Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Killed Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An inactivated vaccine (or killed vaccine) is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then killed using a method such as heat or formaldehyde.
Recombinant vesiculoviruses and their uses
The invention provides recombinant replicable vesiculoviruses. The invention provides a method which, for the first time, successfully allows the production and recovery of replicable vesiculoviruses, as well as recombinant replicable vesiculoviruses, from cloned DNA, by a method comprising expression of the full-length positive-strand vesiculovirus antigenomic RNA in host cells. The recombinant vesiculoviruses do not cause serious pathology in humans, can be obtained in high titers, and have use as vaccines. The recombinant vesiculoviruses can also be inactivated for use as killed vaccines.
Owner:YALE UNIV
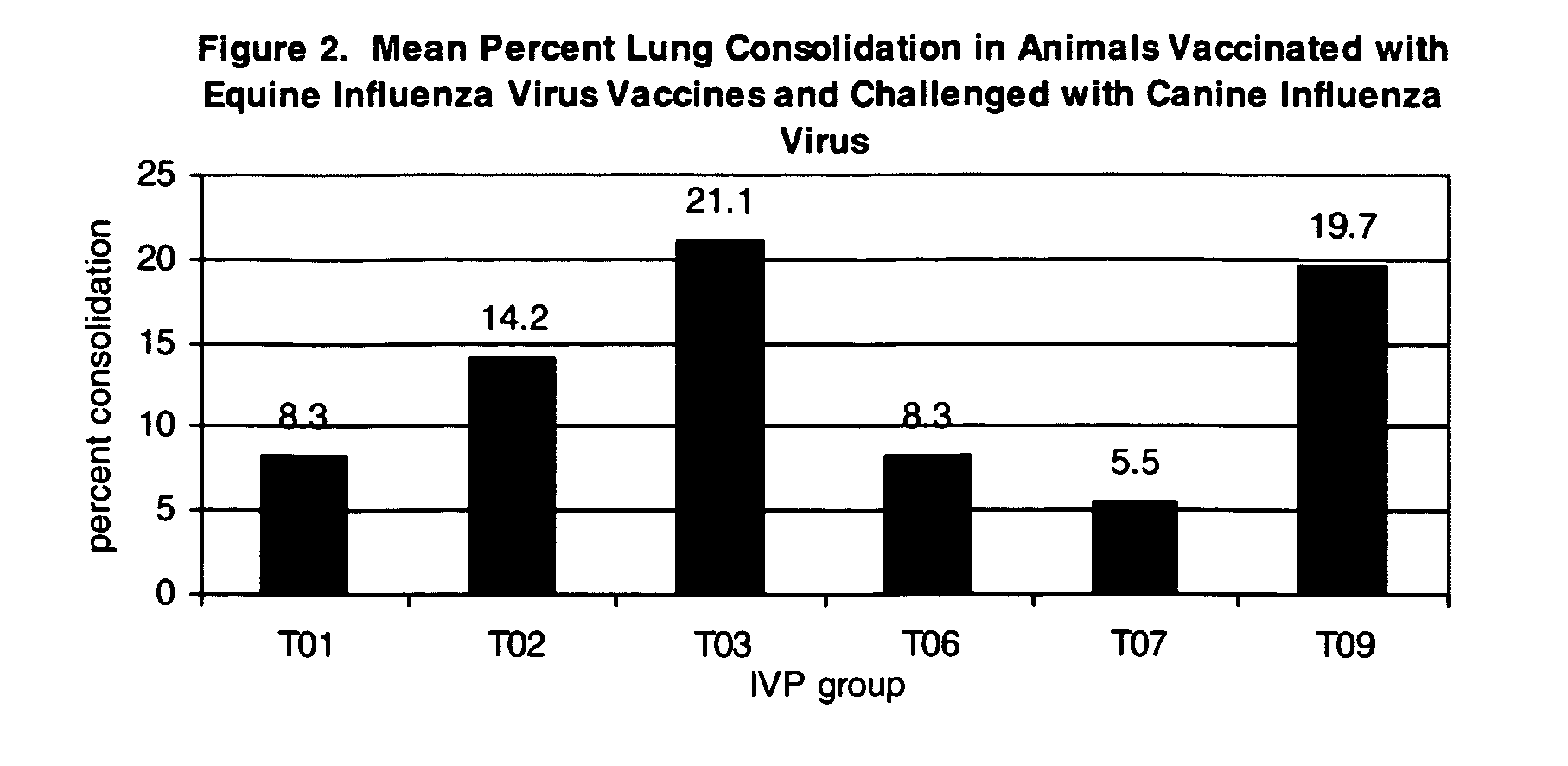
Vaccines and methods to treat canine influenza
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Novel vaccine adjuvant
ActiveCN101428145AGood immune boosterWide range of usePowder deliveryViral antigen ingredientsEmulsionBody fluid
The invention discloses a nanometer particle adjuvant, a preparation method thereof, and the application thereof in the preparation of inactivated vaccines. According to the invention, a novel nanometer particle adjuvant is obtained through preparation by the emulsion preparation and the nanometer treatment following the dissolving of different immunopotentiator and surfactant in water and oil respectively. The adjuvant provided by the invention not only can remarkably improve the humoral immune response and the cellular immune response of various bacterial and viral inactivated vaccines, butalso has unique advantages in the preparation process, the stability, the immunity duration and the side reaction of vaccines.
Owner:BEIJING CENT BIOLOGY
Pseudo-rabies gE/gI-gene loss poison strain, killed vaccine containing it and use
ActiveCN1940063AStrong targetingFacilitate chemical processingViral antigen ingredientsViruses/bacteriophagesRabiesPseudorabies Virus PRV
A recombinant Pseudorabies virus PrV gene engineering strain WKQ-001, inactivated vaccine containing the poisonous strain and its use are disclosed. It can be used to discriminate and diagnose artificial immunity pig or natural infectious pig. It's safe and doesn't contain exogenous gene.
Owner:HUAZHONG AGRI UNIV
Preparation method of mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine
ActiveCN103479995AImproving immunogenicityAvoid infectionAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma synoviae
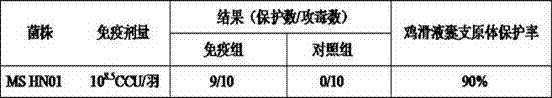
The invention relates to a preparation method of a mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine. The method comprises the steps as follows: a mycoplasma gallisepticum virulent CR strain and a mycoplasma synoviae HN01 strain which have good immunogenicity are inoculated on a proper culture medium for cultivation, so that a culture is acquired; and the culture is inactivated through a formaldehyde solution and then is mixed with an oil emulsion adjuvant and emulsified, so that the mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine is prepared. The mycoplasma gallisepticum and mycoplasma synoviae bivalent inactivated vaccine is used for preventing mycoplasma gallisepticum and mycoplasma synoviae diseases, and can realize immunization, prevent two pathogens at the same time and reduce the workload of immunization; and the prepared vaccine is stable in performance, good in immune effect, and more suitable for actual production of China.
