Group I avian adenovirus type 8 strain and application thereof
A technology of poultry adenovirus and strains, applied in the application field of bivalent inactivated vaccines, can solve the problems of wrong type of cross-protection vaccine and poor protection effect, and achieve long duration of immunity, stable immune effect and good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Isolation and Screening of Virus Strains
[0041] 1. Isolation and identification of avian adenovirus strains
[0042] Since 2014, the inventor has collected multiple suspected poultry adenovirus-infected tissue samples in Liaoning, Shandong, Henan, Jiangsu and other places. The main apparent necropsy lesions of the sick chickens: it can be seen that about 5-10mL of adenovirus has accumulated in the pericardial cavity. Light yellow clear water or jelly-like liquid; the liver is lighter in color, with local necrotic lesions, and the kidneys are pale, swollen, and bleeding.
[0043]Collect the liver and spleen tissues of diseased chickens with typical symptoms and pathological changes, cut them into pieces and grind them, add sterilized PBS at a weight ratio of 1:3 after weighing, centrifuge at 4°C, 6000r / min for 10min, and centrifuge at 4°C, 8000r / min. Centrifuge for 10 min, transfer the supernatant to another sterile 1.5 mL centrifuge tube, add double antibod...
Embodiment 2
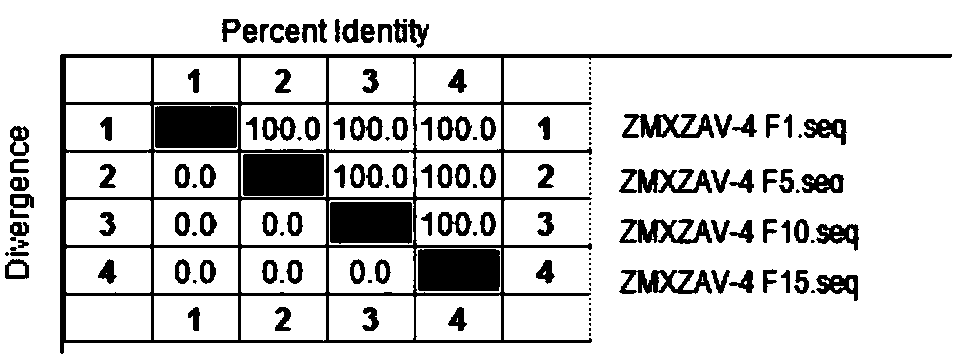
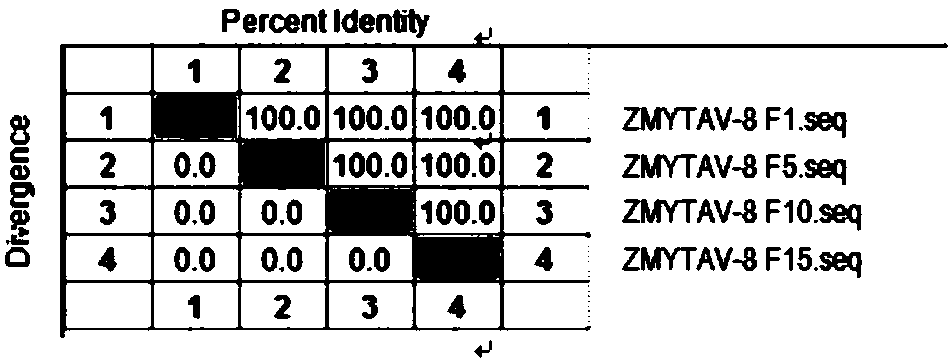
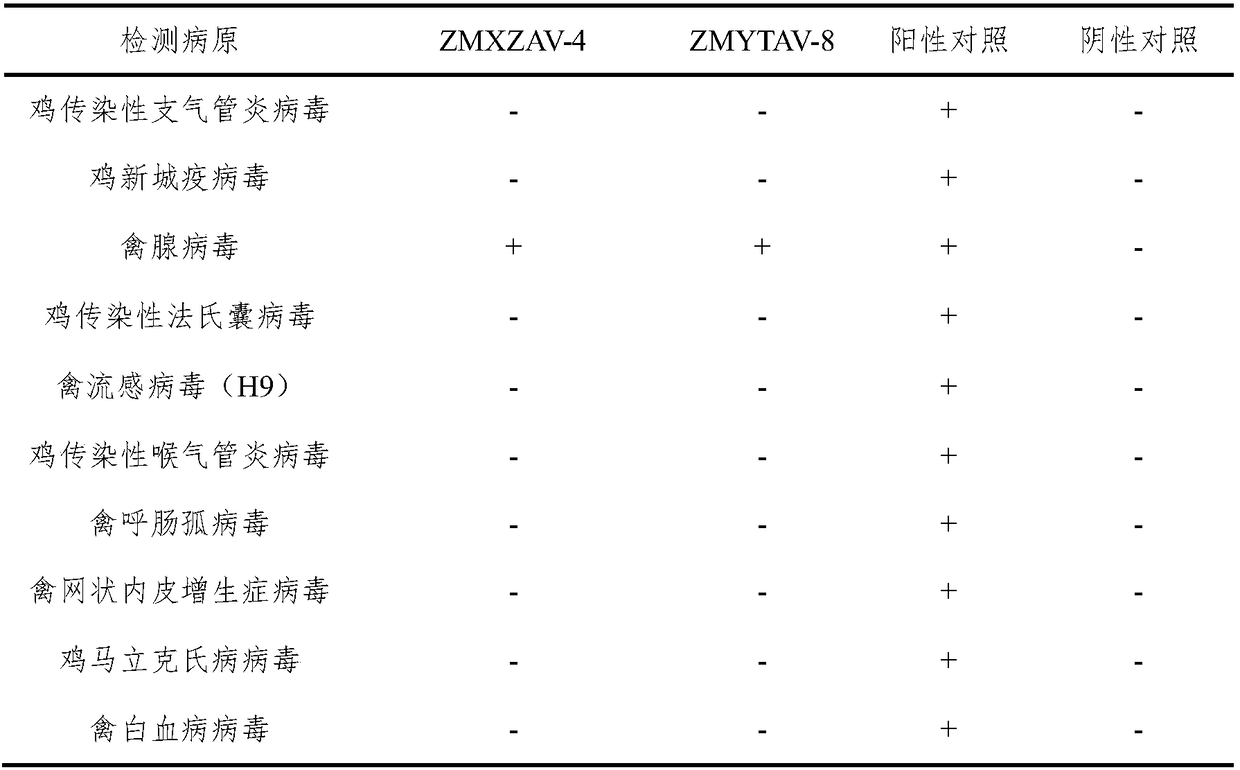
[0051] Example 2 ZMXZAV-4 strain, ZMYTAV-8 strain identification and properties
[0052] 1. Sterility test
[0053] Tested according to the appendix of the current "Chinese Veterinary Pharmacopoeia", ZMXZAV-4 strain and ZMYTAV-8 strain F1 generation seed virus samples grew aseptically after inoculation respectively.
