Quantitative real-time assay for noroviruses and enteroviruses with built in quality control standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of a Quantitative Multiplex RT-PCR Assay for the Detection of Noroviruses and Enteroviruses
[0078] The use of RT-PCR based assays has increased detection sensitivity of Enteroviruses and reduced analysis time. The predominant human Noroviruses, which are placed into two genogroups, GI and GII, have not been cultured. Thus, Norovirus detection is based primarily upon non-quantitative RT-PCR assays. To date, no single assay has been capable of simultaneous detection and enumeration of GI genogroup Norovirus and GII genogroup Norovirus.
[0079] In the present study, a multiplex qRT-PCR assay using the Cepheid SmartCycler® (Cepheid, Sunnyvale, Calif.) system has been developed for the simultaneous detection and quantification of viruses from the Enterovirus genus. The following example demonstrated that the multiplex qRT-PCR can simultaneously detect Enterovirus, Norovirus genogroup I, and Norovirus genogroup II. The assay also incorporated a novel quantitative universal inte...
example 2
Quantitative Multiplex RT-PCR Assay for the Detection of Noroviruses and Enteroviruses
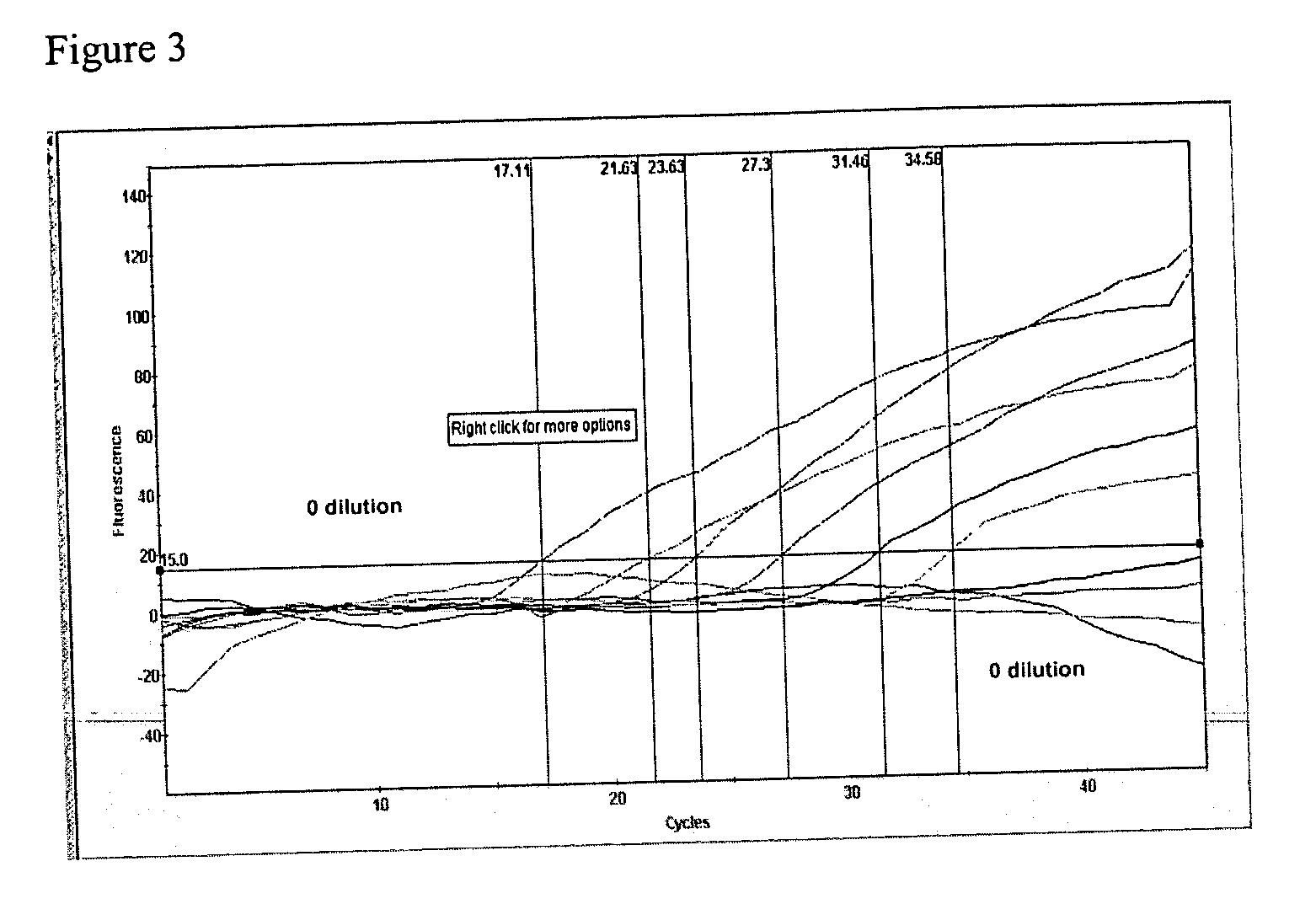
[0093] RNA template was serially diluted 1:10 until a dilution of 10−9. Using the different concentrations of RNA template tested the effect of template overload and the assay's threshold of detectable levels of RNA.
[0094] Methods
[0095] The cDNAs from the RT-PCR of Example I were purified using a NucleoSpin® Extraction Kit (BD Biosciences Clontech, Palo Alto, Calif.) and cloned using a TOPO TA Cloning® Kit (Invitrogen Corp., Carlsbad, Calif.) by known methods. Clones with full-length inserts and proper orientation were subjected to a QIAprep® Miniprep Kit (Qiagen) to purify their plasmids. Plasmids were purified and sequenced in both the forward and reverse direction to determine and / or verify their sequences. Each plasmid was linearized by restriction digest with BamHI (New England BioLabs®, Inc., Beverly, Mass.) and run on a 1% agarose gel to confirm linearization. The linearized plasmid was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com