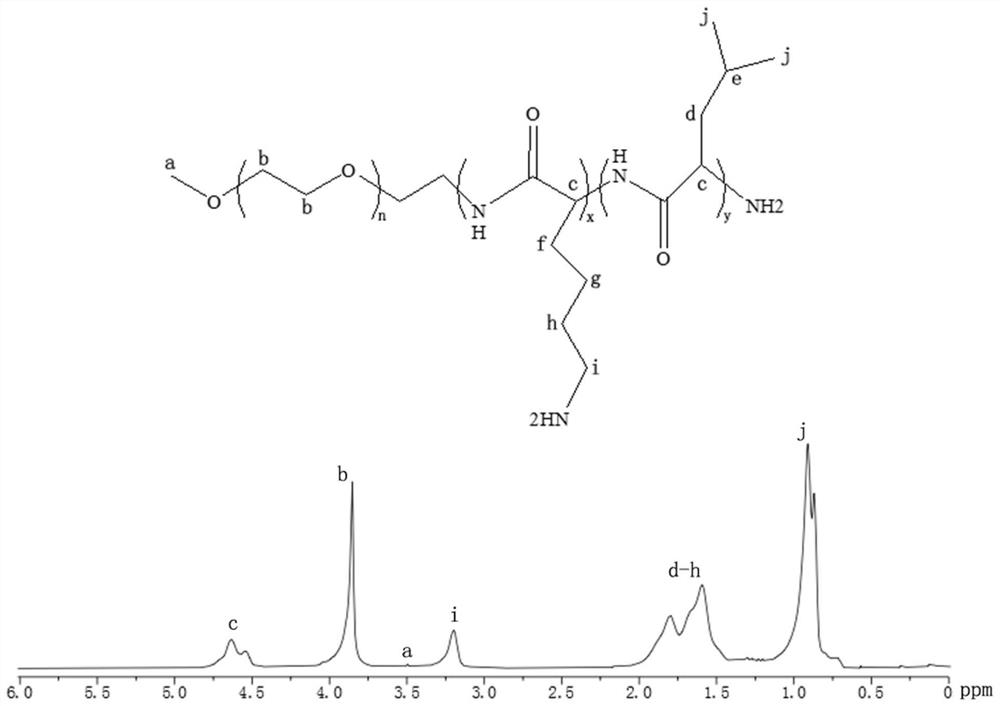
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Vaccine Stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparation of freeze-dried chickenpox vaccine
The invention relates to the preparative way of a kind of freeze-drying varicella vaccine, which has something to do with the biotechnology. The main making procedures include the following steps: the amplification and passage of Varicellavirus strain Oka to the MRC-5 strain in human's diploid cells; the harvest of the vaccine liquids; freeze-drying. The main property of the vaccine is that it adopts 0.5-1.2%(g / ml) gelatine, 0.6-1.2%(g / ml) glutamic sodium, 0.3-0.9%(g / ml) carbamide and 0.05-0.2%(g / ml) arginine as the freeze-drying protective agents, the previous concentrations stand for the final concentration of the protective agents. The good quality of the vaccine lies in that: the freeze-drying protective agents are developed by the producers themselves to make sure the stability of the vaccine. The result of storage stability test at 2-8DEG C shows that: there's no evident difference between the samples that stored for 18 months and the new-produced samples.
Owner:长春百克生物科技有限公司
Vaccine freeze-drying protective agent containing no gelatin and human albumin
ActiveCN105267971AImprove securityReduce adverse reactionsPowder deliveryAntiviralsVaccine StabilitySucrose
The present invention relates to the field of biological products, and in particular relates to a vaccine freeze-drying protective agent containing no gelatin and human albumin. The vaccine freeze-drying protective agent comprises 1.5%-10% of sucrose, 1.5%-3.5% of dextran, 1%-2% of sorbitol, 0.8%-1.2% of sodium glutamate and 0.2% to 1% of L-arginine in a vaccine semi-finished product, a matrix liquid for preparation of the vaccine freeze-drying protective agent is 199 culture medium or PBS buffer solution or water for injection, the vaccine freeze-drying protective agent contains no gelatin and human albumin, and the vaccine semi-finished product is a liquid vaccine before freeze-drying. The present invention also provides the use of the vaccine freeze-drying protective agent. When the vaccine freeze-drying protective agent is used for the preparation of a freeze-dried vaccine, the vaccine freeze-drying protective agent can improve the vaccine stability during freeze-drying and storage processes, greatly improves the freeze-dried vaccine safety for human, and reduces adverse effects of the vaccine.
Owner:SINOVAC DALIAN VACCINE TECH
Compound adjuvant, vaccine containing the same, and its preparation method
InactiveCN102600470ASolve the difficult problem of preparationSolve side effectsAntibody medical ingredientsVaccine StabilityAntigen
The invention relates a compound adjuvant, which comprises mixed oil (white oil and palm oil) and mixed surfactant (polyoxyethylene oleate, polyoxyethylene alkyl ether and mannitol oleate), specifically (by weight percentage) white oil 70-90, palm oil 1-15, polyoxyethylene oleate 1-10, polyoxyethylene alkyl ether 1-10, and mannitol oleate 1-10. The compound adjuvant is mixed with antigen aqueous solution, and emulsified under stirring to obtain W / O / W type vaccine. The vaccine has the advantages of good stability, little side effects, and high practical application value.
