Combined vaccine for preventing hand-foot-mouth disease
A combined vaccine and hand, foot and mouth disease technology, applied in vaccines, multivalent vaccines, viruses, etc., can solve the problems of unknown immune interference, reduce vaccine single-component immunogenicity, affect immune effect, etc., and achieve good process consistency , Improving vaccination efficiency and simplifying vaccination procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 virus liquid
[0047] 1. Cell culture and recover SLF-1 cells, use MEM solution containing 20% bovine serum, culture at 37±1°C, replace the medium after adherence, and use MEM solution containing 10% bovine serum. Wash the monolayer, digest with 0.25% trypsin, and divide into culture flasks or cell factories at a ratio of 1:4, transfer to 40-layer cell factories, grow to a monolayer, and prepare for inoculation of viruses.
[0048] 2. Virus culture
[0049] The cells were inoculated with EV71 virus at an MOI of 0.0001, cultured at 36±1°C with a culture medium containing 2% bovine serum, and the virus was harvested after 8 days.
[0050] The CA16 virus was inoculated into the cells at an MOI of 0.001, cultured at 35±1°C with a culture solution containing 1% bovine serum, and the virus was harvested after 10 days.
[0051] The CA10 virus was inoculated into the cells at an MOI of 0.001, cultured at 35±1°C in culture medium without bovin...
Embodiment 2
[0052] The inactivation of embodiment 2 virus
[0053] (1) Clarification: Filter the virus liquid through a filter element with a continuous two-stage pore size of 3-0.8 μm and 0.65-0.22 μm or centrifuge (including continuous flow centrifugation) with a rotation speed of 3000 rpm and a centrifugation time of 0.5 hours to obtain a clarified virus liquid;
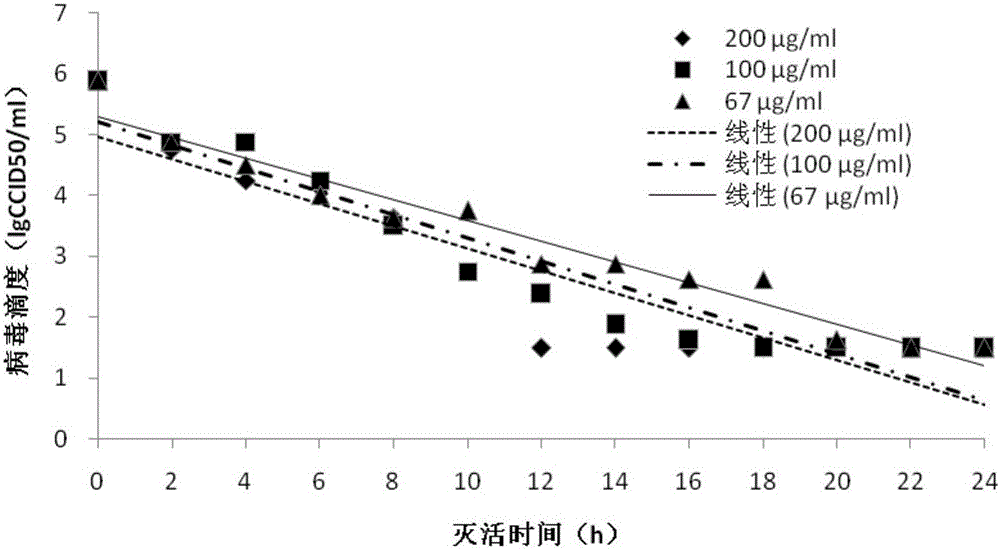
[0054] (2) Inactivation Divide EV71, CA16, and CA10 virus clarified liquid into 9 groups respectively, add different volumes of 1:200 formalin solution to each group, so that the theoretical final concentration is 200, 100, 67 μg / ml, each Concentrations of 3 groups of parallel samples were inactivated at 37±1°C. See Table 1 for grouping information. Sampling was carried out at 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 hours of inactivation, and immediately after the sampling was completed, formaldehyde was neutralized with sodium bisulfite solution, and virus titration was carried out. degree measurement. The virus ...