Owner:兆丰华生物科技(南京)有限公司
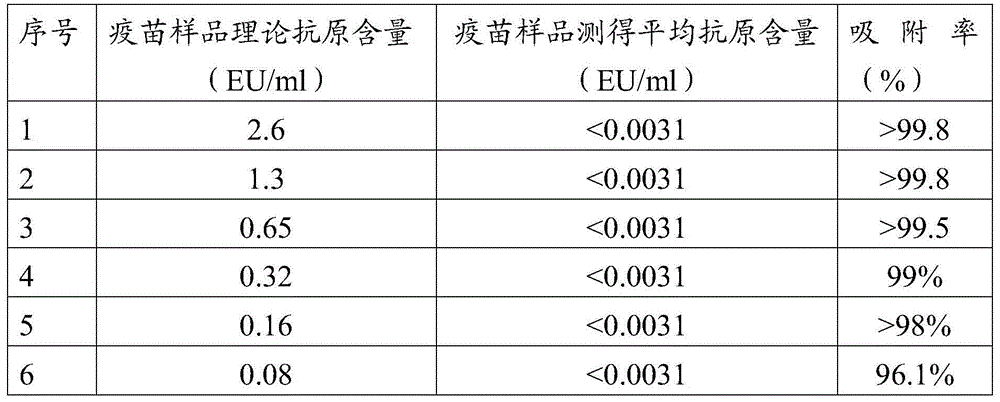
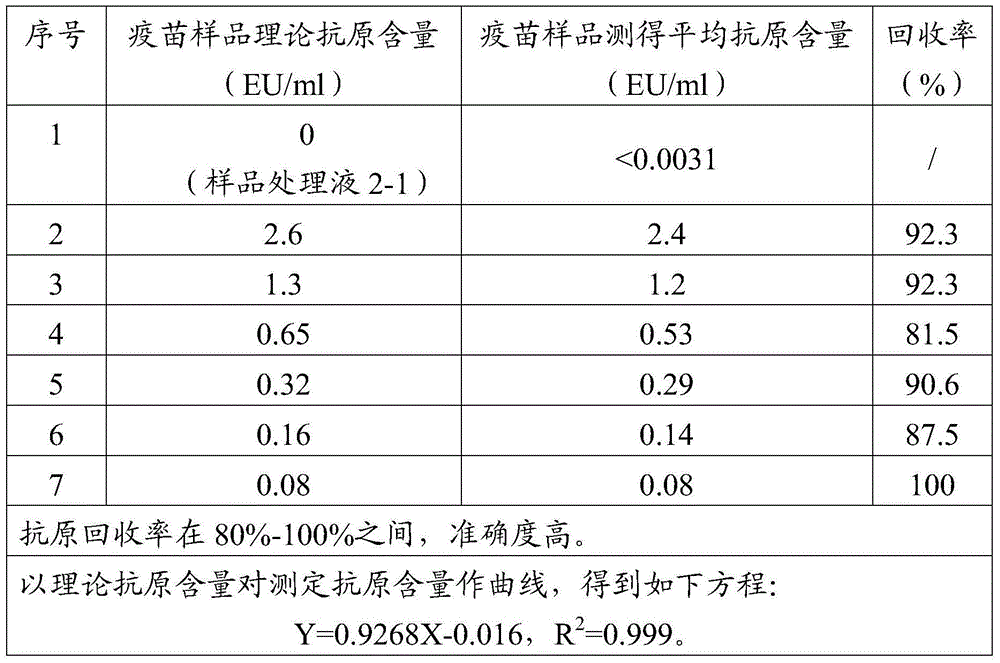
Treatment liquid and method using same to measure antigen content of aluminum salt adsorption type vaccines
ActiveCN104634959AAccurate detectionEfficient detectionMaterial analysisJapanese encephalitis vaccineAntigen
The invention discloses a treatment liquid for desorbing antigens in an aluminum salt adsorption type vaccine. The treatment liquid is a phosphate buffer solution or a citrate buffer solution, wherein the buffer solution contains proteins and at least one acid and / or salts of the acids. The invention further discloses a method using the provided treatment liquid to measure the antigen content of an aluminum salt adsorption type vaccine. The provided method reduces the interference brought by the aluminum adjuvant in Japanese encephalitis vaccine, is capable of rapidly and precisely measuring the antigen content in an adsorption type Japanese encephalitis inactivated vaccine, has the characteristics of good durability, high accuracy, and high precision, and can provide references for quality control of aluminum adjuvant adsorption type Japanese encephalitis inactivated vaccines.
Owner:LIVZON GROUP VACCINE ENG
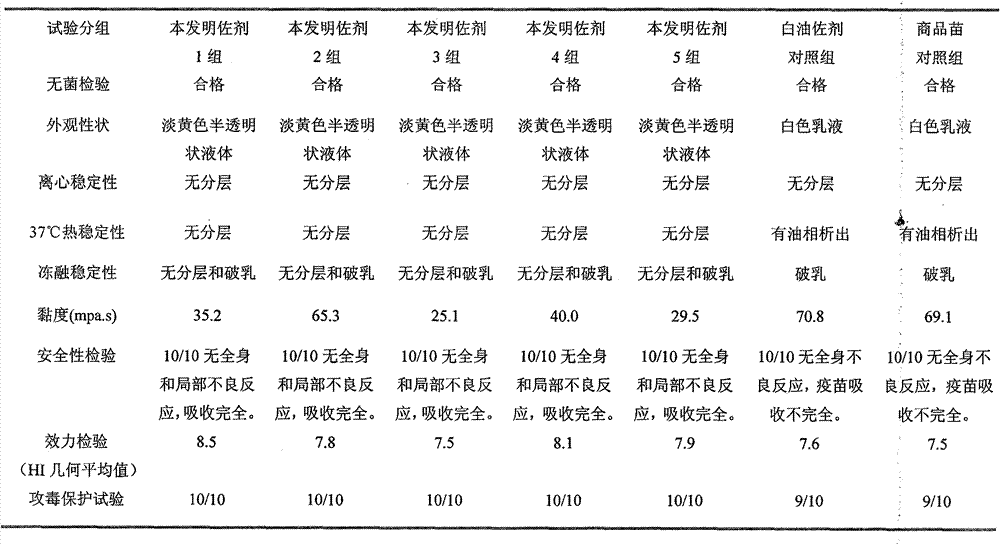
Preparation method and application of novel oil-free adjuvant
ActiveCN103083659AImprove product qualityReduce manufacturing costAntibacterial agentsAntiviralsImmune effectsAdjuvant
The invention relates to a preparation method of a novel oil-free adjuvant (adjuvant 605) compound and an application of the novel oil-free adjuvant in veterinary vaccines. The adjuvant prepared by using the method provided by the invention is capable of completely taking the place of mineral oil adjuvants and alumina gel adjuvants for inactivated vaccine production; the adjuvant can be completely absorbed by organisms within short time without residual; the adjuvant is low in side reaction, and capable of improving the safety of vaccines, and simultaneously simplifying the vaccine production process and reducing the vaccine production cost. The adjuvant prepared by using the method provided by the invention can be also taken as live vaccine diluting protection liquid, and has the characteristics of protecting the biological activity of the vaccine antigen, enhancing the immune effect of the vaccine, prolonging the persistent period of the vaccine antibody and the like.
Owner:BEIJING HUAXIA XINGYANG BIOLOGICAL SCI & TECH
Oil-in-water type compound vaccine adjuvant and method for preparing same
InactiveCN101703771AFully absorbedEarly Attack ProtectionImmunological disordersAntibody medical ingredientsPropolisPhosphate
The invention discloses an oil-in-water type compound vaccine adjuvant and a method for preparing the same. The oil-in-water type compound vaccine adjuvant comprises an oil phase and a water phase, wherein the oil phase part comprises vitamin E, lecithin, propolis, mineral oil and a surface active agent; and the water phase part comprises an aqueous carrier, wherein the aqueous carrier comprises phosphate buffer solution and a hydrophilic surface active agent. The oil-in-water type compound vaccine adjuvant has the advantages of lowering viscosity of inactivated vaccines, making the vaccines more easily injected, making animals quickly generate immunoprotection with little local response, along with simple preparation method, low cost and the suitability for mass production of the vaccines.
Owner:WENS FOOD GRP CO LTD
Preparation of tetravalent wheel shaped virus inactivated vaccine and application
ActiveCN1686540APrevent infectious diseasesViral antigen ingredientsAntiviralsBacteroidesRotavirus RNA
A deactivated tetravalent rotavirus vaccine for preventing the infantile rotavirus infections diseases is prepared from the calf kidney cells digested and dispersed by pancreatin or cultured Vero cells through inoculating rotaviruses G1, G2, G3 and G4, culturing in non-serum culture liquid D-MEM, concentrating, purifying, deactivating, mixing and adding aluminium hydroxide.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Method for making new, branch and flow H9 sub-type tri-combined inactived vaccine
InactiveCN101112617AImprove the inspection methodUnique characteristicsViral antigen ingredientsAntiviralsInfectious bronchitisDisease
The present invention relates to a preparation method of a triple inactivated vaccine for the prevention of H9 subtype newcastle disease, infectious bronchitis and avian influenza, which is characterized in that: the content of the using NDVLaSota stain virus liquid is 10 to 22 percent, the content of IBVM41 stain virus liquid is also 10 to 22 percent and the content of the AIVHL stain virus liquid is 8 to 15 percent; the present invention uses the concentration and inactivation methods; the white oil adjuvant and the immune auxiliaries of Tween, Span and aluminum stearate are added to prepare the triple inactivated vaccine; the present invention realizes the multiple vaccine prevention and achieves the purpose of prevention a plurality of diseases by one needle, which can be used for greatly developing the husbandry, so as to benefit the mankind and improve the living standard of the people.
