Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58results about How to "Long-lasting immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
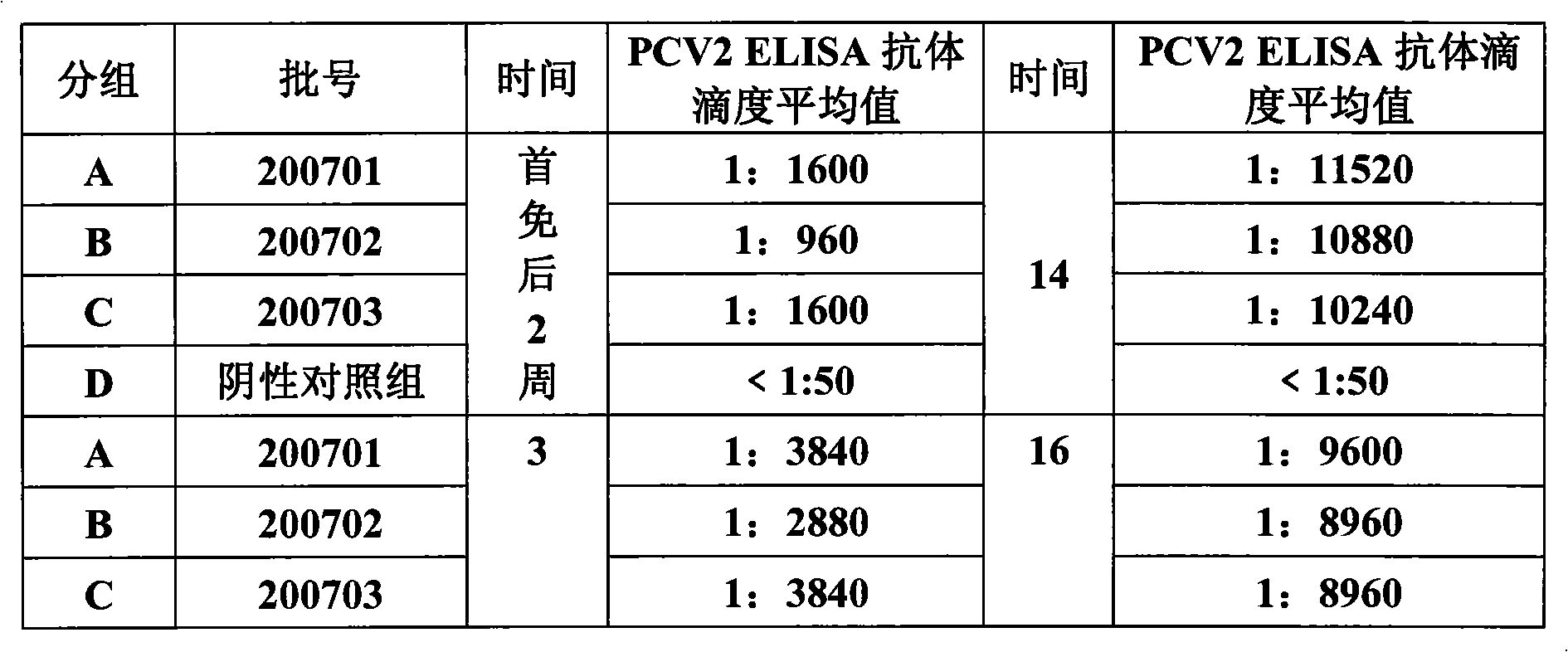
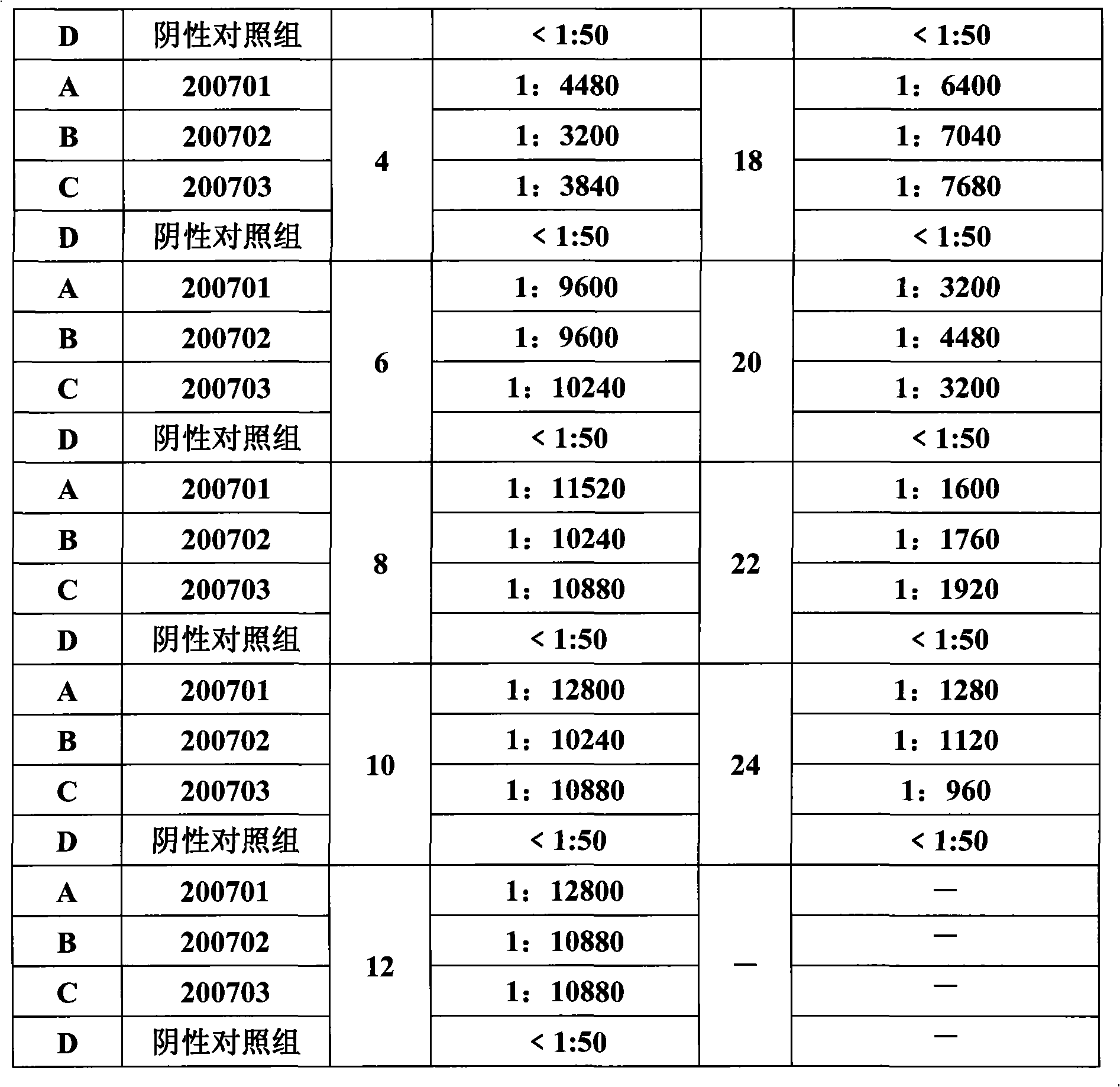
Porcine circovirus type II inactivated vaccine of and method for preparing same
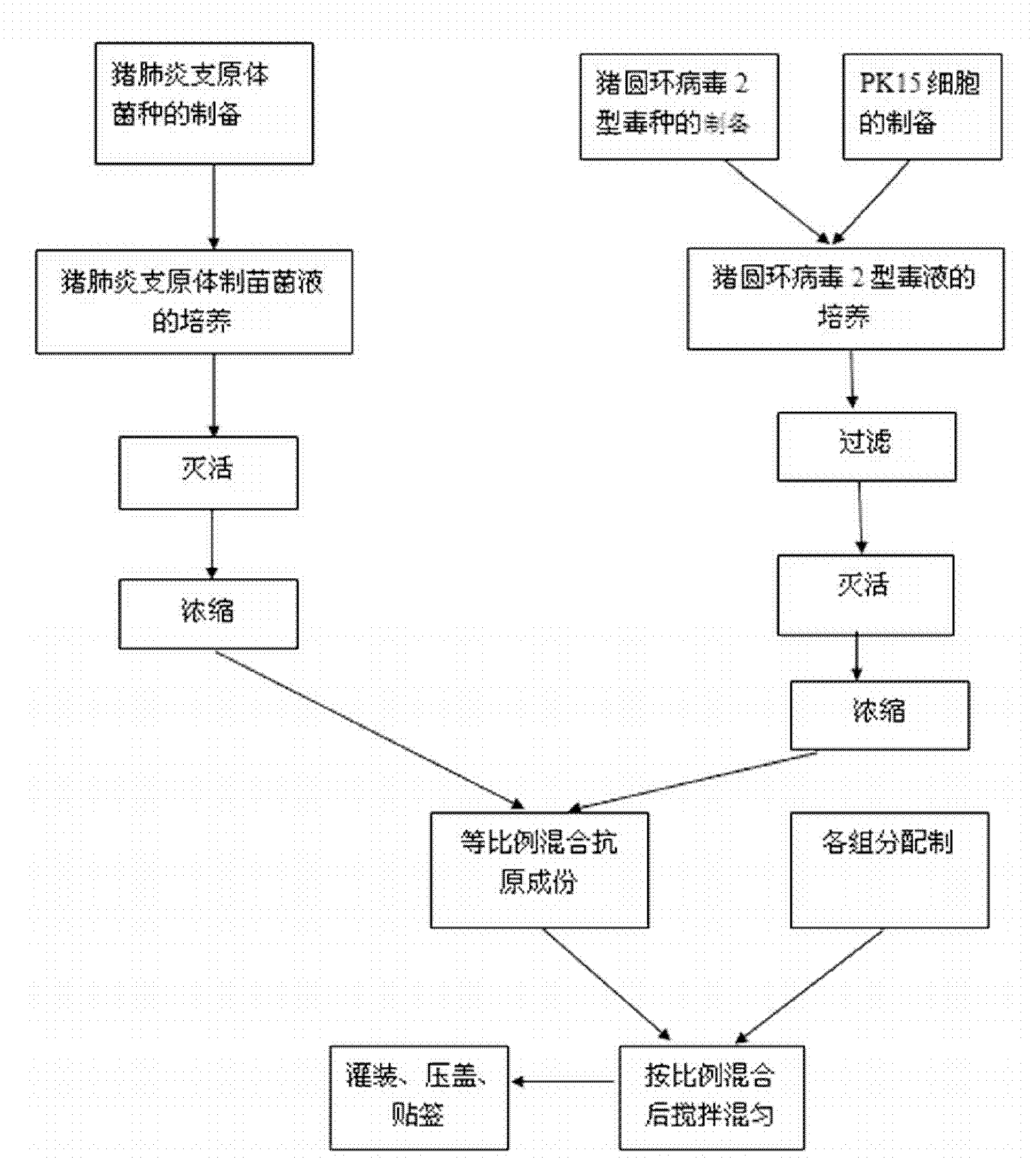
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
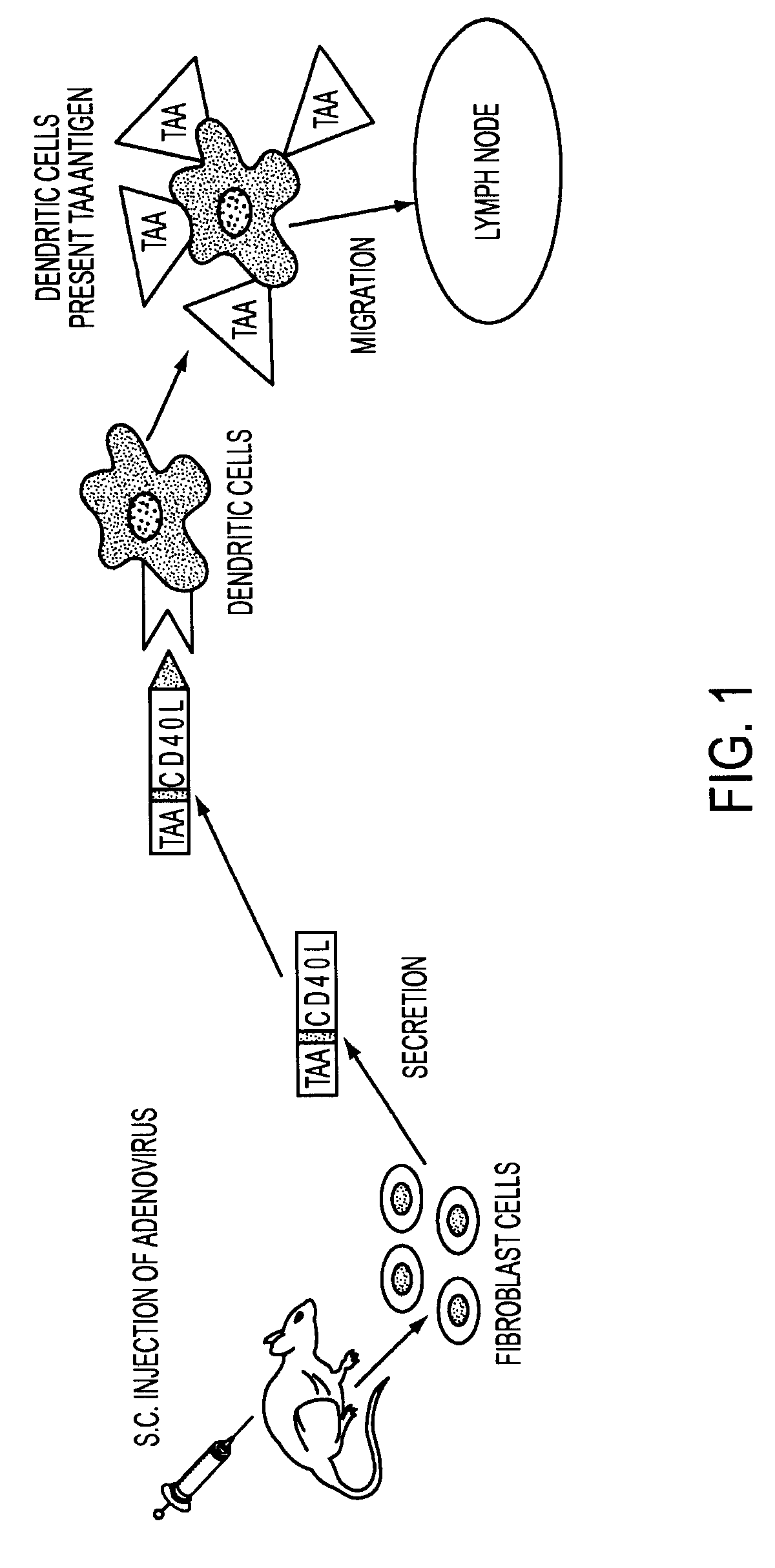
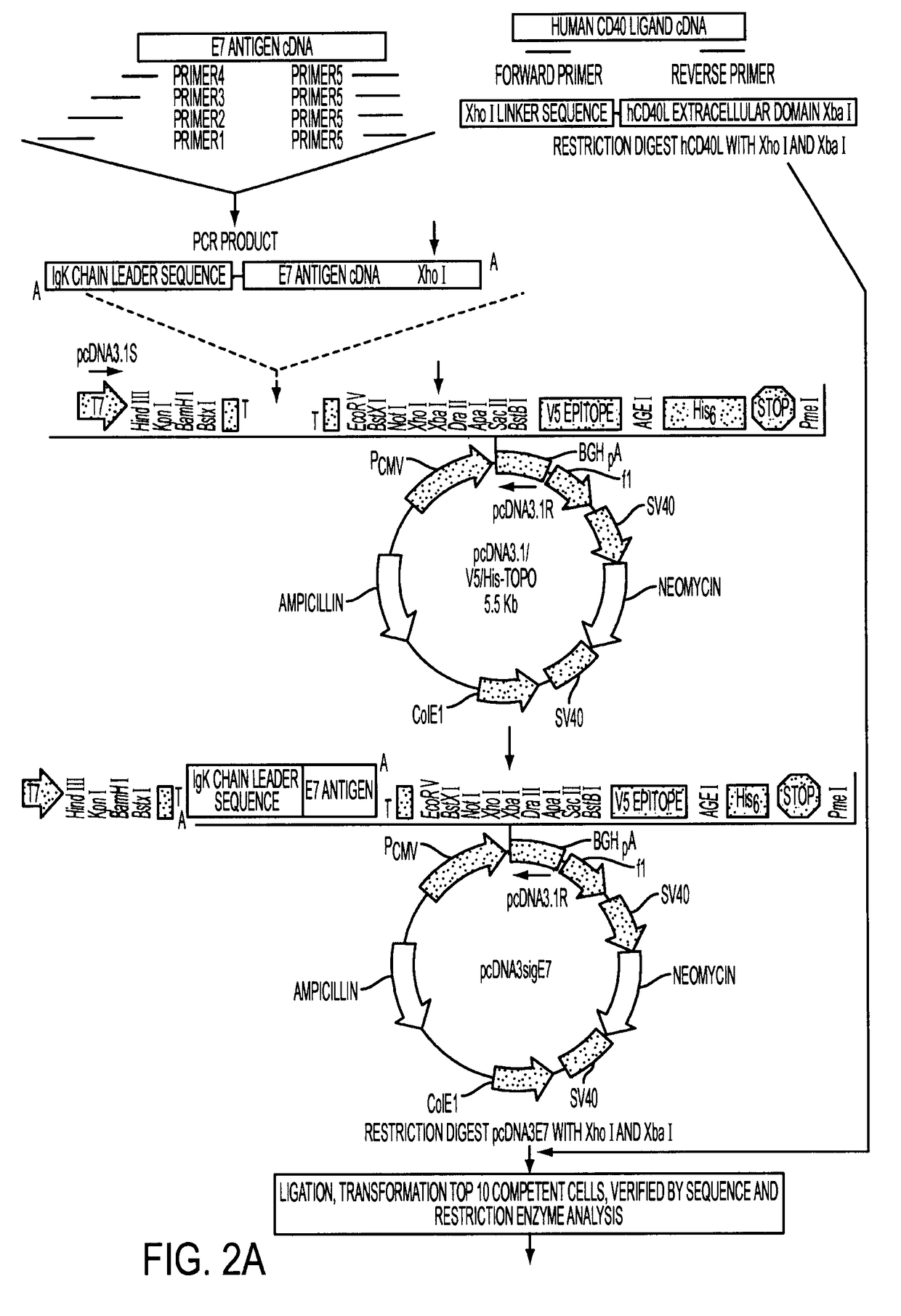
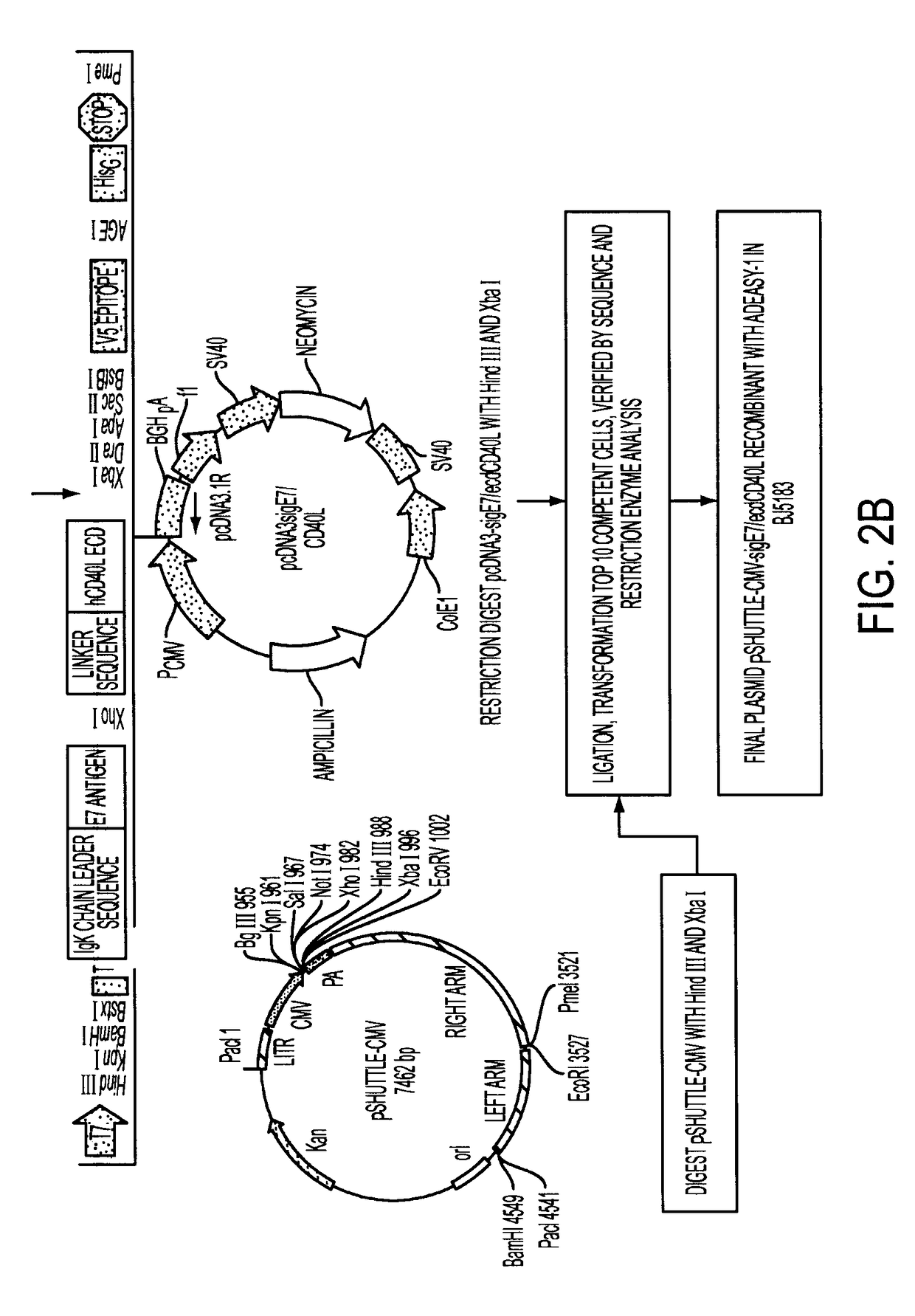
Adenoviral expression vector comprising a CD40L fusion protein adapted to elicit cellular immunity
ActiveUS8119117B2Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsTransmembrane domainTumor antigen
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
Novel Anti-fibroblast activation protein (FAP) binding agents and uses thereof
InactiveUS20180022822A1Enhance tumor targeting tumorEnhance tumor tumor selectivityAntibody mimetics/scaffoldsAntipyreticAntigenAntigen receptors
Provided are novel human-derived antibodies specific for Fibroblast Activation Protein (FAP), preferably capable of selectively inhibiting the enzymatic activity of FAP, and chimeric antigen receptors (CARs) directed against the human FAP antigen as