Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
245 results about "Type Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Four types of vaccines are currently available: Live virus vaccines use the weakened (attenuated) form of the virus. The measles, mumps, and rubella (MMR) vaccine and the varicella (chickenpox) vaccine are examples.
Streptococcus pneumoniae SP042 polynucleotides
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
Streptococcus pneumoniae antigens and vaccines
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
Vaccine adjuvant as well as preparation method and application thereof
ActiveCN104147599AHelp with immunityImprove the effectiveness of anti-virus protectionAntiviralsEmulsion deliveryImmune effectsMedicine
The invention discloses an oil-in-water type vaccine adjuvant as well as a preparation method and application thereof. The oil-in-water type nano-emulsion vaccine adjuvant comprises the following components in percentage by mass: 0.1-10 percent of oil phase, 0.1-10 percent of emulsifier, 0.1-3 percent of stabilizer, 0.1-3 percent of complexing agent and 0.01-10 percent of immunopotentiator. The preparation method comprises the following steps: (1) uniformly dispersing the immunopotentiator, the stabilizer and the complexing agent in water, thereby obtaining an aqueous phase; (2) mixing an oil phase and an emulsifier, thereby obtaining an oil phase; (3) slowly adding the oil phase into the aqueous phase, and continuously stirring, thereby forming a stable emulsion; (4) regulating the pH value of the emulsion, and fixing the volume to obtain a primary emulsion; and (5) performing high-speed shearing and high-pressure homogenizing on the primary emulsion. The vaccine adjuvant provided by the invention is simple in preparation, convenient to use and small in side reactions, is used for diluting vaccines, particularly swine fever live vaccines and is high in stability and good in immune effect.
Owner:HUAZHONG UNIV OF SCI & TECH +1
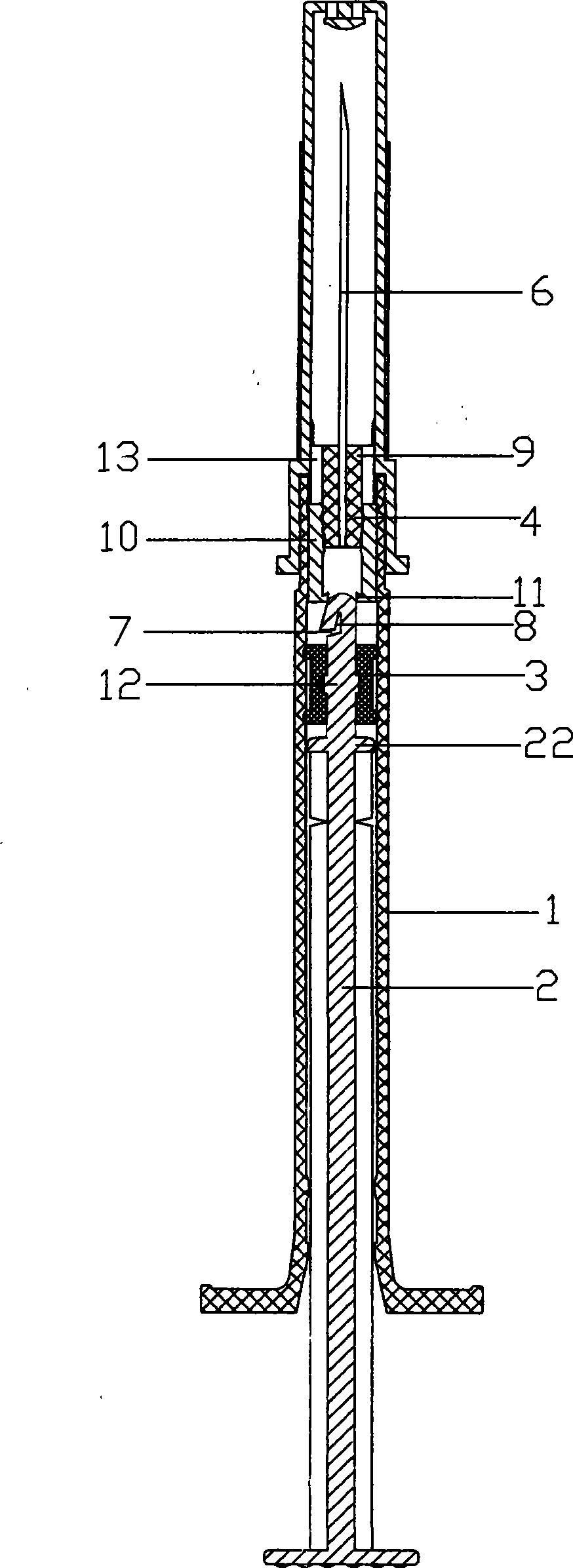
Safe self-destruction type vaccine injector
InactiveCN101411908AEasy to controlAvoid accidental injuryInfusion syringesInfusion needlesManufacturing cost reductionInternational standard
The invention discloses a safe self-destroying vaccine syringe, which is characterized in that the side wall of a rod body at the end part of a core rod is provided with a transverse gap which is communicated with a longitudinal gap at the rod body of the end part of the core rod; the transverse gap is matched with the longitudinal gap; a needle consists of an upper hub and a lower hub which are sleeved with each other; an inner cavity of the lower hub is hollow; the lower hub is of a cylindrical structure; and the inner wall of the lower hub relative to the other end opening for mounting the upper needle stand is provided with a convex rid of the lower hub. The safe self-destroying vaccine syringe has the advantages that the whole vaccine syringe has a reasonable structure, completely accords with various international standards, controls liquid medicine accurately, can achieve high degree automatic mass production during assembling, effectively improves production efficiency, reduces manufacturing cost, has high working stability in actual use experiments, can effectively prevent the syringe from accidentally injuring medical staff and other correlative staff during operation, and also puts an end to the secondary recycle of a disposable vaccine syringe.