[0054] 2. Determination of virus content
[0055] After ZMXZAV-4 strain, ZMYTAV-8 strain F1, F5, F10, F15 generation virus solution was diluted 10 times, take 10 -5 、10 -6 、10 -7 、10 -8 、10 -9 、10 -10 Six dilutions were inoculated into well-growing 96-well culture plates of LMH cells, and placed at 37°C and 5% CO 2 After 2 hours of incubation, maintenance solution was added and culture continued for 168 hours. After 168 hours, the cell lesions were observed hole by hole, and the typical lesions of the inoculated cells were judged as infection, and the virus content was calculated according to the Reed-Muench method. The results show that the virus conten...
Embodiment 3
[0072] Embodiment 3 ZMXZAV-4 strain, ZMYTAV-8 strain virus seed preparation
[0073] 1. Optimal inoculum volume
[0074] ZMXZAV-4 strain and ZMYTAV-8 strain seed virus were inoculated on well-growing monolayer LMH cells according to 1:100, 1:1000, and 1:10000 respectively. Each sample was repeated 3 times, and placed at 37 ° C, 5% CO 2 Cultivate and observe the cell changes. When more than 80% of the cells are damaged, harvest the virus solution. After repeated freezing and thawing twice, use the real-time PCR method to measure the virus particles in the culture, and select the one with the highest concentration to determine the optimal inoculation dose.
[0075] 2. The best time to harvest
[0076]According to the results of the optimal inoculation dose test, select the optimal inoculation dose, inoculate on the well-growing monolayer LMH cells, freeze and thaw after 24, 48, 72, 96, 120, 144, 168, and 192 hours after inoculation, respectively Three times, the cell culture w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com