well as methods related thereto. In addition, methods of diagnosing and / or monitoring diseases and treatments thereof which are associated with FAP are provided. Assays and kits related to antibodies specific for FAP are also disclosed. The novel anti-FAP antibodies can be used in pharmaceutical and diagnostic compositions for FAP-targeted immunotherapy and diagnostics.
Owner:MABIMMUNE DIAGNOSTICS AG
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Adjuvant used for vaccine and application thereof
ActiveCN106177939ALow costEasy to usePharmaceutical non-active ingredientsImmunological disordersAdjuvantProtein stabilization
The invention provides an adjuvant used for vaccine. The adjuvant comprises alumina gel, levamisole or its derivative, and also polyacrylate. The invention also discloses a vaccine composition containing the adjuvant and an application thereof. The adjuvant can be used as the adjuvant for inactivated vaccine, and also can be used for preparing protein stabilization liquid. The vaccine composition containing the adjuvant can generate humoral immunity for human body and can generate cellular immunity, and can generate good immune response under condition of low antigen content and single immune, and can stabilize protein, degradation and irreversible change cannot be generated.
Owner:PU LIKE BIO ENG
Adenoviral Vector Vaccine
ActiveUS20070269409A1Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsHuman papilloma virus infectionVector vaccine
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
Bivalent inactivated vaccine for bovine multocida pasteurellosis and preparation method of bivalent inactivated vaccine
ActiveCN107569681AImprove securityImprove packaging utilizationAntibacterial agentsBacterial antigen ingredientsImmune effectsCapsular type
The invention relates to a bivalent inactivated vaccine for bovine multocida pasteurellosis, belonging to the technical field of preparation of veterinary biological products. The bivalent inactivatedvaccine provided by the invention is composed of antigens and a vaccine adjuvant, wherein the antigens are a bovine multocida pasteurellosis capsular type-A Pm-TJ strain and a bovine multocida pasteurellosis capsular type-B C45-2 strain. For the bivalent inactivated vaccine prepared by adopting the method provided by the invention, the concentration purifying process is adopted, the fermentationculture process of the bovine multocida pasteurellosis capsular type-B C45-2 strain is optimized, so that the fermentation time is shortened by one half, and the production efficiency is improved. Forthe bivalent inactivated vaccine provided by the invention, the bovine fibrinous and suppurative pneumonia and bovine hemorrhagic septicemia caused by bovine pasteurella multocida infection can be prevented through one-time injection immunization, and the bivalent inactivated vaccine has the features of safety, reliability and good immune effect.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Anti-foot-and-mouth disease vaccine composition and preparation method and application thereof
ActiveCN105566449ASafe preparationNo pathogenic effectMicroorganism based processesAntiviralsFoot-and-mouth disease virusAdjuvant
The present invention relates to the field of veterinary biological products, and more specifically discloses a type-A foot-and-mouth disease virus-like particle and an anti-foot-and-mouth disease vaccine composition and a preparation method and application thereof in prevention and / or treatment of infections-related diseases caused by FMD (foot-and-mouth disease) virus. The vaccine composition comprises a type-O FMD virus-like particle and / or type-Asia-I FMD virus-like particle and / or the type-A FMD virus-like particle and an adjuvant.