Owner:BIOTOP TECH SHANGHAI
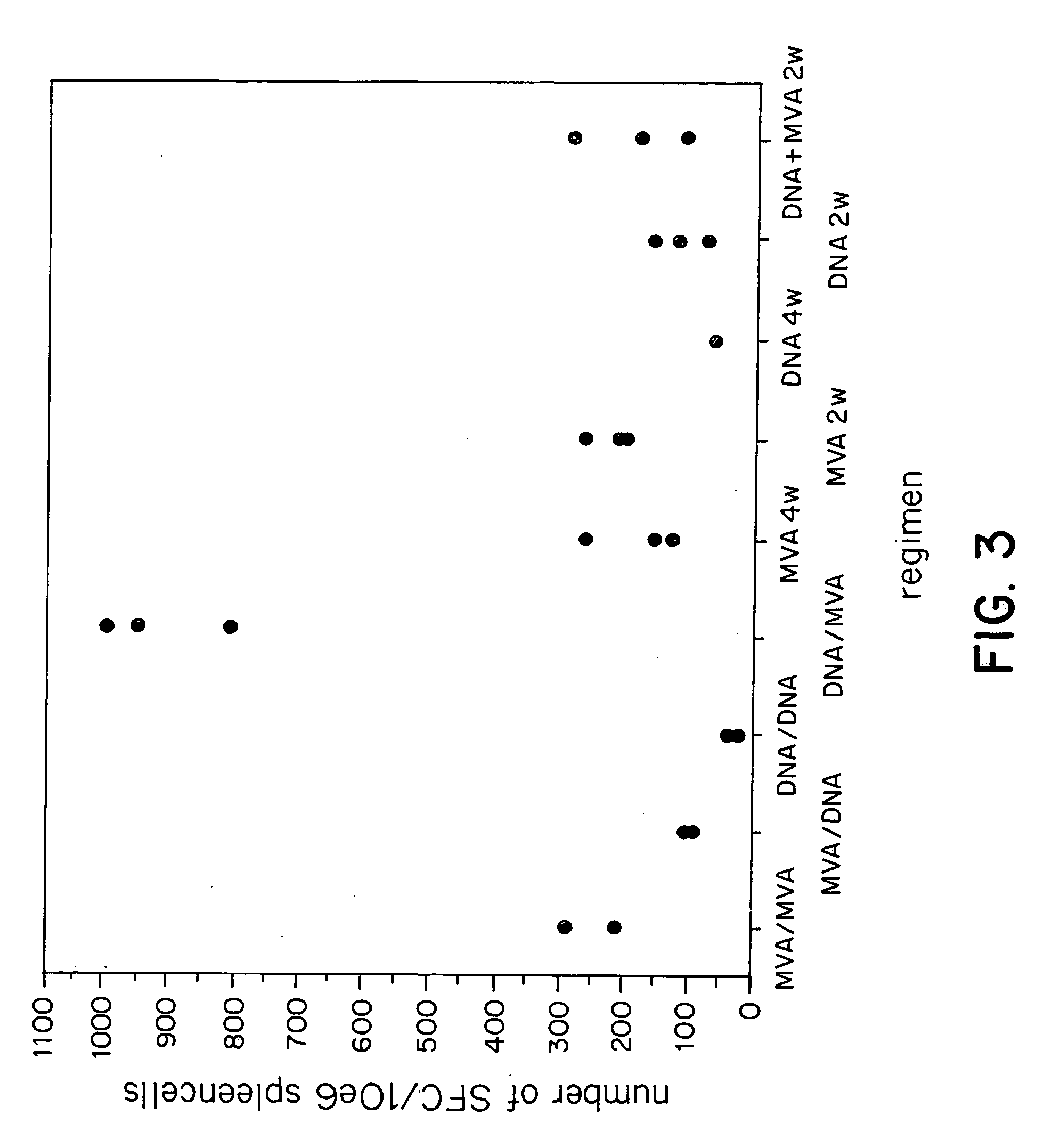
Methods and reagents for vaccination which generate a CD8 T cell immune response
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
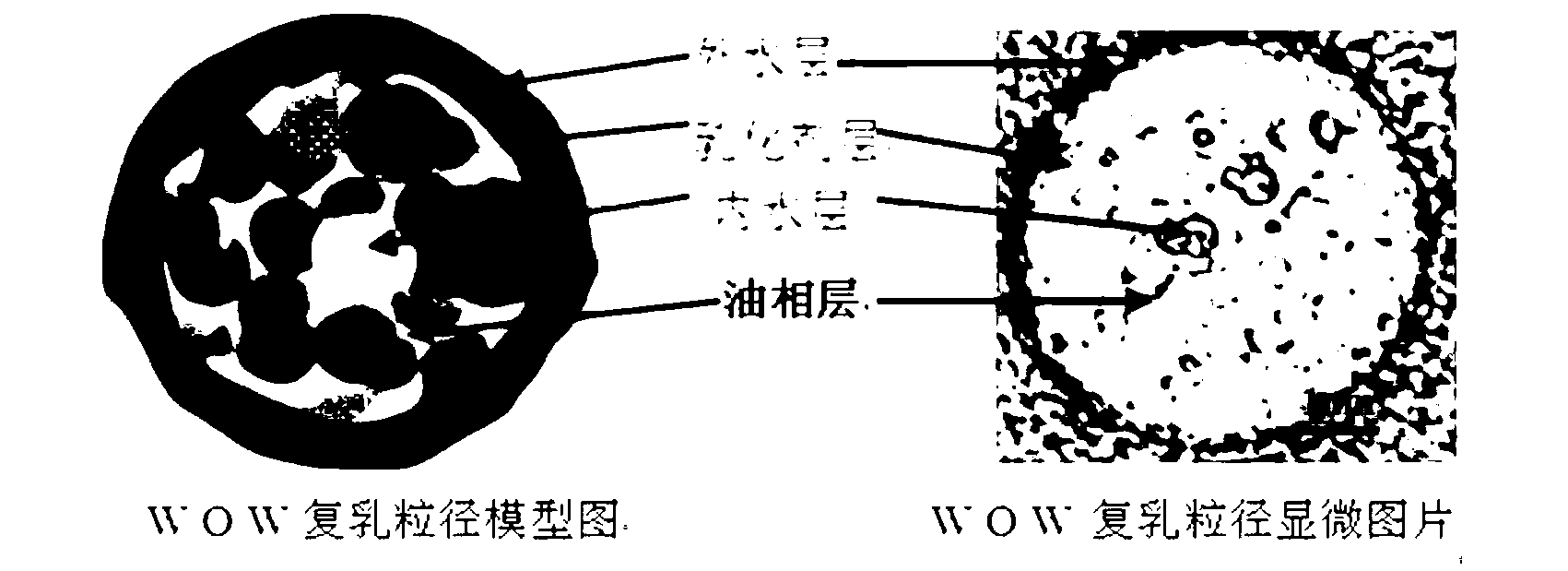
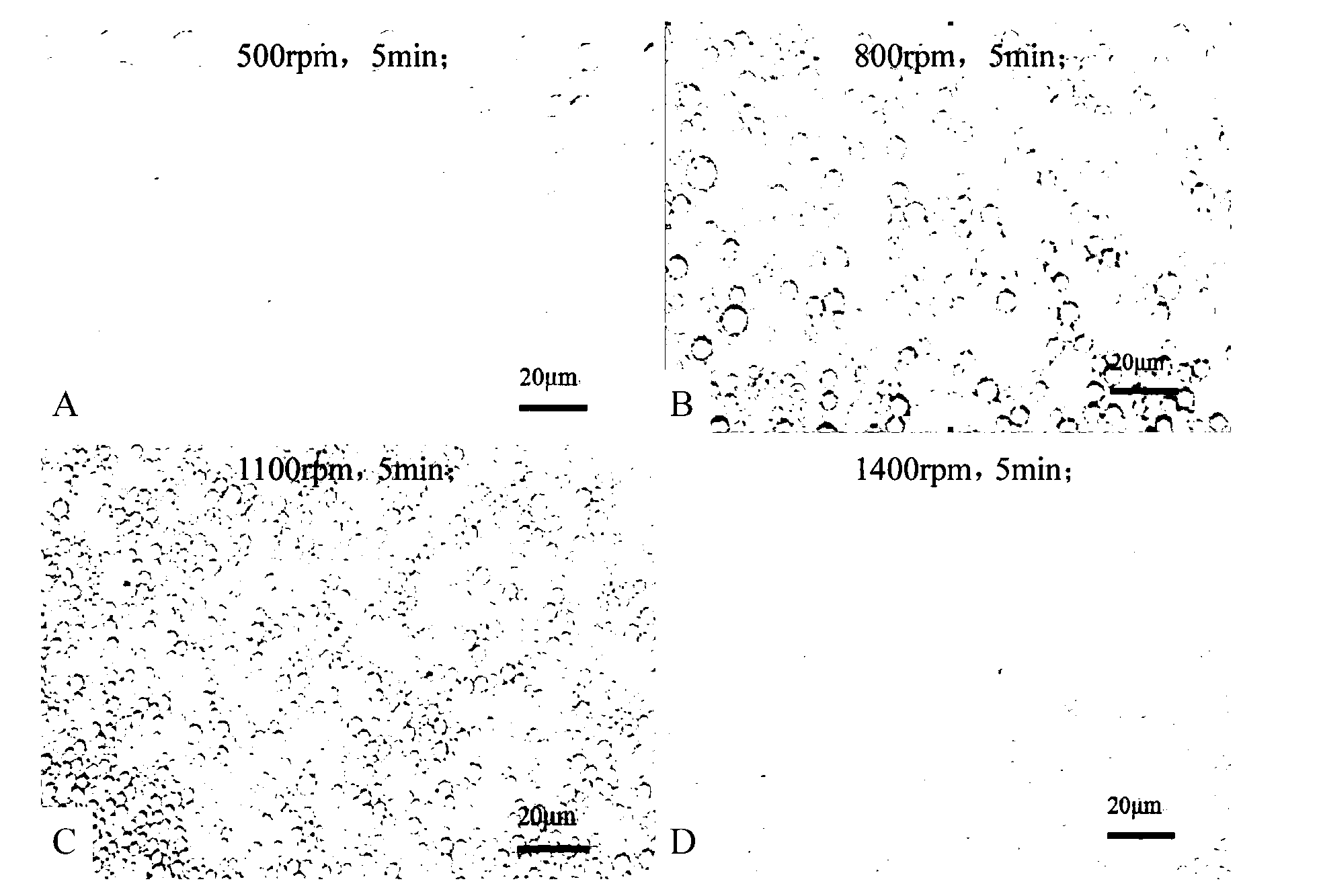
Water-in-oil-in-water adjuvant vaccine and preparation method thereof
ActiveCN103223164APromote absorptionGenerate fastAntiinfectivesAntibody medical ingredientsEngineeringImmunity response
The invention discloses a water-in-oil-in-water adjuvant vaccine and a preparation method thereof. The adjuvant vaccine is composed of an internal water phase, a middle oil phase used for cladding the internal water phase, and an external water phase for cladding the middle oil phase. The external water phase comprises: a composite surfactant, a hydrophilic surfactant and mormal saline. The middle oil phase consists of: a lipophilic surfactant, a stabilizer and mineral oil. The internal water phase comprises: an immunopotentiator solution, the hydrophilic surfactant, and an inactivated antigen solution. The W / O / W (water-in-oil-in-water) type adjuvant vaccine provided in the invention can induce an immunoreaction earlier than traditional W / O (water-in-oil) type adjuvant vaccines, and can make experimental animals obtain earlier protective antibodies. The vaccine has good absorption, and causes a small inflammatory response at an inoculation site. The adjuvant vaccine disclosed in the invention has better stability than existing W / O / W type vaccines, and the antibody generation speed is fast.
Owner:SOUTH CHINA AGRI UNIV +1
Novel vaccine for preventing COVID-19 and preparation method thereof
ActiveCN111939250AHighly conservativeAntibody induction ability is weakSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleotideReceptor
Provided is a novel vaccine for preventing COVID-19, the nucleotide sequence of an antigen of the novel vaccine is SEQ NO: 1, the amino acid sequence of the antigen of the novel vaccine is SEQ NO: 2,and the antigen of the vaccine comprises two functional parts: an S protein receptor binding structural domain capable of inducing a specific neutralizing antibody and a T cell related N protein truncated peptide fragment capable of inducing and activating effector T cells; The vaccine disclosed by the invention has the characteristics that the T cell related N protein truncated peptide fragment has weak capability of inducing the generation of the N protein antibody, so that a vaccine inoculator and a COVID-19 infected patient can be identified by using the N protein antibody, and the vaccineantigen does not induce the generation of the N protein antibody, so that lung injuries can be reduced, and the vaccine is safer. The cell vaccine disclosed by the invention is low in manufacturing cost, and can induce generation of virus-specific neutralizing antibodies and T cell immune response.