Owner:PU LIKE BIO ENG
Method for preparing pathogenicity hydrosphere unit cell bacterium killed vaccine
InactiveCN101181635AAvoid spreadingSimple and fast operationAntibacterial agentsBacterial antigen ingredientsSide effectPathogenicity
The invention discloses a method for preparing pathogenicity aeromonas hydrophila bacteria inactivating vaccine, which comprises the following steps: firstly, inoculating the pathogenicity aeromonas hydrophila bacteria separated from the brown paralichthys olivaceus with abdominal dropsy through PCR detecting to the common LB culture medium to be cultured so as to reach a certain density; secondly, inactivating the bacteria by using formaldehyde solution; thirdly, collecting the thalli by using centrifugal culture and then diluting the thalli by using physiological saline so as to get the pathogenicity aeromonas hydrophila bacteria inactivating vaccine. The preparing method is rapid, simple and convenient in operation, and easy in batch preparing; the gotten vaccine is a pure biological agent which does not has any toxic and side effect and does not cause pollution to the meat quality of the paralichthys olivaceus and the surrounding water. The immunity protecting rate reaches 80 percent by using the vaccine prepared by the method to inoculate brown paralichthys olivaceus so as to radically curb the spreading of the pathogeny. The invention provides new approach for preventing and curing the epidemic abdominal dropsy of brown paralichthys olivaceus.
Owner:HEBEI NORMAL UNIV
Method for preparing nano aluminum hydroxide gel adjuvant
ActiveCN102988982AImprove immune efficiencyHigh purityAntibody medical ingredientsAluminium oxides/hydroxidesAluminium hydroxideSalt solution
The invention relates to a method for preparing a nano aluminum hydroxide gel adjuvant, belonging to the technical field of processes for preparing immunologic adjuvant of biological products for animals. The method comprises the following steps: mixing an aluminum salt solution and a soda solution which serve as raw materials, and adding a certain amount of stabilizing agent during mixing; shearing by a high-shearing emulsifying machine to obtain the nano aluminum hydroxide solution preliminarily; precipitating, washing and re-precipitating to obtain nano aluminum hydroxide gel; preparing a solution from the nano aluminum hydroxide; and compounding the solution with an inactivated bacterial solution to obtain an inactivated vaccine. The nano aluminum hydroxide gel adjuvant prepared by adopting the method is uniform in particle size distribution, has high purity, strong adsorbability and no side effect and has a good application prospect in the aspect of preparation of immunologic adjuvant.
Owner:山东滨州沃华生物工程有限公司
Method for producing quadruple inactivated vaccine for newcastle disease, infectious bronchitis, avian influenza (H9 subtype) and infectious bursal disease
ActiveCN102068695AProduced fastReduce generationOrganic active ingredientsViral antigen ingredientsEscherichia coliAdjuvant
The invention relates to a method for producing a quadruple inactivated vaccine for newcastle disease, infectious bronchitis, avian influenza (H9 subtype) and infectious bursal disease. In the method, the inactivated vaccine is prepared by adopting a newcastle disease virus La Sota strain, an infectious bronchitis virus M41 strain, an avian influenza virus (H9 subtype) YBF003 strain, and an Escherichia coli genetic engineering bacteria E. coil BL21 / pET28a-VP2 strain which expresses an infectious bursal disease virus VP2 protein serving as production bacteria toxic species, a super concentration process and a high-quality adjuvant. After immunizing animals, antibodies are produced rapidly; the potency is high; the protection period is long; and the outbreak and the spreading of epidemic diseases are reduced.
Owner:YEBIO BIOENG OF QINGDAO
Oil emulsion inactivated vaccine for parvoviral diseases of pigs
InactiveCN1704119AGood physical propertiesQuality improvementAntiviralsEmulsion deliveryAluminiumKilled Vaccine
The invention relates to an oil emulsion inactivated vaccine for parvoviral diseases of pigs, which is prepared by charging deactivated porcine parvovirus (PPV) into oil adjuvant for emulsion, wherein the oil adjuvant is prepared through charging aluminum tristearate into the stable solution of 94 wt% white oil and 6 wt% sorbitan mono-oleate-80, the charging amount being 2g of aluminum tristearate into each 100ml of the stable solution.
Owner:上海佳牧生物制品有限公司
Bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma hyopneumoniae and preparation method of bigeminy inactivated vaccine
ActiveCN103127497ALong-lasting immunityImprove efficiencyAntibacterial agentsViral antigen ingredientsMycoplasma hyopneumoniaeKilled Vaccine
The invention provides a bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma pneumoniae and a preparation method of the bigeminy inactivated vaccine. The bigeminy inactivated vaccine comprises inactivated porcine circovirus type 2, inactivated swine mycoplasma pneumoniae, a vaccine adjuvant, and an excipient, wherein the content of the porcine circovirus type 2 is at least105.5 tissue culture inoculated dose (TCID) 50 / head, and the content of the swine mycoplasma pneumoniae is at least 2*109 MHDCE / head. The bigeminy inactivated vaccine has the advantages that the immune effect is equal to or better than the effect of sum of commodity single vaccines in the market, two antigens do not interfere each other, immune persistent period is long, potency is lasting, and due to the fact that one time immunization only needs, cost is lowered, and stress reaction of animals is also reduced. The bigeminy inactivated vaccine can be used for preventing porcine circovirus disease and at the same time preventing the swine mycoplasma pneumoniae.
Owner:PU LIKE BIO ENG +1
Method of preparing newcastle disease, infectiousness bronchitis bigeminy killed vaccine
InactiveCN101108248AReduce usageReduce stepsViral antigen ingredientsRespiratory disorderInfectious bronchitisAdjuvant
The invention relates to a method to prepare a combined inactive vaccine of newcastle disease and infectious bronchitis vaccine for chicks. The invention is characterized in that: firstly, concentrate and inactivate the NDVLaSota virus strains (10 per cent to 22 per cent weight ratio) and IBVM41 virus strains (10 per cent to 22 per cent weight ratio) respectively; and then add white oil and immune additives including tween, span and stearic acid to prepare the combined vaccine.
