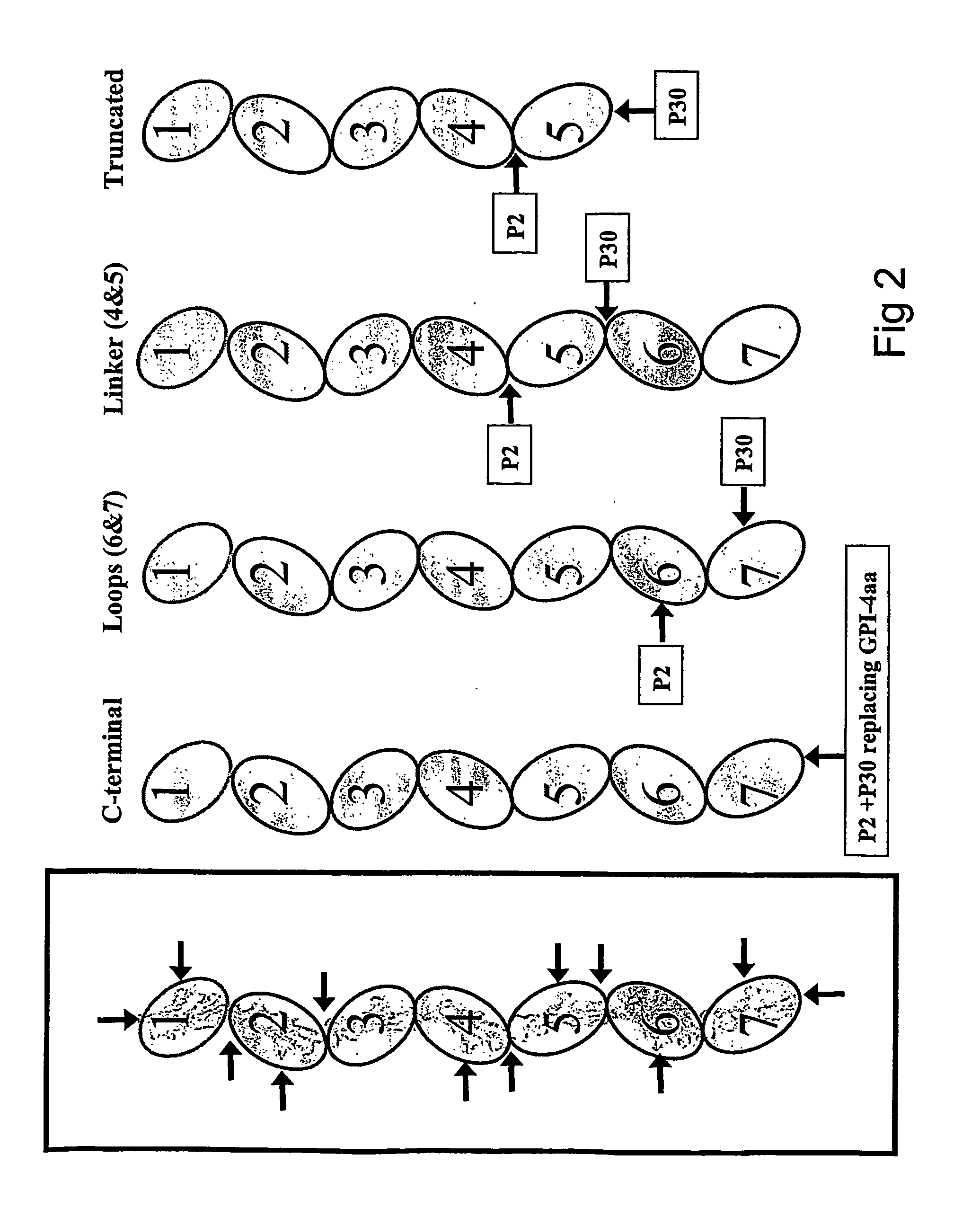
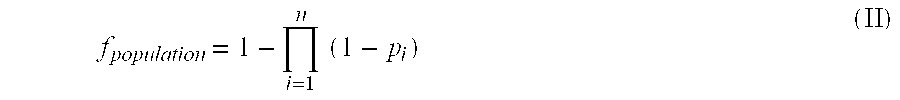Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
277 results about "Nucleic Acid Vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nucleic acid vaccines have been shown to elicit both antibody and cytotoxic T-lymphocyte responses to diverse protein antigens. Advantages of nucleic acid-based vaccines include the simplicity of the vector, the ease of delivery, the duration of expression, and, to date, the lack of evidence of integration.
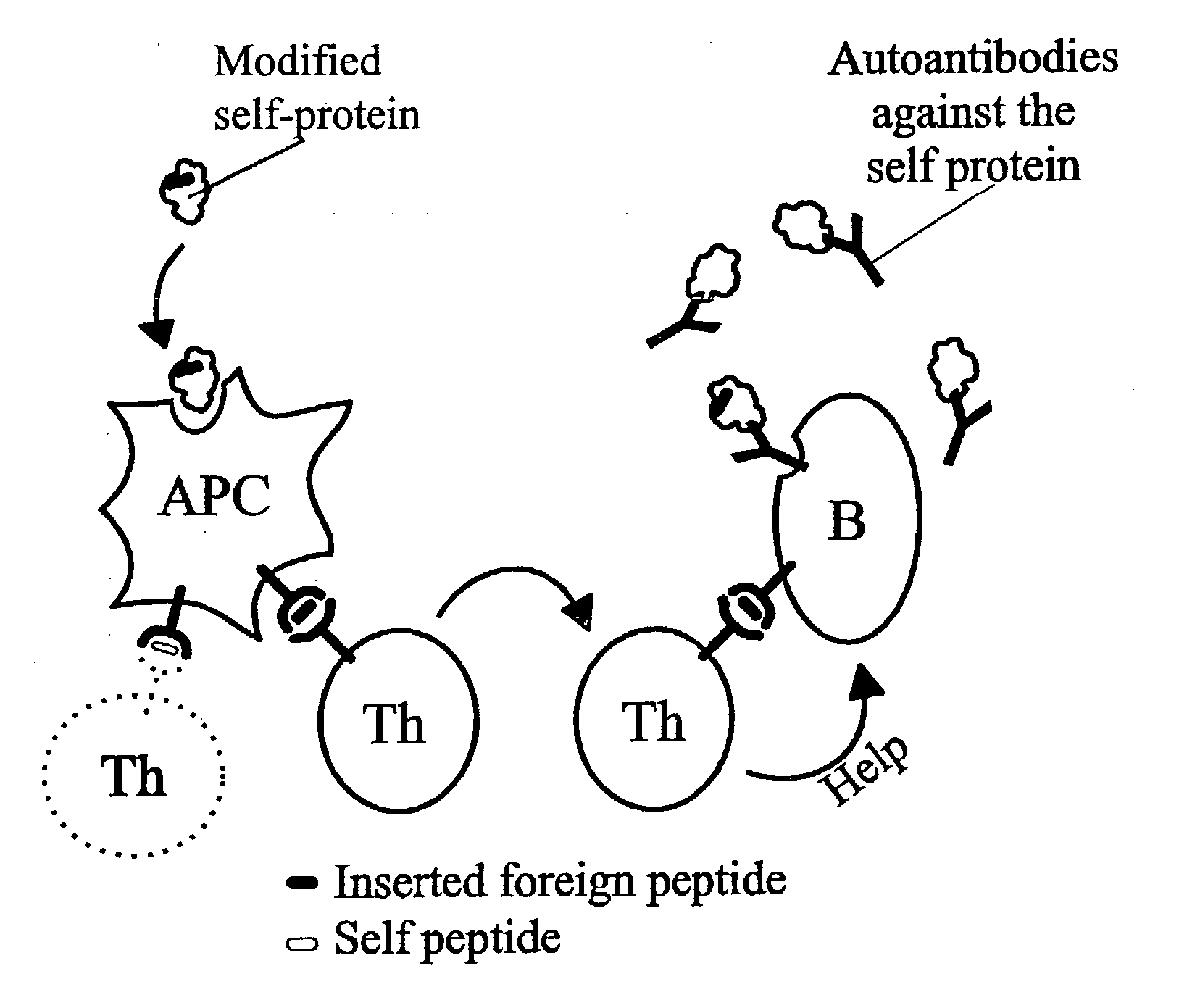
Novel methods for therapeutic vaccination
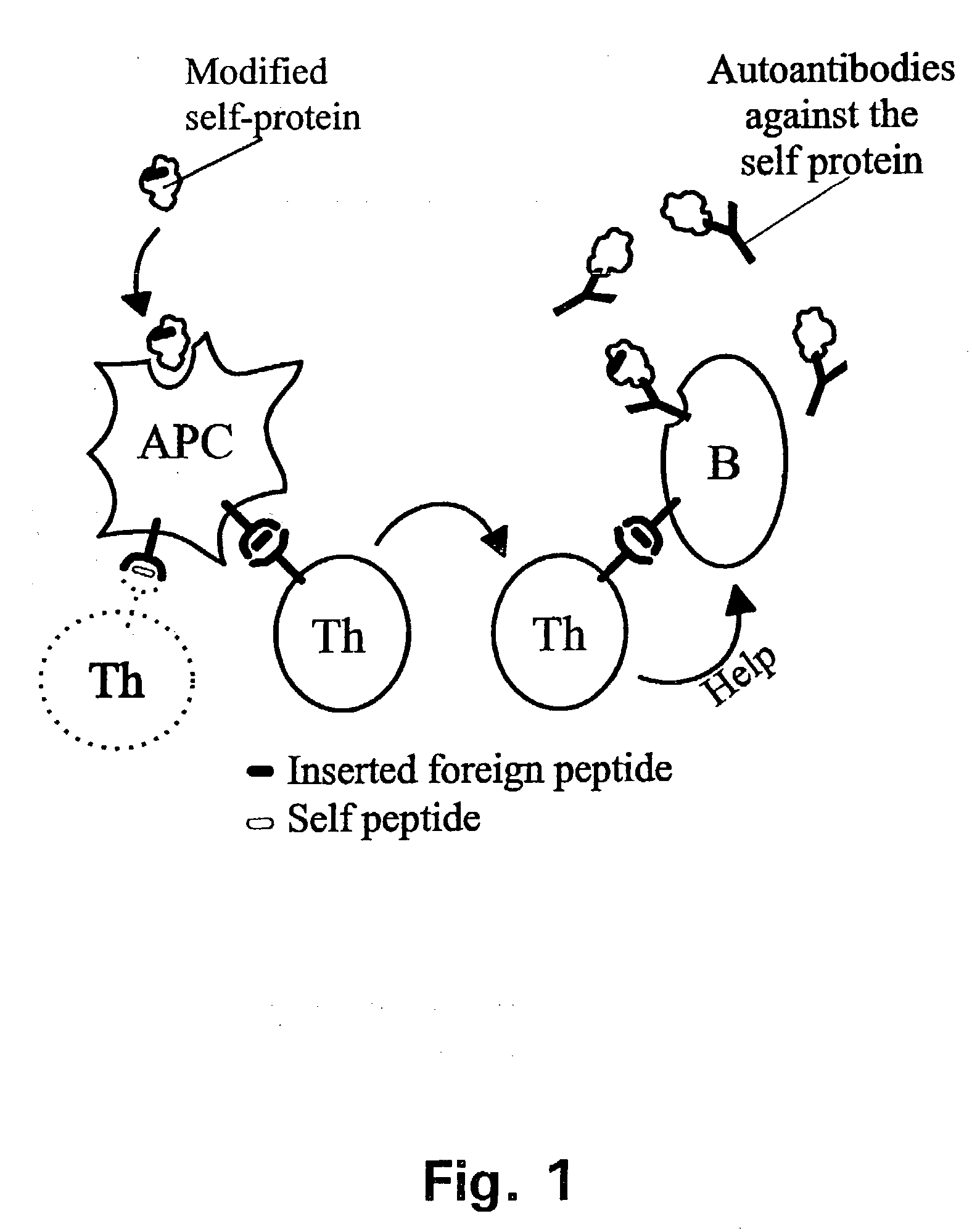
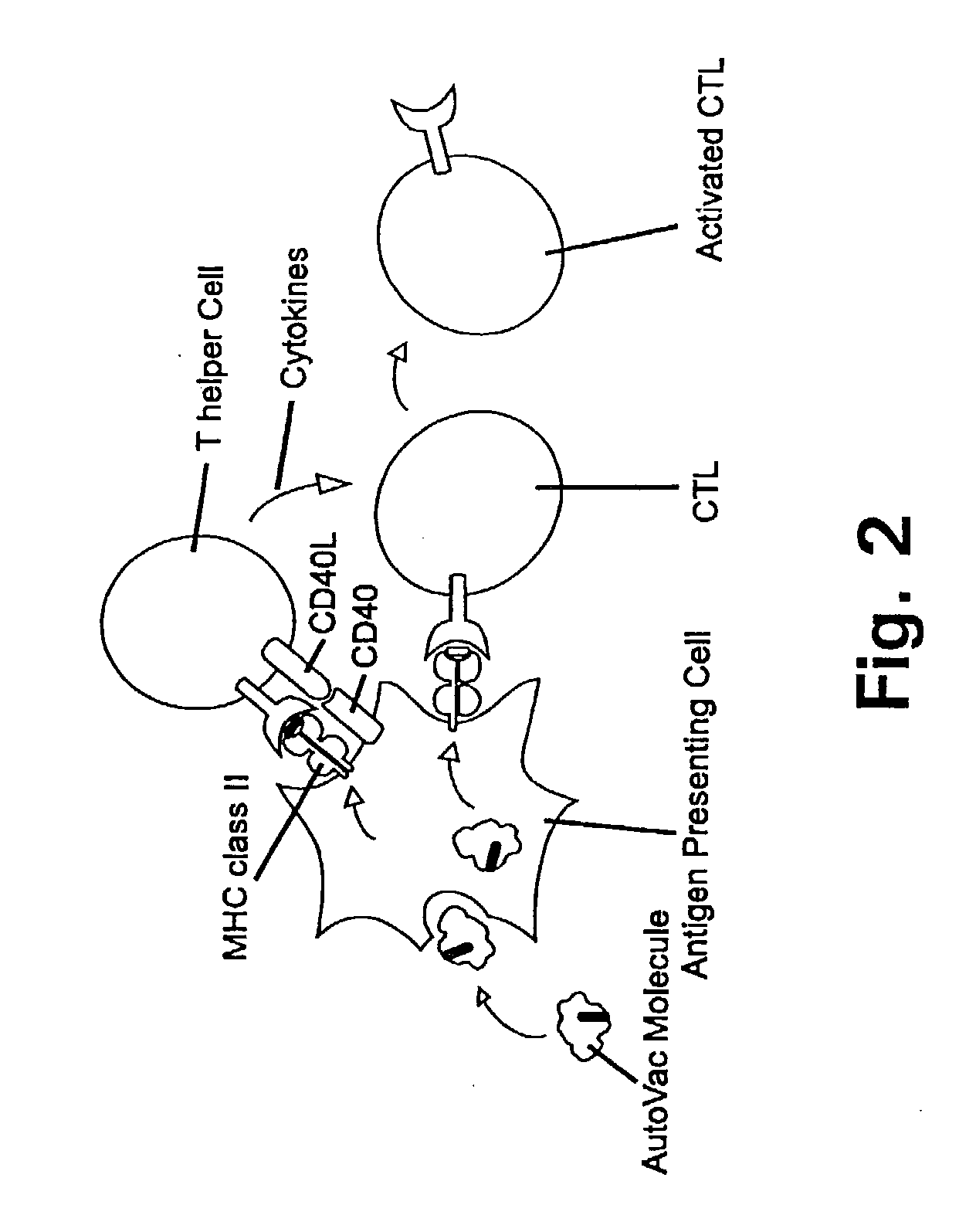
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
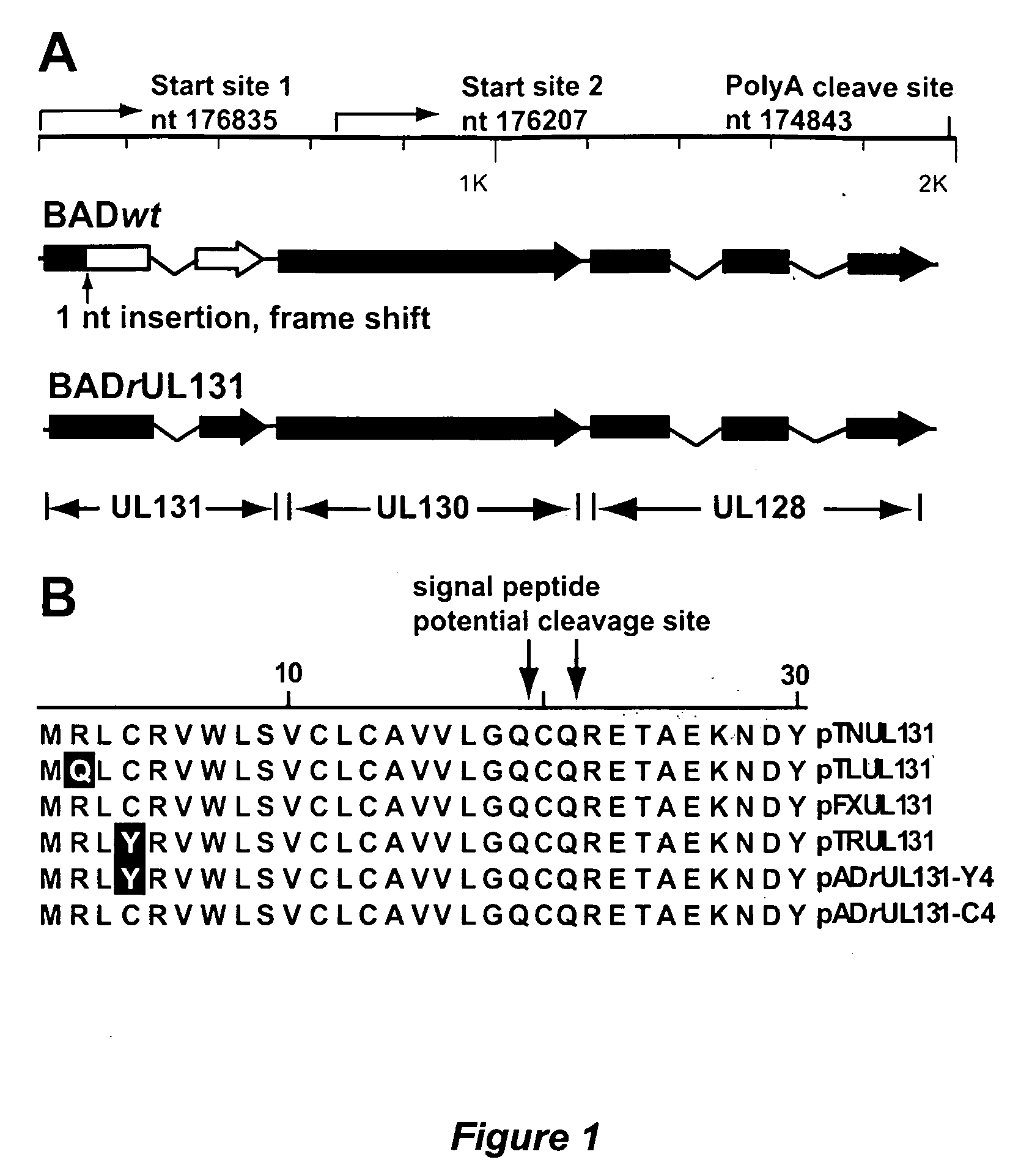
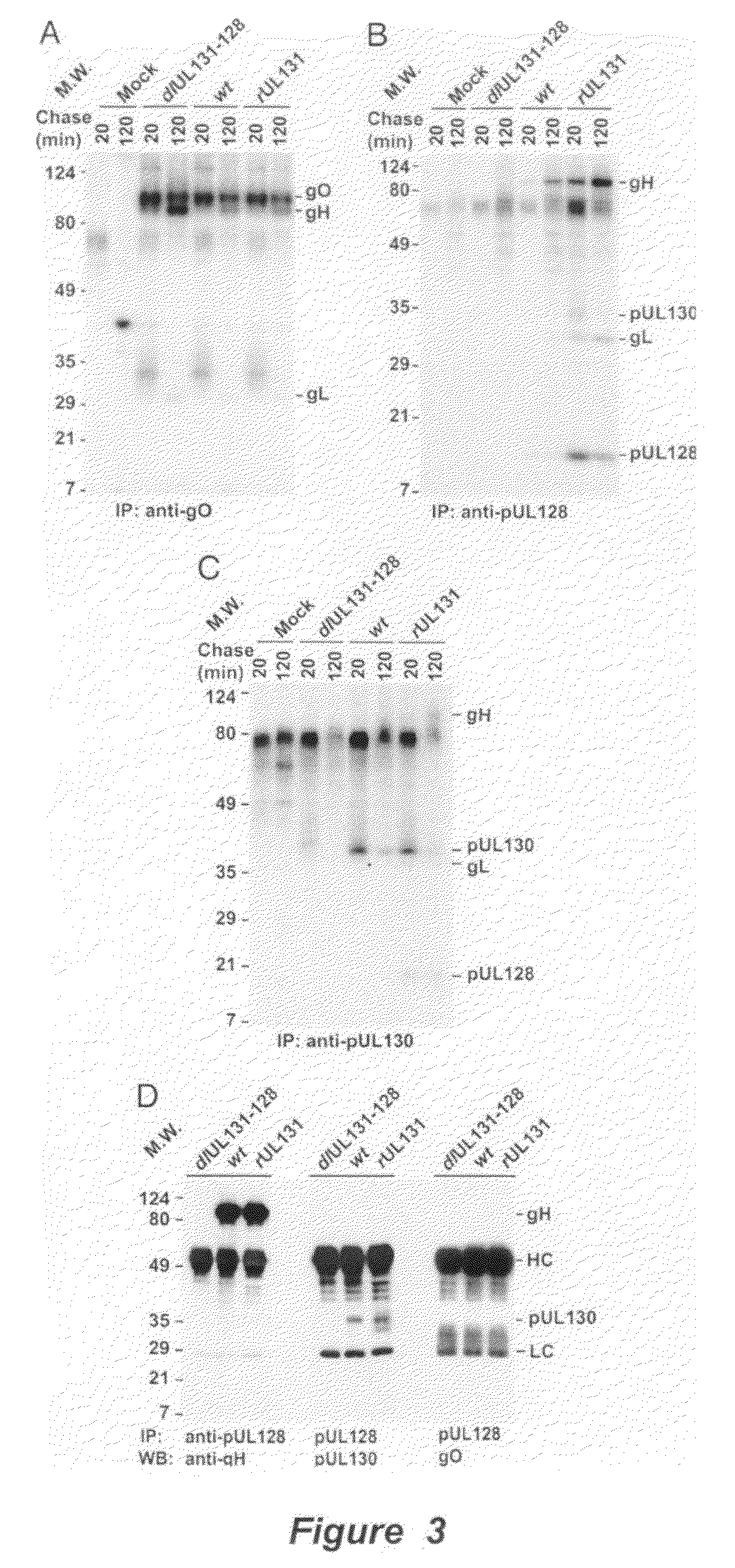
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
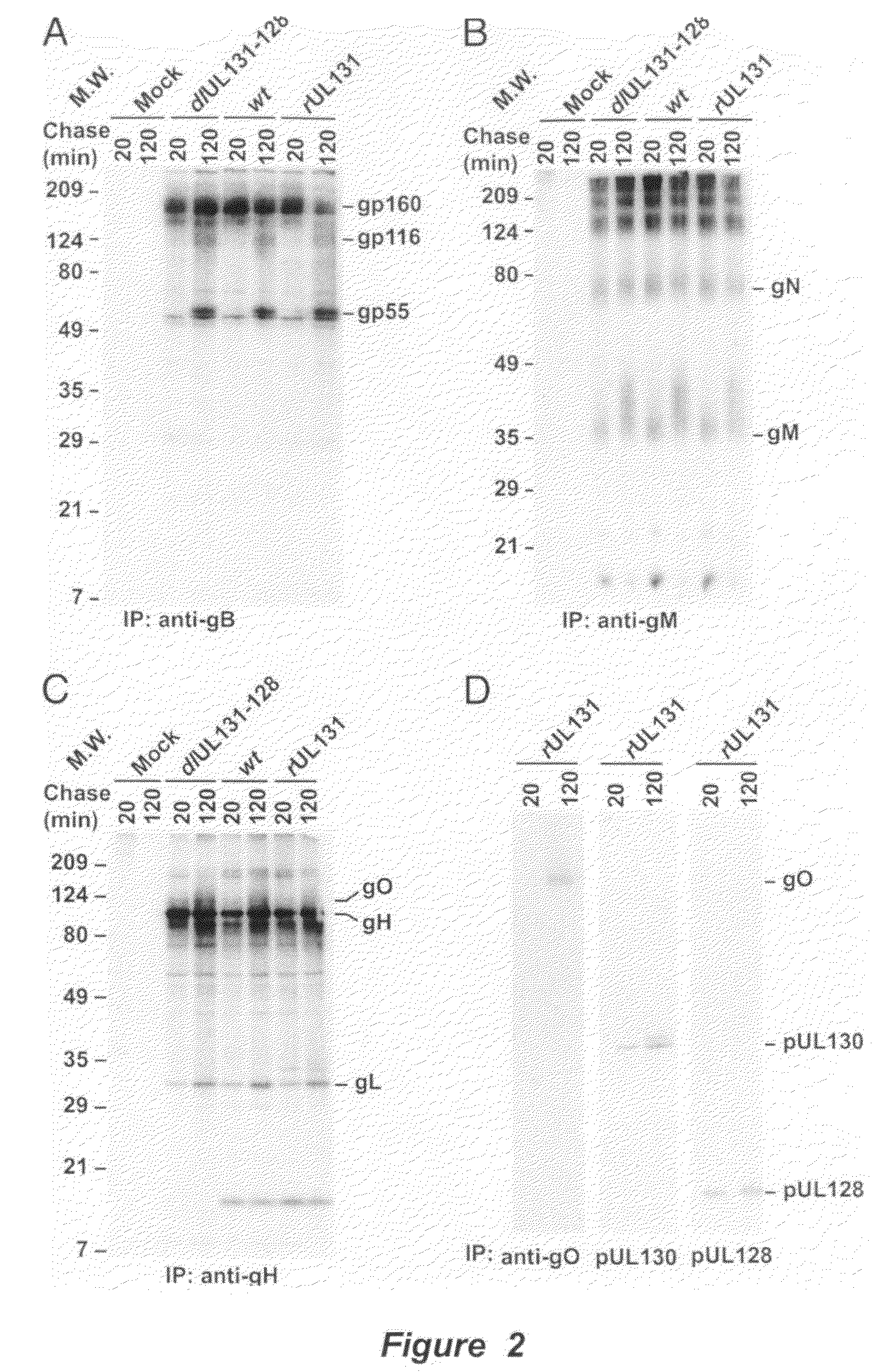
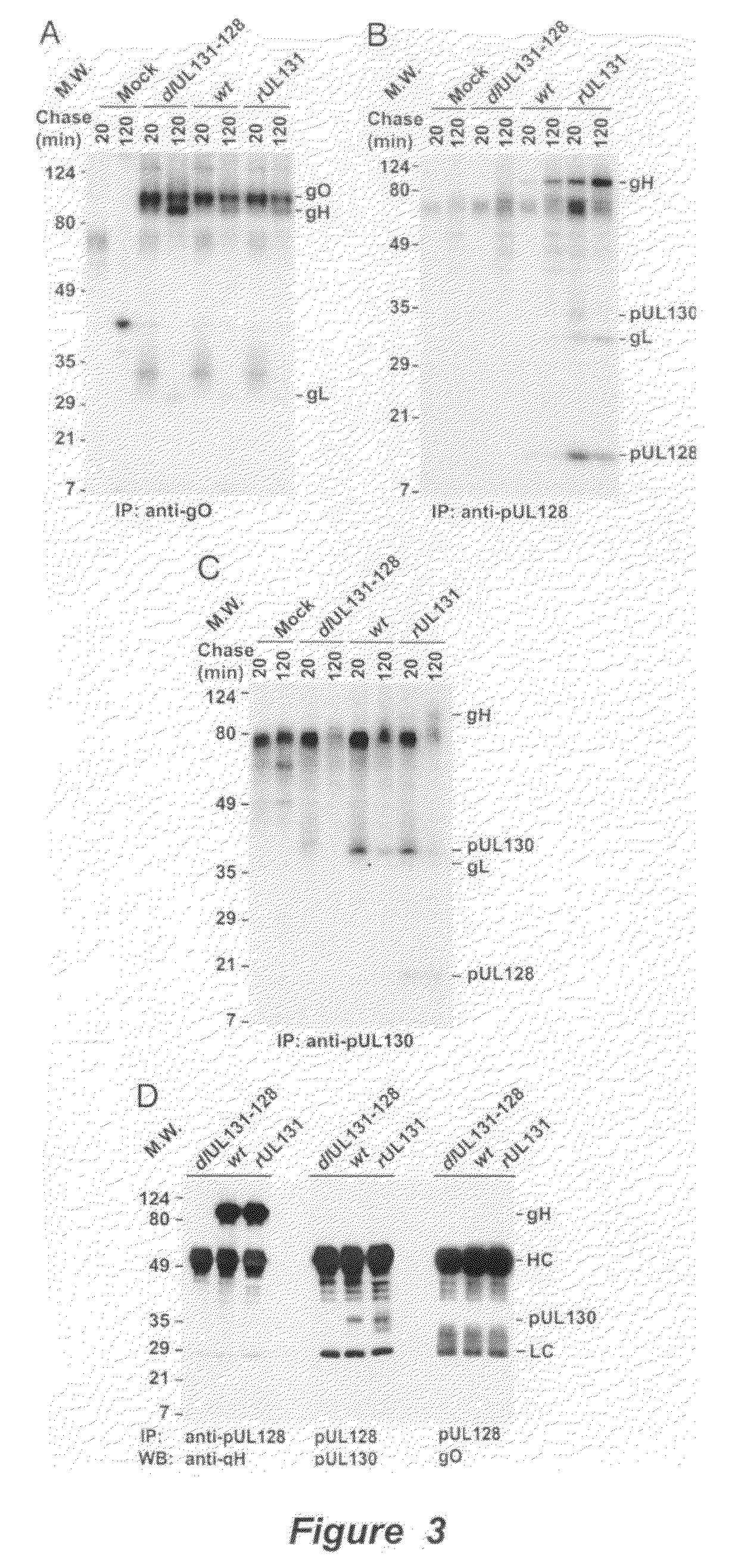
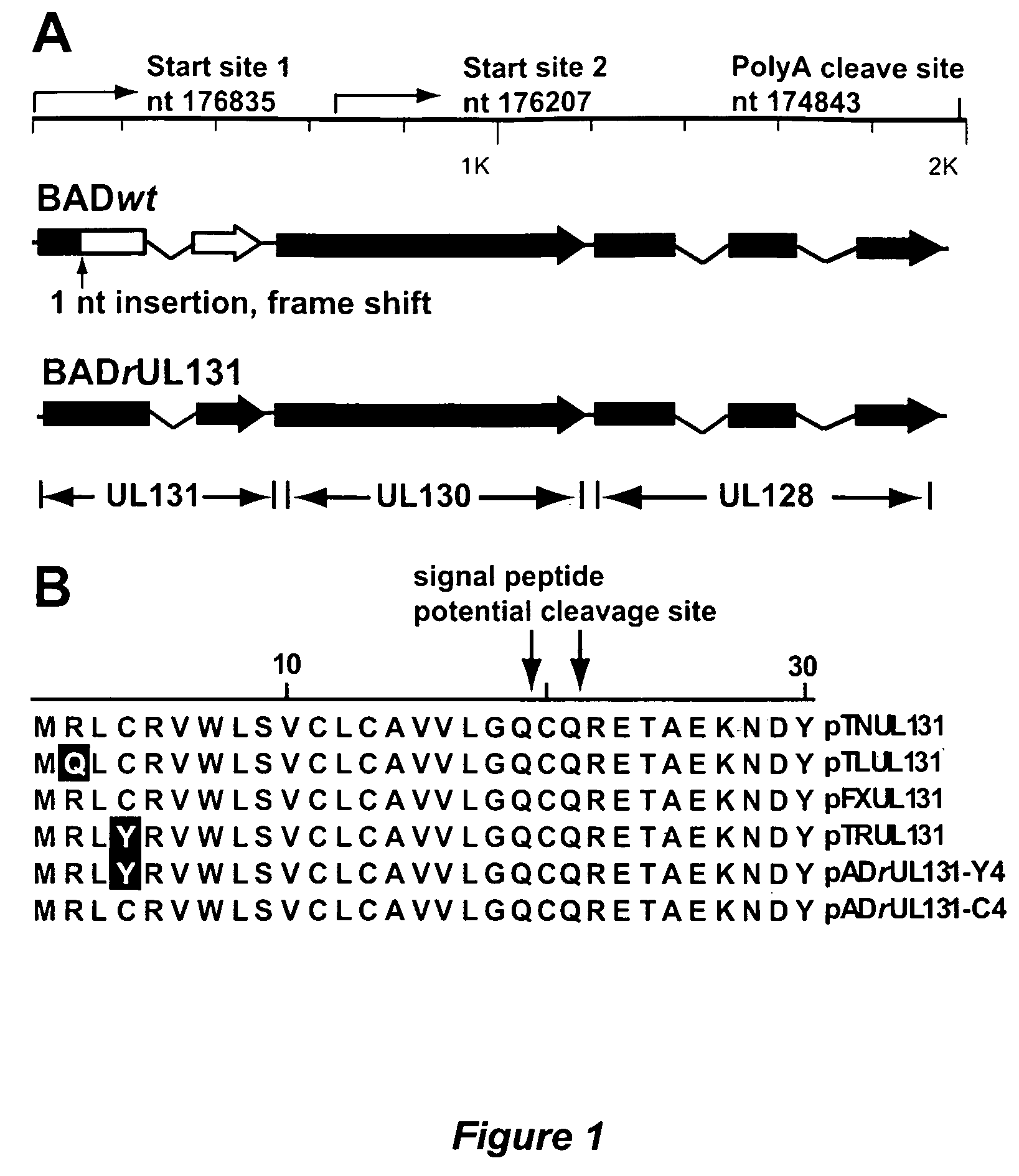
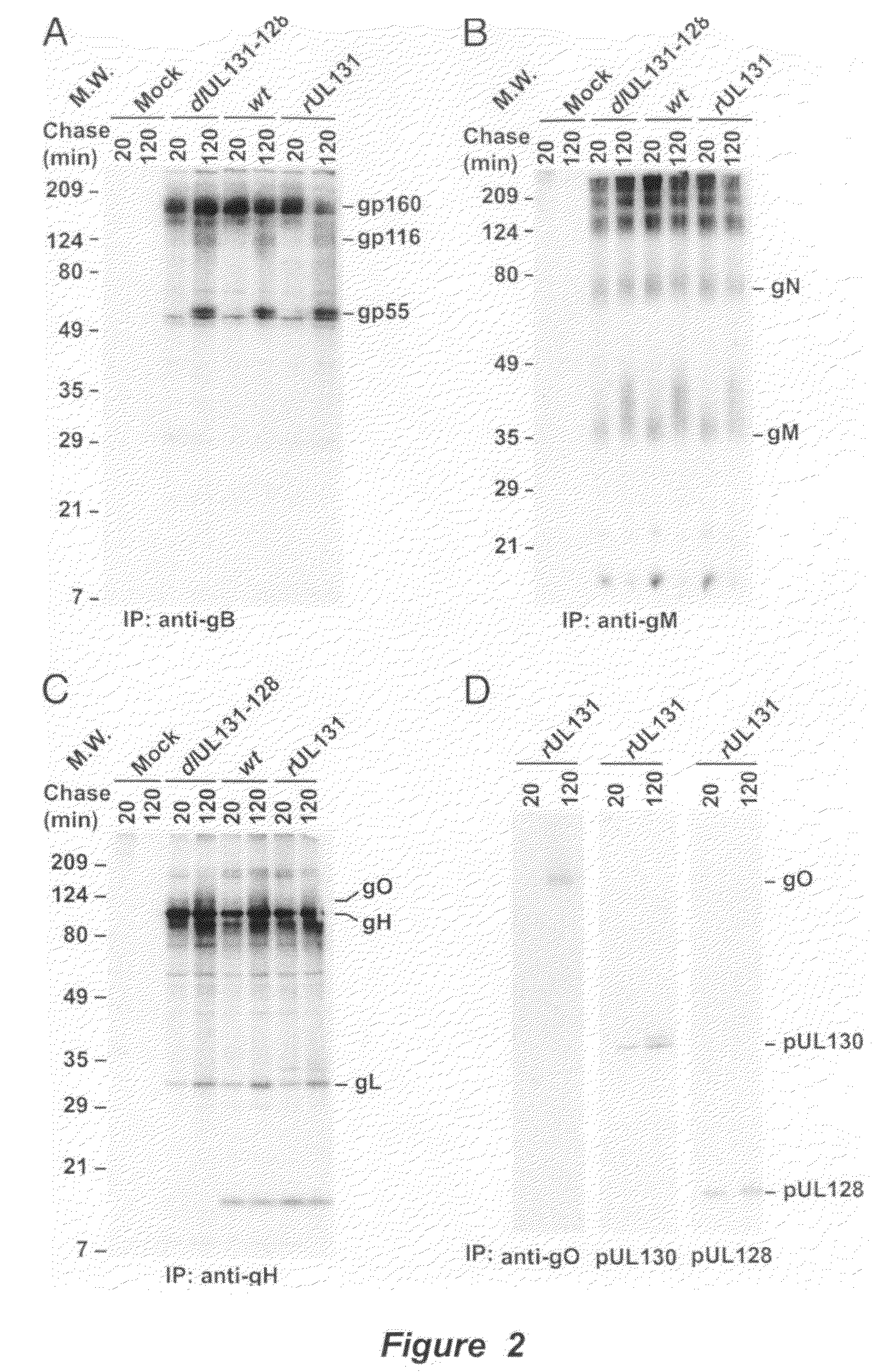
Immunogenic compositions and prophylactic or therapeutic vaccines for use in protecting and treating against human cytomegalovirus (CMV) are disclosed. Subunit vaccines comprising a human CMV protein complex comprising pUL128 or pUL130, and nucleic acid vaccines comprising at least one nucleic acid encoding a CMV protein complex comprising pUL128 or pUL130 are described. Also disclosed are therapeutic antibodies reactive against a CMV protein complex comprising pUL128 or pUL130, as well as methods for screening compounds that inhibit CMV infection of epithelial and endothelial cells, methods for immunizing a subject against CMV infection, methods for determining the capability of neutralizing antibodies to inhibit human CMV infection of cell types other than fibroblasts, and methods of diminishing an CMV infection.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Owner:THE TRUSTEES FOR PRINCETON UNIV
Method for preparing II-type pig's ring-virus nucleic vaccine and the use thereof
InactiveCN1579553AShorten the production cycleAvoid pollutionViral antigen ingredientsGenetic material ingredientsPeripheral blood mononuclear cellBiology