Owner:ZHENGZHOU UNIV
Vaccine for hand-foot-and-mouth disease viruses
ActiveCN101897963AEnsure safetyGood immune effectMicroorganism based processesAntiviralsAdjuvantInfectious Disorder
The invention relates to preparation of a vaccine for hand-foot-and-mouth disease viruses and an application method thereof, belonging to a novel vaccine for preventing infectious diseases. The vaccine of the invention mainly comprises the components of high-purified inactivated human enteropathogenic virus 71 (EV 71) and an aluminum adjuvant. The vaccine, which is prepared according to the method of the invention, has excellent immunogenicity, and after immunity, organisms can selectively generate a high titer serum neutralization antibody, thereby preventing infectious diseases caused by the human EV 71.
Owner:BEIJING LUZHU BIOTECH +1
Ready-to-use adjuvant of livestock vaccines, preparation and applications thereof
ActiveCN103071153ASolve side effectsReduce dosageImmunological disordersEmulsion deliveryBiotechnologyAdjuvant
The invention provides a ready-to-use adjuvant of livestock vaccines, preparation and applications thereof, belonging to the field of the adjuvant of livestock vaccines. The ready-to-use adjuvant is prepared from the following substances: 5-30 percent of oil, 0.1-10 percent of hydrophilic surfactant, 0.1-10 percent of oleophilic surfactant, 0.1-10 percent of polymeric micelle substance and 40-90 percent of water. The invention also provides a preparation method of the ready-to-use adjuvant, and the method comprises the following steps: respectively weighing the substances, mixing and then emulsifying, and degerming to obtain the ready-to-use adjuvant of the livestock vaccines. The invention also provides an oil-in-water type vaccine with the ready-to-use adjuvant. The ready-to-use adjuvant is an oil-in-water type adjuvant and has the advantages of simple ingredients, stable dosage form, convenience in use and easiness in injection. The preparation method of the ready-to-use adjuvant has a simple process and is low in cost, and the obtained dosage form is stable. The oil-in-water type vaccine has the advantages of long stable period, easiness in storage, good immune effect, protection period prolonging, easiness in injection and small side reaction.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method for determining cross antigen region initiating human antigens in human enterovirus 71-type total protein
The invention belongs to the field of biomedicine, and provides a method for determining a cross antigen region initiating human antigens in human enterovirus 71-type (EV71) total protein. In the method, the human enterovirus 71-type total protein is identified through segmental expression; and cross reaction between immunogenic and inductive antibodies and the human antigens in each segment is performed, and different segments of the human enterovirus 71-type total protein are divided into three classes: (1) strongly-crossed immunogens initiating the human antigens; (2) weakly-crossed immunogens initiating the human antigens; and (3) no crossed immunogens existing between the antibodies and the human antigens. Thus, the method plays a guidance role in developing and preparing human enterovirus 71-type vaccines, and hand-foot-and-mouth disease vaccines having no cross reaction or weak cross reaction with human bodies can be designed according to the method.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Vaccine for the prevention of bacterial infection of the bovine mammary gland
InactiveUS6984381B2Readily apparentBiocideBacterial antigen ingredientsMastitisStaphylococcus aureus bacteria
A novel vaccine for immunizing animals against Staphylococcus aureus-induced mastitis is disclosed. The vaccine is comprised of whole killed cells of S. aureus in a dosage effective to immunize an animal against the organism, in combination with a pharmaceutically acceptable carrier.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
O type foot-and-mouth disease virus variant as well as coding gene and application thereof
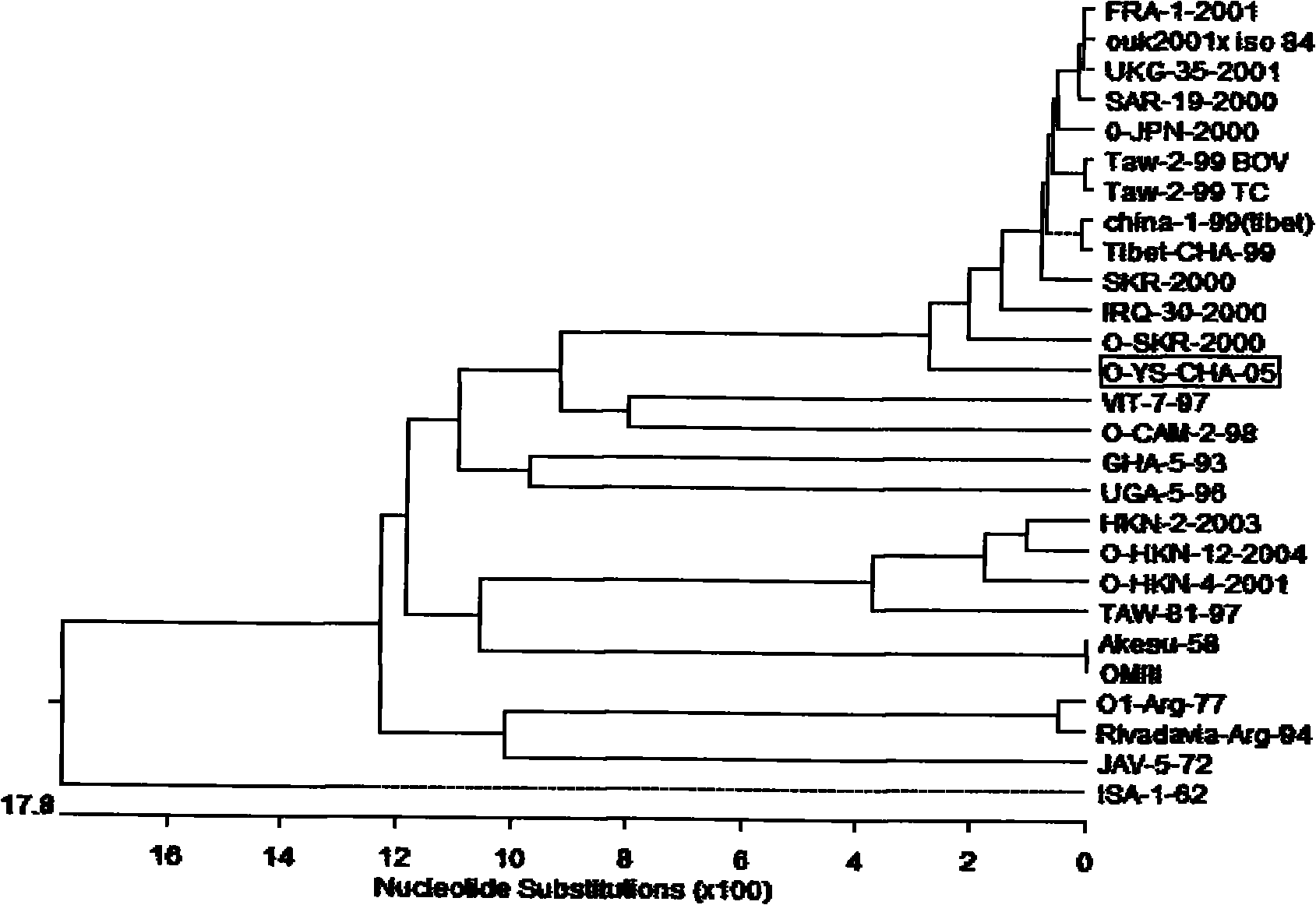