Owner:JIANGSU ACAD OF AGRI SCI
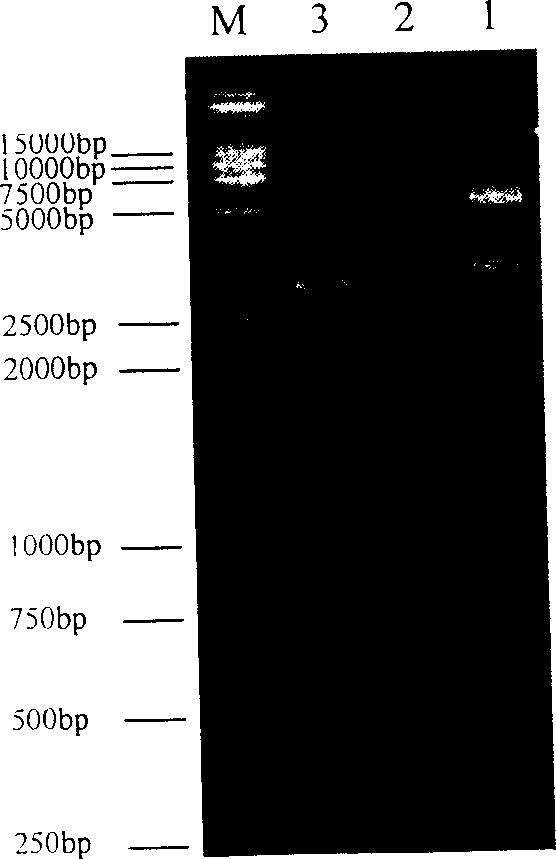
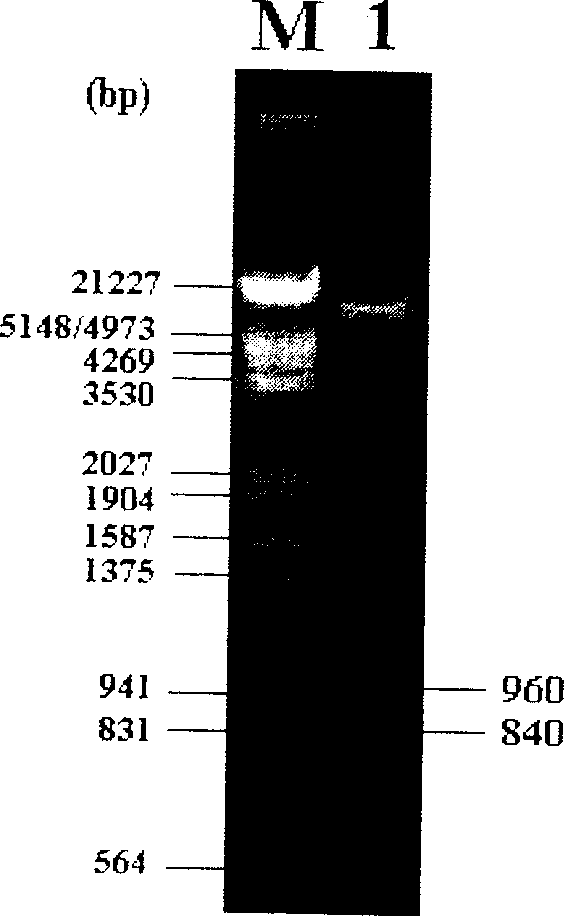
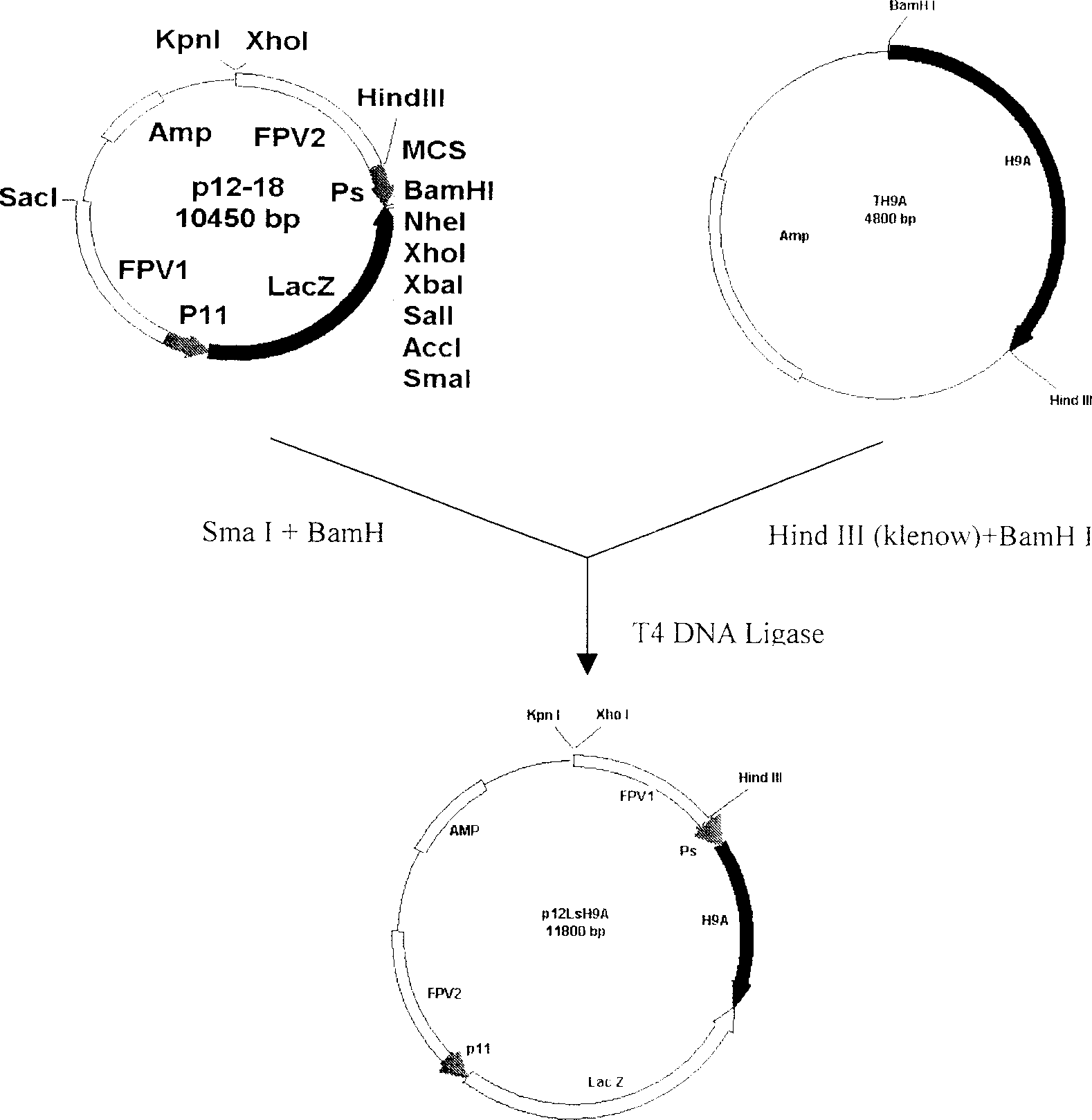
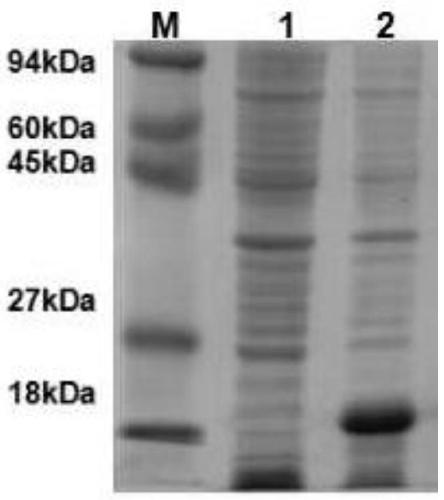
Recombined chicken pox virus vaccine rFPV-12LSH5H9, its construction method and use
InactiveCN1736482AMaintain and enhance immunogenicityImprove practicalityAntiviralsAntibody medical ingredientsVaccine StabilityEmbryo
Disclosed is a recombined chicken pox virus vaccine rFPV-12LSH5H9, its construction method and use, wherein the construction process consists of placing AIV HA gene fragment containing H5 hypotype and H9 hypotype onto the downstream of right promotors, inserting into chicken poxviridae expression vector p12-18 containing FPV duplicated non-indispensable fragment FPV1-2 and reported genes through backside cascade connection to construct transition vector p12LSH5H9, then transforming chick embryo desmocyte infected with wt-FPV with the transition vector p12LSH5H9, and obtaining co-expression H5 and H9 hypotype AIV HA gene rFPV-12LSH5H9 through consanguinity recombination.
Owner:YANGZHOU UNIV
Heatproof freeze-drying protecting agent for recombined pseudorabies virus vaccines and preparation method of heatproof freeze-drying protecting agent
ActiveCN105561317AImprove protectionImprove securityViral antigen ingredientsAntiviralsVaccine StabilitySaccharum
The invention relates to a heatproof freeze-drying protecting agent for recombined pseudorabies virus vaccines. The heatproof freeze-drying protecting agent comprises, by weight, 4-8% of trehalose, 0-3% of cane sugar, 0.2-0.8% of sorbitol, 1-3% of polyvinylpyrrolidone K-30, 4-7% of soybean protein isolate, 0.5-1% of wheat proteolysis product and the balance injection water. The invention further provides a preparation method of the heatproof freeze-drying protecting agent for the recombined pseudorabies virus vaccines. The heatproof freeze-drying protecting agent is simple in formula, easy to prepare and suitable for mass production, has the advantages of high vaccine stability, prolonged vaccine shelf life and the like, and does not contain gelatin and animal source ingredients. Safety of the vaccines is high when the protecting agent is applied to the vaccines.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA
Recombined chicken pox virus vaccine rPFV-12LSH9A, preparation process and use thereof
InactiveCN1736481AMaintain and enhance immunogenicityImprove practicalityAntiviralsRecombinant DNA-technologyVaccine StabilityEmbryo
Disclosed is a recombined chicken pox virus vaccine rPFV-12LSH9A, preparation process and use, wherein the construction process consists of placing AIV HA gene fragment containing H9 hypotype onto the downstream of right promotors, inserting into chicken poxviridae expression vector p12-18 containing FPV duplicated non-indispensable fragment FPV1-2 and reported genes to construct transition vector, then transforming chick embryo desmocyte infected with wt-FPV with the transition vector, and obtaining rFPV expressing H9 hypotype AIV HA gene through consanguinity recombination, i.e. recombinant chicken poxviridae vaccine rFPV-12LSH9A.
Owner:YANGZHOU UNIV
Freezedrying type vaccine of AIDS recombined poxvirus with Ankara gene stock carrier, and preparation method
InactiveCN1695737AImprove protectionImprove stabilityPowder deliveryAntiviralsVaccine StabilityFreeze-drying
Owner:JILIN UNIV
Fusion protein, and canine toxoplasma subunit vaccine and vaccine composition thereof
InactiveCN111234035AStimulate humoral immune responseHigh expressionProtozoa antigen ingredientsAntibody mimetics/scaffoldsVaccine StabilityCellular antigens
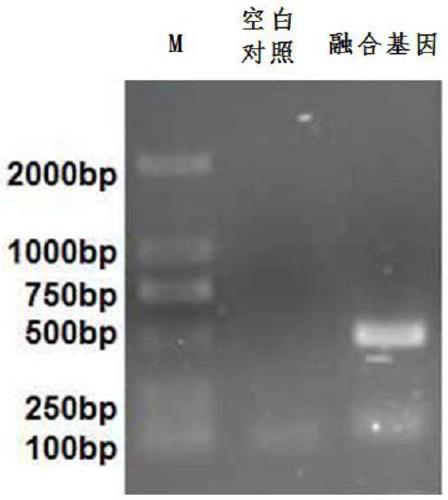
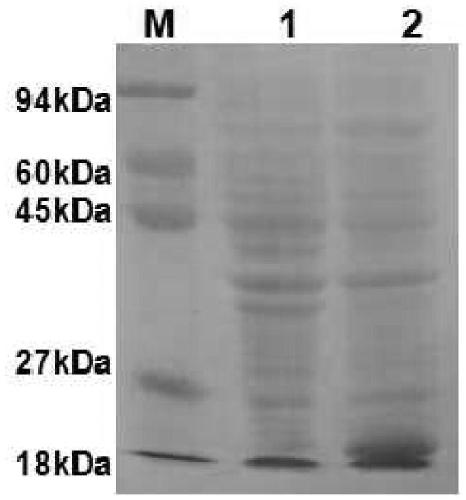
The invention provides a fusion protein and a nucleotide sequence thereof. The fusion protein comprises a toxoplasma cyclophilin protein subunit and a T cell epitope. The invention also provides a vaccine for preventing toxoplasmosis of dogs by using the fusion protein and a vaccine composition of the vaccine. The invention has the beneficial effects that the fusion protein has the characteristicsof high expression quantity and high immunogenicity; and the vaccine and the vaccine composition can effectively prevent canine toxoplasmosis and have high vaccine stability.