The invention discloses a manufacturing method and application of II type pig ring virus nucleic acid vaccine. The method is: 1) designs the specific primer, uses PCV2 Hangzhou (HZ201) gene group as template, closes ORF1, ORF2, ORF3 and ORF4 genes of PCV-2 with PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 2) separates the pig external blood single nucleus cell, clones the genes IFN, IL-2 and IL-4 with RT-PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 3) based on above mentioned reconstructed carrier, constructs the fused expressing carrier of the other genes of PCV2, ORF2 and PCV2 or pig cell factor gene; the merits of the invention lie in: (1) it needs not to culture virus, the producing period is short; (2) it needs not cell culture, thus can prevent the contamination from other pig source virus; (3) it does not express the pathogenesis protein which is harmful to body, the safety is high. (4) it can activates body secretion and cell immunity replay at the same time.
Owner:ZHEJIANG UNIV
Preparation method and use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors
InactiveCN102485274AGood monodispersityImprove stabilityAntibacterial agentsGenetic material ingredientsMicrosphereGluconic acid
The invention discloses a preparation method and a use of poly(lactic-co-glycolic acid) (PLGA) microspheres as nucleic acid vaccine vectors. A result of an animal immunization experiment shows that the PLGA microspheres can be utilized as gene vaccine vectors. Principles of the PLGA microspheres comprise that 1, the PLGA microspheres have core-shell structures; surface polymers comprise polymine, PLGA, glucose, chitosan, polylysine, FeCl3 and FeCl2; and through static electricity, dewatering interaction and hydrogen bond-nucleic acid vaccine interaction, a nucleic acid vaccine is concentrated to form a compact nucleic acid vaccine so that nucleic acid vaccine degradation is reduced in vivo; 2, the PLGA microspheres have magnetism and thus after immunization injection, in a strong magnetic field, the distribution of the PLGA microspheres in muscular tissue is improved and the defect of limited contact between the PLGA microspheres and target cells is overcome; and 3, through long-term strong magnetic field induction, a magnetic PLGA microsphere / nucleic acid vaccine complex can enter into the skin; and because of rich antigen presenting cells in the skin, a strong and fast immune response can be induced.
Owner:JILIN UNIV
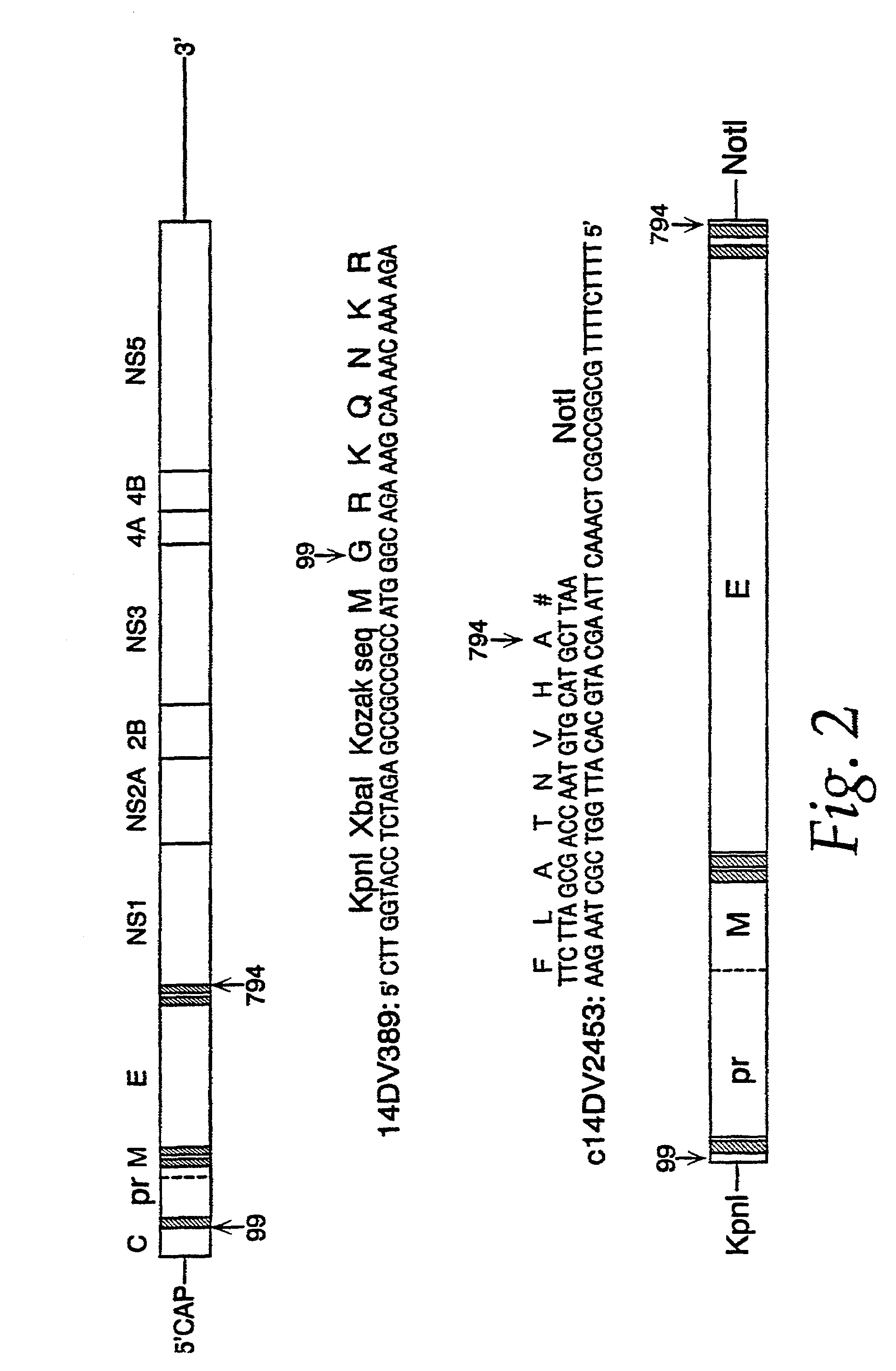
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7227011B2Improve translationLarge structureOrganic active ingredientsFungiImmunogenicityVirology
The present invention encompasses isolated nucleic acids containing transcriptional units which encode a signal sequence of one flavivirus and an immunogenic flavivirus antigen of a second flavivirus. The invention further encompasses a nucleic acid and protein vaccine and the use of the vaccine to immunize a subject against flavivirus infection. The invention also provides antigens encoded by nucleic acids of the invention, antibodies elicited in response to the antigens and use of the antigens and / or antibodies in detecting flavivirus or diagnosing flavivirus infection.