Owner:PU LIKE BIO ENG
Inactivated vaccine of cow chlamydia, its preparation and inspection method
ActiveCN1698892AFight infectionInfection fromChlamydiaceae ingredientsAntiinfectivesMicrocosmic saltOil adjuvant
The invention relates to an inactivated vaccine of cow chlamydia, its preparation and the related inspection method during the vaccine preparation. The preparing process comprises diluting the Chlamydia psittaci SX 5 or NX with microcosmic salt buffering liquid or physiological saline, vaccinating to healthy chick embryo hatched at 37 deg. C for 6-7 days, harvesting vitelline membrane and allantois liquid of dead chick embryo after 72 hours as antigens, triturating the antigens, diluting and charging formaldehyde for deactivation, mixing the deactivated antigens with oil adjuvant by the proportion of 1:1, stirring homogeneously, carrying out homogeneous emulsion to obtain the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
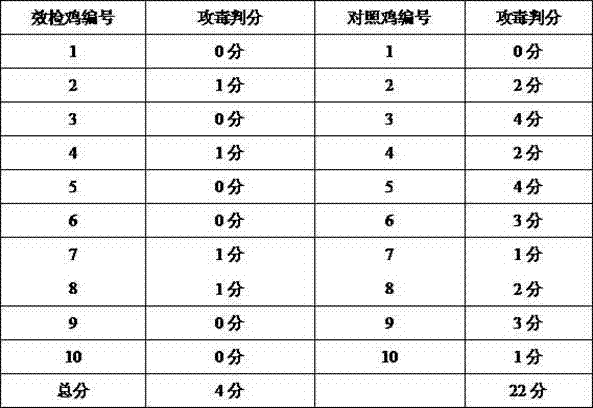
Efficacy test method of mycoplasma gallisepticum inactivated vaccine and application thereof
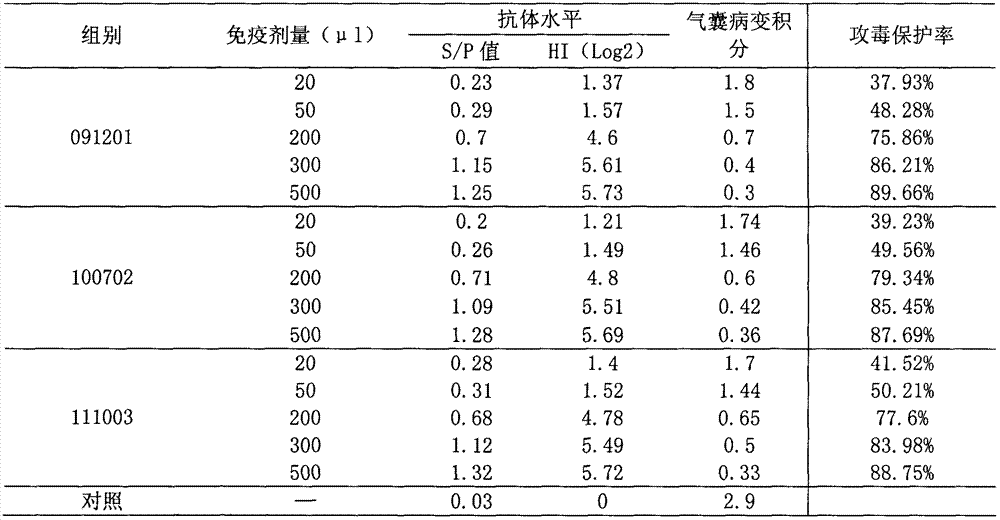
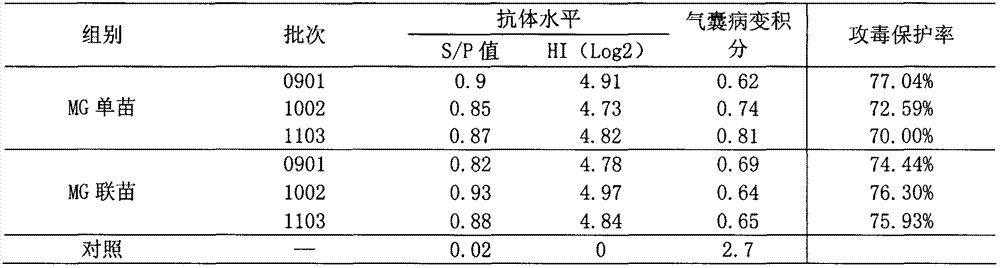
The invention relates to the technical field of animal biological products and discloses an efficacy test method of mycoplasma gallisepticum inactivated vaccine. Simultaneously, the efficacy test method disclosed by the invention can also be applied to quality control of the vaccine, in particular comprising the determination of immune toxicity attack protection rate and vaccine quality standard, measurement of immunization deadline and clinical monitoring. Experiments prove that the animal toxicity attack test is replaced by a serology detection method (comprising ELISA (Enzyme-Linked Immuno Sorbent Assay) and HI (Hemagglutination Inbition)); the efficacy test method is simple, convenient, rapid, accurate in result and good in repeatability and specificity; due to the establishment of the efficacy test method, subjectivity for checking airbag pathological change integrals after attacking toxicity is reduced; toxicity dispersion is avoided; a basis for establishing a rational immune program is also provided; and the efficacy test method has general popularization significance.
Owner:兆丰华生物科技(南京)有限公司
Duck infectious serositis inactivated vaccine emulsification method
ActiveCN101991845AIncreased qualification rate of dosage formsHigh viscosity pass rateAntibacterial agentsBacterial antigen ingredientsALUMINUM STEARATESOil phase
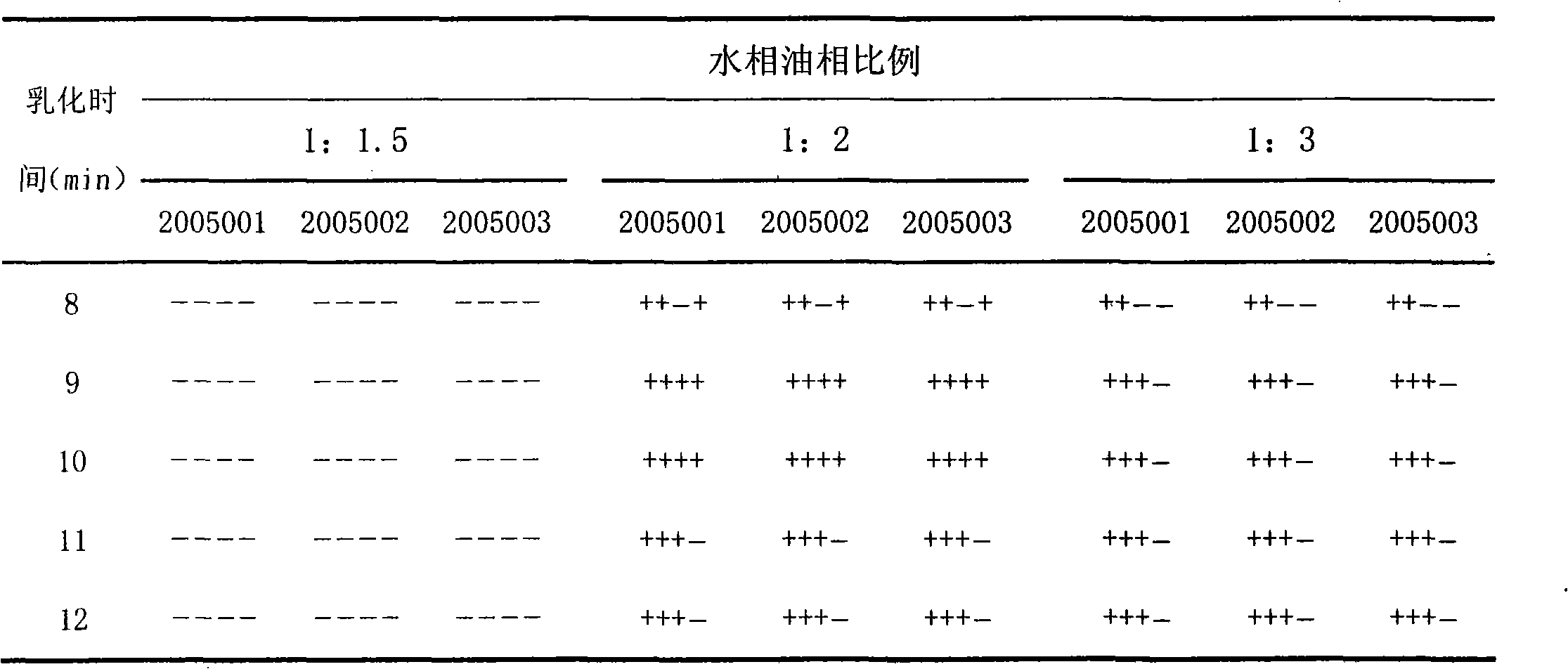
The invention discloses a duck infectious serositis inactivated vaccine emulsification method which comprises the following steps of: water phase preparation: loading four parts of Tween-80 into a bottle to be sterilized, adding 96 parts of germ liquid after cooling and sufficiently vibrating and shaking until the Tween-80 is completely dissolved; oil phase preparation: adopting 93 parts of white oil for injection, 5 parts of spun-80 and 2 parts of aluminum stearate, firstly taking a small amount of white oil for injection to be mixed with the aluminum stearate while preparing the oil phase, heating and dissolving the mixture, then, uniformly mixing the total amount of spun-80 with the white oil for injection, stirring the mixture until in the transparent state, and sterilizing for 30 minutes at 116 DEG C for use; and emulsifying the water phase and the oil phase according to a proportion of 1:1.5 to 1:1.3 at the rotating speed of 3200 to 4800 r / min for 8 to 12 minutes.