Owner:海木集团有限公司 +1
RNA vaccine for treating non-small cell lung cancer and construction method thereof
PendingCN112501201AImprove stabilityImprove translation efficiencyTumor rejection antigen precursorsTumor specific antigensVaccine StabilityNucleotide
The invention provides an RNA vaccine for treating non-small cell lung cancer. The nucleotide sequence of the RNA vaccine is shown as SEQ ID NO. 1. The RNA vaccine comprises two parts, namely a basicskeleton sequence (backstone) and a tandem vaccine sequence. The invention further provides a construction method of the RNA vaccine for treating the non-small cell lung cancer. According to the RNA vaccine for treating the non-small cell lung cancer, provided by the invention, a nucleotide sequence of a new antigen is optimally designed by utilizing a codon, connection is carried out by utilizinglinker, insertion of sequences such as SEC and MITD and construction of poly A tails are perfected, plasmids are constructed after sequence design is finished, and a target RNA sequence, namely the new antigen RNA vaccine, is obtained through linearization and in-vitro transcription. The vaccine is good in stability, high in translation efficiency and good in treatment effect, and expression products are easy to transfer.
Owner:WUXI PEOPLES HOSPITAL
Preparation of triple inactivated vaccine for Newcastle disease and infectious bronchitis as well as avian flu
ActiveCN105833264AReduce usageImprove stabilitySsRNA viruses negative-senseSsRNA viruses positive-senseVaccine StabilityInfectious bronchitis
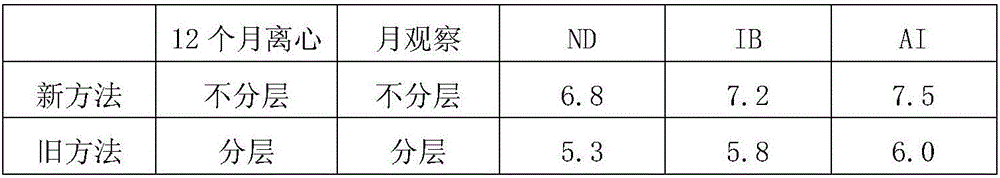
The invention relates to a preparation method of a triple inactivated vaccine for a Newcastle disease and infectious bronchitis as well as avian flu. The preparation method of the triple inactivated vaccine for the Newcastle disease and the infectious bronchitis as well as the avian flu comprises the following steps of (1), preparing a coexisting seed virus for the Newcastle disease and the infectious bronchitis as well as the avian flu; (2), preparing an antigen; (3), centrifuging the antigen; (4), inactivating the antigen; (5), carrying out emulsification, so as to finally obtain the finished product triple vaccine. According to the preparation method of the triple inactivated vaccine for the Newcastle disease and the infectious bronchitis as well as the avian flu, through optimizing a virus culturing process, the usage amount of chick embryos is decreased; the production cost is reduced; the labor efficiency is improved; the titer of the antigen for three viruses is improved; the concentration is needed for the finished product antigen; the stability and the usage effect of the vaccine are improved.
Owner:兆丰华生物科技(南京)有限公司 +1
Fusion protein as well as toxoplasma subunit vaccine and vaccine composition thereof
ActiveCN111138553AAvoid miscarriageImprove stabilityProtozoa antigen ingredientsAntibody mimetics/scaffoldsVaccine StabilityCellular antigens
The invention provides fusion protein and a nucleotide sequence thereof. The fusion protein comprises a toxoplasma P30 protein subunit and a T cell antigenic epitope. The invention further provides avaccine using the fusion protein to prevent abortion of pregnant sows due to toxoplasmosis and a vaccine composition thereof. The fusion protein has the beneficial effects of the characteristics of high expression quantity and high immunogenicity. The vaccine and the vaccine composition can effectively prevent abortion of the pregnant sows due to the toxoplasmosis and are high in vaccine stability.
Owner:JILIN UNIV FIRST HOSPITAL
Preparation method for freeze-dried b-type haemophilus influenza conjugate vaccines
InactiveCN102600086AImprove stabilityLong validity periodAntibacterial agentsPowder deliveryVaccine StabilityHaemophilus
The invention discloses a preparation method for freeze-dried b-type haemophilus influenza conjugate vaccines, which comprises the following steps of: preparing and separately packaging semi-finished product solution of the conjugate vaccine; putting the semi-finished product solution of the conjugate vaccine into a freeze-drying machine to be freeze-dried: firstly prefreezing for 2-4 hours, vacuumizing after prefreezing, regulating the temperature of the vacuum freeze-drying machine to be 30-35 DEG C below zero after vacuumizing, keeping the temperature for 10 hours, raising the temperature of a shelf to 30 DEG C at the speed of 5 DEG C / hour, and keeping the temperature of 30 DEG C for 3.8-4.2 hours; and taking out the freeze-dried conjugate vaccine to obtain a finished product. The vaccine prepared in the method disclosed by the invention meets the requirement, and conventional liquid dosage form is replaced by the freeze-dried Hib conjugate vaccine which has the characteristics of convenience in storage, transportation and use, good stability, long period of validity and the like.
Owner:CHENGDU OLYMVAX BIOPHARM
Method and device for predicting stability of new crown vaccine through quantum gating recurrent neural network
PendingCN114496297AMeet the need to establish long-term and short-term dependencies of sequencesMeet the needs of long-term and short-term dependenciesEpidemiological alert systemsProteomicsVaccine StabilityAlgorithm
The invention provides a method for predicting stability of a new crown vaccine based on a quantum gating recurrent neural network, and belongs to the technical field of quantum computing. According to the method, a quantum gating circulating unit is used for resetting and updating a basic group in an RNA sequence and a corresponding hidden state, a new hidden state is generated, and the reaction rate and degradation rate of the RNA sequence at different positions are output, so that the stability of the new crown vaccine is predicted, and therefore, the stability of the new crown vaccine is predicted. The method meets the requirement for establishing the long-term and short-term dependency relationship of the sequence by a gated recurrent neural network algorithm, and is low in computing resource consumption, so that the method is widely applied to cooperative work of a quantum chip and an electronic chip.