Owner:HEALTH & HUMAN SERVICES THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC +1
Method for down-regulating GDF-8 activity using immunogenic GDF-8 analogues
InactiveUS7070784B1Small sizeQuality improvementAntibody mimetics/scaffoldsGenetic material ingredientsMyostatinVaccination
Disclosed are novel methods for increasing muscle mass by means of immunization against Growth Differentiation Factor 8 (GDF-8, myostatin). Immunization is preferably effected by administration of analogues of GDF-8 which are capable of inducing antibody production against homologous GDF-8. Especially preferred as an immunogen is homologous GDF-8 which has been modified by introduction of one single or a few foreign, immunodominant and promiscuous T-cell epitopes while substantially preserving the tertiary structure of the homologous GDF-8. Also disclosed are nucleic acid vaccination against GDF-8 and vaccination using live vaccines as well as methods and means useful for the vaccination. Such methods and means include methods for identification of useful immunogenic GDF-8 analogues, methods for the preparation of analogues and pharmaceutical formulations, as well as nucleic acid fragments, vectors, transformed cells, polypeptides and pharmaceutical formulations.
Owner:PHARMEXA
Medicine-carrying nanometer polymer particle and its prepn and use
InactiveCN1608675AGenetic material ingredientsPharmaceutical non-active ingredientsWater insolublePolyethylene glycol
The present invention discloses one medicinal polymer (PELGE / PELGA) nanometer particle carrier and its preparation process and use. The carrier material is PELGE material of different molecular weights, different LA / GA ratios and different PEG contents, and the nanometer PELGE / PELGA carrier particle is prepared through evaporation process. The said copolymer is self-assembled in water into nanometer particle or micelle, and its hydrophobic PLGA segment coagulates into the core while the hydrophilic polyglycol forms hydrophilic shell. The carrier may be used for the nanometer preparation of plasmid, nucleic acid vaccine, antisense oligodeoxynucleotide or ribozyme for genetic treatment; the nanometer preparation of various chemical medicines; and the nanometer preparation of polypeptide and protein medicines.