Owner:SICHUAN AGRI UNIV
Method for preparing porcine circovirus inactivated vaccine
InactiveCN105797150AStrong antigen stabilityGood antigen stabilityViral antigen ingredientsAntiviralsCell growthPorcine Circoviruses
The invention discloses a method for preparing a porcine circovirus inactivated vaccine.The method comprises the steps that, PK15 and ST or BHK-21 are subjected to EDTA-trypsin digestion and then subjected to passage, and continue to be cultured with a cell growth solution, and the number of cells is maintained with a cell maintaining solution when the cells grow to reach 85-100%; a porcine circovirus 2b type strain is inoculated to a medium with the cells growing on 85-100% of the medium, and the strain is cultured and collected; toxins of the strain are collected and inoculated to the medium with the cells growing on 85-95% of the medium, and culture and collection are carried out to obtain a porcine circovirus 2b type strain antigen solution; an immunologic adjuvant is added after inactivation is carried out, and the inactivated vaccine is obtained through emulsification.The vaccine obtained through the method is high in antigen stability, immunogenicity, antigen recycling rate and vaccine safety.The inactivating method and a methanal inactivating method are compared, after inactivated vaccines prepared through the two inactivating methods are used for immunity of pigs, the serum antibody valence is high, and an inactivated vaccine obtained through the method of carrying out inactivating for 48 h with 0.002 mol / L BEI has the best antigen stability and immunogenicity and the highest antigen recycling rate and vaccine safety.
Owner:HUAZHONG AGRI UNIV
DNA loaded Brucella ghost composite vaccine
InactiveCN108690823ALittle side effectsImprove securityAntibacterial agentsBacterial antigen ingredientsSide effectA-DNA
The invention discloses a DNA loaded Brucella ghost composite vaccine. The preparation method comprises following steps: introducing a suicide plasmid that contains a nucleic acid molecule encoding atemperature sensitive regulatory protein cI857, a nucleic acid molecule encoding a bacteriophage splitting protein E, and a nucleic acid molecule encoding a bacterial nuclease protein A into Brucella;utilizing a homologous recombination technology to obtain recombinant Brucella; culturing the recombinant Brucella to obtain a bacterial solution, processing the bacterial solution at a high temperature, collecting bacterial cells, and adding target DNA to obtain the DNA loaded Brucella ghost composite vaccine. The composite vaccine has following advantages: (1) the vaccine has the characteristics of bacterial ghost, compared with a conventional killed vaccine or an attenuated live vaccine, the side effect of the composite vaccine is small, the safety is high, and the protection effect is good; and (2) the bacterial ghost is a safe and effective carrier for delivering DNA vaccines, can introduce nucleic acid vaccines into antigen presenting cells, and performs high efficient expression. The composite vaccine has an important meaning for controlling the epidemic spreading of brucellosis and has a wide application range.