The invention discloses an O type foot-and-mouth disease virus variant as well as a coding gene and application thereof. In the invention, an O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 is firstly separated out, the nucleotide sequence of the O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 SEQ ID NO: 1, and the amino acid sequence is SEQ ID NO: 2. In comparison with a VP1 amino acid sequence, the virus strain has 7 variable sites, five of which are centralized in a G-H ring. Mutation of the sites ensures that the virus variant has the capability of escaping from host immunity so as to have the superiority for becoming a popular virus strain. Therefore, the variant can be employed to prepare an inactivated vaccine for prevention and treatment of the variant and relevant strains, dominant antigen epitope of the variant can be employed to prepare a synthetic peptide vaccine, and the variant can be employed to develop novel O type foot-and-mouth disease virus vaccines such as VLP vaccine and the like. Therefore, the invention has important value in controlling the popularity of O / YS / CHA / 05 and relevant variable strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
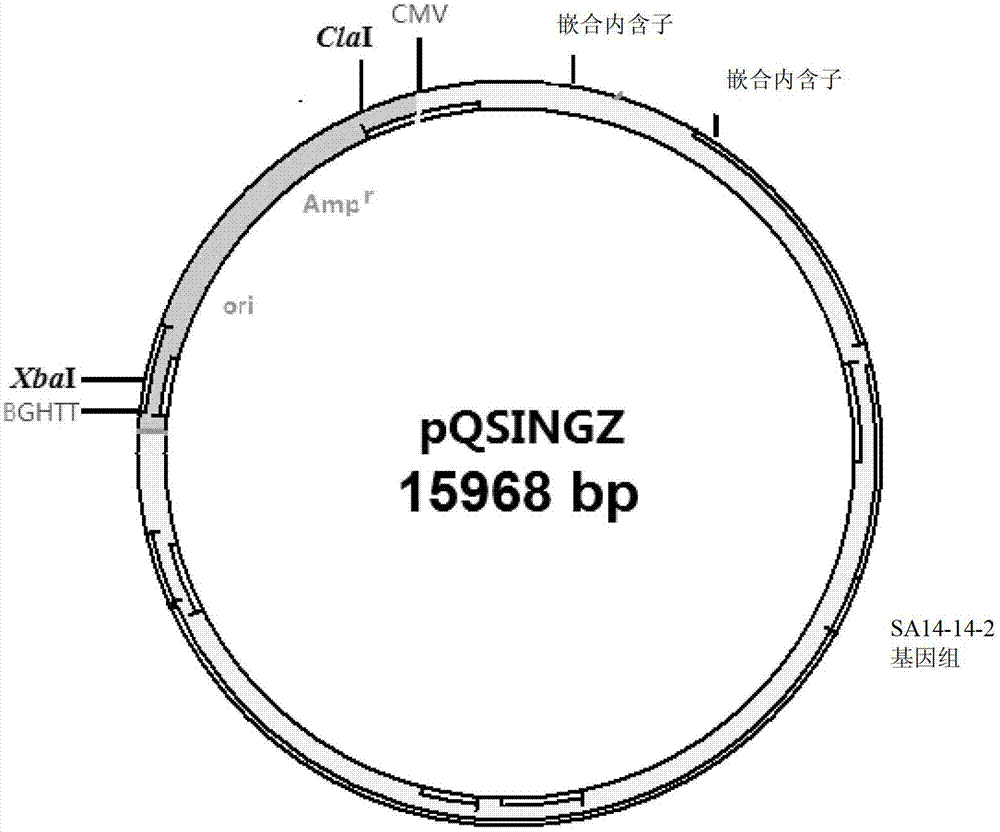
DNA (Deoxyribose Nucleic Acid)-based infectious clone of a Japanese encephalitis virus SA14-14-2 strain, as well as construction method and application thereof
InactiveCN103088049AInfectiousViral antigen ingredientsMicroorganism based processesPolyadenylationJapanese encephalitis viruses
The invention relates to a DNA (Deoxyribose Nucleic Acid)-based infectious clone of a Japanese encephalitis virus SA14-14-2 strain and a construction method of the infectious clone. The infectious clone is constructed by adopting a pBR322 plasmid as a framework vector and then inserting the full-length cDNA of the Japanese encephalitis virus SA14-14-2 vaccine strain; the 5' end of the full-length cDNA of the SA214-14-2 vaccine strain is connected with a CMV (Cucumber Mosaic Virus) promoter, a BGH (Bovine Growth Hormone) polyadenylation sequence is added at the 3' end, and gomphosis intron sequences for stabilizing are respectively added on the 356 locus and the 2217 locus of the genome cDNA. The invention also provides application of infectious clone serving as a novel viral vector, and thus a solid foundation is provided for developing a plurality of novel vaccines for preventing and treating tumours and viral diseases.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE +1
Recombinant protein coded by grass carp reovirus (GCRV) type-II S10 gene, polyclonal antibody prepared from recombinant protein and application of recombinant protein
InactiveCN103539842AImproving immunogenicityGood immune protectionViral antigen ingredientsVirus peptidesNucleotideStructural protein
The invention discloses a recombinant protein coded by a grass carp reovirus (GCRV) type-II S10 gene, a polyclonal antibody prepared from the recombinant protein and an application of the recombinant protein. The amino acid sequence of the GCRV type-II S10 gene-coded protein is shown by SEQ ID No:2, and the nucleotide sequence coding the protein is shown by SEQ ID No:1. The recombinant protein disclosed by the invention has good immunogenicity; compared with the proteins of other structures of GCRV, the specific antibody valence generated by inducing an immune animal is higher. Further tests indicate that by immunizing the grass carp with the S10 recombinant protein, the grass carp can be induced to generate high specific antibody, and certain immune protection effect can be realized against the attack of a virulent strain of GCRV. Therefore, the study and application of the GCRV type-II S10 gene-coded protein are of vitally important significance to the development of a novel vaccine for the hemorrhagic disease of grass carp and an immunological detection kit, and a new effective solution is provided for the hemorrhagic disease of grass carp.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
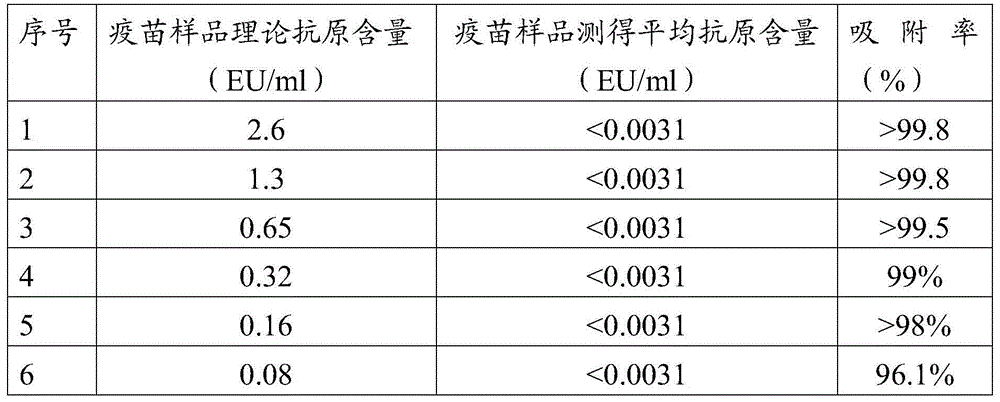
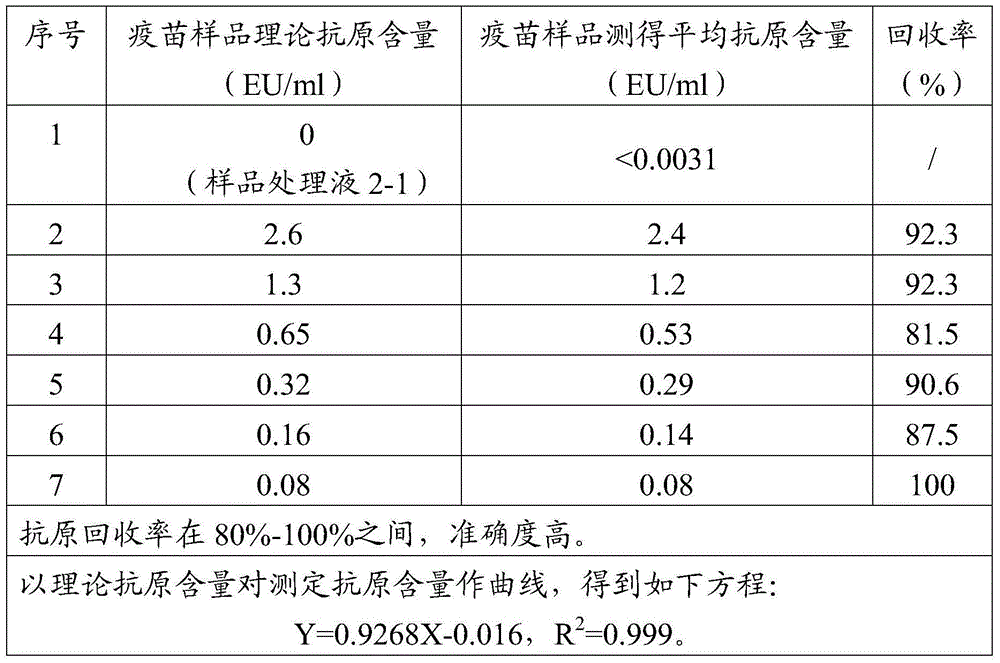
Treatment liquid and method using same to measure antigen content of aluminum salt adsorption type vaccines
ActiveCN104634959AAccurate detectionEfficient detectionMaterial analysisJapanese encephalitis vaccineAntigen
The invention discloses a treatment liquid for desorbing antigens in an aluminum salt adsorption type vaccine. The treatment liquid is a phosphate buffer solution or a citrate buffer solution, wherein the buffer solution contains proteins and at least one acid and / or salts of the acids. The invention further discloses a method using the provided treatment liquid to measure the antigen content of an aluminum salt adsorption type vaccine. The provided method reduces the interference brought by the aluminum adjuvant in Japanese encephalitis vaccine, is capable of rapidly and precisely measuring the antigen content in an adsorption type Japanese encephalitis inactivated vaccine, has the characteristics of good durability, high accuracy, and high precision, and can provide references for quality control of aluminum adjuvant adsorption type Japanese encephalitis inactivated vaccines.