Owner:SICHUAN UNIV +1
DNA vaccine formulations
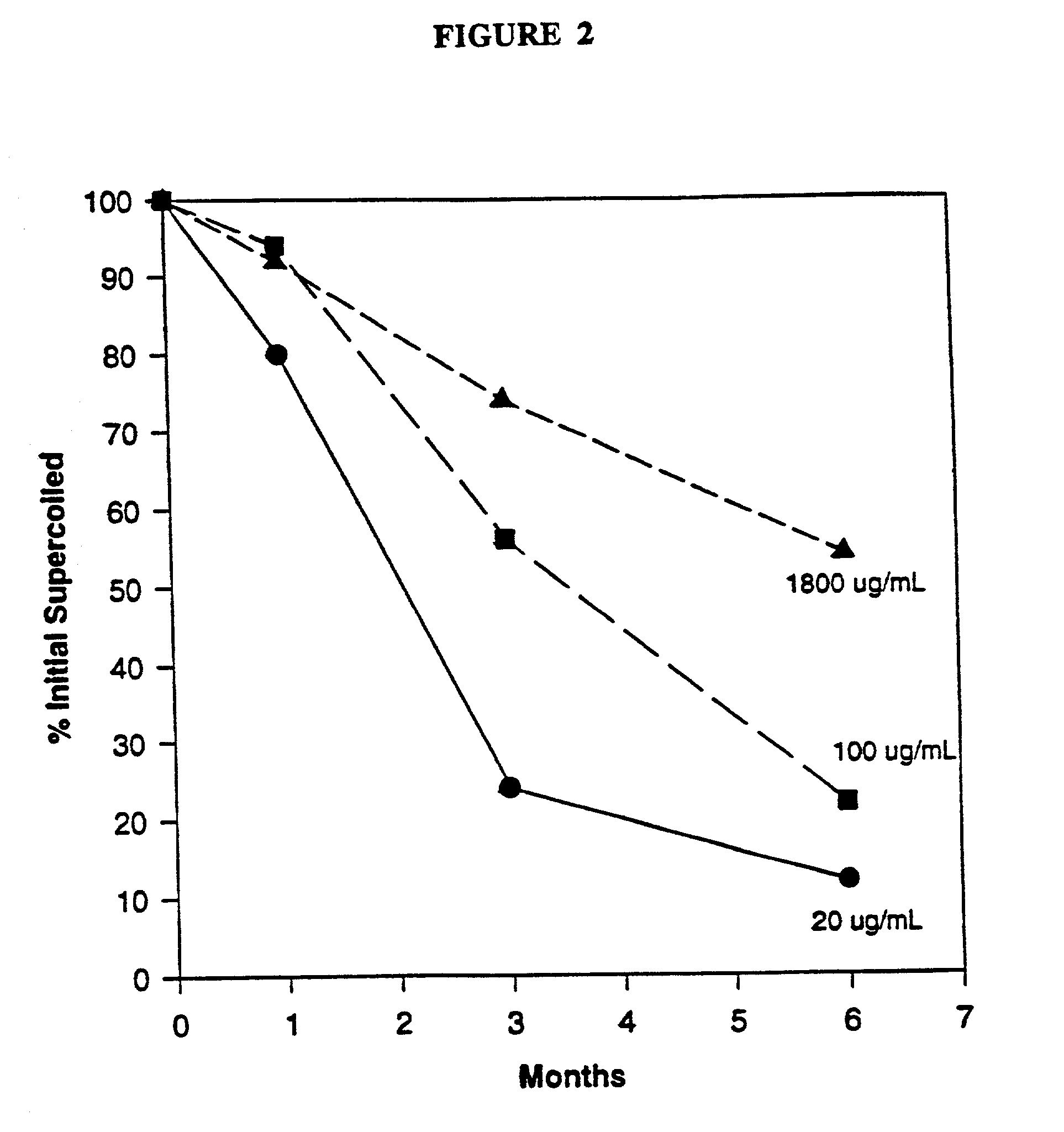
This invention relates to novel methods and formulations of nucleic acid pharmaceutical products, specifically formulations of nucleic acid vaccine products and nucleic acid gene therapy products. The formulations of the disclosure stabilize the conformation of DNA pharmaceutical products.
Owner:MERCK & CO INC
Nucleic acid vectors for immunization
InactiveUS20100098718A1Rapid and high antibody responseEfficient transportSsRNA viruses positive-senseBacteriaVaccinationNucleic Acid Vaccines
A dimeric protein comprising a first fusion protein and a second fusion protein, wherein the first fusion protein comprises a targeting domain, a leucine zipper domain, and an antigen; and wherein the second fusion protein comprises a targeting domain, a leucine zipper domain, and optionally an antigen. Nucleic acid vectors encoding proteins of the invention are provided, particularly for use in nucleic acid vaccination.
Owner:CHIRON CORP
Immunogenic CEA
InactiveUS20050063952A1Effective immune responseBiocideGenetic material ingredientsVaccinationCarcinoembryonic antigen
The present invention provides for methods for immunizing actively against autologous carcinoembryonic antigen (CEA). The method encompasses that the immune system is engaged with variant CEA which is either administered as a protein vaccine, or is effected expressed by nucleic acid vaccination or live-viral vaccination. Preferred embodiments include immunization with variants that include at least one foreign T-helper epitope introduced in the CEA sequence. Also disclosed is variant proteins, DNA, vectors, and host cells useful for practising the method of the invention.
Owner:PHARMEXA
HIV-1gp120 gene consensus sequence optimized by codon and gp120 nucleic acid vaccine
InactiveCN101885760AEfficient expressionEffective stimulationGenetic material ingredientsVirus peptidesEscherichia coliFhit gene
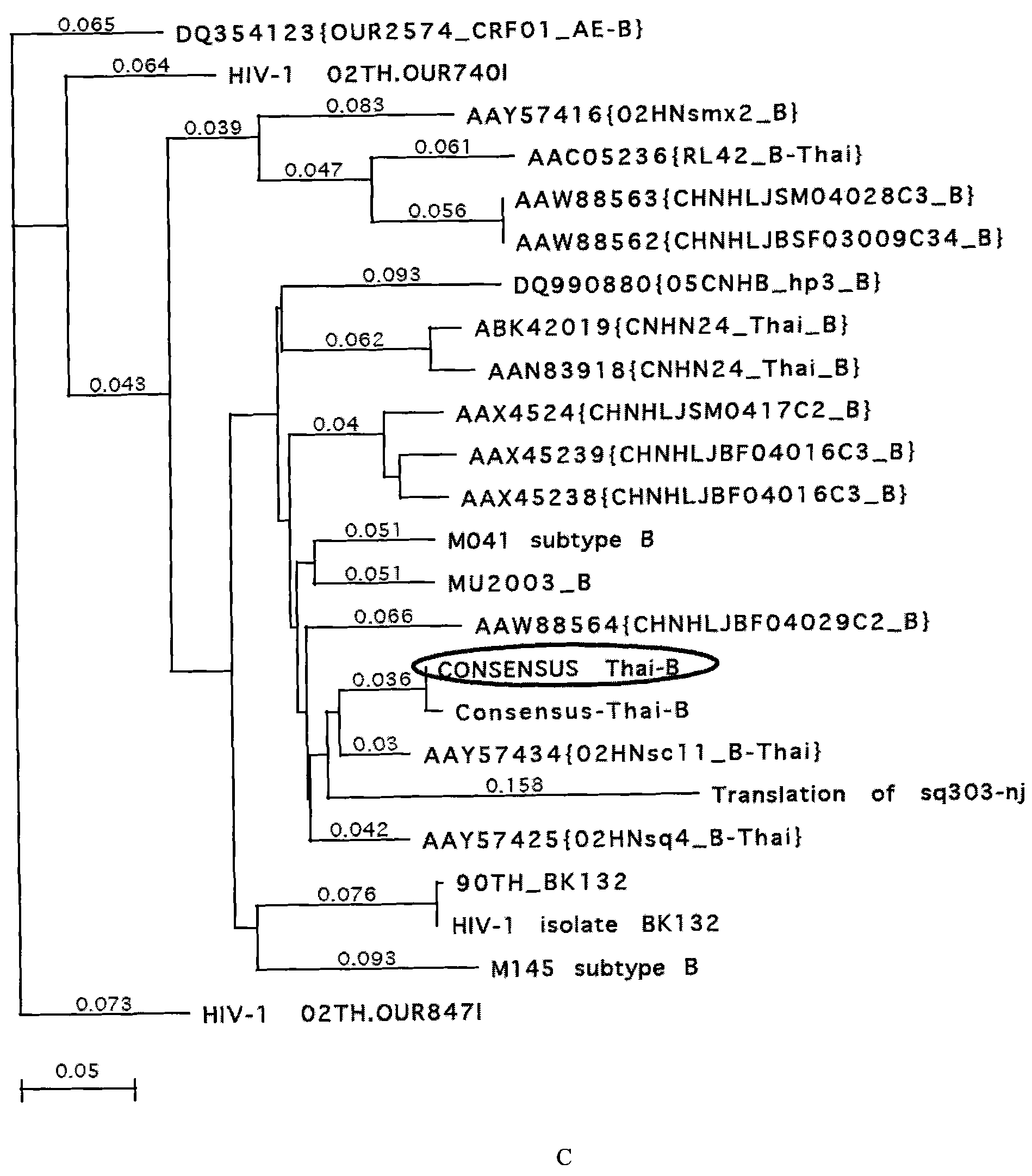
The invention belongs to the technical field of biological medicine, relating to an HIV-1gp120 gene consensus sequence optimized by codons and a gp120 nucleic acid vaccine. In the invention, a Chinese HIV-1 epidemic strain (A / E recombinant subtype, B / C recombinant subtype and ThB subtype) envelope protein gp120 consensus amino acid sequence is obtained by applying multi-sequence comparative analysis; a gp120 gene segment encoding the consensus amino acid sequence is prepared by using an artificially synthesized method, and the gene is optimized by the codons and combines preferences of mammalian cells and escherichia coli codons; and on the basis, HIV-1gp120 nucleic acid vaccine consisting of the gp120 gene and eukaryotic expression vector pJW4303 is constructed. The nucleic acid vaccine can be applied to animal and human immunization and expression and production of the gp120 protein.
Owner:王世霞 +3
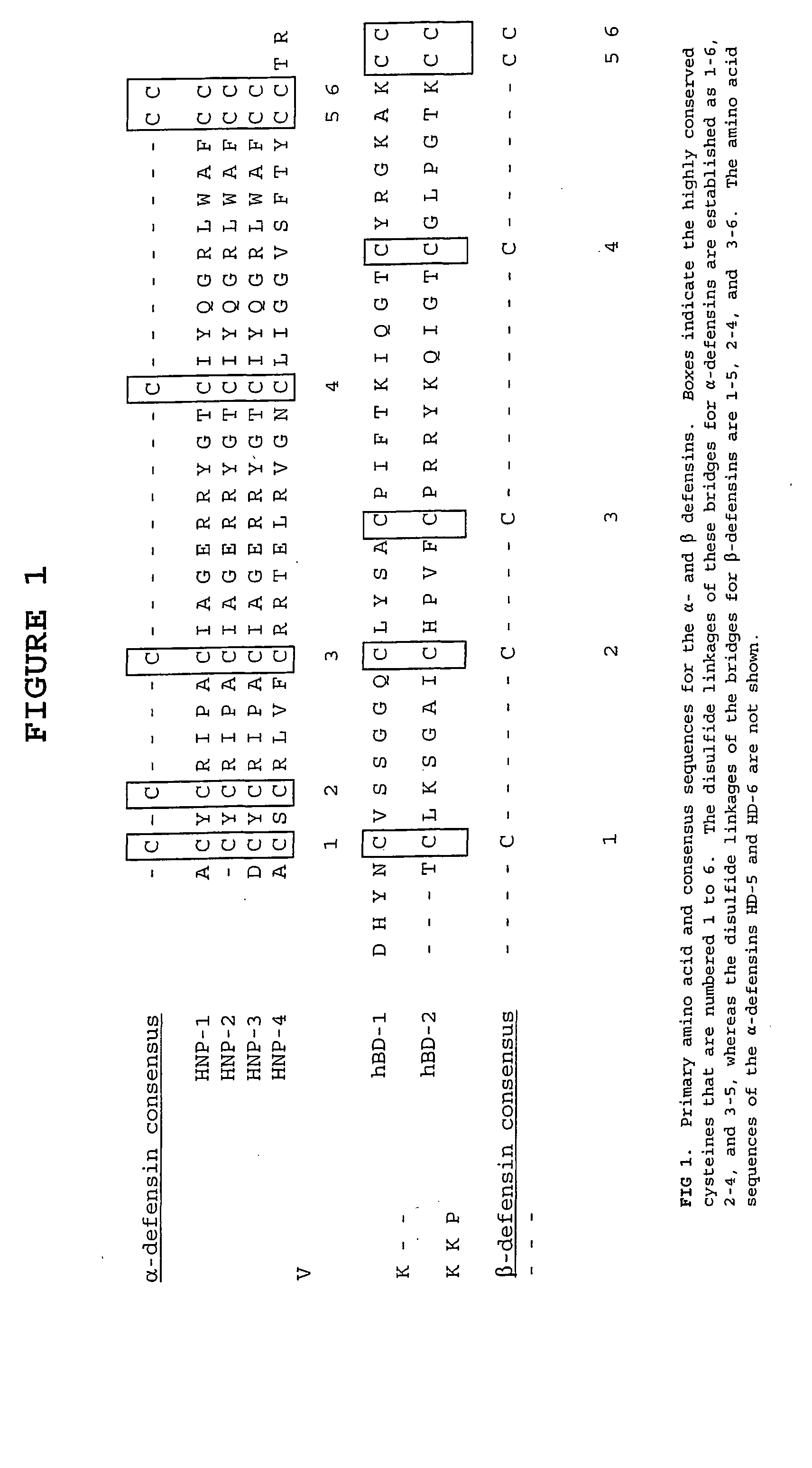
Defensin-antigen fusion proteins
The present invention relates to a vaccine for increasing the immunogenicity of a tumor antigen thus allowing treatment of cancer, as well as a vaccine that increases the immunogenicity of a viral antigen, thus allowing treatment of viral infection, including immunodeficiency virus (HIV) infection. In particular, the present invention provides a fusion protein comprising a defensin fused to either a tumor antigen or viral antigen which is administered as either a protein or nucleic acid vaccine to elicit an immune response effective in treating cancer or effective in treating or preventing viral infection.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER
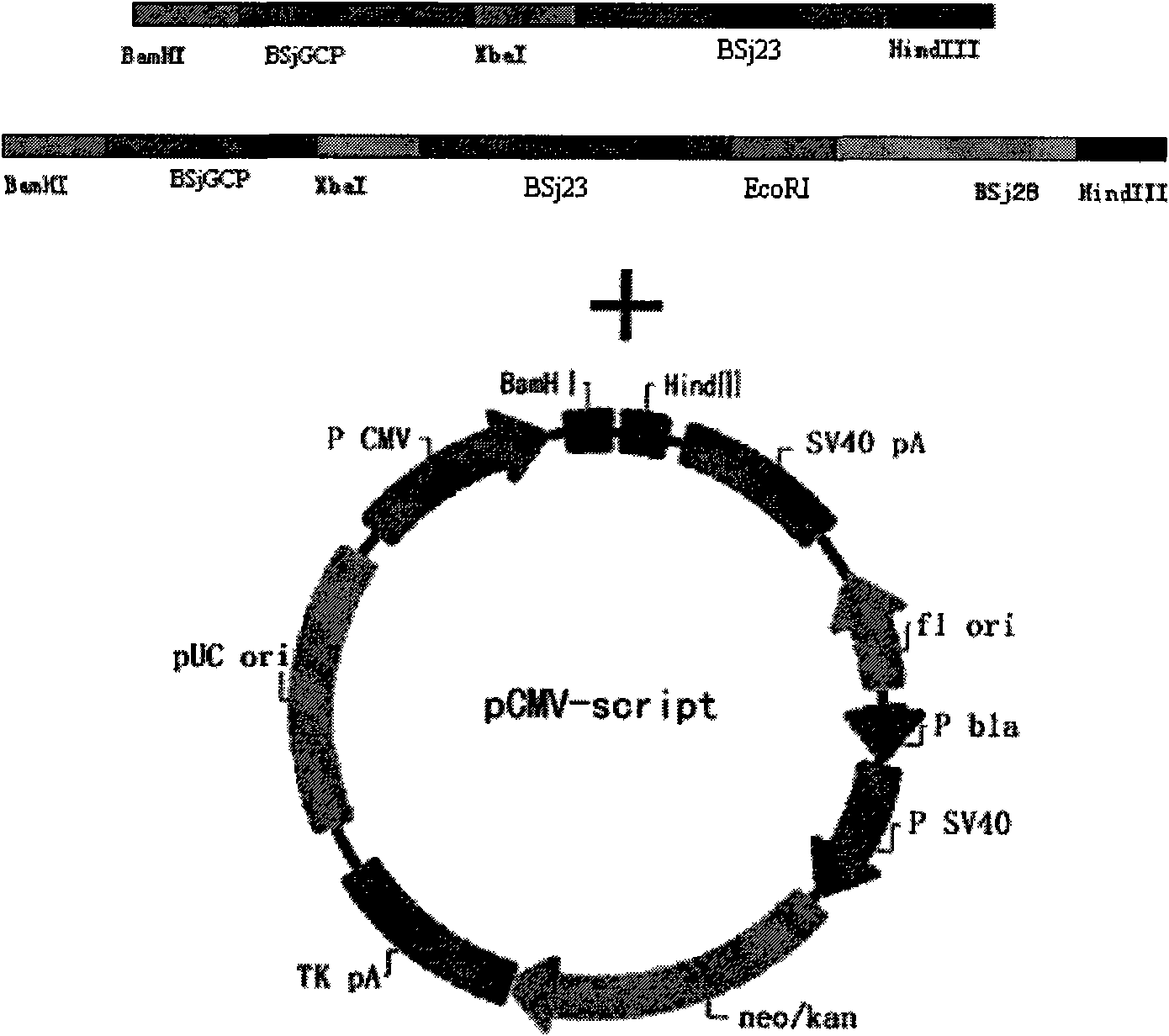
Schistosoma japonicum recombinant multi-epitope antigens, method for expressing and purifying same and application thereof
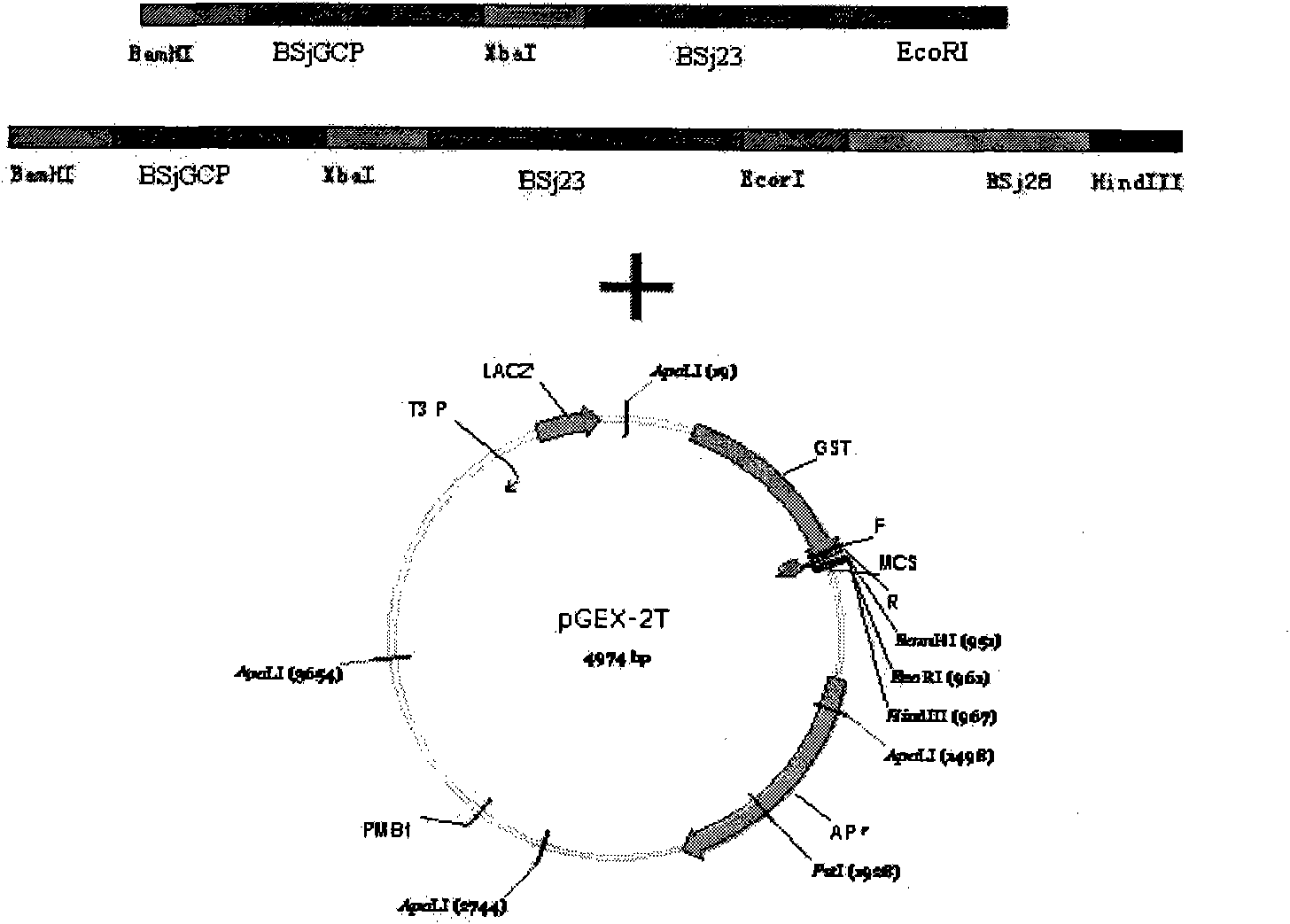
The invention discloses gene orders of schistosoma japonicum recombinant multi-epitope antigens BSjGCP-BSj23 and BSjGCP-BSj23-BSj28, a method for expressing and purifying the same, and application thereof in preparing schistosomiasis japonica immunity prevention vaccines and diagnostic reagents. Recombinant multi-epitope nucleic acid vaccines pCMV-BSjGCP-BSj23 and pCMV-BSjGCP-BSj23-BSj28 obtain 14.76 percent and 64.95 percent of worm reduction rates respectively in Kunming mice. The recombinant multi-epitope antigens pGEX-BSjGCP-BSj23 and pGEX-BSjGCP-BSj23-BSj28 obtain 15.7 percent and 57.99 percent of worm reduction rates in immunizing BalB / c mice, and obtain 91.0 percent and 89.9 percent of sensitivities as well as 97.8 percent and 93.4 percent of specificities respectively as diagnostic antigens.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS20050163804A1Easy to prepareEasy to manageOrganic active ingredientsFungiImmunogenicityViral infection