Owner:上海图灵智算量子科技有限公司
Immunogenic composition containing rabbit staphylococcal antigen, and preparation method and application thereof
ActiveCN110420324AFacilitated DiffusionGood for dispersion releaseAntibacterial agentsPill deliveryVaccine StabilityAdjuvant
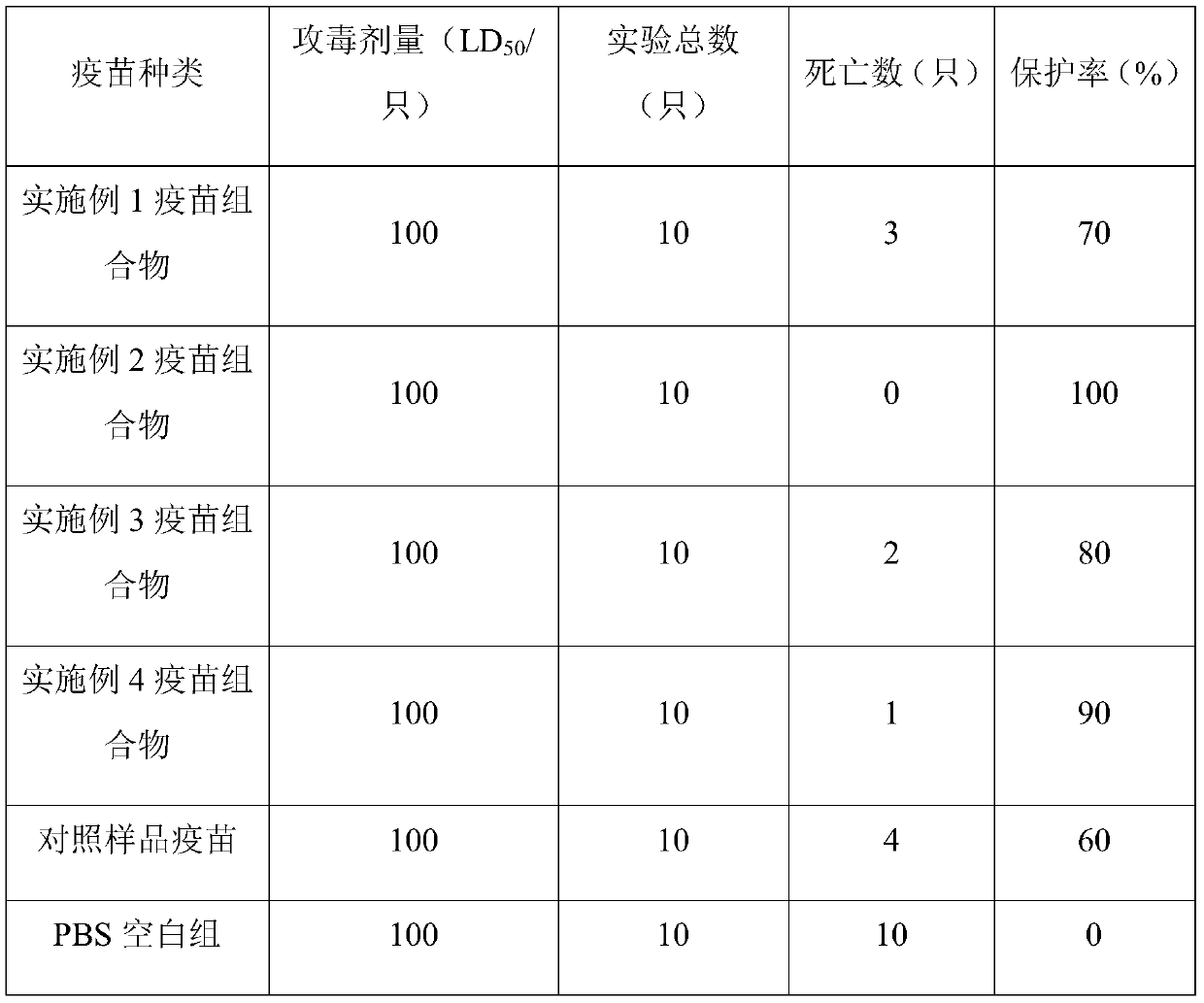
The present invention discloses an immunogenic composition containing a rabbit staphylococcal antigen, and a preparation method and an application thereof. The immunogenic composition uses a mixture of a rabbit staphylococcal antigen, a dispersion carrier and an aqueous printing adjuvant as printing spray liquid and freeze-dried protective powder as accessory powder, and the printing spray liquidis repeatedly sprayed on each layer of the accessory powder by a 3D printing technique to obtain the immunogenic composition containing the rabbit staphylococcal antigen with a three-dimensional structure. The immunogenic composition prepared by the method has good immunogenic activity and immunoprotection, can be effectively used for prevention and treatment of rabbit staphylococcus aureus, and has an immune protection rate up to 100%, and the vaccine is good in stability and not liable to be inactivated.