Owner:INNER MONGOLIA HUAXI BIOTECH
Preparation method and application of flagellin FliC from salmonella abortus equi
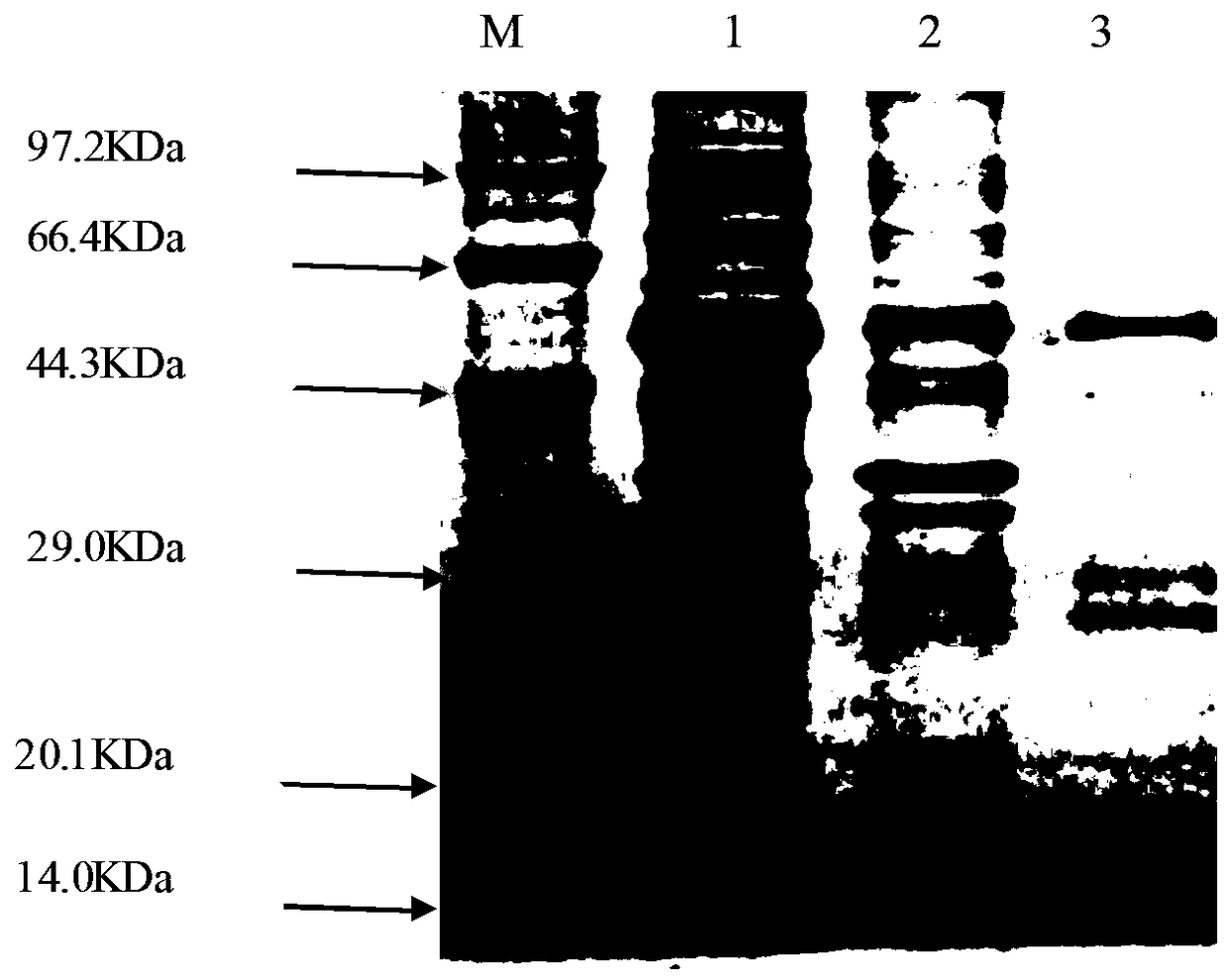
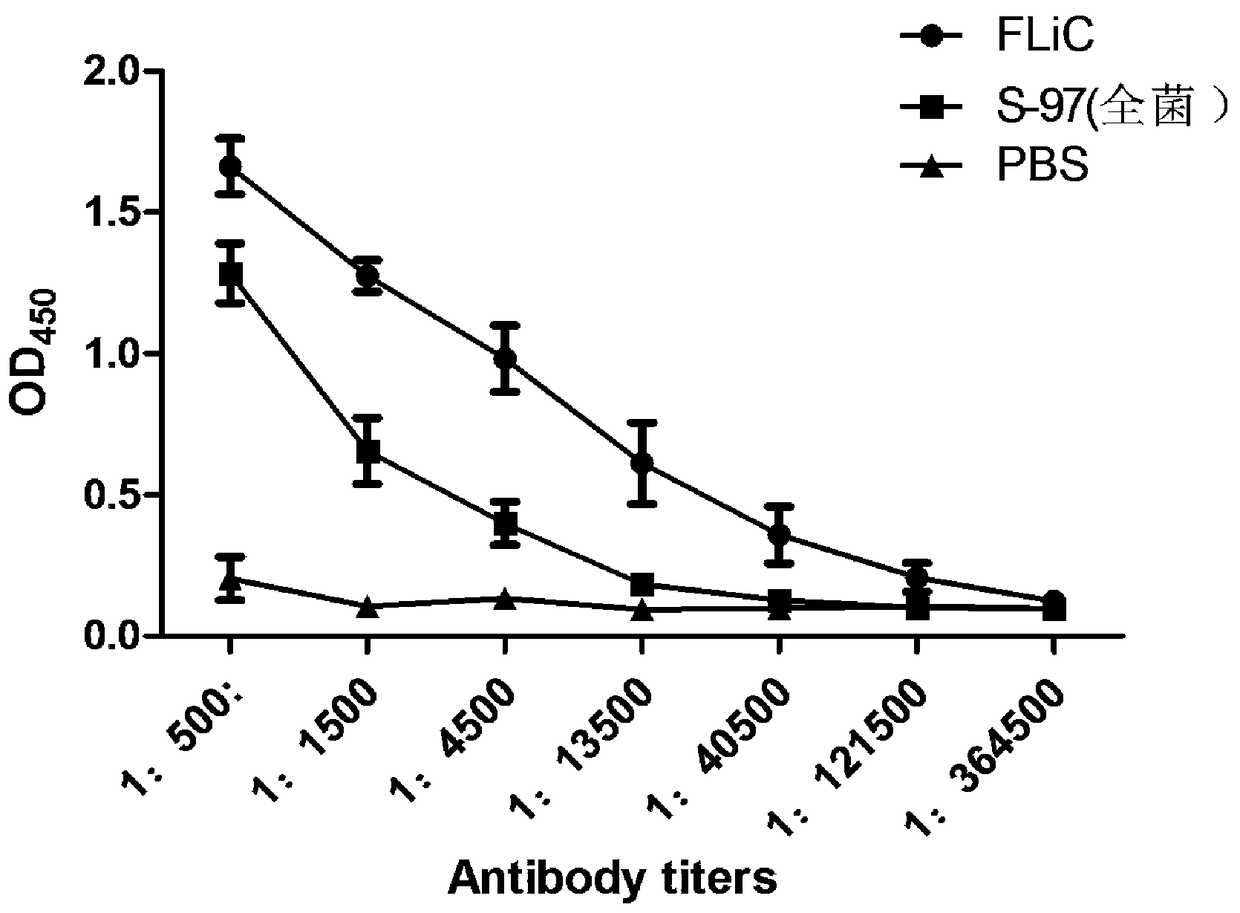
ActiveCN108218965AImproving immunogenicityExcellent levelBacteriaMicroorganism based processesAdjuvantAnimals vaccines
The invention discloses a preparation method and possible application of flagellin FliC from salmonella abortus equi to salmonella abortus equi immunity. Through cloning and connecting a flagellin FliC gene from the salmonella abortus equi to a prokaryotic expression vector pGEX-4T-2, a prokaryotic recombinant of a protein is obtained; the prokaryotic recombinant is transformed into engineering bacteria BL-21, and the protein is subjected to induced expression and purification; the protein has an amino acid sequence in a sequence table 1; the purified FliC recombinant protein has good immunogenicity after being used for the immunization of experimental animals, can overcome the defects of low antibody level and poor safety generated by inactivated vaccines and can be used for preparing subunit vaccines for preventing and treating equine paratyphoid infection and can be used as equine animal vaccine adjuvants, thereby having good development and application prospect.
Owner:XINJIANG AGRI UNIV
Influenza virus vaccine strain
ActiveCN103614345AEasy to combineWide range of choicesMicroorganism based processesAntiviralsInfluenza virus vaccineTGE VACCINE
The invention discloses an influenza virus vaccine strain. The invention discloses a virus. The amino acid sequence of each of PB2 protein, PB1 protein, PA protein, NP protein, NA protein, M1 protein, M2 protein, NS1 protein, NS2 protein and HA protein are respectively shown as follows: SEQ ID No. 26, SEQ ID No. 34, SEQ ID No. 33, SEQ ID No. 21, SEQ ID No. 29, SEQ ID No. 27, SEQ ID No. 28, SEQ ID No. 31, SEQ ID No. 32 and SEQ ID No. 16. The recombinant virus disclosed by the invention can be rapidly and stably proliferated in high yield. Compared with the method for preparing the vaccine by utilizing the chicken embryo, the recombinant virus has obvious advantage, and the inactivated vaccine of the recombinant virus can be used for protecting animals against the infection of influenza virus.
Owner:CHINA AGRI UNIV
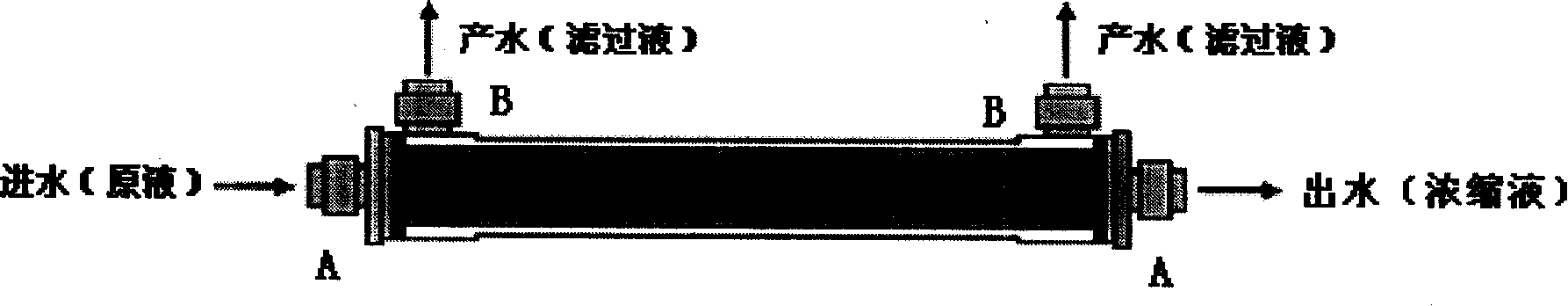
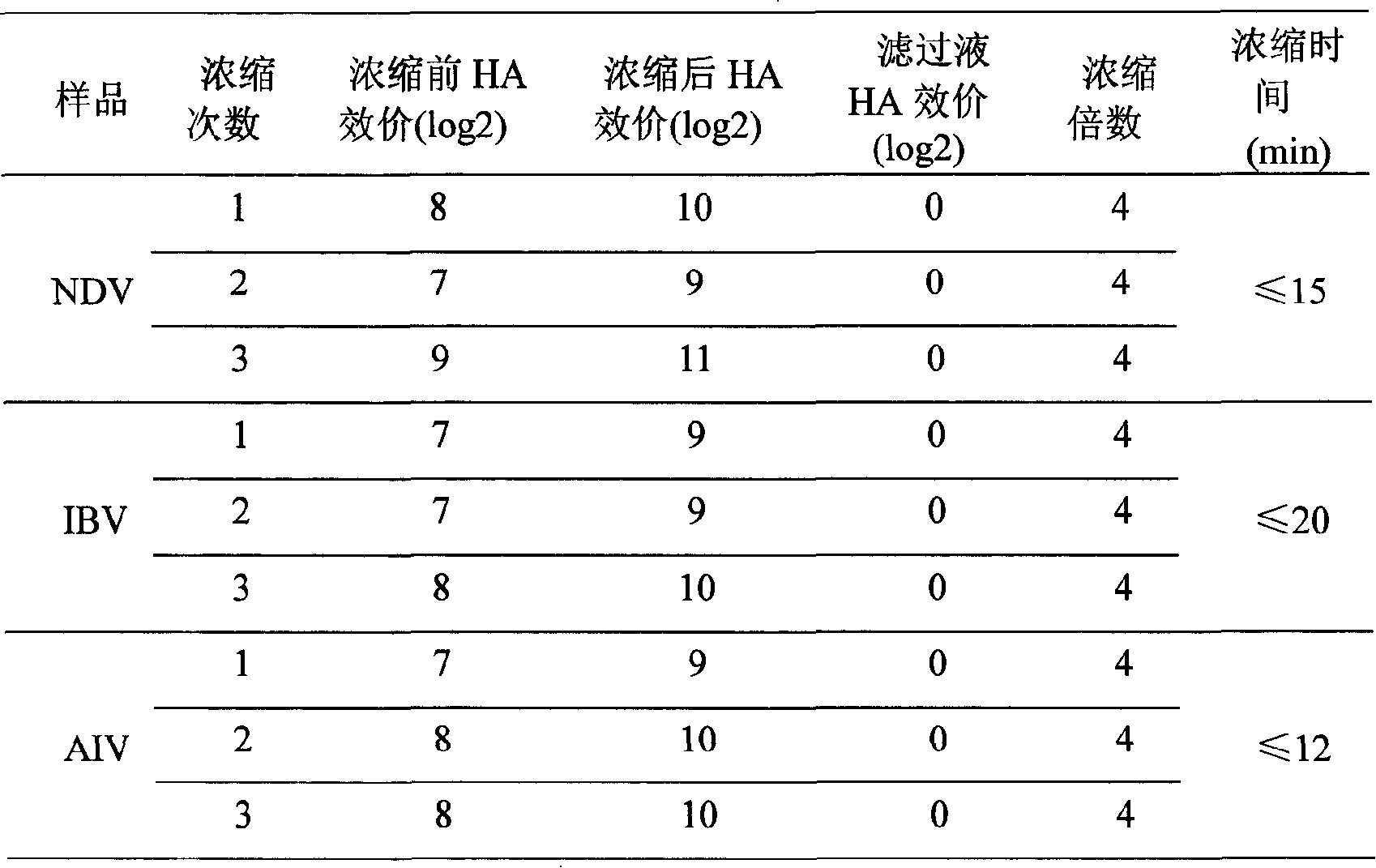
Technique for concentrating chicken ND-IB-AI-EDS tetrad oil emulsion killed vaccine antigen
The invention provides a concentration technology of virus antigen during the preparation process of a chicken ND-IB-AI-EDS quadruple inactivated oil-emulsion vaccine. The main contents of the method are that: newcastle disease virus (NDV), infectious bronchitis virus (IBV) and H9 subtype avian influenza virus (AIV) are concentrated by four steps of rough filtration, fine filtration, ultrafiltration and material collection via an internal pressure hollow fiber ultrafilter. After the concentration, the three virus antigens are mixed with egg drop syndrome virus (EDSV) for the preparation of the chicken ND-IB-AI-EDS quadruple inactivated oil-emulsion vaccine.
