Inactivated vaccine for duck viral hepatitis as well as preparation method and application thereof
A technology for duck viral hepatitis and duck hepatitis A, which is applied in the field of inactivated duck viral hepatitis vaccine and its preparation, can solve the problems of susceptibility to virulence, long survival time, immunization failure, etc., and achieves the duration of immunization. Long, stable immune effect, easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, the acquisition of DHAV-SH strain
[0058] 1. Discovery of DHAV-SH strain
[0059] In a duck farm in Shanghai in December 2013, Liu Wenjun and Li Jing isolated a strain of duck viral hepatitis type Ⅰ virus from the diseased duck, and named it DHAV-SH strain.
[0060] 2. Identification of DHAV-SH strain
[0061] 1. Virus form
[0062] Virus particles of the DHAV-SH strain are icosahedral and about 40 nm in diameter.
[0063] 2. Molecular biological identification
[0064] Extract the RNA of the DHAV-SH strain, obtain the DNA after reverse transcription, design a pair of specific primers (P1 and P2) according to the DHAV complete gene sequence published by GENBANK, and use P1 and P2 to perform RT-PCR amplification on the obtained DNA , to obtain the amplified product. Sequencing of the amplified product showed that the target fragment with a size of 229 bp was amplified, and its sequence is shown in sequence 1 of the sequence list, which has a high homo...
Embodiment 2
[0082] Embodiment 2, the preparation of duck viral hepatitis bivalent inactivated vaccine
[0083] 1. Antigen preparation
[0084] 1. Preparation of virus liquid
[0085] The DHAV-SH strain was inoculated into 11-day-old susceptible duck embryos through the allantoic cavity, 0.1 mL per embryo, incubated at 37°C for 120 hours, then aseptically collected embryo liquid and embryo body, homogenized at high speed (14000r / min), and then used Filter through 8 layers of sterile gauze, collect the filtrate, and freeze and thaw once at -20°C to obtain the DHAV-SH strain virus liquid. Virus content=10 of the DHAV-SH strain virus liquid of every 0.1mL 6.25 ELD 50 (Duck embryo half lethal dose). In actual application, the virus content of every 0.2mL of DHAV-SH strain virus liquid is ≥10 6.25 ELD 50 can be.
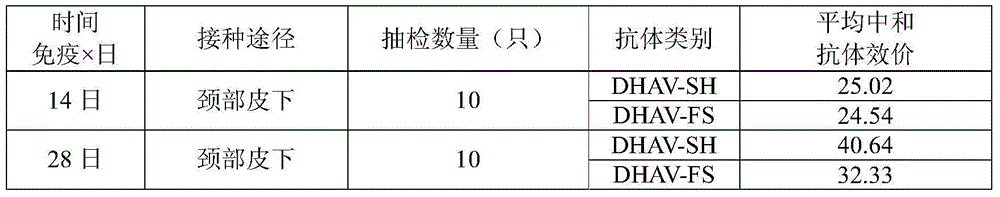
[0086] The DHAV-FS strain was inoculated into 11-day-old susceptible duck embryos through the allantoic cavity, 0.1 mL per embryo, and incubated at 37°C for 120 hours, then ase...
Embodiment 3
[0097] Embodiment 3, the stability of duck viral hepatitis bivalent inactivated vaccine
[0098] Take 3 batches of duck viral hepatitis bivalent inactivated vaccine and store them at 4°C. After 3, 6, 9, 12, and 15 months of storage, take samples for physical property testing, and compare the test results with those after production .
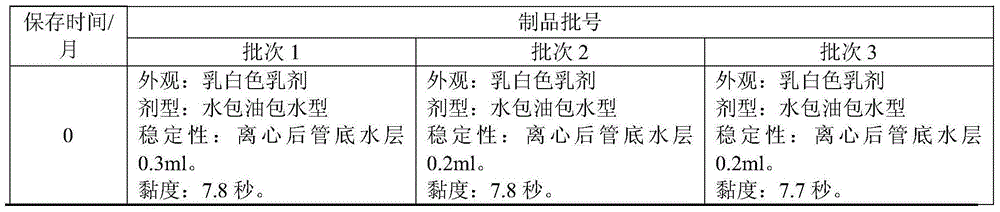
[0099] The results showed that after 3, 6, 9, 12, and 15 months of storage at 2-8°C, the physical properties of the three batches of laboratory products remained stable, and the appearance, dosage form, stability, and viscosity all conformed to the "Trial Regulations" (draft) ) in the quality standards. See Table 1 for detailed results.
[0100] Table 1. Test results of physical properties of 3 batches of laboratory products at different storage times
[0101]
[0102]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com