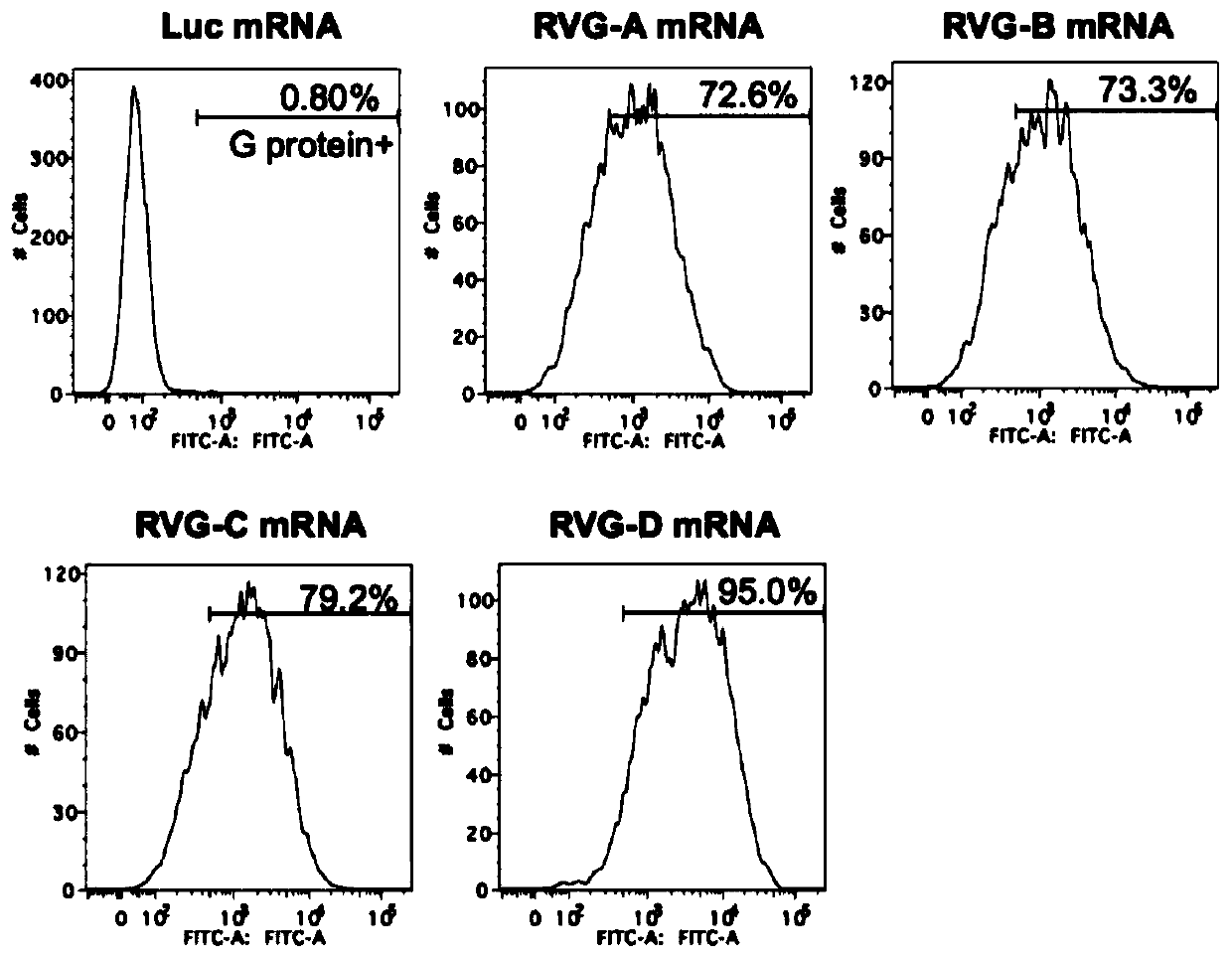
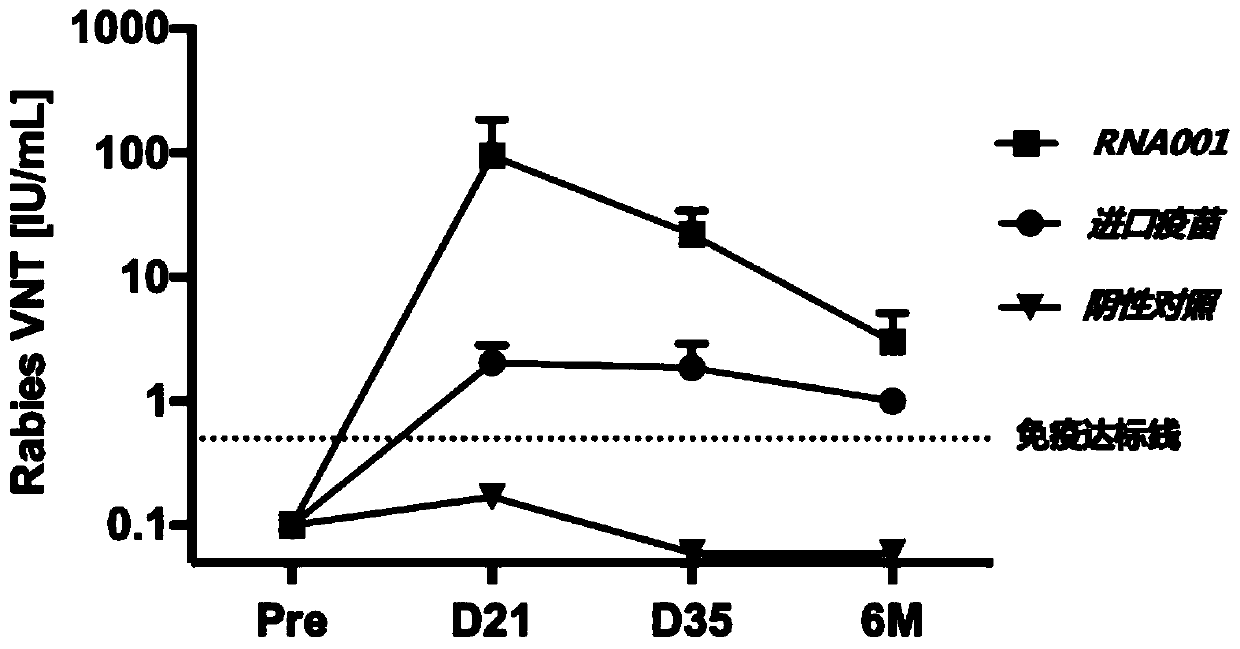
mRNA rabies vaccine
A rabies virus and nucleic acid vaccine technology, applied in DNA/RNA vaccination, antisense single-stranded RNA virus, vaccines, etc., can solve problems such as poor translation efficiency and low antigenic activity, and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] The invention provides a method for preparing the above-mentioned rabies virus nucleic acid vaccine. The vaccine carrier and the optimized RVG mRNA provided by the invention are mixed to obtain the rabies virus nucleic acid vaccine. The method is simple and easy to operate, and the production cost is greatly reduced.
[0091] In a preferred embodiment, the vaccine carrier is the cationic lipid nanoparticle in the rabies virus nucleic acid vaccine provided by the invention, and the preparation method of the rabies virus nucleic acid vaccine comprises:
[0092] (a) dissolving protonatable cationic lipids, structural lipids, auxiliary lipids and surfactants in an organic solution according to the formula ratio to obtain an organic phase;
[0093] (b) dissolving the optimized RVG mRNA in PBS or citrate solution to obtain an aqueous phase;
[0094] (c) mixing the organic phase obtained in (a) and the aqueous phase obtained in (b) to obtain a mixed solution, and replacing th...
Embodiment 1
[0106] The preparation method of embodiment 1 rabies virus nucleic acid vaccine