Owner:TIANJIN INST OF ANIMAL HUSBANDRY & VETERINARY
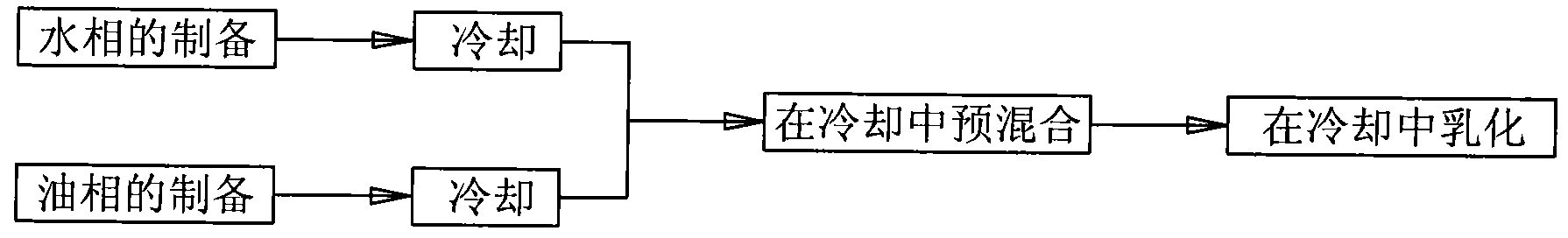
Low-temperature emulsification process of animal oil emulsion inactivated vaccines
InactiveCN101978948AAvoid destructionReduce initial dosagePharmaceutical product form changeEmulsion deliveryAntigenAdjuvant
The invention relates to a low-temperature emulsification process of animal oil emulsion inactivated vaccines, which belongs to the field of vaccine preparation methods. The low-temperature emulsification process comprises the following steps of: 1) preparing a water phase in a water-phase tank and preparing an oil phase in an oil-phase tank, and cooling the water phase and the oil phase to 5 to 8 DEG C after the preparation; 2) pre-cooling an emulsification tank to control the temperature in the tank body to be 5 to 8 DEG C; 3) adding the cooled water phase and oil phase into the emulsification tank together for premixing and keeping the temperature in the emulsification tank at 5 to 8 DEG C; and 4) stirring at a high speed in the emulsification tank for emulsification to make water drops further broken and uniformly dispersed in the oil phase, wherein the temperature in the emulsification tank is kept at 5 to 8 DEG C during emulsification. When the process is used for preparing the inactivated vaccines, damage to an active antigen is small, yield is high, and product stability is stable. The process makes the different adjuvant types of inactivated vaccines achieve the same effect and can be used for preparing various animal oil emulsion inactivated vaccines.
Owner:SINOPHARM YANGZHOU VAC BIOLOGICAL ENG CO LTD
ELISA (Enzyme Linked Immuno-Sorbent Assay) knit based on Nsp10 protein in PRRSV (porcine reproductive and respiratory syndrome virus)
The invention discloses an ELISA (Enzyme Linked Immuno-Sorbent Assay) knit based on Nsp10 protein in PRRSV (porcine reproductive and respiratory syndrome virus), which comprises 50-200 ml of recombined protein peridium, 50-200 ml of positive reference, 50-200 ml of negative reference, 50-200 ml of sealing liquid, 50-200 ml of enzyme labeled secondary antibody, 50-200 ml of substrate liquid and 50-200 ml of stop solution. The purified Nsp10 recombined protein is taken as envelope antigen, and an ELISA method is established to optimize the reaction condition and research an ELISA knit based on Nsp10 protein in PRRSV; via comparing the ELISA detection result with the positive reference and the negative reference, a detection conclusion is obtained; a detection conclusion is obtained through the comparison between the ELISA detection result and the negative reference and the positive reference; the conclusion can basically distinguish PRRS inactivated vaccine immune swinery from inapparent infected swinery; therefore, reference data is provided for the clinical diagnosis and the prevention and control of the PRSS; according to the detection result, the uninfected swinery is selectively immune; therefore, the immune aimlessness can be reduced, and the economic loss is effectively avoided. The ELISA knit has the characteristics of good specificity, sensitivity, repeatability, and the like, and is good in use effect.
