A fusion protein, a subunit vaccine of toxoplasma gondii and vaccine composition thereof
A fusion protein, Toxoplasma gondii technology, applied in the fields of immunology and biology, can solve the problems of virulence reversion, high price, lack of immune protection in mice, etc., and achieve the effect of high expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Construction of the prokaryotic expression vector of Toxoplasma gondii recombinant protein
[0042] According to the open reading frame of Toxoplasma gondii P30 gene sequence and the physical map of prokaryotic expression vector pET28a, primers were designed and restriction sites BamH I and Hind III were introduced.
[0043] Upstream: GGATCCatgtcggtttcgctgcacca
[0044] Downstream: AAGCTTtggctcctttggaaag
[0045] In Escherichia coli, under normal circumstances, protein expression with a relatively large molecular weight generally exists in the form of inclusion bodies, and the codons of the natural sequence are not conducive to expression in Escherichia coli, and codon optimization is required to facilitate the preference of the Escherichia coli expression system sex. At the same time, in the research process of the present invention, we found that the main antigenic epitope of the P30 gene is mainly located in the first 480 nucleotides (that is, compare...
Embodiment 2
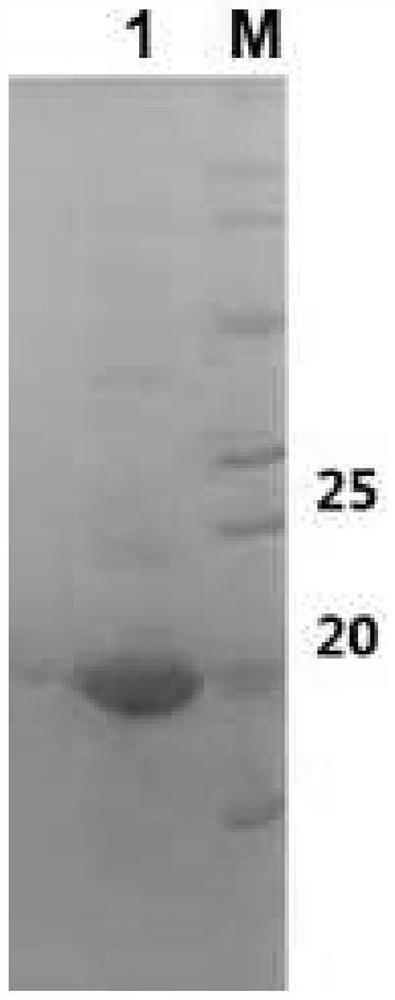
[0046] Example 2: Expression and purification of expressed protein
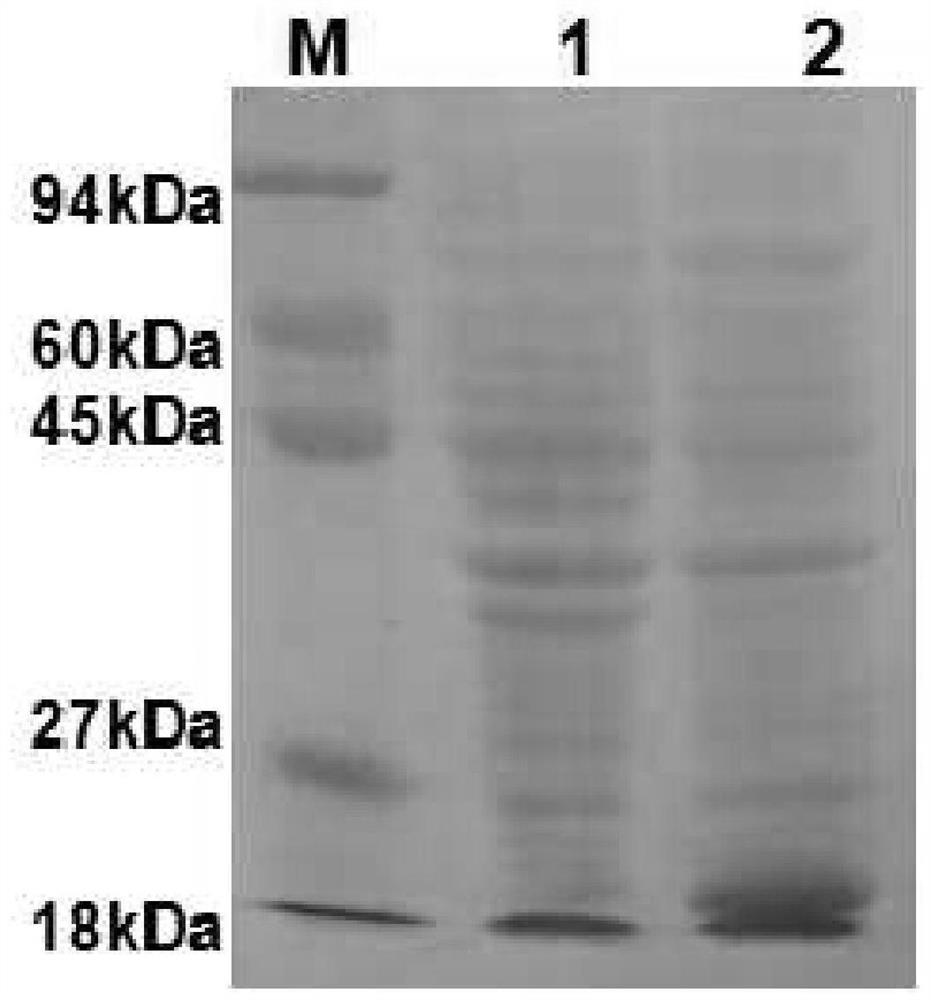
[0047] (1) Extraction and solubility verification of expressed protein
[0048] A single colony of E. coli BL21 (DE3) transformed with the recombinant plasmid pET-28a-P30 was used as the engineering strain of Toxoplasma gondii vaccine (BL21 (DE3)-pET-P30), and E. A single colony of coli BL21(DE3) was used as a blank control strain (BL21(DE3)-pET-28a). The above two strains were inoculated in 8 mL LB liquid medium respectively, and incubated overnight at 37°C with shaking. The next day, the seed solution was transferred to 400mL LB liquid medium for propagation at 37°C. When the OD600 of the bacterial solution was monitored to 0.6-0.7, the final concentration of IPTG was added to 0.1Mm / L, and the expression was induced at 18°C for 16 hours. Collect the bacteria by centrifugation, suspend the bacteria with 10mL soluble protein lysate, add 100μL 0.1M PMSF, freeze and thaw 3 times (-80℃, 1h / 37℃, 10min), after ...
Embodiment 3
[0051] Embodiment 3: western blot identification
[0052] Using Toxoplasma gondii-positive serum as the primary antibody, horseradish peroxidase-labeled rabbit anti-dog IgG as the secondary antibody, and DAB as the substrate chromogenic reagent, the fusion of BL21(DE3)-pET-P30 engineered bacteria induced expression Proteins were identified by western blot. BL21(DE3)-pET-28a served as a negative control. as a result of Figure 4 , Figure 4 Middle: M: pre-stained with Marker III; 1, BL21(DE3)-pET-P30; 2, BL21(DE3)-pET-28a. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com