The present invention encompasses isolated nucleic acids containing transcriptional units which encode a signal sequence of one flavivirus and an immunogenic flavivirus antigen of a second flavivirus or of a chimeric immunogenic flavivirus antigen comprising sequence from more than one flavivirus. The invention further encompasses a nucleic acid and protein vaccine and the use of the vaccine to immunize a subject against flavivirus infection. The invention also provides antigens encoded by nucleic acids of the invention, antibodies elicited in response to the antigens and use of the antigens and / or antibodies in detecting flavivirus or diagnosing flavivirus infection.
Owner:THE GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICE CENTS FOR DISEASE CONTROL & PREVENTION
Strain-Independent Amplification of Pathogens and Vaccines Thereto
ActiveUS20080311155A1Sugar derivativesMicrobiological testing/measurementAntigen-presenting cellRetrovirus
This in invention relates to methods for the nucleic acid amplification of multiple variants (strains) of any pathogen present in a sample, and preferably in a sample from a pathogen infected individual. In preferred embodiments, the pathogen is a retrovirus, such as HIV. The amplified pathogen nucleic acid can be used to identify the pathogen variants present in a sample, to quantitate the pathogen present in a sample, and as a nucleic acid vaccine, or in the preparation of antigen presenting cell vaccines. Nucleic acids produced by the methods of the invention or the proteins encoded thereby can be used to transfect / load antigen presenting cells. The loaded antigen presenting cells can then be used as a vaccine for the treatment of pathogen infection. In another embodiment, nucleic acids produced by the methods of the invention can be used directly as nucleic acid vaccines without prior loading into antigen presenting cells.
Owner:KIRIN BREWERY CO LTD +1
Epitope screening method capable of exciting anti-mycobacterium tuberculosis protective immunological reaction of body and uses
ActiveCN101289496AHelp predictHelp determineAntibacterial agentsPeptide preparation methodsScreening methodPeptide vaccine
The invention relates to a selection method for epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof, in particular to the molecule mimic peptide of the epitope with vaccine development prospect from the mycobacterium tuberculosis and the coding DNA thereof. The selection method of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof provides T lymphocyte epitope contained in one important gene Ag85B in the research of mycobacterium tuberculosis and the method of deducting or selecting the epitope, which is beneficial to further developing novel multivalent and poly epitope tuberculosis vaccine and prevent and control the happening and development of tuberculosis; the method makes a foundation of the future development of synthesizing peptide vaccine epitope vaccine and dna vaccine by using epitope and provides molecule mimic peptide of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the peptide has the amino acid sequence of FVRSSNLKFQDAYNA(SEQ ID NO:1).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
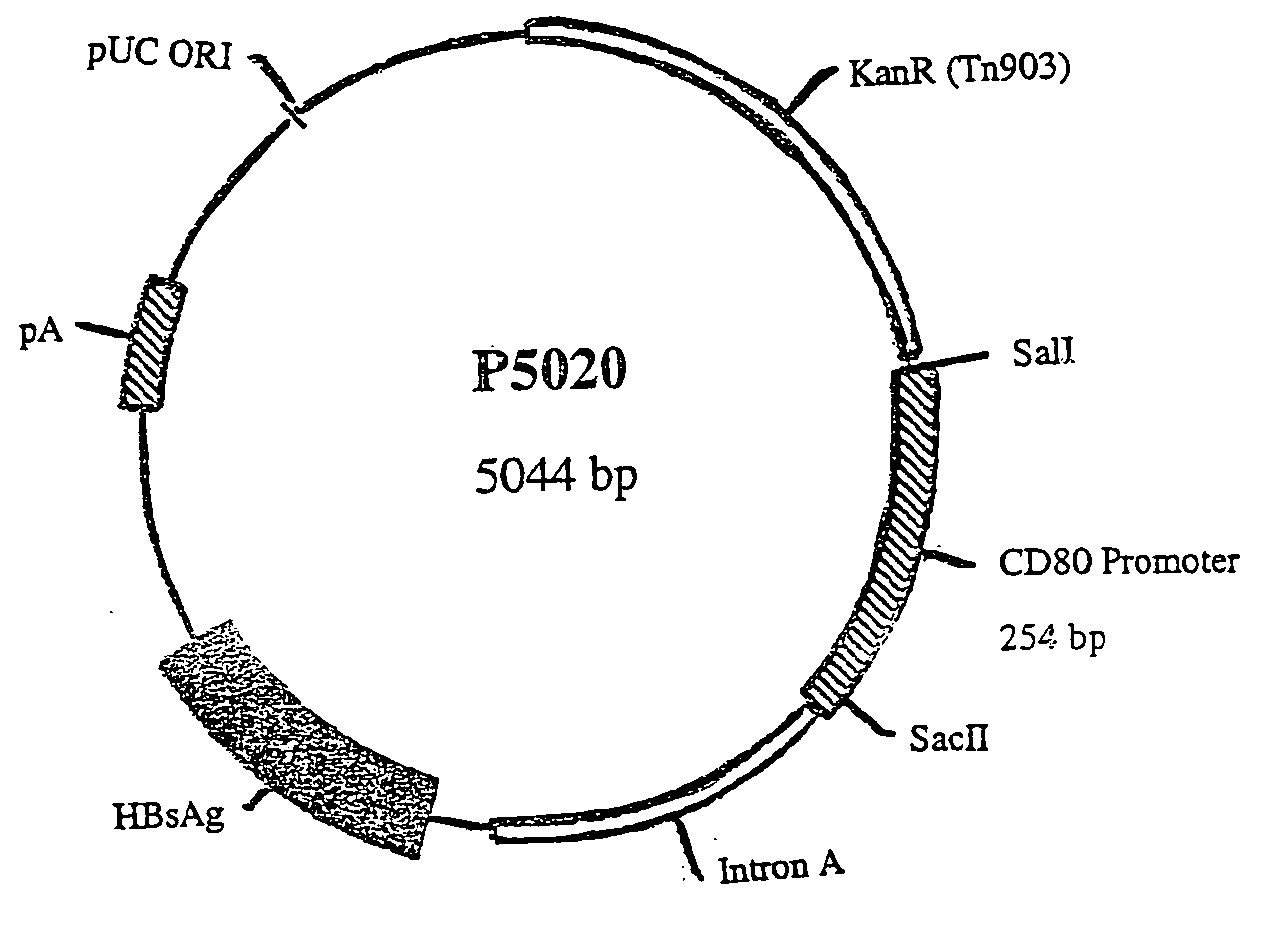
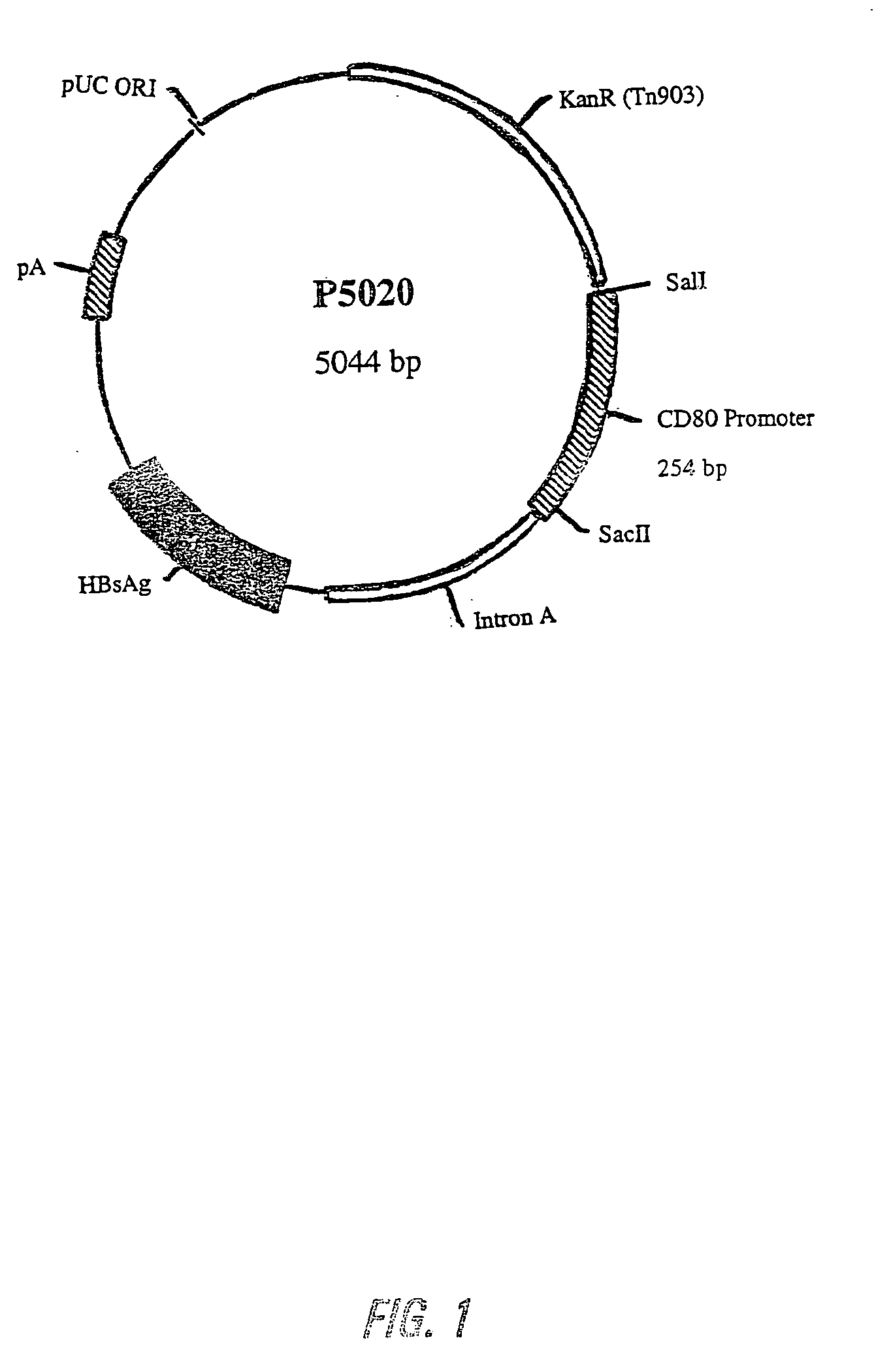
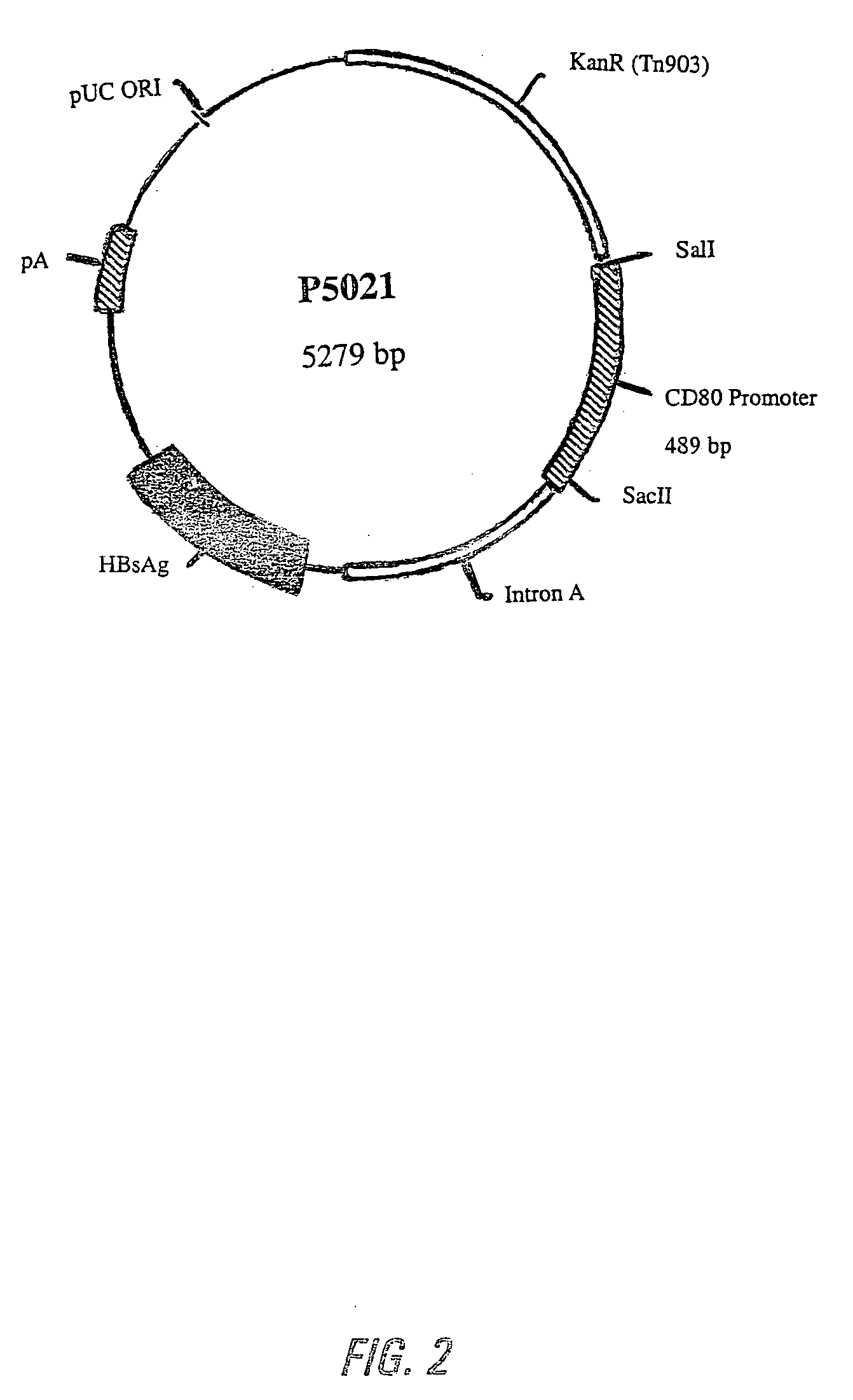
Nucleic acid vaccine compositions having a mammalian CD80/CD86 gene promoter driving antigen expression
Polynucleotides encoding at least one immunizing antigen whose expression is controlled by a promoter derived from a gene encoding a co-stimulatory molecule are provided. The polynucleotides may also encode adjuvants. Compositions comprising at least one immunizing agent and at least one cytokine that enhance dendritic cell stimulation and / or survival are also provided. Methods for eliciting an immune response against the immunizing agent are also provided. The method includes the steps of administering the polynucleotides and, optionally, co-administering an adjuvant.