Owner:LIVZON GROUP VACCINE ENG
Nanometer vaccine and preparation method thereof
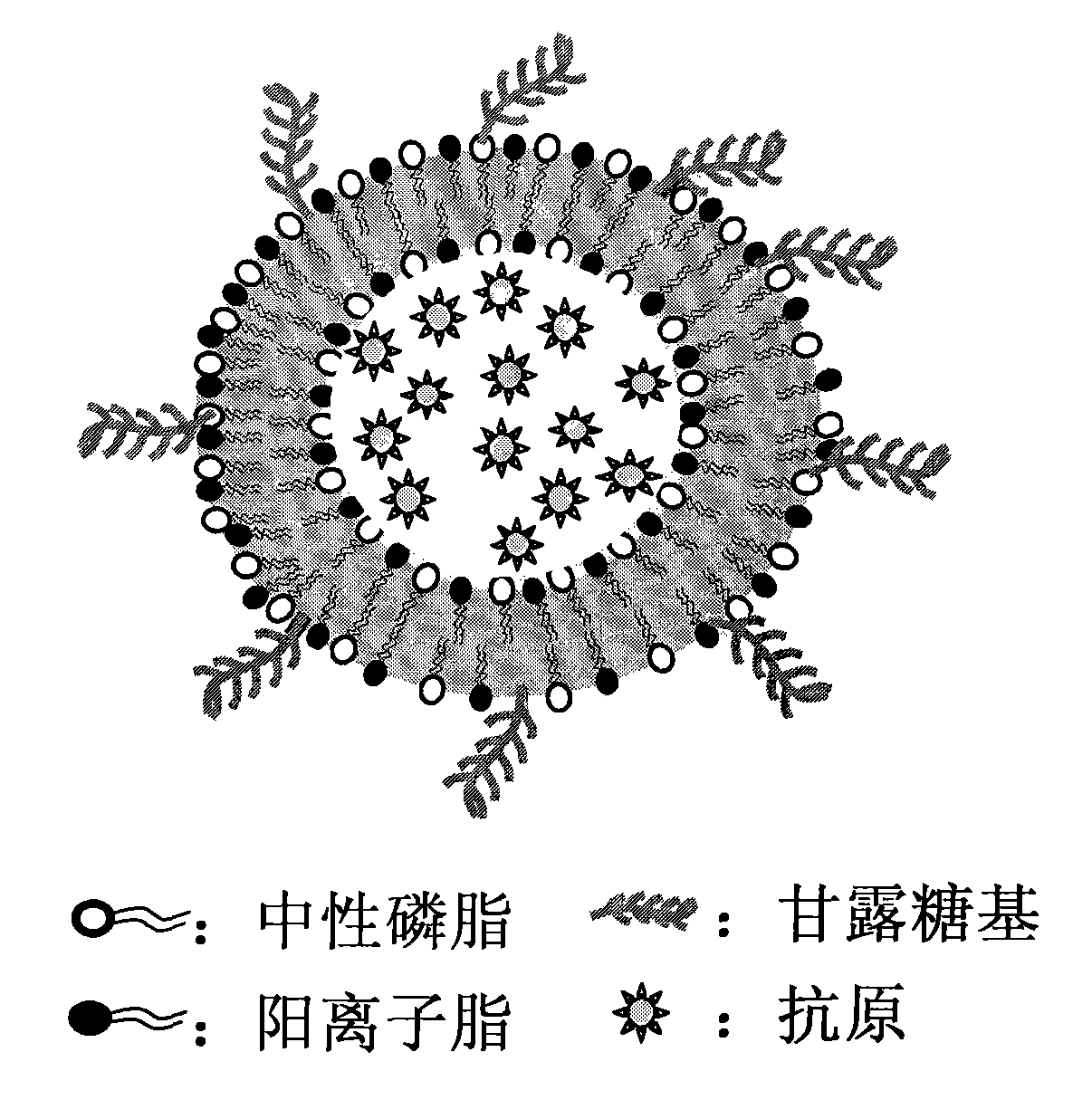
InactiveCN102068698AImprove abilitiesEnhance immune adjuvant effectAntiinfectivesPharmaceutical non-active ingredientsCompound sLiposome
The invention provides a nanometer vaccine and a preparation method thereof. A new mannose glycosylated-cationic liposome complex can be used as a vaccine vector and can be applied to the vaccine. In the new liposome complex, neutral phospholipid and mannose are mixed into cationic lipid, so the immunologic adjuvant effect and the antigen-presenting cell targeting property of a liposome are improved, and the cytotoxic effect of the liposome is reduced obviously. The invention provides a novel high-efficiency vaccine vector and an adjuvant which do not have side effects and can improve the immunologic effect of the vaccine obviously.
Owner:SHENZHEN INST OF ADVANCED TECH
Porcine reproductive and respiratory syndrome virus-like particle vaccine and preparation method thereof
A porcine reproductive and respiratory syndrome virus-like particle vaccine and a preparation method thereof relate to the field of biological medicines and aims at disclosing the porcine reproductive and respiratory syndrome virus-like particle vaccine (PRRS VLP vaccine) and the preparation method the vaccine. The PRRS VLP vaccine contains a VLP comprising three structure proteins of porcine reproductive and respiratory viruses M, N and GP5 and can excite favorable dual cell and humoral immune response. By adding or not adding an adjuvant into the formed VLP protein antigen, the pharmacodynamic test shows the prepared injection type, nasal drop type and water agent type vaccines are immunized with different animal groups so as to safely and effectively prevent the PRRSV infection and provide ideal vaccines for safely and effectively immunizing, preventing and controlling the PRRSV infection on different groups of sows, piglets and fat pigs.
Owner:CHONGQING UNIV
Grass carp reovirus II type vaccine strain and wild strain diagnostic primer, kit employing diagnostic primer and diagnostic detection method
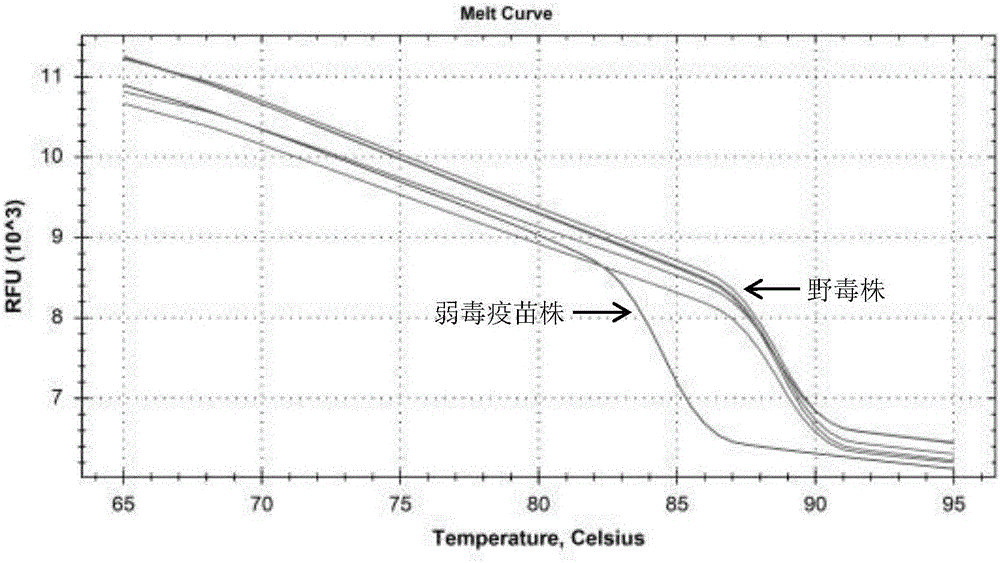
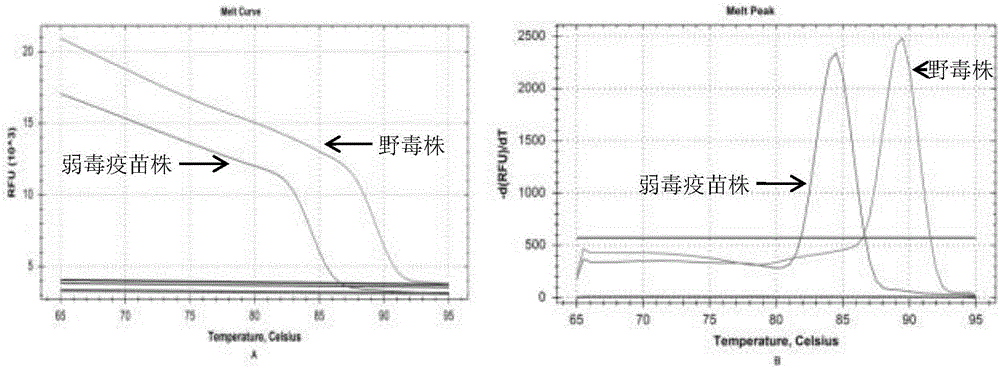
ActiveCN106811550AAchieving Differential DiagnosisImprove throughputMicrobiological testing/measurementMicroorganism based processesMicrobiologyType Vaccine