Owner:PU LIKE BIO ENG +1
Bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma hyopneumoniae and preparation method of bigeminy inactivated vaccine
ActiveCN103127497ALong-lasting immunityImprove efficiencyAntibacterial agentsViral antigen ingredientsMycoplasma hyopneumoniaeKilled Vaccine
The invention provides a bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma pneumoniae and a preparation method of the bigeminy inactivated vaccine. The bigeminy inactivated vaccine comprises inactivated porcine circovirus type 2, inactivated swine mycoplasma pneumoniae, a vaccine adjuvant, and an excipient, wherein the content of the porcine circovirus type 2 is at least105.5 tissue culture inoculated dose (TCID) 50 / head, and the content of the swine mycoplasma pneumoniae is at least 2*109 MHDCE / head. The bigeminy inactivated vaccine has the advantages that the immune effect is equal to or better than the effect of sum of commodity single vaccines in the market, two antigens do not interfere each other, immune persistent period is long, potency is lasting, and due to the fact that one time immunization only needs, cost is lowered, and stress reaction of animals is also reduced. The bigeminy inactivated vaccine can be used for preventing porcine circovirus disease and at the same time preventing the swine mycoplasma pneumoniae.
Owner:PU LIKE BIO ENG +1
Apple vinegar health drink capable of improving immunity, and preparation method thereof
ActiveCN103211260AHigh synergistic effectRealize industrializationFood preparationSugar intakeFruit juice
The invention discloses an apple vinegar health drink capable of improving immunity. The apple vinegar health drink is prepared by the following raw materials: 0.2-0.5% of dioscoreae rhizome extract, 0.05-0.2% of achyranthes bidentata extract, 0.1-0.3% of radix rehmanniae extract, 0.1-0.3% of poria cocos extract, 1.5-4% of original apple vinegar, 2-4% of concentrated apple juice, 0.4-1% of concentrated haw juice, 0.2-0.8% of honey, 0.01-0.025% of sucralose and the balance of water. The apple vinegar health drink is prepared by the five extracts of traditional Chinese medicines including dioscoreae rhizome, achyranthes bidentata, radix rehmanniae, poria cocos and chrysanthemum which have the function of improving immunity according to the scientific proportion, the extracts of traditional Chinese medicines are supplemented by the original apple vinegar, the apple juice, the haw juice and the honey, reasonable compatibility is carried out on the raw materials, and the natural sweetener-sucralose is added into the raw materials for seasoning. The apple vinegar health drink not only can meet the demand of the people with low immunity for the delicious mouthfeel under the condition of controlling excessive sugar intake, but also more importantly utilizes the traditional Chinese medicines to carry out symptomatic treatment and comprehensive conditioning on the human body and utilizes the comprehensive functions such as nutritional supplement of fruit juice and fruit vinegar, thus achieving the health care effect of improving immunity.
Owner:河南省淼雨饮品股份有限公司
Specific targeting receptor protein MAGEA and construction method of CAR-T carrier thereof
InactiveCN107446938ASolve problems without a targetLong-lasting immunityTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsHeavy chainMonoclonal antibody
The present invention provides a construction method for specifically targeting the receptor protein MAGEA and its CAR-T carrier. The construction method includes first extracting the RNA of monoclonal hybridoma cells against osteosarcoma, and then using the specific monoclonal antibody RT-PCR with specific primers, clone the variable region DNA fragments of the heavy and light chains of the antibody into the T vector, sequence, obtain the receptor protein MAGEA that specifically recognizes tumor cells, and clone its expression sequence into CAR-T On the carrier, a new type of CAR‑T carrier specifically targeting MAGEA was obtained. The present invention can provide an effective target for solid tumors treated by CAR-T cells, especially in the immunotherapy of osteosarcoma.
Owner:赵蔚
Triple inactivated vaccine for newcastle disease, infectious bronchitis and H9 subtype avian influenza
InactiveCN104922665AReliable production processImprove immunityViral antigen ingredientsAntiviralsInfectious bronchitisInfectious bronchitis virus
The invention discloses a triple inactivated vaccine for newcastle disease, infectious bronchitis and H9 subtype avian influenza. The triple inactivated vaccine comprises inactivated newcastle disease virus La Sota strain, infectious bronchitis virus M41 strain and H9 subtype avian influenza virus SY strain; the preservation number of the H9 subtype avian influenza virus SY strain is CGMCC No. 5968. The triple inactivated vaccine for newcastle disease, infectious bronchitis and H9 subtype avian influenza is good in immune effect, and stable and reliable in preparation technology.
Owner:YANGLING LVFANG BIOLOGICAL ENG CO LTD
Porcine circovirus 2 and porcine circovirus 3 bivalent inactivated vaccine and preparation method thereof
ActiveCN107854688AImprove immunityNo mutual interferenceBacterial antigen ingredientsViral antigen ingredientsImmune effectsPorcine circovirus
The invention provides a porcine circovirus 2 and porcine circovirus 3 bivalent inactivated vaccine and a preparation method thereof. The bivalent inactivated vaccine comprises inactivated porcine circovirus 2 and 3 and a vaccine adjuvant, wherein the content of the porcine circovirus 2 and 3 is respectively at least 105.5 TCID50 / piece. The bivalent inactivated vaccine disclosed by the invention has the following advantages that the immune effect of the porcine circovirus 2 is equivalent to or better than that of a commercial vaccine, a porcine circovirus 3 commercial vaccine does not exist inthe market, and two antigens do not interfere with each other; the immune duration is long, and the efficacy is lasting; and only one shot immunization is needed, the cost is reduced, and stress reactions of animals are reduced. The bivalent inactivated vaccine disclosed by the invention can be applied to simultaneously preventing the porcine circovirus 2 and 3.
Owner:陕西诺威利华生物科技有限公司
Mucin antigen vaccine
ActiveUS20050226887A1Enhance immune responseLong-lasting immunityAntibody mimetics/scaffoldsNGF-receptor/TNF-receptor superfamilyAntigenCancer cell
Provided are expression vectors for generating an immune response to a mucin. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal, a mucin antigen and CD40 ligand. Also provided are methods of generating an immune response against cells expressing a mucin by administering an effective amount of the vector. Further provided are methods of generating an immune response against cancer cells expressing a mucin in an individual by administering an effective amount of the vector. Still further provided are methods of overcoming anergy to a mucin self antigen by administering an effective amount of the vector.