Owner:POWDERJECT RES LTD OXFORD (GB)
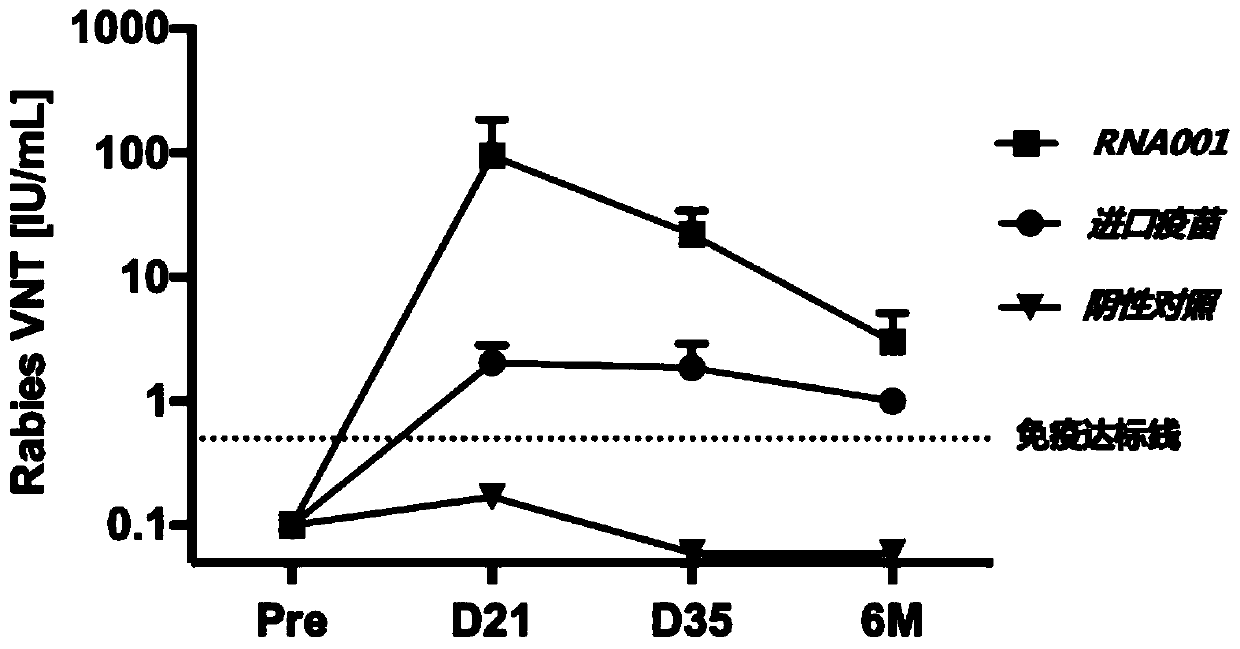
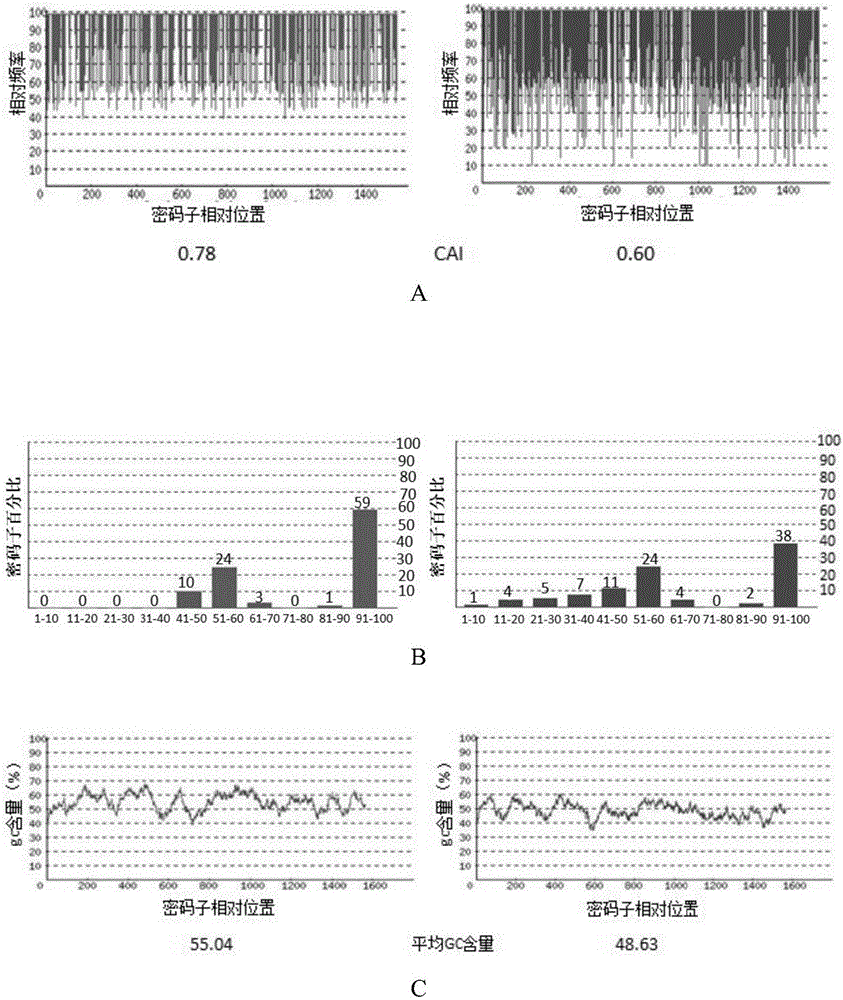
mRNA rabies vaccine
ActiveCN110714015AStable structureIncrease GC contentSsRNA viruses negative-sensePowder deliveryNucleotideNucleotide sequencing
The present invention relates to the field of nucleic acid vaccines and particularly provides an mRNA rabies vaccine. A nucleotide sequence for transcribing the provided optimized RVG mRNA is shown asSEQ ID NO.1. A coding region of a rabies virus CTN-1 strain G protein (RVG) is optimized and an obtained nucleotide sequence is the optimized RVG mRNA sequence shown in the SEQ ID NO.1. The nucleotide sequence enables structure of the transcribed mRNA to be more stable and the target protein is more efficiently translated in mammals and humans. The provided rabies virus nucleic acid vaccine comprises a vaccine vector and the optimized RVG mRNA, achieves sufficient protection effects by using an extreme small dose, and is superior to existing rabies vaccine technologies in terms of safety andeffectiveness.
Owner:珠海丽凡达生物技术有限公司
Hepatitis C virus nucleic acid vaccine
ActiveUS9056090B2Reduce capacityReduce loadSsRNA viruses positive-senseViral antigen ingredientsMolecular biologyNucleic Acid Vaccines
The present invention features nucleic acid constructs that can be used as a HCV nucleic acid vaccine, vaccine component, or in the production of a HCV vaccine. Described constructs include those: (1) encoding for a chimeric HCV polypeptide containing a NS3-4A region based on a first HCV strain and an NS3-NS4A-NS4B-NS5A or an NS3-NS4A-NS4B-NS5A-NS5B region based on a second strain; and (2) a chimpanzee based adenovector encoding an HCV polypeptide.
Owner:MSD ITAL
Molecular marker for cancer stem cell
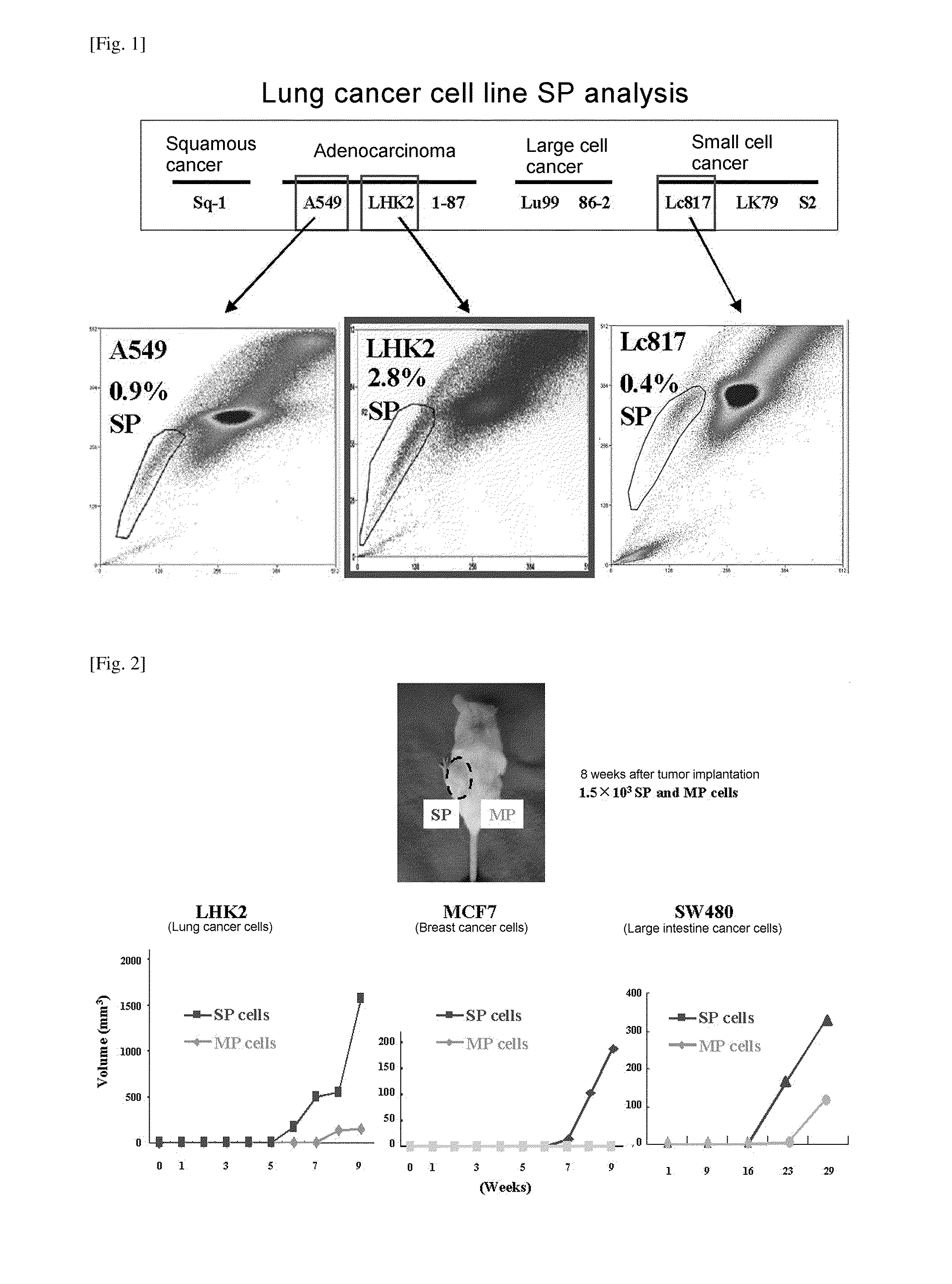
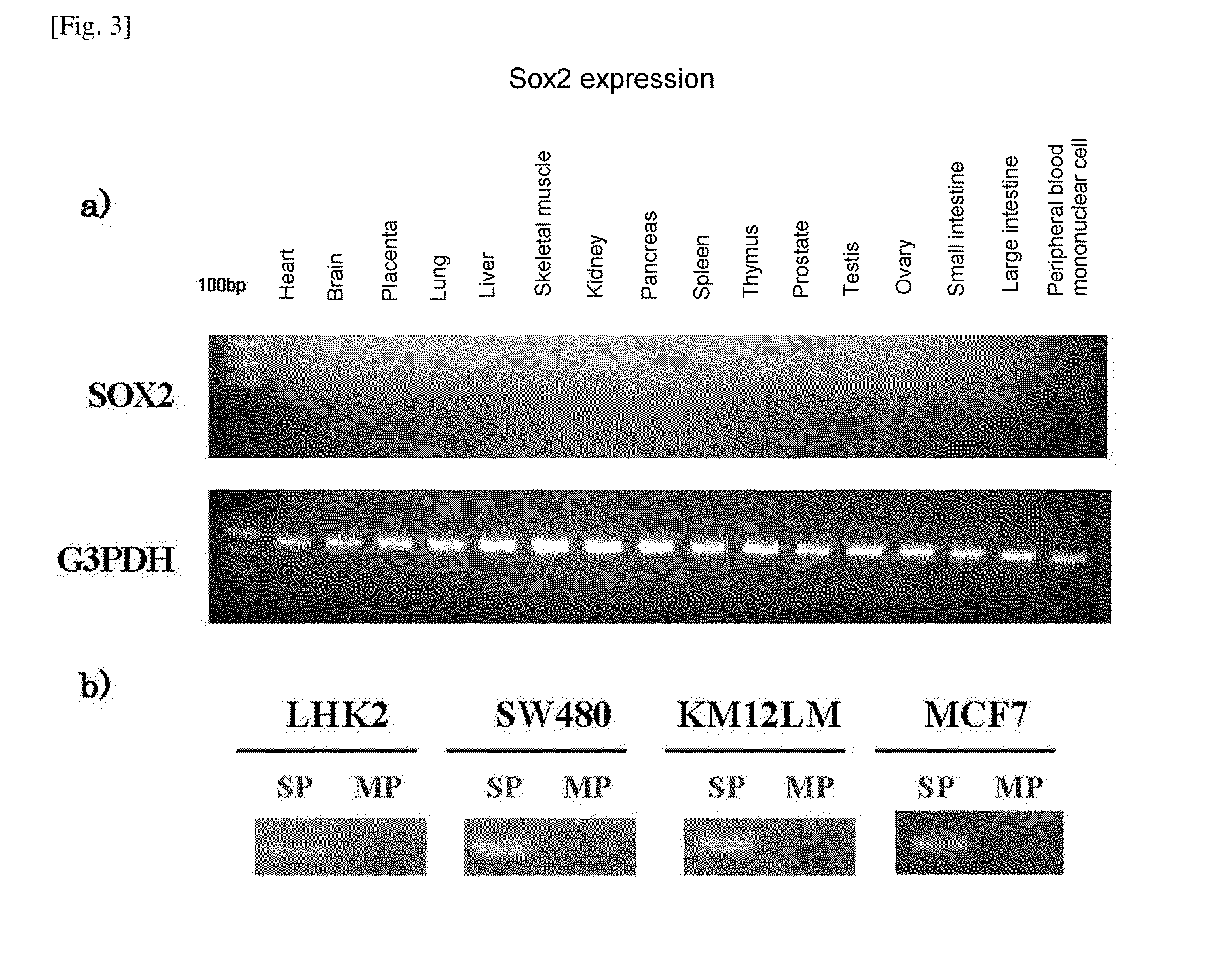
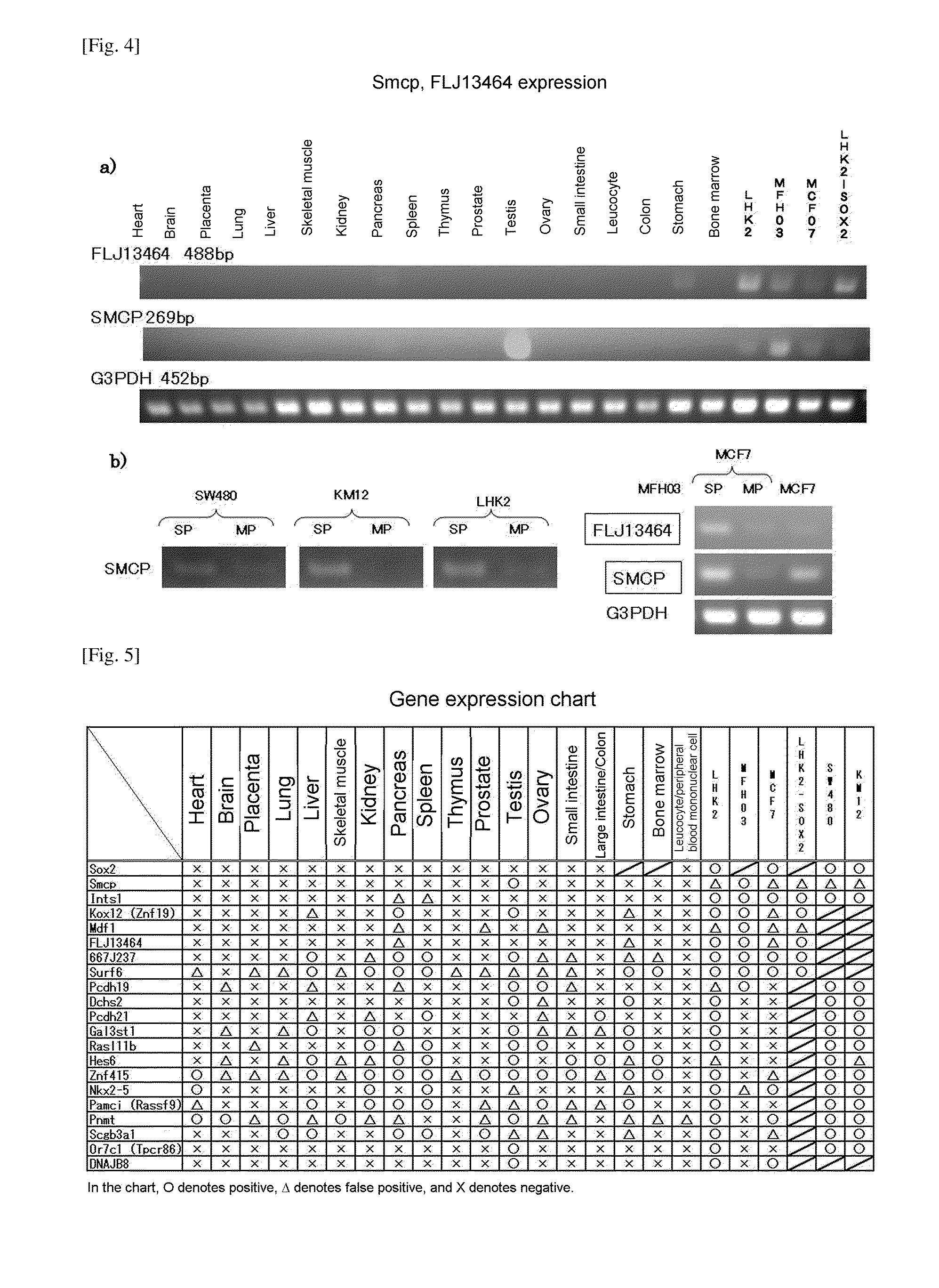
ActiveUS20110262358A1Easily discriminatedHigh relevance to tumorigenicityOrganic active ingredientsPeptide/protein ingredientsEpitopeCancer cell
A molecular marker for detecting a cancer stem cell in a cell mass which is a subject of interest, wherein the molecular marker can be detected in a cancer stem cell contained in the subject of interest but cannot be detected in a normal cell and a cancer cell that is different from a cancer stem cell; a method for determining the presence or absence of a cancer stem cell in a subject of interest by using the molecular marker as an measure; a kit for determining the presence or absence of a cancer stem cell, which comprises at least a reagent for detecting the molecular marker; a polypeptide encoded by the molecular marker; an antibody capable of recognizing an epitope of an expression product of a gene derived from the molecular marker; a nucleic acid capable of inhibiting the expression of the molecular marker; and a nucleic acid vaccine comprising a gene derived from the molecular marker.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
Condon optimized African swine fever virus P54 gene, nucleic acid vaccine and application thereof
InactiveCN103805615ATo solve the immune prevention and controlSame immune effectViral antigen ingredientsGenetic material ingredientsImmune effectsAfrican swine fever