Owner:LINYI UNIVERSITY
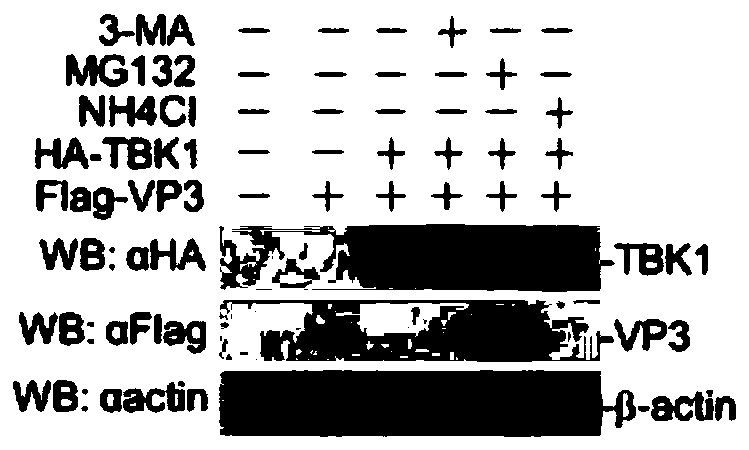
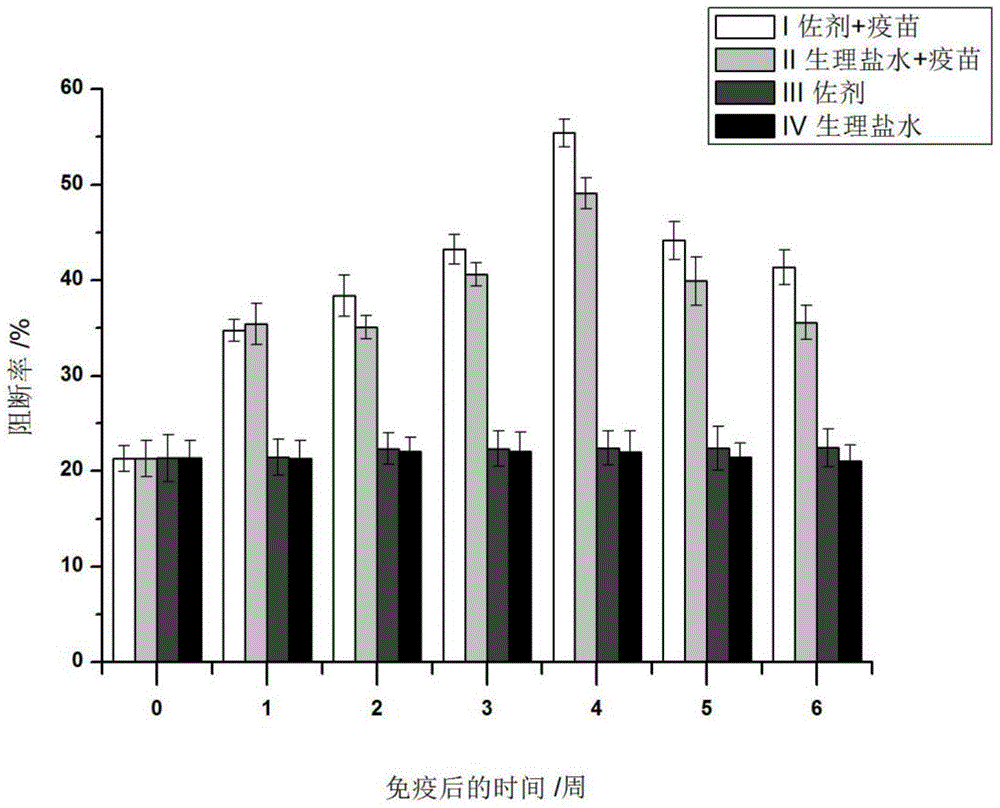
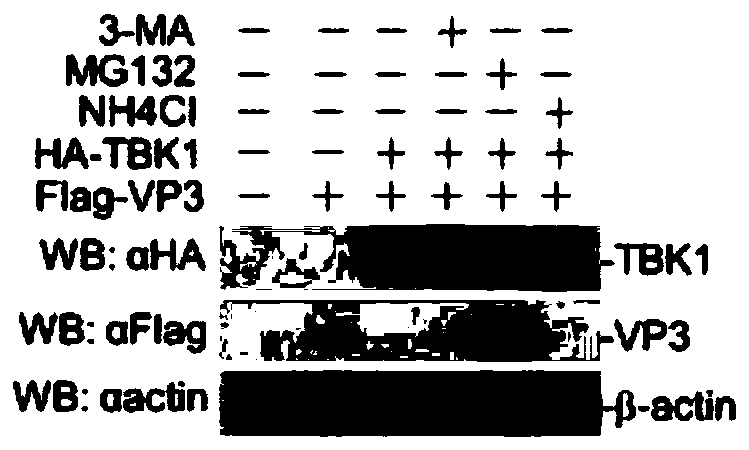
Application of MG132 as vaccine production synergist and stabilizing agent
ActiveCN110093322AAvoid degradationHigh expressionSsRNA viruses positive-senseVirus peptidesVaccine StabilityVirus-like particle
The invention belongs to the field of biomedicine, and particularly relates to an application of MG132 as a vaccine production synergist and a stabilizing agent. The MG132 can increase the expressionlevel of ribonucleic acid virus structural protein VP3, further promotes the assembly of virus-like particles, and can be applied to virus-like particle expression synergists, virus-like particle production synergists and the stabilizing agents of related vaccines to promote vaccine production and improve vaccine stability.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Freezedrying type vaccine of AIDS recombined poxvirus with Ankara gene stock carrier, and preparation method
InactiveCN1321694CImprove protectionImprove stabilityPowder deliveryAntiviralsVaccine StabilityFreeze-drying
Owner:JILIN UNIV
A fusion protein, a subunit vaccine of toxoplasma gondii and vaccine composition thereof
ActiveCN111138553BAvoid miscarriageImprove stabilityProtozoa antigen ingredientsAntibody mimetics/scaffoldsVaccine StabilityCellular antigens
The invention provides fusion protein and a nucleotide sequence thereof. The fusion protein comprises a toxoplasma P30 protein subunit and a T cell antigenic epitope. The invention further provides avaccine using the fusion protein to prevent abortion of pregnant sows due to toxoplasmosis and a vaccine composition thereof. The fusion protein has the beneficial effects of the characteristics of high expression quantity and high immunogenicity. The vaccine and the vaccine composition can effectively prevent abortion of the pregnant sows due to the toxoplasmosis and are high in vaccine stability.