The invention provides a high-resolution-melting (HRM)-based grass carp reovirus (GCRV) II type vaccine strain and wild strain differential diagnosis and detection primer. The differential diagnosis and detection primer comprises the following pair of primers: HRM-II-F: 5'CACCGGAGTTAAACTTCATCGC-3'(SEQ ID NO: 1) and HRM-II-R: 5'CCTGTGGCAGACCGGCAGATAA 3'(SEQ ID NO: 2), and is obtained through designing according to a GCRV II type vaccine strain and a known II type wild strain S2 gene 5' end and 3' end conserved domains, one or more stationary base mutation sites exist or exists between the vaccine strain and a wild strain amplified product, the mutation sites are unique for the vaccine strain, identification and analysis of HRM to the GCRV II type vaccine strain and the wild strain are achieved, moreover, a corresponding detection agent and a corresponding detection method are designed according to the HRM-based GCRV II type vaccine strain and wild strain differential diagnosis and detection primer, and the practicability of the technology is improved.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
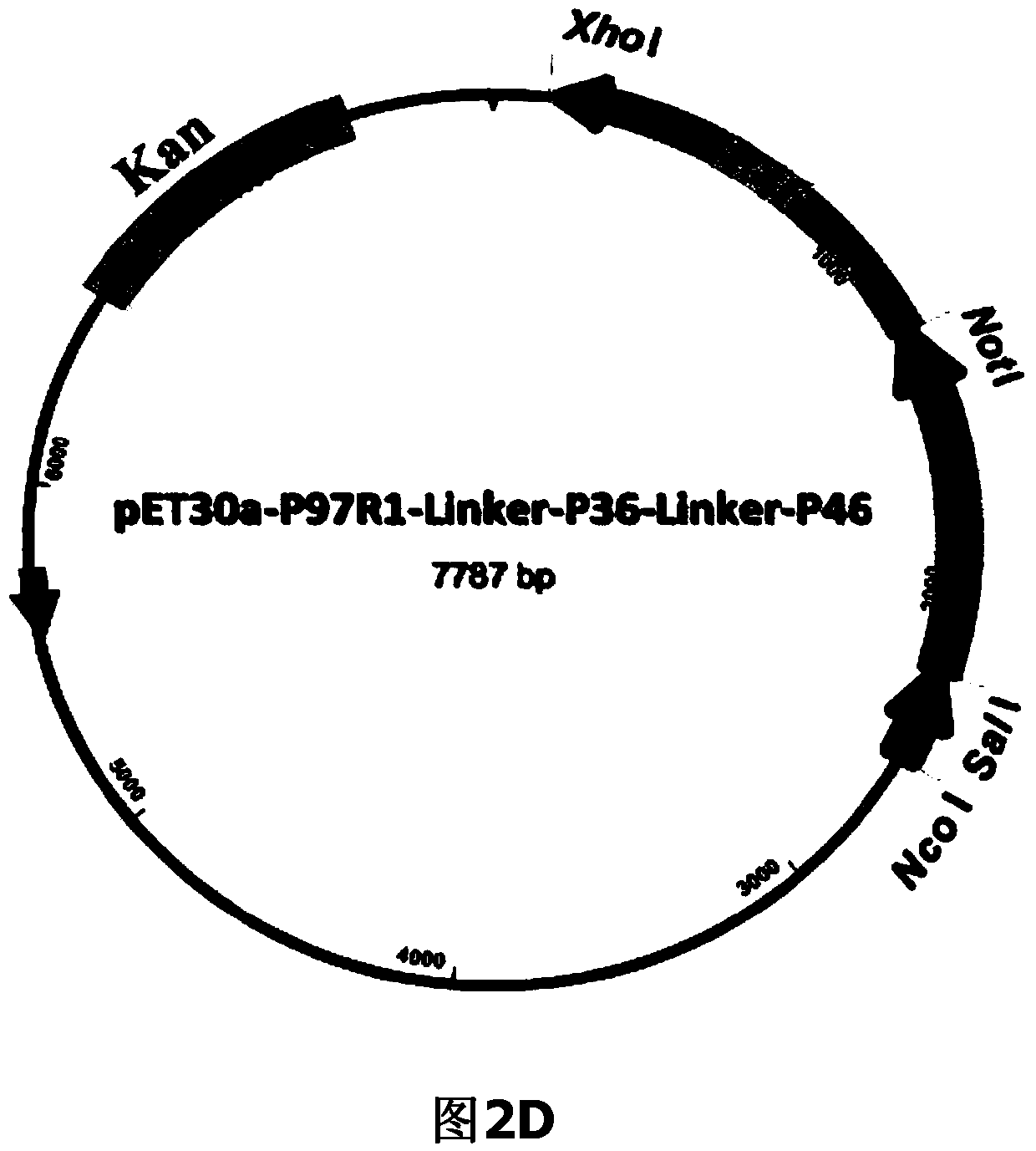
Mycoplasma hyopneumoniae fusion gene and application
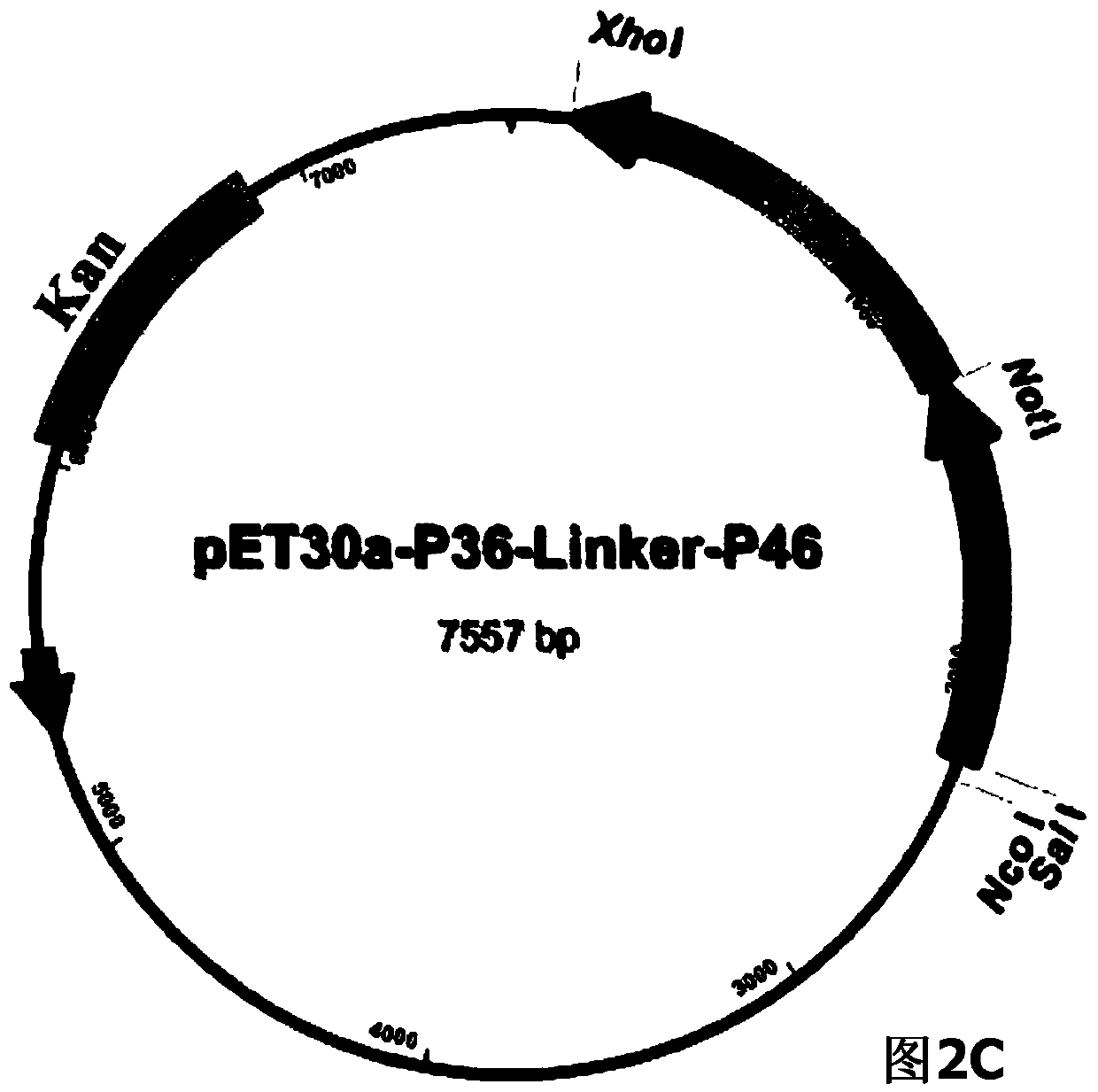
ActiveCN104293816AImproving immunogenicityHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliNucleotide
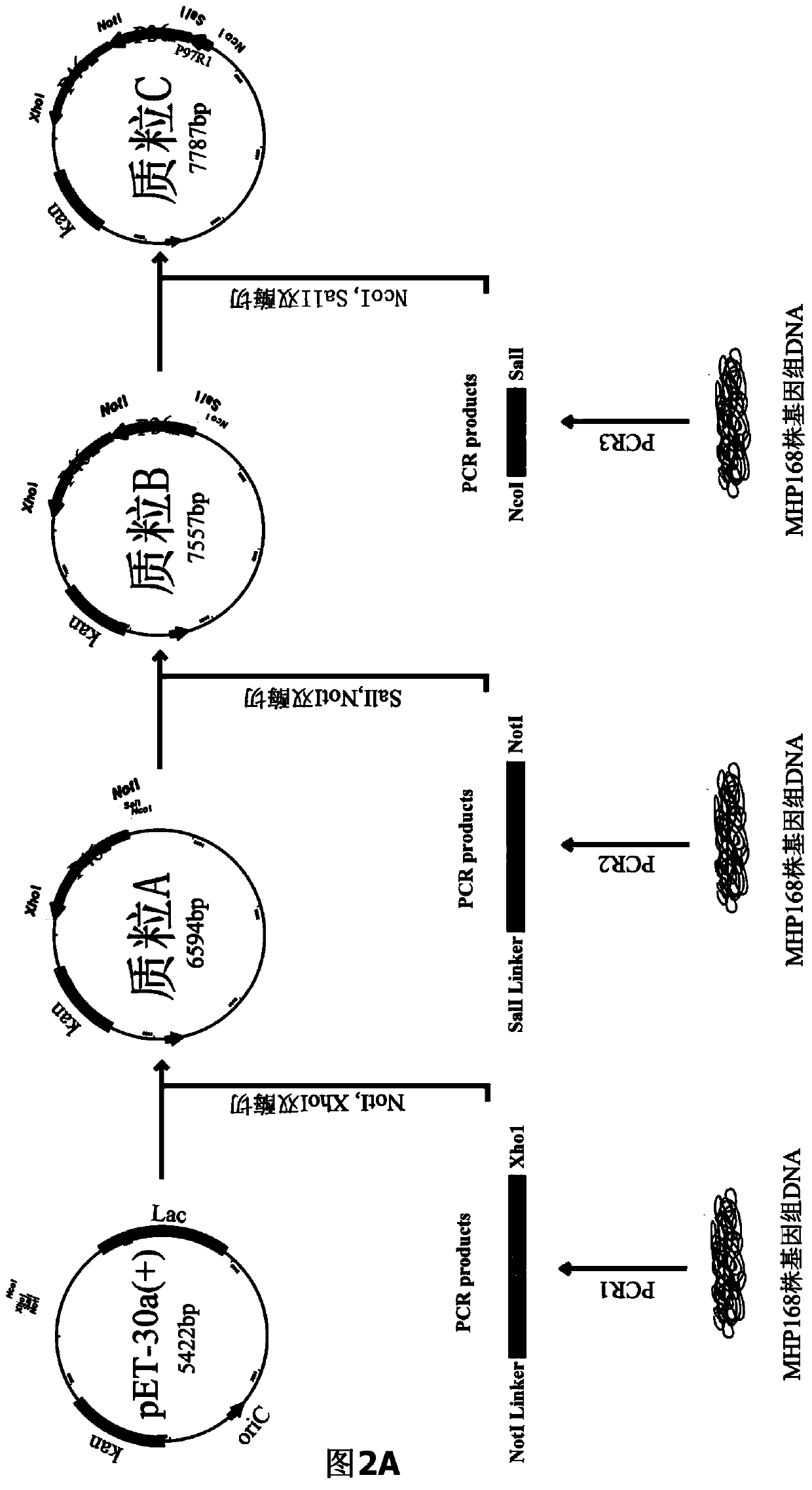
The invention belongs to the field of animal gene engineering, and in particular relates to a synthetic fusion gene for expressing mycoplasma hyopneumoniae and application. The fusion gene is characterized in that a mycoplasma hyopneumoniae P97R1 gene is connected in series with a P36 gene through a Linker, the termination codon of the P36 gene is deleted, the P46 gene with signal peptide removed is fused at the C end of the P36 gene through Linker, and then the fusion gene P97R1-P36-P46 is obtained, wherein the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The fusion gene is included in a prokaryotic expression plasmid, and escherichia coli BL21 / pET30a-P97R1-Linker-P36-Linker-P46 containing the plasmid is collected in CCTCC with the collection number CCTCC NO: M2014269. The invention further discloses immune efficacy evaluation of the fusion gene and application of the fusion gene in a novel vaccine.