The invention relates to a condon optimized African swine fever virus P54 gene and a nucleic acid vaccine based on the gene, wherein the nucleic acid vaccine consists of an eukaryotic expression vector and the condon optimized African swine fever virus P54 gene. The nucleic acid vaccine is constructed through the following steps of optimizing the condon of P54protein, designing a specific primer, transferring sequence of the artificially synthesized African swine fever virus P54 gene into a cloning vector and recombining the purified P54 gene into the eukaryotic expression vector. The nucleic acid vaccine can be used for preventing the occurrence of African swine fever and is a powerful tool for solving the immune prevention and control of the African swine fever. Compared with the traditional vaccines, the nucleic acid vaccine has the advantages of small quantity, security, long-term effect, stability and convenience in operation when reaching same immune effect.
Owner:孙洁
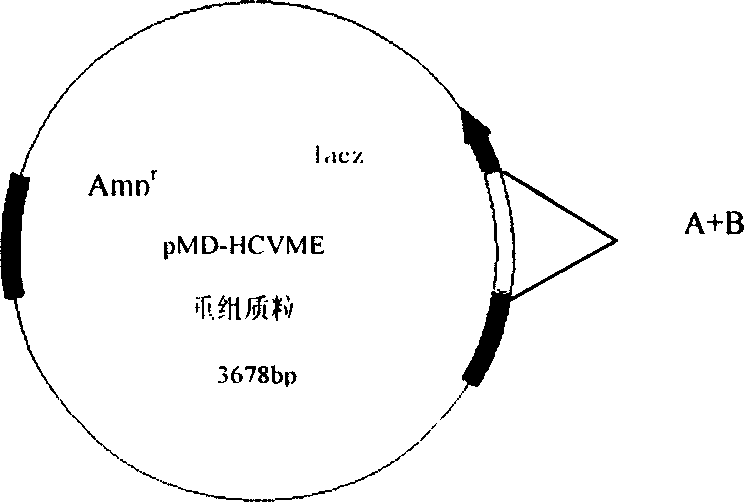
Gene cloning of polyepitope antigen of hepatitis C virus and its coding sequence
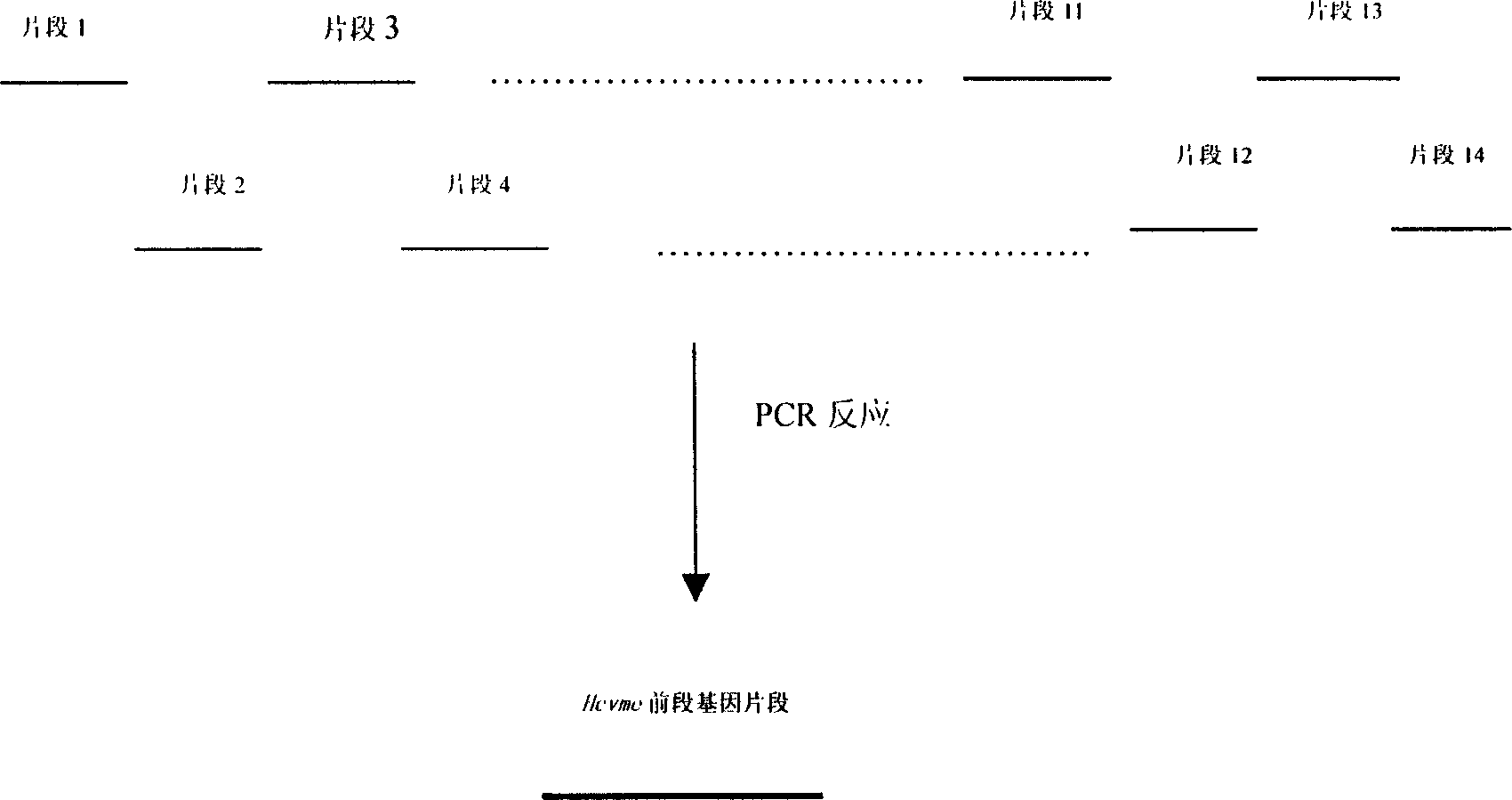
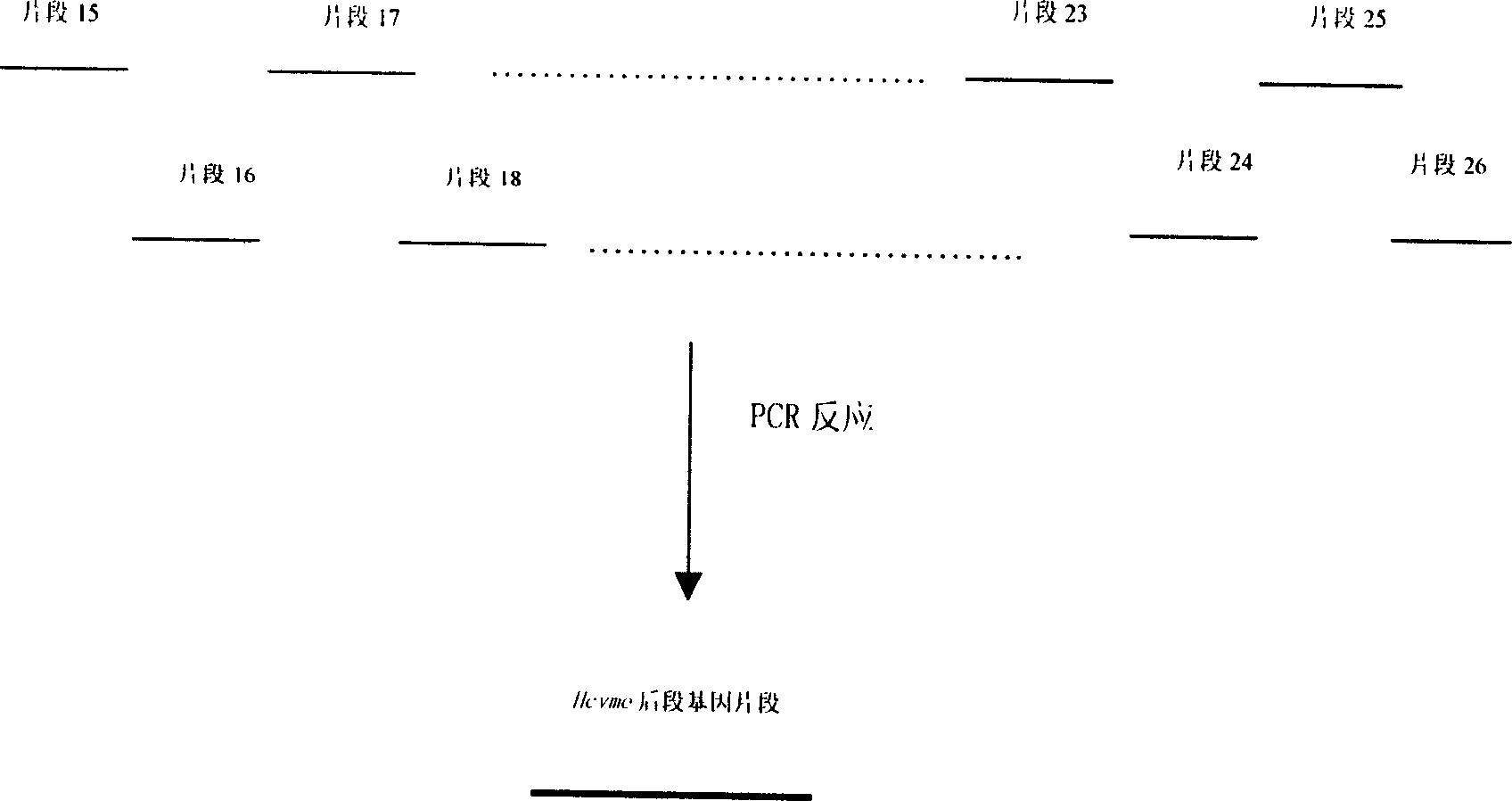
InactiveCN1609213AAvoid the disadvantage of being prone to mutationValid submissionGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCtl epitopeGene clone
The present invention relates to biomedicine engineering technology, and is the gene cloning of polyepitope antigen of hepatitis C virus hcvme and the coded polyepitope antigen HCVME. The hcvme includes 9 epitope genes of HVR1 simulating B cell and 4 epitope genes of T cell in E2 region of HCV genome, and the 4 epitope genes of T cell includes 2 of conservative CTL epitope in region C, 1 of conservative CTL epitope in region NS3 and 1 of conservative Th epitope in region NS3. Serial tests show that hcvme has high cross reaction with karyogamy expressed product GST-HCVME of gst gene and positive hepatitis C antibody serum, and the GST-HCVME immunized male rabbit serum has high distinction frequency on natural HVR1 synthetic peptide. Therefore, hcvme and its expressing product HCVME may be used in preparing nucleic acid vaccine and polypeptide vaccine for hepatitis C and the test reagent for HCV antigen and antibody.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine
InactiveCN107760716AInducible propertiesImproving immunogenicityViral antigen ingredientsAntiviralsEscherichia coliDisease
The invention discloses a grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine, and belongs to the technical field of gene engineering and molecular immunology. The preparation method disclosed by the invention is characterized by comprising the steps of extracting viral genome RNA, performing reverse transcription to convert the viral genome RNA into cDNA, amplifying a corresponding DNA sequence out, constructing the DNA sequence to pcDNA-3.1(+) plasmid, converting Escherichia coli DH5alpha, screening out positive clone bacteria containing recombinant plasmid, culturing a lot of the positive bacteria and extracting recombinant plasmid S11-pcDNA3.1 contained in the bacteria. The recombinant plasmid is utilized as nucleic acid vaccine to perform intramuscular injection on the grass carps, and the nucleic acid vaccine enters the muscle cells and expresses VP35 protein of the grass carp reovirus in the muscle cells; thus, fish body immune cell proliferation is stimulated, antiviral related gene expression is up regulated, fish bodies are stimulated to generate antiviral antibodies, and capability of grass carps in resisting grass carp reovirus infection is effectively improved; furthermore, the grass carp reovirus S11 gene eukaryotic expression recombinant plasmid can be used for preventing a grass carp hemorragic disease caused by the grass carp reovirus in aquaculture.
