highly effective universal antiviral natural medicine. Total peptides account to 15-25 percent of the chemical structures of dark brown total sulfate peptidoglycan phenolic-acidic components, and include totaling 17 amino acids by proportion: aspartic acid, glutamic acid, serine, histidine, cystine, methionine, isoleucine; the polysaccharides essentially consist of glucose, galactose and arabinose and form sulfate polysaccharides. The composition can be used for treating virus diseases, including AIDS virus, hepatitis virus, respire virus such as influenza virus including highly pathogenic bird flu virus, para influenza virus and adenovirus, and papovavirus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus, and varicella-zoster virus, and on the like, does not show toxicity, and can be prepared into more than 10 medicinal dosage forms and healthcare products, and the production process does not cause pollution to the environment.
Owner:朱耕新
Combined EV71 (enterovirus 71)-HA (hepatitis A) vaccine
ActiveCN102210858AImproving immunogenicityImprove securityAntiviralsAntibody medical ingredientsDiseaseAdjuvant
The present invention discloses a combined EV71 (enterovirus 71)-HA (hepatitis A) vaccine which comprises inactivated enterovirus 71 viruses, inactivated hepatitis A viruses, and a aluminium adjuvant. The invention also provides a method for preparing the combined EV71-HA vaccine; and in the method, the protective agents such as gelatins and the like are not required to be added, the pH adjustment is not required to be performed, and the absorption effect and stability of the prepared vaccine are good. After the vaccine is vaccinated, the hepatitis A virus has an adjuvant effect on EV71 (enterovirus 71), therefore, the immunogenicity of the EV71 (enterovirus 71) is enhanced, and the immunity effect of the combined EV71-HA vaccine is equal to or greater than the immunity effect of a univalent vaccine. The vaccine disclosed by the invention has EV71-HA double immunities and protection performances; when the vaccine disclosed by the invention is vaccinated, the inoculating needle time can be reduced, and the immunization process can be simplified; and meanwhile, the diseases of people and animals arising from enterovirus 71 viruses and hepatitis A viruses can be effectively prevented.
Owner:SINOVAC BIOTECH
Enterovirus real-time fluorescent quantitative detection kit
InactiveCN105238880AMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
The invention discloses an enterovirus real-time fluorescent quantitative detection kit, which is used for distinguishing EV71 enterovirus from CA16 enterovirus, wherein the detection kit comprises a forward primer EV71F1 and a reverse primer EV71R1 for amplifying the EV71 enterovirus, a probe EV71P1 for detecting the EV71 enterovirus, a forward primer CA16F2 and a reverse primer CA16R2 for amplifying the CA16 enterovirus, a probe CA16P2 for detecting the CA16 enterovirus and a real-time fluorescent quantitative PCR (polymerase chain reaction) buffer solution, wherein a first fluorescence group and a first fluorescence quencher are respectively bonded to two ends of the probe EV71P1; a second fluorescence group and a second fluorescence quencher are respectively bonded to two ends of the probe CA16P2; and the first fluorescence group is different from the second fluorescence group. The enterovirus real-time fluorescent quantitative detection kit, when being used, can distinguish the EV71 enterovirus from the CA16 enterovirus by respectively detecting signals of the first fluorescence group and signals of the second fluorescence group.
Owner:FAPON BIOTECH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com