Owner:HUAZHONG AGRI UNIV
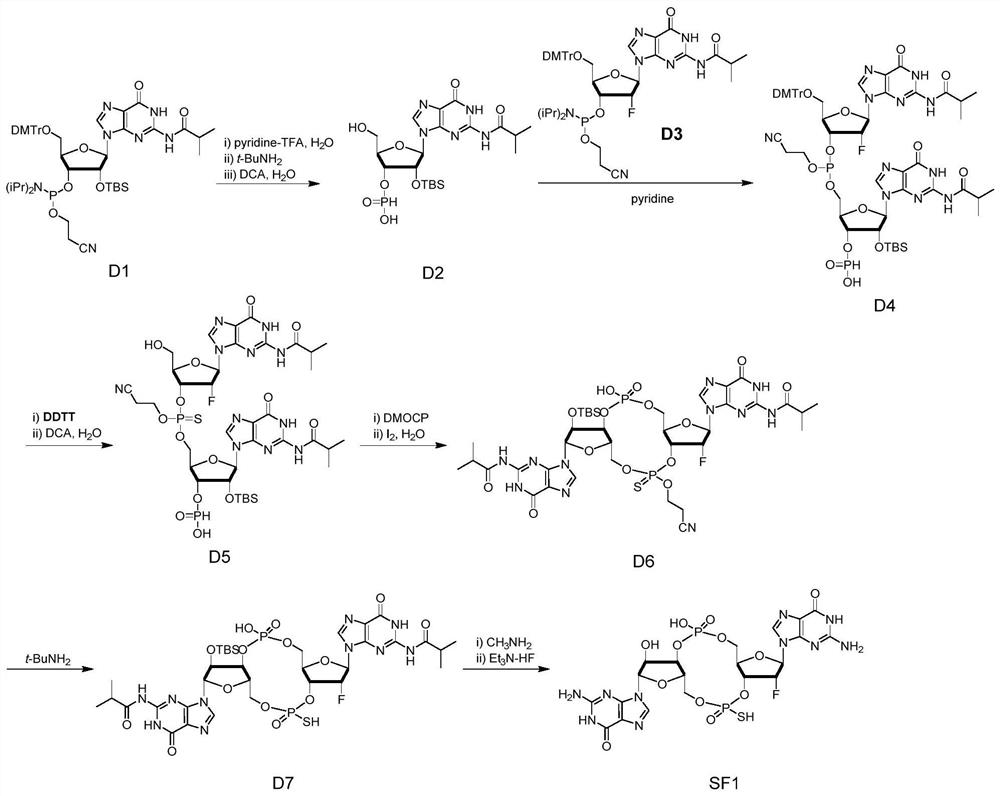
Novel vaccine adjuvant and application thereof in novel coronavirus pneumonia vaccine and other vaccines
ActiveCN111956797ASsRNA viruses positive-senseViral antigen ingredientsAntiendomysial antibodiesCoronavirus vaccination
The invention relates to the field of biological medicines, in particular to a novel vaccine adjuvant and an application thereof in a novel coronavirus pneumonia vaccine and other vaccines. Chemicallymodified cyclic dinucleotide, namely an SF compound, is used as the vaccine adjuvant and is used in cooperation with the novel coronavirus vaccine, so that the SARS-CoV-2 virus antigen specific antibody titer and the generation of T cells can be remarkably improved; and the SF compound as the vaccine adjuvant is obviously superior to an aluminum adjuvant in immunopotentiation effect.
Owner:TSINGHUA UNIV
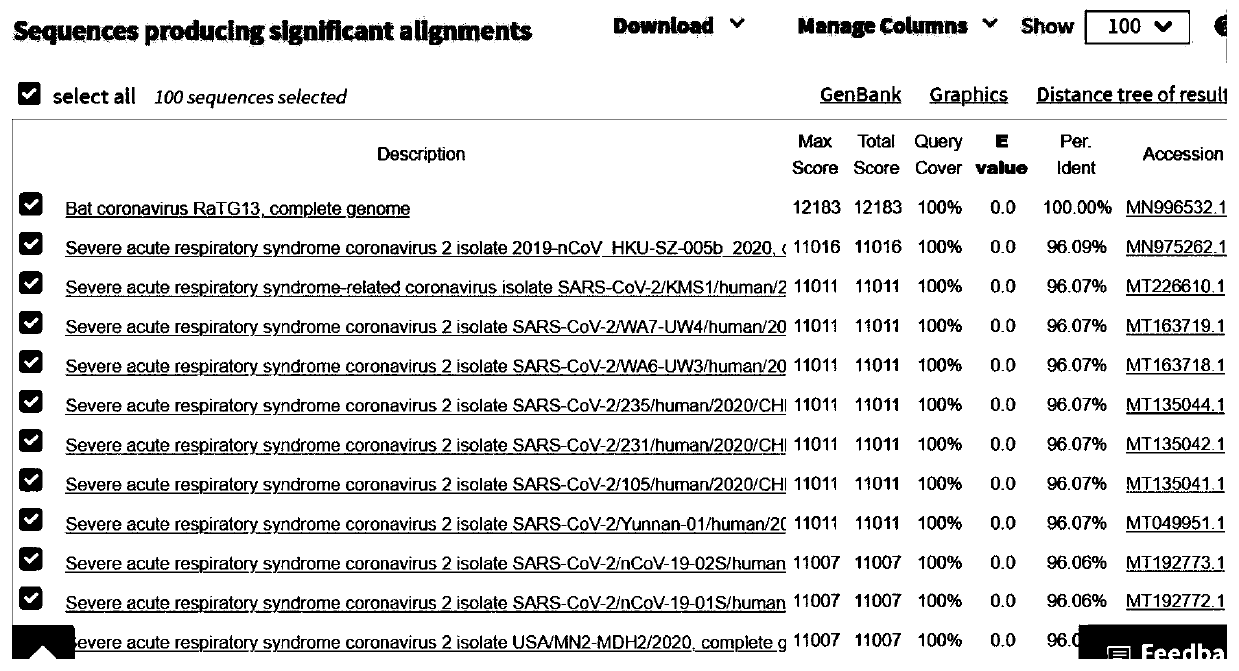
Bat-derived coronavirus vaccine for preventing COVID-19
PendingCN111437384ASsRNA viruses positive-senseViral antigen ingredientsDiseaseCoronavirus vaccination
The invention discloses a bat-derived coronavirus vaccine for preventing COVID-19. The bat-derived coronavirus Bat / CovRaTG13 is adopted to produce the vaccine so as to control and prevent the ongoingCOVID-19 pandemic and the future epidemic of the disease. The genetic fingerprint of the Bat / CovRaTG13 is closest to the genetic fingerprint of the 2019 new coronavirus (SARS-CoV-2). Homology of all amino acid sequences not only determines the genetic relationship, but also determines the similarity of biological characteristics, and antigenicity is one of main factors for determining specificityand effectiveness of the vaccine. The vaccine strain comes from a Bat / CovRaTG13 family member, and has sequence homology. The bat coronavirus-derived vaccine can be prepared into three types of vaccines, namely a live vaccine, an inactivated vaccine and / or a recombinant vaccine, through corresponding construction and manufacturing method procedures so as to prevent COVID-19 pandemic.