Embodiment 3
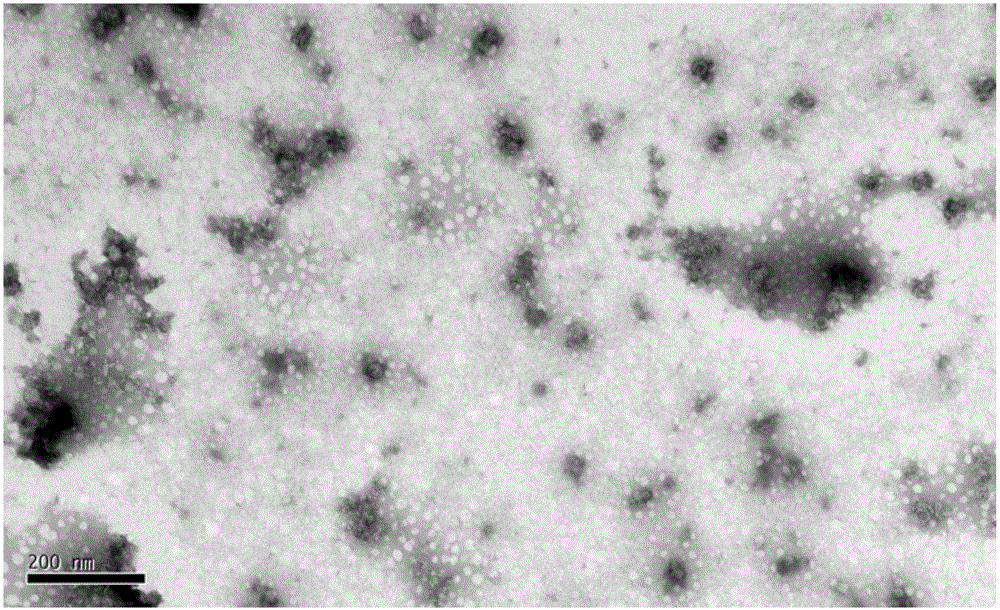
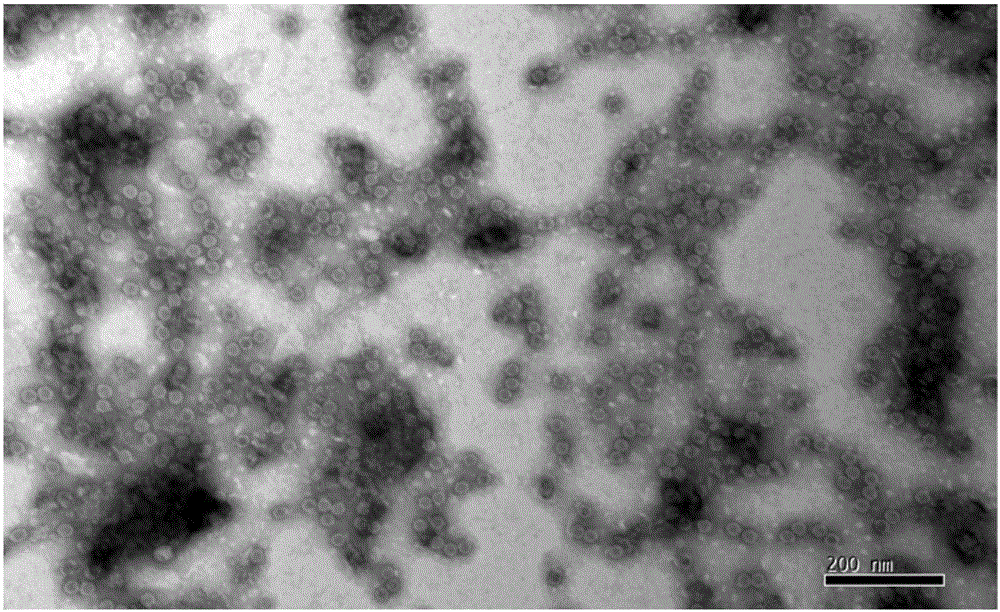
[0064] The purification of embodiment 3 virus
[0065] (1) Ultrafiltration concentration of virus liquid Select ultrafiltration membrane packs with molecular weight cut-offs of 100kD, 300kD, and 500kD three pore sizes, respectively carry out ultrafiltration concentration of EV71 (or CA16 or CA10) with a batch of inactivation liquid, and fix the ultrafiltration pressure , concentrate the same multiple (100 times), fix the number of times of diafiltration (8 times of diafiltration), compare the ultrafiltration time and the antigen recovery rate of three membrane packs, the removal rate of impurity protein and the activity ratio of the product before and after ultrafiltration concentration (antigen content / protein content), and detect the antigen content of each filtrate. Determine the pore size of the ultrafiltration membrane package according to the test results. The results are shown in Table 4.
[0066] Table 4 Comparison results of ultrafiltration effects with different m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com