[0107] The mRNA of SEQ ID NO.2 was dissolved in citrate buffer at pH 4, and the concentration was adjusted to 0.1 mg / ml to obtain an aqueous phase.
[0108] The formula ratio of Dlin-MC3-DMA, cholesterol, DSPC and PEG-DMG was dissolved in absolute ethanol, and the total lipid concentration was adjusted to 6 mg / ml to obtain an organic phase.
[0109] The organic phase and the aqueous phase were mixed at a volume ratio of 1:3 by means of microfluidic equipment mixing. The flow rate is 12.0ml / min when using the microfluidic equipment for mixing.
[0110] The mixture was immediately diluted 50-100 times with a PBS solution of pH 7.4, and the ethanol component in the solution was removed by tangential flow filtration (TFF) and concentrated to an mRNA concentration of about 100 μg / ml to obtain lipid nanoparticles encapsulating mRNA, namely For the rabies virus nucleic acid vaccine.
Embodiment 2
[0111] Embodiment 2 freeze-dried preparation
[0112] The rabies virus nucleic acid vaccine in Example 1 is prepared into a freeze-dried preparation, and the freeze-drying protective agent sucrose and the surfactant Tween 20 are added. The process includes pre-freezing, primary freeze-drying and secondary freeze-drying: the pre-freezing temperature is -50 °C, and the temperature was maintained for 5 hours. The first freeze-drying temperature was -40°C for 24 hours, the second freeze-drying temperature was 10°C and kept for 17 hours, and the vacuum degree during the freeze-drying process was 40 μbar.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com