Owner:MICROVAX
O-type foot-and-mouth disease virus-like particle antigen, vaccine composition containing O-type foot-and-mouth disease virus-like particle antigen, and preparation method and application of vaccine composition
ActiveCN111233984AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsDiseaseParticulate antigen
The invention provides a foot-and-mouth disease virus-like particle antigen. The foot-and-mouth disease virus-like particle antigen consists of a VP0 antigen protein, a VP1 antigen protein and a VP3 antigen protein through assembling, wherein the VP0 antigen protein is coded by a nucleotide sequence as shown in Seq ID No.1 or coded by a degenerate sequence of the nucleotide sequence as shown in Seq ID No.1, the VP1 antigen protein is coded by a nucleotide sequence as shown in Seq ID No.3 or coded by a degenerate sequence of the nucleotide sequence as shown in Seq ID No.3, and the VP3 antigen protein is coded by a nucleotide sequence as shown in Seq ID No.2 or coded by a degenerate sequence of the nucleotide sequence as shown in Seq ID No.2. The foot-and-mouth disease virus-like particle antigen has favorable immunogenicity, a vaccine composition prepared from the foot-and-mouth disease virus-like particle antigen can protect current epidemic O-type Ind strains through one-time immunization, and the immunoprotection period is long. The invention further provides the vaccine composition prepared from the foot-and-mouth disease virus-like particle antigen, and a preparation method andan application of the vaccine.
Owner:PU LIKE BIO ENG
Group I avian adenovirus type 8 strain and application thereof
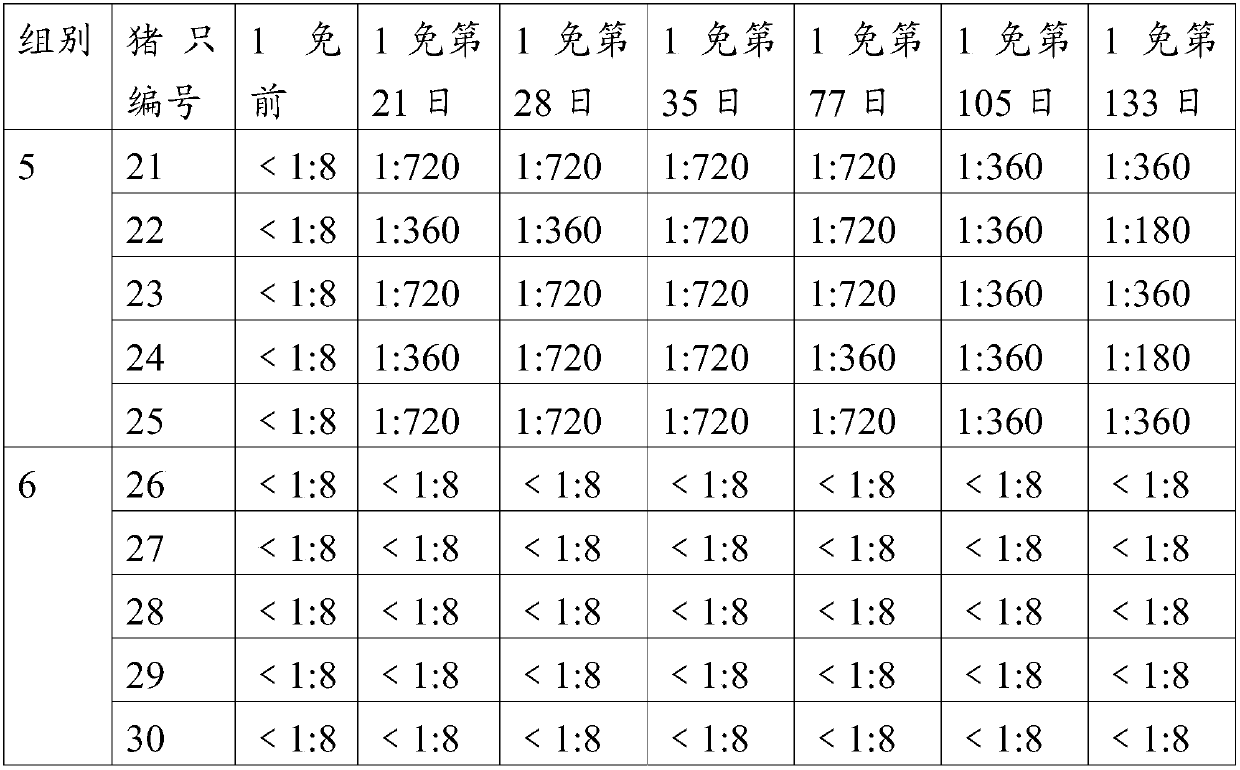
ActiveCN109207437AImprove securityImprove protectionViral antigen ingredientsDigestive systemDiseaseInclusion bodies
The invention relates to the technical field of veterinary biological products, in particular to a group I avian adenovirus type 8 strain and application thereof. The group I avian adenovirus type 8 strain ZMYTAV-8 strain, with an accession number of CGMCC No. 14297 has the characteristics of high toxin production and good immunogenicity. The strain can effectively prevent chicken inclusion body hepatitis caused by serum avian adenovirus type 8. Therefore, the invention also provides application of the avian adenovirus type 8 strain ZMYTAV-8 in the preparation of drugs for the prevention of inclusion body hepatitis. Further, the present invention provides a bivalent inactivated vaccine of avian adenovirus serotype 4, serotype 8 for the prevention of pericardial effusion-hepatitis syndromeand inclusion body hepatitis. The vaccine has good immunogenicity and can prevent avian diseases caused by adenovirus, such as inclusion body hepatitis and pericardial effusion-hepatitis syndrome, which are popular in recent years. The vaccine has 100% protection rate against local isolates, and has high safety, rapid antibody production and stable efficacy.