Owner:HENAN NORMAL UNIV
Hepatitis B nucleic acid vaccine with optimized codon
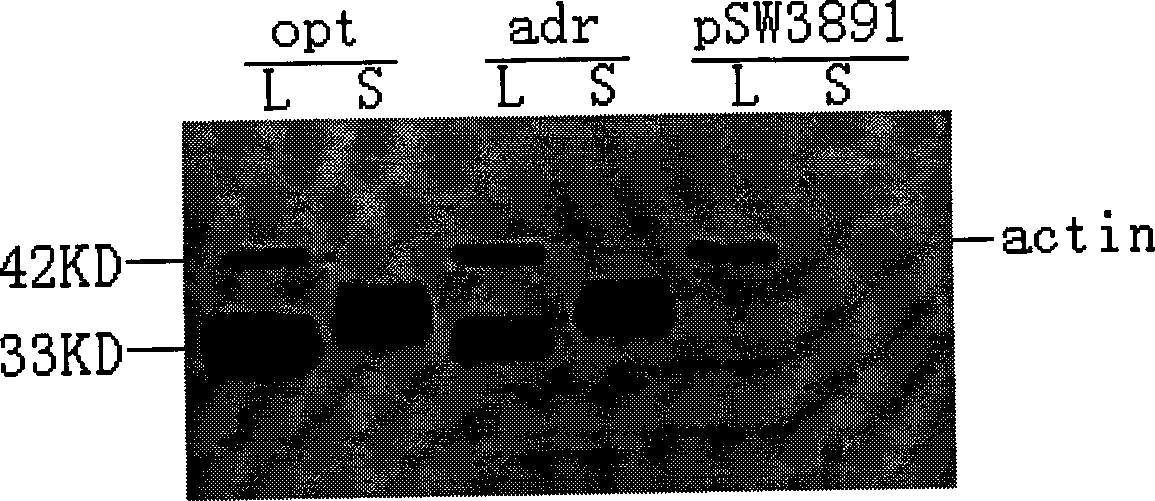
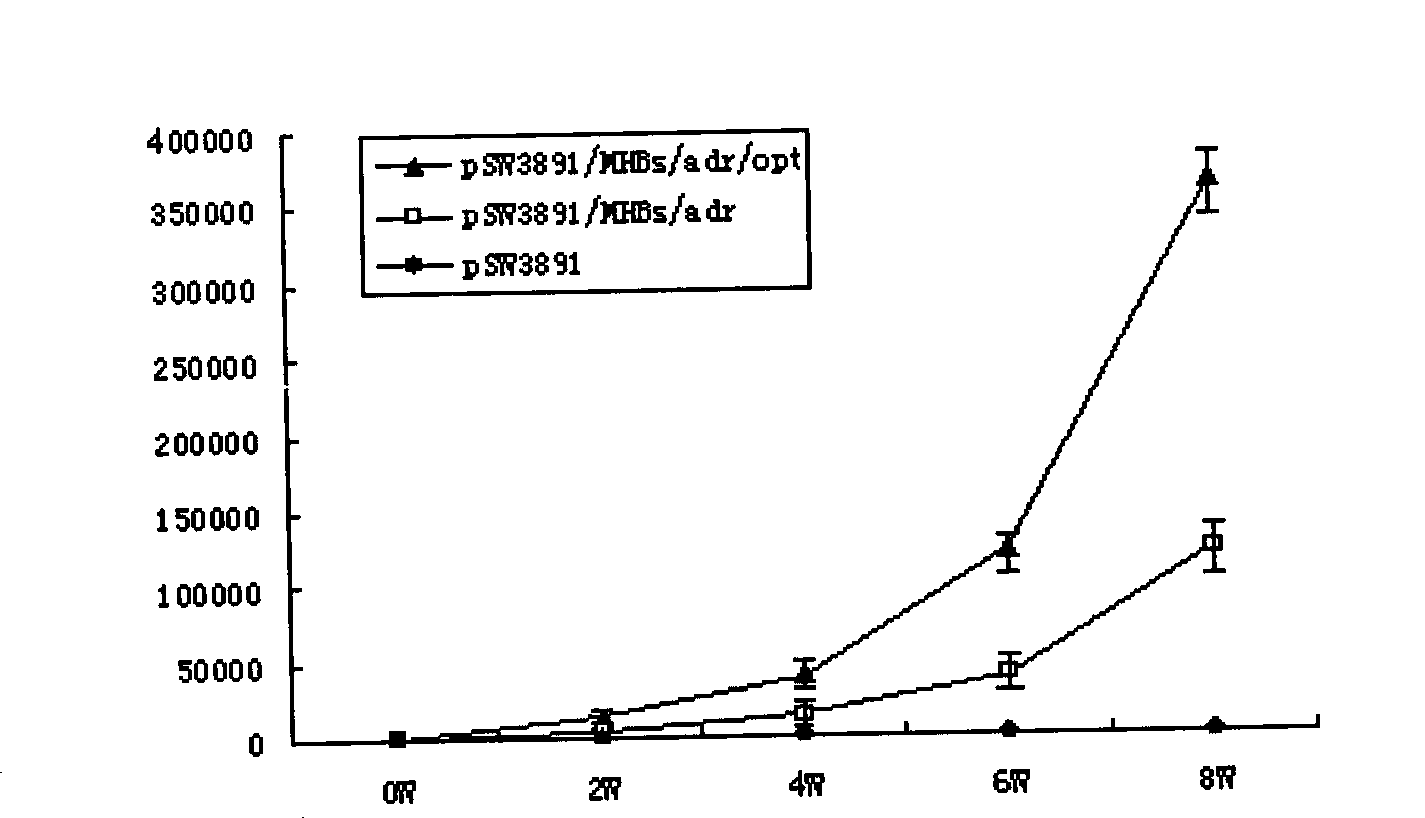
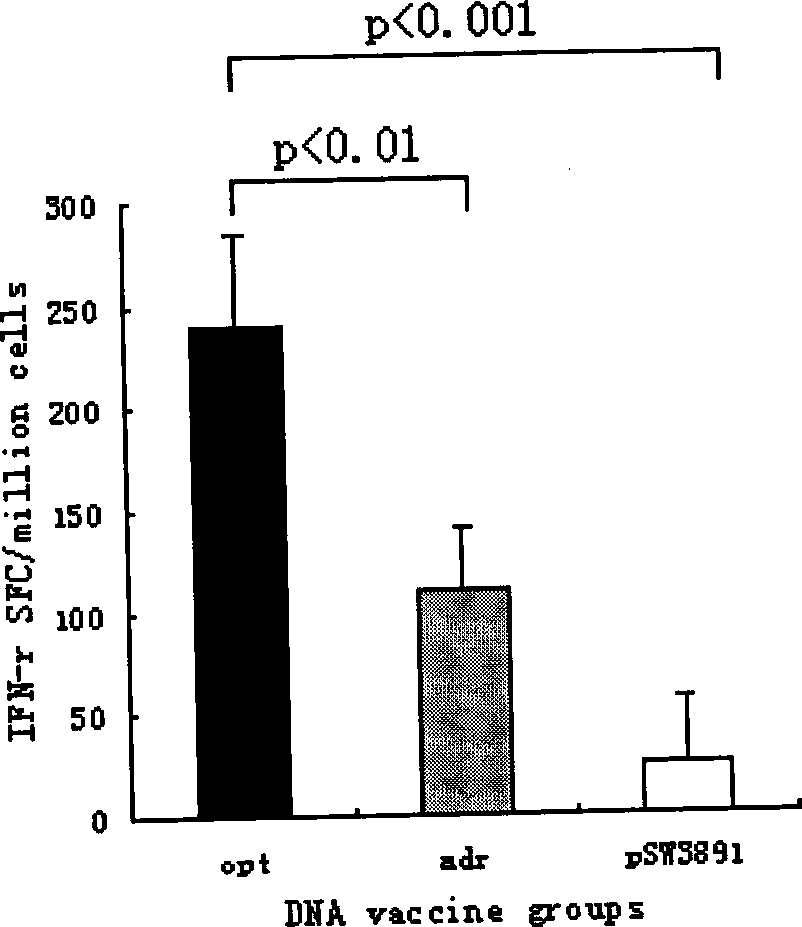
InactiveCN101502650AHigh protein expressionImprove responseDigestive systemAntiviralsMammalDigestion
The invention relates to a hepatitis B virus nucleic acid vaccine optimized by codon. In the invention, hepatitis B surface antigen (HBs) gene order (adr subtype) is analyzed to find codon locus which tells the differences between the codon preferences of the gene order and the codon preferences of the mammal; the codon of the HBs gene order is replaced to obtain the surface antigen gene; the gene order is combined and expanded to obtain MHBs, Pst I, BamH I, double digestion MHBs gene and carrier pSW3891 plasmid optimized by the codon; 10ul connection system is configured to obtain a middle protein gene. The nucleic acid vaccine of the invention overcomes the defects that the differences between prokaryote and eukaryote in terms of codon preferences cause that the foreign gene can not be expressed effectively in mammal reservoir and can not generate relatively good immune sheltering effect; in addition, the invention remarkably improve protein expression of the foreign gene in the mammal reservoir, effectively stimulates immune system of the reservoir to generate relatively good immunological reaction of human body fluids and cellular immune response.
Owner:邢益平 +1
Method for preparing chicken genetic engineering immunopotentiator by akirin2 gene sequence
InactiveCN102240402AImprove immune efficiencyReduce manufacturing costGenetic material ingredientsAntibody medical ingredientsPhosphateRestriction site
The invention provides a method for preparing a chicken genetic engineering immunopotentiator by an akirin2 gene sequence, relating to a method for preparing a chicken genetic engineering immunopotentiator. The invention solves the problem that the existing genetic engineering immunoenhancement nucleic acid vaccines can not extensively improve the immunity effects of vaccines and bacterins. The method comprises the following steps of: acquiring a chicken akirin2 gene coded sequence; acquiring a recombinant pMD18-T vector containing the chicken akirin2 gene; designing a primer and enabling the 5'- terminals to have HindIII and EcoRI restriction sites, subcloning the chicken akirin2 gene in the recombinant pMD18-T vector into an expression vector to construct a recombinant expression vector; and diluting the recombinant expression vector by a sterile PBS (phosphate buffer solution). The chicken genetic engineering immunopotentiator provided by the invention can improve the expression levels of multiple immunity related factors such as IL-1, IL-6, toll-like receptors and the like, and has the characteristics of wide applicable range and the like for various vaccines and bacterins.
Owner:HARBIN NORMAL UNIVERSITY
Codon optimized severe fever with thrombocytopenia syndrome virus (SFTSV) glycoprotein Gn gene sequence carrying tPA signal peptide and nucleic acid vaccine thereof
InactiveCN106011155AEfficient removalEffective combinationSsRNA viruses negative-senseViral antigen ingredientsSequence signalWild type
The invention belongs to the technical field of biological medicines, and relates to a codon optimized severe fever with thrombocytopenia syndrome virus (SFTSV) glycoprotein Gn gene sequence carrying tPA signal peptide and a nucleic acid vaccine thereof. The SFTSV glycoprotein Gn nucleic acid vaccine is composed of the codon optimized SFTSV glycoprotein Gn gene sequence and a eukaryotic expression vector pJW4303, and the 5'end of the SFTSV glycoprotein Gn gene sequence is connected with a tPA signal peptide sequence. Compared with a nucleic acid vaccine in a wild type state, the nucleic acid vaccine can express objective protein more efficiently and can effectively secrete the objective protein out of cells in gene expression, and an immune system can be effectively stimulated to produce good humoral immune response after a mammal is inoculated with the nucleic acid vaccine.
Owner:李军 +6
Construction and application of recombinant human interleukin 12 eukaryotic expression vector
InactiveCN101638657AFully extendedFully foldedGenetic material ingredientsAntibody medical ingredientsLaboratory mouseClinical trial
The invention provides a construction and an application of recombinant human interleukin 12 eukaryotic expression vector and relates to the development of recombinant human interleukin 12 (rhIL-12) eukaryotic expression vector, the screening of stable expression cell lines and the adjuvant function of nucleic acid vaccine gene and the therapeutical effect of tumor gene. Ten hydrophobic flexible amino acid joints are used to connect P40 and P35 to obtain fusion gene to construct rhIL-12 eukaryotic expression plasmid (pCDNA6-IL-12), then the expression plasmid is transferred to CHO cell and finally stable expression cell lines are screened. The expression product has good biological activity. Nucleic acid vaccine and pCDNA6-IL-12 are used for coimmune so as to significantly increase the immune effect. Strong antitumor effect can be exerted when the expression vector of the invention is directly injected inside the tumor of laboratory mouse tumor model or is first used to perform gene modification to tumor cells and then implanted in tumor. The expression vector of the invention can be used in the search of clinical trial stage after further perfecting.
Owner:张文卿
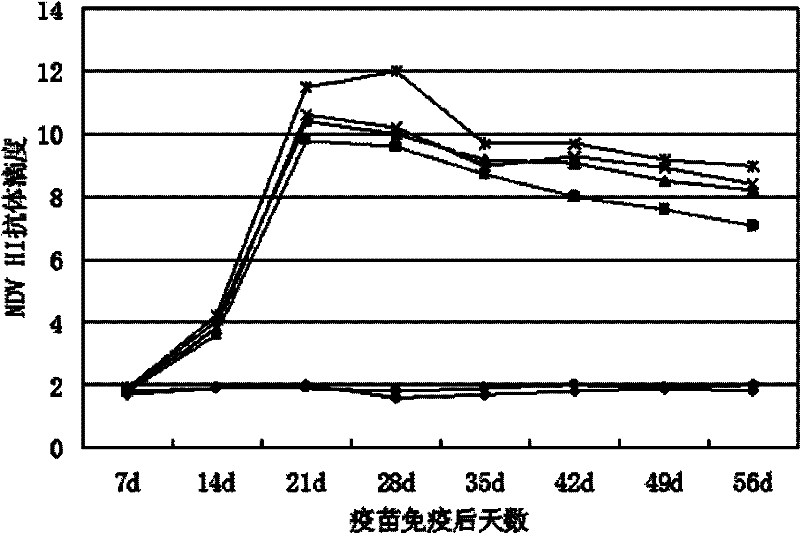
Preparation method of avian influenza virus HA gene recombinant adenovirus
InactiveCN104404005ASolve technical problems with low expression efficiencyViruses/bacteriophagesGenetic engineeringHemagglutininAvian influenza virus
The invention provides a preparation method of avian influenza virus HA gene recombinant adenovirus, which creatively comprises the following steps: carrying out a series of intermediate processes on plasmid pCAGGS, adenovirus shuttle plasmid pShuttle, adenovirus framework plasmid pAdEasy-1 and the like to obtain a gene expression plasmid and other intermediate products, and transfecting the obtained recombinant adenovirus plasmid with 293 cell; and carrying out immunohistochemical screening on the recombinant virus according to the adenovirus-infected cytopathy and specific cells. By using the CAG as the promoter to express the target gene, the method obviously enhances the expression level of the target gene. The hemagglutinin recombinant adenovirus for respectively expressing H5N1 and H9N2 subtype avian influenza viruses provides a virus model for development of the H5 / H9 subtype avian influenza virus bivalent nucleic acid vaccine, and also lays the foundation for development of the AIV (avian influenza virus) adenovirus live vector vaccine.
Owner:TIANJIN RINGPU BIO TECH
Pine cone extracts and uses thereof
A method of producing a pine cone extract and the pine cone extract produced there from, wherein the pine cone extract is useful in increasing the effects of nucleic acid vaccines and medicaments; and useful in the production of phenotypically immature and / or mature dendritic and / or fibrocyte cells.
Owner:TAMPA BAY RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com