Owner:GUIZHOU UNIV
Cyprinus carpio var. jian cerebral line and establishment and application methods thereof
ActiveCN109971710AIncreased sensitivityImprove researchCompound screeningApoptosis detectionCyprinusDigestion
The invention provides a Cyprinus carpio var. jian cerebral line and application thereof. Through primary isolation on Cyprinus carpio var. jian cerebral tissues with pancreatin of an appropriate concentration and reasonable digestion conditions, Cyprinus carpio var. jian cerebral cells can be obtained, and through subculture, the Cyprinus carpio var. jian cerebral line CCB-J is established for the first time; the Cyprinus carpio var. jian cerebral line is mainly represented as epithelioid cells and can be passaged for more than 60 generations, thereby be high in research and application the fields such as virology, toxicology, physiology, molecular genetics and biology of evolution. The Cyprinus carpio var. jian cerebral line can be applied to virus identification and virus strain isolation, to preparation of Cyprinus carpio var. jian virus preventing or killing vaccines, as biological models of drug screening, preparation and evaluation and the like.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Combined inactivated vaccine of photobacterium damselae and vibrio splendidus of pampus argenteus
PendingCN109865135ASimple preparation processStable outputAntibacterial agentsBacterial antigen ingredientsImmune effectsVibrio splendidus
The invention provides a combined inactivated vaccine of photobacterium damselae and vibrio splendidus of pampus argenteus. The preservation number of the photobacterium damselae is CGMCC NO.16907. The combined inactivated vaccine is simple in preparation process, stable in yield and low in cost, and a large amount of inactivated vaccines with a high immune effect can be obtained. The immune protection rate of the inactivated vaccine prepared by the method reaches 92.5%; the vaccine is a pure biological preparation, and is safe and environmentally friendly, drug residues and environmental pollution are avoided, the occurrence and prevalence of the photobacterium damselae and vibrio splendidus diseases of the pampus argenteus can be fundamentally restrained, and technical support is provided for large-scale preparation of the bigeminy inactivated vaccine for the photobacterium damselae and vibrio splendidus diseases of the pampus argenteus.
Owner:NINGBO UNIV
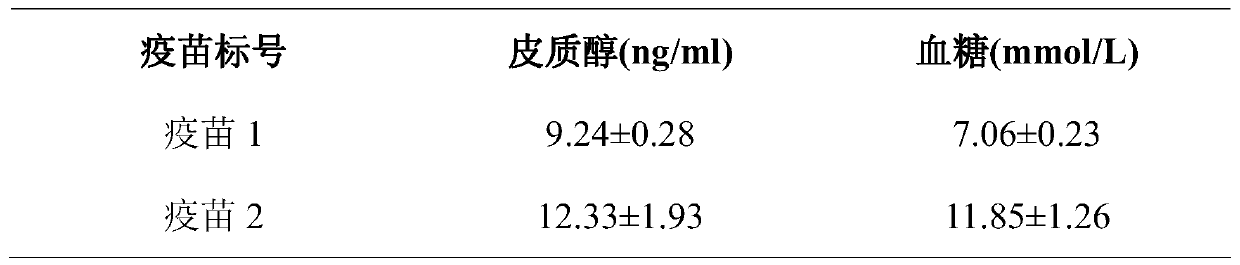
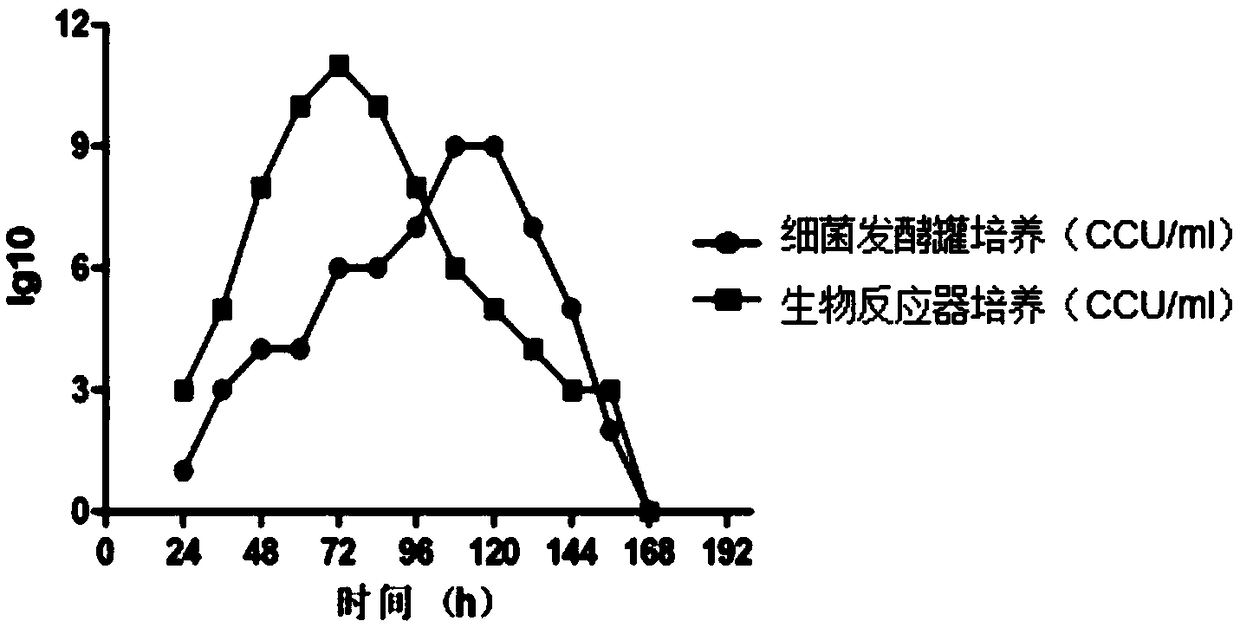
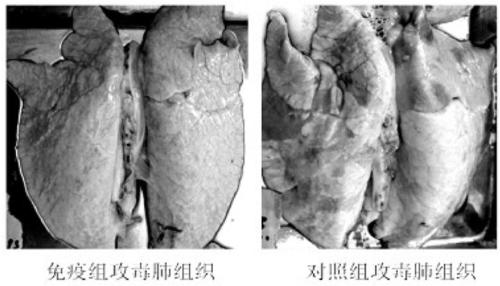
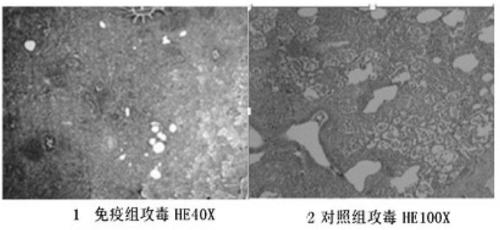
Production method of haemophilus parasuis/mycoplasma hyopneumoniae bivalent inactivated vaccine
ActiveCN109010814AEasy to controlReduce controlAntibacterial agentsBacterial antigen ingredientsSerum igeHaemophilus
The invention belongs to the technical field of vaccine production, and particularly discloses a production method of a haemophilus parasuis / mycoplasma hyopneumoniae bivalent inactivated vaccine. Themethod includes: performing fermenting culture of mycoplasma hyopneumoniae in a bioreactor; performing fermenting culture of serum IV type haemophilus parasuis and serum V type haemophilus parasuis ina fermentation tank; inactivating, concentrating and purifying the microbial liquids after the fermenting cultivation, mixing the microbial liquids according to certain ratio, and adding an immuno-enhancer and a vaccine adjuvant to obtain the haemophilus parasuis / mycoplasma hyopneumoniae bivalent inactivated vaccine. The vaccine composition has excellent specificity and good immunity, can achievethe object of prevention from the two diseases by one injection, is more economical and practicability, can avoid repeated inoculation and reduces vaccine cost and manpower cost. The bivalent inactivated vaccine is very suitable for prevention and treatment on breeding farms with mixed infection of the diseases.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Permanent cell line for ecthyreosis virus multiplication and establishment method of permanent cell line
ActiveCN107287149AReduce usageCell dissociation methodsViral antigen ingredientsSerum igeVirus multiplication
The invention relates to a permanent cell line for ecthyreosis virus multiplication. The name of the cell line is a goat testis cell GT26, the cell line is preserved at the China Center for Type Culture Collection on March 1, 2017, and the preservation number is CCTCC NO:C201734. An establishment method of the permanent cell line comprises the steps of (1) permanent establishment of the goat testis cell; (2) low-serum domestication of the goat testis cell; and (3) cultivation of an ecthyreosis virus in a newborn goat testis cell line. The ecthyreosis virus is grafted by using the goat testis cell line, thereby achieving the target of stable virus multiplication and providing a stable cell environment and a standardized culture method for development and production of an attenuated vaccine and an inactivated vaccine of the ecthyreosis virus.
Owner:陕西博德越生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com