Owner:乾元浩生物股份有限公司
Pseudorabies virus passage attenuated strain and application thereof
ActiveCN110387354ALow toxicityImprove securityViral antigen ingredientsInactivation/attenuationAttenuated strainToxin
The invention provides a pseudorabies virus passage attenuated strain and an application thereof, and belongs to the field of vaccines of animal medicine. The pseudorabies virus passage attenuated strain is a pseudorabies virus LA2017 strain, and the preservation number is CGMCC No.18170. The invention also provides an application of the pseudorabies virus passage attenuated strain to preparationof pseudorabies vaccines, and a vaccine using the pseudorabies virus passage attenuated strain as an active component. The LA2017 strain is a natural deletion attenuated strain, the virulence is notably reduced, and when new-born piggies are inoculated with the LA2017 strain, no adverse reactions are caused. The strain is obtained by a passage attenuated method, so that the biological security risk is low. After weaned highly-susceptible piggies are subjected to primary inoculation of the vaccines prepared from the LA2017 strain for 7 days, 100% of protective effects can be achieved, and the immunization persistent period can achieve 5 months. The vaccines not only can prevent pathogenesis but also can prevent toxin expelling, and purification of pseudorabies virus variation strains can beextremely facilitated.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Brucella deoxyribonucleic acid (DNA) vaccine as well as construction method and application thereof
InactiveCN102772793AAvoid defectsEasy to makeAntibacterial agentsBacterial antigen ingredientsCompetent cellBrucella abortus
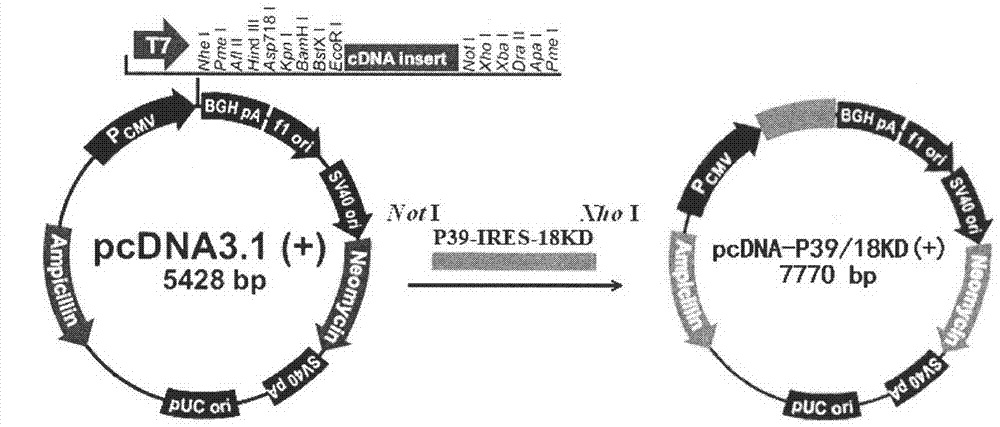
The invention discloses a Brucella deoxyribonucleic acid (DNA) vaccine and a construction method thereof as well as the application of the vaccine. The Brucella DNA vaccine disclosed by the invention contains Brucella P39 and 18KD genes which can also be co-expressed in eukaryotic cells by internal ribosome entry site (IRES) mediation. The Brucella DNA vaccine disclosed by the invention has an SEQ1 gene sequence. According to the Brucella DNA vaccine and the construction method thereof, which are disclosed by the invention, the total DNA of Brucella abortus 2 type CVCC 12 is taken as a template, a polymerase chain reaction is carried out by using P39S primers SEQ2 and SEQ3 and 18KDS primers SEQ4 and SEQ5 respectively so as to obtain a target gene, and then the target gene is converted into a JM109 competent cell after being cleaved so as to obtain positive plasmid named as pQC-P39 / 18KD. Then, the pQC-P39 / 18KD and pcDNA3.1 plasmid are connected by using a T4DNA ligase after being cleaved, and afterwards are converted into E.coli DH5 alpha so as to obtain pcDNA-P39 / 18KD recombinant plasmid.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Nucleic acid and recombinant protein co-immune vaccine based on classical swine fever virus gene as well as preparation method and application of co-immune vaccine
PendingCN111973738AImproving immunogenicityImmunization blank period is shortSsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVAdjuvant
The invention discloses a nucleic acid and recombinant protein co-immune vaccine based on a classical swine fever virus gene as well as a preparation method and application of the co-immune vaccine. The preparation method comprises the steps that firstly, optimized recombinant plasmids and recombinant classical swine fever vaccine antigen protein are obtained by combining gene engineering and cellengineering; then the nucleic acid sequence and the recombinant protein are compounded into different liquid phase layers of a biphasic adjuvant, so that the obtained co-immune vaccine is good in immunogenicity and short in immune blank period, can rapidly induce an organism to generate high-level, high-affinity and durable antibody response and immune memory, effectively protects a target animalfrom being attacked by classical swine fever virulent viruses, and the method can be applied to preparation of products for preventing / treating classical swine fever.
Owner:天康生物制药有限公司
Canine distemper virus attenuated vaccine strain and application thereof
ActiveCN109735504AGenetically stableImprove immunityAntibacterial agentsBacterial antigen ingredientsDiseaseMicroorganism
The invention discloses a canine distemper virus attenuated vaccine strain and application thereof. The canine distemper virus is named as an HBa strain, and the microorganism has the preservation number of CGMCC No.15036. The separated canine distemper virus is subjected to passage and cloning on a Vero cell, the attenuated vaccine strain (HBa strain) is cultured, and the results of a pathogenicity test and an immunogenicity test show that the canine distemper virus HBa strain is not pathogenic to both minks or dogs and can provide quite good protecting force for other sources of strong poison attacks. The attenuated vaccine strain has good immunogenicity, can be prepared into a single vaccine or combined vaccine, can effectively prevent diseases caused by the canine distemper virus, andcan also be used for preparing reagents or kits for treating, diagnosing or detecting the diseases caused by the canine distemper virus. The attenuated vaccine strain has the advantages of being stable in hereditary, lasting in immune, good in effect, safe, reliable, long in storage life and the life.
Owner:兆丰华生物科技(南京)有限公司 +3
Immunopotentiator for swine atrophic rhinitis inactivated vaccine and preparation method of immunopotentiator
ActiveCN107261134ASuppress latent infectionAvoid latent infectionAntibacterial agentsBacterial antigen ingredientsPig farmsVitamin C
The invention discloses an immunopotentiator for a swine atrophic rhinitis inactivated vaccine. The immunopotentiator comprises a liquid containing dry powder immunity particles, and a solvent, wherein the liquid containing the dry powder immunity particles mainly comprises an aluminium hydroxide aqueous solution, ginsenoside, cholesterol, a vitamin C, diethylaminoethyl glucan, potassium iodide and polyethylene glycol according to an appropriate ratio by compatibility. Compared with the prior art, the prepared immunopotentiator for the swine atrophic rhinitis inactivated vaccine can effectively inhibit establishment and reactivation of latent infection of bordetella bronchiseptica and pasteurella multocida, can shorten a window phase of antibody production, improves an immunity effect of the swine atrophic rhinitis vaccine, prolongs the immunity duration, effectively avoids latent infection of swine atrophic rhinitis, provides guarantee for prevention and elimination of the swine atrophic rhinitis, can be widely applied to a pig farm for prevention and treatment of the swine atrophic rhinitis, and increases an economic benefit of the pig farm.
Owner:SHANGHAI CHUANG HONG BIOTECH
Fast and efficient preparation method of pig A type clostridium perfringens inactivated vaccine
ActiveCN108273051AInhibitory activityAvoid problems such as toxicity dropAntibacterial agentsBacterial antigen ingredientsLiver stomachAdjuvant
The invention belongs to the technical field of veterinary biological products, and relates to a fast and efficient preparation method of a pig A type clostridium perfringens inactivated vaccine. Themethod comprises the following steps of picking a suspected sample of an A type clostridium perfringens lethal diseased pig, scraping an intestinal content, and refrigerating or freezing; autoclavinga culture medium in an autoclave, after temperature reducing, pouring the suspected sample in the autoclave, carrying out anaerobic culture, and carrying out passage for three times; inoculating bacteria liquid subjected to three-time passage into a meat liver-stomach membrane digestion soup, culturing, and centrifuging after finishing culturing, thus obtaining a supernatant liquor; adding an inactivator into the supernatant liquor, fully shaking out, inactivating, and detoxifying to obtain inactivated bacteria liquid; mixing the inactivated bacteria liquid and an adjuvant to obtain the inactivated vaccine. The method provided by the invention has the advantages of simple process, low cost, and low pollution; the prepared inactivated vaccine has the advantages of high safety, no adverse reaction after being injected, high immunization efficiency and long immunity period.