Owner:JILIN UNIV FIRST HOSPITAL
Recombinant pseudorabies virus vaccine heat-resistant freeze-drying protectant and preparation method thereof
ActiveCN105561317BImprove protectionImprove securityViral antigen ingredientsAntiviralsVaccine StabilityFreeze-drying
The invention relates to a heatproof freeze-drying protecting agent for recombined pseudorabies virus vaccines. The heatproof freeze-drying protecting agent comprises, by weight, 4-8% of trehalose, 0-3% of cane sugar, 0.2-0.8% of sorbitol, 1-3% of polyvinylpyrrolidone K-30, 4-7% of soybean protein isolate, 0.5-1% of wheat proteolysis product and the balance injection water. The invention further provides a preparation method of the heatproof freeze-drying protecting agent for the recombined pseudorabies virus vaccines. The heatproof freeze-drying protecting agent is simple in formula, easy to prepare and suitable for mass production, has the advantages of high vaccine stability, prolonged vaccine shelf life and the like, and does not contain gelatin and animal source ingredients. Safety of the vaccines is high when the protecting agent is applied to the vaccines.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA
Method for protecting viral vaccine and improving thermal stability utilizing heavy water
InactiveCN1704122AImprove stabilityImprove thermal stabilityInorganic non-active ingredientsViruses/bacteriophagesHigh concentrationVaccine Stability
Disclosed is a method for protecting viral vaccine and improving thermal stability utilizing heavy water, which consists of choosing the concentration of heavy water (D2O) by a predetermined proportion, processing vaccines at different stages during vaccine production, deuterating the cells with heavy water, thus obtaining deuterated virus vaccine, or suspending the virus vaccine directly with high concentration heavy water (D2O).
Owner:ZHEJIANG PUKANG BIOTECH +1
A kind of vaccine adjuvant, its preparation method and application
ActiveCN104147599BImprove the effectiveness of anti-virus protectionEnhance immune stimulationAntiviralsEmulsion deliveryOil phaseHigh pressure
The invention discloses an oil-in-water type vaccine adjuvant as well as a preparation method and application thereof. The oil-in-water type nano-emulsion vaccine adjuvant comprises the following components in percentage by mass: 0.1-10 percent of oil phase, 0.1-10 percent of emulsifier, 0.1-3 percent of stabilizer, 0.1-3 percent of complexing agent and 0.01-10 percent of immunopotentiator. The preparation method comprises the following steps: (1) uniformly dispersing the immunopotentiator, the stabilizer and the complexing agent in water, thereby obtaining an aqueous phase; (2) mixing an oil phase and an emulsifier, thereby obtaining an oil phase; (3) slowly adding the oil phase into the aqueous phase, and continuously stirring, thereby forming a stable emulsion; (4) regulating the pH value of the emulsion, and fixing the volume to obtain a primary emulsion; and (5) performing high-speed shearing and high-pressure homogenizing on the primary emulsion. The vaccine adjuvant provided by the invention is simple in preparation, convenient to use and small in side reactions, is used for diluting vaccines, particularly swine fever live vaccines and is high in stability and good in immune effect.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Application of mg132 as synergist and stabilizer in vaccine production
ActiveCN110093322BAvoid degradationHigh expressionSsRNA viruses positive-senseVirus peptidesVaccine StabilityVaccine Production
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Goat pox virus proliferation method, goat pox live vaccine as well as preparation method and application of goat pox live vaccine
ActiveCN112126628AGood for mass reproductionRealize high-density large-scale cultivationViral antigen ingredientsAntiviralsVaccine StabilityEngineering
The invention provides a goat pox virus proliferation method, a goat pox live vaccine and a preparation method and application of the goat pox live vaccine, and relates to the technical field of vaccine preparation. The goat pox virus proliferation method provided by the invention comprises the following steps of carrying out passage domestication on goat pox viruses on a suspension culture cell line to obtain domesticated viruses; carrying out suspension amplification culture on the cell line; and inoculating the domesticated viruses to the cell line subjected to the amplification culture, and performing culture to obtain proliferated viruses. According to the virus proliferation method, large-scale suspension culture of the cell line is realized, the adaptability of the viruses to cellsis improved, and the goat pox viruses with high and stable viral titer can be obtained. The preparation method of the goat pox virus live vaccine provided by the invention is simple in process and lowin cost, and the technical problems of virus damage, poor vaccine stability and short preservation time in a vaccine preparation process are relieved. The goat pox live vaccine provided by the invention is safe and effective, and can be applied to prevention and control of goat pox.
Owner:天康制药股份有限公司
Replication-defective Ad29 adenovirus vector as well as construction method and application thereof
PendingCN113897389ALow popularitySafeSsRNA viruses positive-senseHydrolasesVaccine StabilityEngineering
The invention provides a replication-defective Ad29 adenovirus vector as well as a construction method and application thereof. The replication-defective Ad29 adenovirus vector is more efficiently and conveniently prepared through constructing a shuttle plasmid containing a replicon and a resistance gene, preparing an Ad29 adenovirus cyclized plasmid, finding the most suitable knockout site through a CRISPR / Cas9 technology, designing optimal specific targeting gRNA, and specifically knocking out E1 and E3 genes from the Ad29 adenovirus. The adenovirus is low in popularity and low in average neutralization titer in people, can be used for preparing novel coronavirus vaccines, is high in stability, and can be used for inducing very high humoral immunity and cellular immunity.
Owner:JIAXING ANYU BIOTECH CO LTD
New antigen RNA and immunologic adjuvant poly (I: C) composite vaccine and construction method thereof
PendingCN112516296AImprove stabilityImprove translation efficiencyTumor rejection antigen precursorsTumor specific antigensVaccine StabilityTranslational efficiency
The invention provides a new antigen RNA and immunologic adjuvant poly (I: C) composite vaccine. The composite vaccine comprises PEG-PLL-PLLeu self-assembled nano-micelle, a new antigen RNA vaccine and an immunologic adjuvant poly (I: C), wherein the new antigen RNA vaccine and the immunologic adjuvant poly (I: C) are loaded on the PEG-PLL-PLLeu self-assembled nano-micelle; and the nucleotide sequence of the new antigen RNA is shown as SEQ ID NO. 1. The new antigen RNA and immunologic adjuvant poly (I: C) composite vaccine provided by the invention is good in stability, high in translation efficiency, easy in expression product transfer and good in treatment effect.