Owner:SICHUAN CHENGYU BIOLOGICAL PROD INC
Cattle food-and-mouth disease virus A type synthetic peptide and preparation and application thereof
ActiveCN103193869AImprove the efficacy of immune protectionGood protective effectVirus peptidesAntiviralsAntigenFoot mouth disease virus
The invention discloses a cattle food-and-mouth disease virus A type synthetic peptide. The cattle food-and-mouth disease virus A type synthetic peptide has the following amino acid sequence: acetyl-YDLDF EALKP HFKSL GQTIT PADKS PPS VYNGT CKYSA PATRR GDLGS LAARL AACLP ASFNY GAIRA T-amide. The cattle food-and-mouth disease virus A type synthetic peptide can be used as an effective novel vaccine for the A type food-and-mouth disease in production practice; simultaneously, the realization of the test provides a certain foundation for further perfecting the construction of a food-and-mouth disease virus A type synthetic peptide vaccine and the construction of other food-and-mouth disease subtype synthetic peptide vaccines; and the new ideal proposed in the research of the synthetic peptide vaccine and the construction of a new method of the synthetic peptide vaccine provide theoretical foundation and technical support for further perfecting the development and research of the multiple antigenic peptide, multivalent peptides and joint peptide vaccines in future.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Herpes virus complementing cell line
InactiveUS6841373B2Reduce the possibilityAvoid reorganizationBiocideGenetic material ingredientsICP8Herpes simplex virus DNA
The present invention is directed to a cell line capable of supporting replication of a growth-defective Herpes Simplex Virus strain; specifically a replication-defective HSV-2 double mutant. Particularly disclosed is a cell line that expresses the ICP8 protein and the UL5 protein of Herpes Simplex Virus. This cell line is useful to propagate a replication-defective HSV-2 vaccine strain that contains mutations and / or deletions in the ICP8 and UL5 genes.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Innate immune system-directed vaccines
InactiveCN1549726ABoosts adaptive immune responseDoes not cause inflammation levelsPolypeptide with localisation/targeting motifBacterial antigen ingredientsAntigenCell immune response
The present invention provides novel vaccines, methods for the production of such vaccines and methods of using such vaccines. The novel vaccines of the present invention combine both of the signals necessary to activate native T-cells-a specific antigen and the co-stimulatory signal-leading to a robust and specific T-cell immune response.
Owner:YALE UNIV
Method and Device for Conditional in ovo Injection
InactiveUS20150327521A1Easily and cost-effectively adaptedEasy to adaptCannulasIntravenous devicesNeedle penetrationMedicine
The present invention provides in ovo injection devices, including a novel vaccine / substance conservation valve (VCV), a needle depth adjuster (NDA), and methods for selectively delivering vaccines and other substances to eggs in the context of poultry hatcheries. The valve of the present invention allows for the conditional dispensing of vaccines and other substances dependent upon the presence or absence of an egg. When an egg is present, the valve becomes activated, allowing vaccines or other substances to be injected. When an egg is absent, the valve is not activated, thus vaccines and other valuable substances are conserved. The NDA allows embryonated eggs to be more safely injected by reducing the depth of needle penetration, particularly in smaller eggs.
Owner:MERIAL INC
Vibrio harveyi secretory type vaccine and structure and application thereof
InactiveCN101780273AHigh protection rateEasy to makeAntibacterial agentsBacteriaAdjuvantRoom temperature
The invention relates to the fields of molecular biology and immunology, in particular to a vibrio harveyi secretory type vaccine and a structure and application thereof. Particularly, the secretory type vaccine is a base sequence in a sequence table SEQ ID No.1, and the construction process thereof is as follows: utilizing vibrio harveyi T4 as a template and F11 and R8 as primers for PCR amplification, connecting the product with pBS-T at room temperature, utilizing NdeI / XhoI double-enzyme cleavage to recover 1.2kb segment from plasmids pBSVhP1, simultaneously utilizing NdeI / XhoI double-enzyme cleavage to recover 4.3kb segment of plasmids pBT3, connecting the two segments at room temperature by ligase T4DNA for 2-4h, transforming connection liquid into colon bacillus DH5 alpha, culturing on an LB solid medium containing Ap for 24h to screen out white transformant, i.e. the Vibrio harveyi secretory type vaccine of base sequence in the expression sequence table SEQ ID No.1. The obtained vaccine has immune and protective functions on Vibrio harveyi. The obtained vaccine has the immune and protective effects on the Vibrio harbeyi, and the immune and protective efficiency of the vaccine of the invention on the Vibrio harveyi can reach to 90%. The preparation process is simple, devitalization and other steps do not needed, and no adjuvant is needed.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Virus-like particles for pseudorabies virus and preparation method for same
ActiveCN102533680AAvoid quality risksEasy to design and manufactureInactivation/attenuationAntiviralsRabiesGlycoprotein G
The invention provides virus-like particles for pseudorabies virus. The virus-like particles consist of pseudorabies virus glycoprotein G and pseudorabies virus matrix protein M, and have stable and homogenous spatial structures; and the outer membrane protein of the virus-like particles contains all or partial fragments of the pseudorabies virus glycoprotein G, and can induce an organism to generate protective-level cellular immunity and humoral immunity. The virus-like particles do not comprise any nucleic acid component of chromosome of the pseudorabies virus, and avoid quality risks caused by inactivator addition. The virus-like particles can be conveniently purified by the conventional purification technology, and the quality risks possibly caused by inactivator addition in the traditional inactivated vaccines are avoided on principle. Meanwhile, due to a simple and quick construction and preparation mode, novel vaccines aiming at new strains can be conveniently and quickly designed and prepared. Based on the principle, novel polyvalent vaccines and multi-vaccines are easily formed on the basis of the particles; and the particles can widely replace related critical raw materials in the conventional practical technologies such as related antigen and antibody detection, functional protein vectors and the like.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Compound adjuvant, vaccine containing the same, and its preparation method
InactiveCN102600470ASolve the difficult problem of preparationSolve side effectsAntibody medical ingredientsVaccine StabilityAntigen
The invention relates a compound adjuvant, which comprises mixed oil (white oil and palm oil) and mixed surfactant (polyoxyethylene oleate, polyoxyethylene alkyl ether and mannitol oleate), specifically (by weight percentage) white oil 70-90, palm oil 1-15, polyoxyethylene oleate 1-10, polyoxyethylene alkyl ether 1-10, and mannitol oleate 1-10. The compound adjuvant is mixed with antigen aqueous solution, and emulsified under stirring to obtain W / O / W type vaccine. The vaccine has the advantages of good stability, little side effects, and high practical application value.
Owner:JIANGSU ACAD OF AGRI SCI
ILTV gD glycoprotein nucleotide sequence and amino acid sequence, recombined virus bacterin thereof and application of the bacterin
InactiveCN101210248AReduce economic lossEase of mass productionViral antigen ingredientsGenetic material ingredientsInfectious laryngotracheitisNucleotide
The invention provides a novel vaccine with the same immunoefficiency as the prior infectious laryngotracheitis virus (ILTV) attenuated vaccine and capable of overcoming dormant infection induced by the attenuated vaccine. The invention provides the nucleotide sequence and amino acid sequence of an ILTV glycoprotein D (gD) and a preparation method, as well as a recombinant vaccine containing a viral vector (fowlpox virus vector) and ILTV gD. The fowlpox virus is obtained by homologous arm recombination of ILTV gD. The invention also provides the preparation method of the recombinant vaccine. The invention clones gD gene of ILTV Henan isolate, and performs animal experiments of the immunoprotection of the recombinant fowlpox virus. The novel vaccine has the same immunoefficiency as the prior ILTV attenuated vaccine and is capable of overcoming the shortcomings of the attenuated vaccine. The invention is convenient for large-scale production, and provides a foundation for prevention and elimination of infectious laryngotracheitis (ILT).
Owner:HENAN AGRICULTURAL UNIVERSITY
Recombinant protein coded by GCRV (grass carp reovirus) II type S9 genes and application thereof
InactiveCN106866796AImproving immunogenicityEasy to identifyViral antigen ingredientsVirus peptidesDiseaseNucleotide
The invention discloses a recombinant protein coded by GCRV (grass carp reovirus) II type S9 genes and application thereof. The amino acid sequence of the recombinant protein is shown as SEQ ID NO:2. The nucleotide sequence for coding the protein is shown as SEQ ID NO:1. The obtained recombinant protein has better immunogenicity. Compared with CGRV proteins of other structures, the recombinant protein has the advantage that the valence of specific antibodies generated by immune animals induced by the protein is higher. Further experiments show that when VP6 proteins are used for immunizing grass carps, the grass carps can be induced to generate higher specific antibody; a certain immune protection effect is achieved on the GCRV strong virus plant attack. Therefore the development and the application of the GCRV II type VP6 protein have very important significance on developing novel grass carp hemorragic disease vaccine and immunological detection kits, and a novel effective solution path is provided for grass carp hemorragic diseases.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com