Owner:鄂尔多斯市旭和畜牧有限责任公司
Preparation method of fructus momordicae health wine
InactiveCN108220093AUnique tasteUnique fragranceAnthropod material medical ingredientsDigestive systemForest yamMedicine
The invention discloses a preparation method of fructus momordicae health wine. The preparation method comprises the following steps: preparing fructus momordicae raw liquid; mixing the raw liquid andleaching liquid of maca, ginseng, burdock, medlar, jujubes, gecko tails, male silkworm moths and yam; mixing the mixed raw liquid, honey and crystal sugar, filtering, clarifying and aging to obtain the health wine. The health wine prepared by the preparation method disclosed by the invention has good liver-protecting and kidney-tonifying effects.
Owner:泸州施可富大曲酒厂有限责任公司
Low toxic bacterial strain of Pasteurella multocid B26-T1200
ActiveCN1789410AImprove securityImprove stabilityBacterial antigen ingredientsBacteriaPasteurella aviumImmunity
The invention relates the attenuated strain used for preparing pasteurella multocid vaccine, which is pasteurella multocid B26-T1200. The method comprises the following steps: adding the virulent strain B26 into martin soup containing the 0.1% splitting sheep whole blood to propagate at 37-45Deg.C, measuring the virulence and immunity of passage strain, and getting the pasteurella multocid B26-T1200 at the 1200 generation. The pasteurella multocid vaccine used by the said attenuated strain has high safety, good stability, and long immunity duration. The minimum immunizing dose of the strain is 13,000,000 viable bacteria.
Owner:GUANGXI VETERINARY RES INST
Goat contagious pleuropneumonia subunit vaccine as well as preparation method and application thereof
ActiveCN112870341AReduce spreadReduce the risk of contaminationAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Rabies vaccine for human use and preparation method thereof
ActiveCN105031645AProduce quicklyImprove immune response timeAntiviralsAntibody medical ingredientsZinc hydroxideHuman use
The invention discloses a rabies vaccine for human use. The rabies vaccine is characterized by comprising a rabies virus inactivated vaccine material and a compound adjuvant, wherein the compound adjuvant is composed of zinc hydroxide colloid, squalene, hyaluronic acid and Tween-80; each dosage of the rabies virus inactivated vaccine material uses 0.1-10mg of zinc hydroxide colloid, 10-1000 mug of squalene, and 10-1000 mug of hyaluronic acid, and 10-1000 mug of Tween-80. With the adoption of the compound adjuvant, the immune response time is greatly prolonged, the neutralization is rapidly generated, the immuning capacity lasts for a long time, meanwhile, the preparation technology is simple, the needed equipment is easily available, the industrial production cost is reduced greatly, and the stability of the obtained rabies vaccine is also greatly improved.
Owner:NINGBO RONGAN BIOLOGICAL PHARMA
Long-acting animal rabies vaccine and preparing method thereof
InactiveCN101537179BLong-term immune protectionEasy to useAntiviralsAntibody medical ingredientsViral glycoproteinRecombinant vaccines
The invention relates to a long-acting animal rabies vaccine and a preparing method thereof. Replication-defective retrovirus is used as a carrier. After forming a virus infection organism, the carrier can be integrated into a chromosome of a host cell. An expression cassette for expressing a rabies virus glycosidoprotein is used as an exogenous target gene, and the protein expressed by the target gene can stimulate the organism to produce the immune response to the rabies virus. By converting the packaging cell of the retrovirus, recombinant viruses can be continuously produced to recombineviruses and immune animals, and therefore the animals can obtain long-term immune protection effect on the rabies. The recombinant vaccine has the advantages of wide applicable range for animals, safe use, and long duration time of the induced immune effective period, and is a novel long-effective vaccine for preventing rabies.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
A kind of human rabies vaccine and preparation method thereof
ActiveCN104922667BProduce quicklyImprove immune response timeAntiviralsAntibody medical ingredientsZinc hydroxideAdjuvant
Owner:NINGBO RONGAN BIOLOGICAL PHARMA
Porcine circovirus type 2 and porcine circovirus type 3 bivalent inactivated vaccine and preparation method thereof
ActiveCN107854688BImprove immunityNo mutual interferenceBacterial antigen ingredientsViral antigen ingredientsImmune effectsCircovirus
The invention provides a porcine circovirus 2 and porcine circovirus 3 bivalent inactivated vaccine and a preparation method thereof. The bivalent inactivated vaccine comprises inactivated porcine circovirus 2 and 3 and a vaccine adjuvant, wherein the content of the porcine circovirus 2 and 3 is respectively at least 105.5 TCID50 / piece. The bivalent inactivated vaccine disclosed by the invention has the following advantages that the immune effect of the porcine circovirus 2 is equivalent to or better than that of a commercial vaccine, a porcine circovirus 3 commercial vaccine does not exist inthe market, and two antigens do not interfere with each other; the immune duration is long, and the efficacy is lasting; and only one shot immunization is needed, the cost is reduced, and stress reactions of animals are reduced. The bivalent inactivated vaccine disclosed by the invention can be applied to simultaneously preventing the porcine circovirus 2 and 3.
Owner:陕西诺威利华生物科技有限公司
A kind of bivalent inactivated vaccine of bovine pasteurellosis multocida and preparation method thereof
ActiveCN107569681BImprove securityImprove packaging utilizationAntibacterial agentsBacterial antigen ingredientsImmune effectsCMV Pneumonia
The invention relates to a bivalent inactivated vaccine for bovine Pasteurella multocida and belongs to the technical field of preparation of veterinary biological products. The bivalent inactivated vaccine of the present invention is composed of an antigen and a vaccine adjuvant, wherein the antigen is the Pasteurella multocida capsular type A Pm-TJ strain and the Pasteurella multocida capsular type B C45-2 strain. The bivalent inactivated vaccine prepared by the present invention adopts a concentration and purification process, and optimizes the fermentation and cultivation process of the Pasteurella multocida capsular B type C45-2 strain, which shortens the fermentation time by more than half and improves production efficiency. The invented bivalent inactivated vaccine can simultaneously prevent bovine fibrinous suppurative pneumonia and bovine hemorrhagic sepsis caused by Pasteurella multocida infection through one-shot immunization, and has the characteristics of safety, reliability and good immune effect.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com