Owner:WUXI PEOPLES HOSPITAL
Method for producing live vaccine against pseudorabies in swine from continuous cell line
ActiveCN107224579AGood effectImprove stabilityArtificial cell constructsVertebrate cellsVaccine StabilityFreeze-drying
The invention especially relates to a method for producing a live vaccine against pseudorabies in swine from a continuous cell line, belonging to the technical field of biological products for livestock. The continuous cell line used in the invention is Vero cells. The method comprises the following steps: passage and culture of cells for vaccine preparation; propagation of virus seeds of the cells; propagation of virus liquid used for vaccine preparation; and vaccine preparation, split charging and freeze-drying. Porcine pseudorabies viruses obtained by using the method re high in content and titer; and the eventually prepared vaccine has high stability and long shelf life.
Owner:广州渔跃生物技术有限公司
A method for preparing a serum-free cultured suspension mammalian cell line, the prepared cell line and application thereof
ActiveCN104726392BExtended growth timeHigh densityArtificial cell constructsVertebrate cellsBiotechnologyVaccine Stability
The invention provides a method for preparing a serum-free cultured suspension mammal cell line, which comprises the following steps: 1)recovering and culturing mammal cells to obtain adherent cultured mammal cells, digesting by pancreatin; 2)culturing the adherent cultured mammal cells by a serum-free medium added with a certain amount of serum, continuously culturing the mammal cells so as to adapt to the serum-free medium with the certain amount of serum; and 3)repeating the continuous cultivation process in the step 2), and gradually reducing the serum addition amount in the serum-free medium until the serum addition in the serum-free medium is 0 to obtain the suspension mammal cell line. According to the method, equalization nutrition of cells is obtained, vaccine stability and uniformity can be guaranteed, and the problems of side effect due to serum addition can be solved, risk due to exogenous pollution is reduced, stress reaction of animal is reduced, and subsequent processing is easy and simple.
Owner:PU LIKE BIO ENG
A vaccine lyoprotectant without gelatin and human albumin
ActiveCN105267971BImprove securityReduce adverse reactionsPowder deliveryAntiviralsVaccine StabilitySaccharum
The present invention relates to the field of biological products, and in particular relates to a vaccine freeze-drying protective agent containing no gelatin and human albumin. The vaccine freeze-drying protective agent comprises 1.5%-10% of sucrose, 1.5%-3.5% of dextran, 1%-2% of sorbitol, 0.8%-1.2% of sodium glutamate and 0.2% to 1% of L-arginine in a vaccine semi-finished product, a matrix liquid for preparation of the vaccine freeze-drying protective agent is 199 culture medium or PBS buffer solution or water for injection, the vaccine freeze-drying protective agent contains no gelatin and human albumin, and the vaccine semi-finished product is a liquid vaccine before freeze-drying. The present invention also provides the use of the vaccine freeze-drying protective agent. When the vaccine freeze-drying protective agent is used for the preparation of a freeze-dried vaccine, the vaccine freeze-drying protective agent can improve the vaccine stability during freeze-drying and storage processes, greatly improves the freeze-dried vaccine safety for human, and reduces adverse effects of the vaccine.
Owner:SINOVAC DALIAN VACCINE TECH
A kind of method of producing porcine pseudorabies live vaccine with passage cell line
ActiveCN107224579BImprove stabilityLong retention periodArtificial cell constructsAntiviralsVaccine StabilityFreeze-drying
The invention especially relates to a method for producing a live vaccine against pseudorabies in swine from a continuous cell line, belonging to the technical field of biological products for livestock. The continuous cell line used in the invention is Vero cells. The method comprises the following steps: passage and culture of cells for vaccine preparation; propagation of virus seeds of the cells; propagation of virus liquid used for vaccine preparation; and vaccine preparation, split charging and freeze-drying. Porcine pseudorabies viruses obtained by using the method re high in content and titer; and the eventually prepared vaccine has high stability and long shelf life.
Owner:广州渔跃生物技术有限公司
Immunogenic composition containing rabbit staphylococcus antigen, preparation method and application thereof
ActiveCN110420324BThree-dimensional structureReduce volumeAntibacterial agentsPill deliveryBiotechnologyVaccine Stability
The invention discloses an immunogenic composition containing rabbit staphylococcus antigen, a preparation method and application thereof. The immunogenic composition comprises a mixture of rabbit staphylococcus antigen, dispersion carrier and aqueous printing adjuvant as a printing spray liquid , and the freeze-dried protective powder is used as the coating powder, and the printing spraying liquid is repeatedly sprayed on each layer of the coating powder by using the 3DP printing technology to obtain a three-dimensional structure of the immunogenic composition containing the rabbit staphylococcus antigen. The immunogenic composition prepared by the method of the invention has good immunogenic activity and immune protection, can be effectively used for the prevention and treatment of rabbit Staphylococcus aureus, the immune protection rate is as high as 100%, and the vaccine has good stability and is not easy to be inactivated.
Owner:LINYI UNIVERSITY
Preparation of Newcastle Disease, Infectious Bronchitis and Avian Influenza Triple Inactivated Vaccine
ActiveCN105833264BReduce usageImprove stabilitySsRNA viruses negative-senseSsRNA viruses positive-senseVaccine StabilityInfectious bronchitis
The invention relates to a preparation method of a triple inactivated vaccine for a Newcastle disease and infectious bronchitis as well as avian flu. The preparation method of the triple inactivated vaccine for the Newcastle disease and the infectious bronchitis as well as the avian flu comprises the following steps of (1), preparing a coexisting seed virus for the Newcastle disease and the infectious bronchitis as well as the avian flu; (2), preparing an antigen; (3), centrifuging the antigen; (4), inactivating the antigen; (5), carrying out emulsification, so as to finally obtain the finished product triple vaccine. According to the preparation method of the triple inactivated vaccine for the Newcastle disease and the infectious bronchitis as well as the avian flu, through optimizing a virus culturing process, the usage amount of chick embryos is decreased; the production cost is reduced; the labor efficiency is improved; the titer of the antigen for three viruses is improved; the concentration is needed for the finished product antigen; the stability and the usage effect of the vaccine are improved.
Owner:兆丰华生物科技(南京)有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com