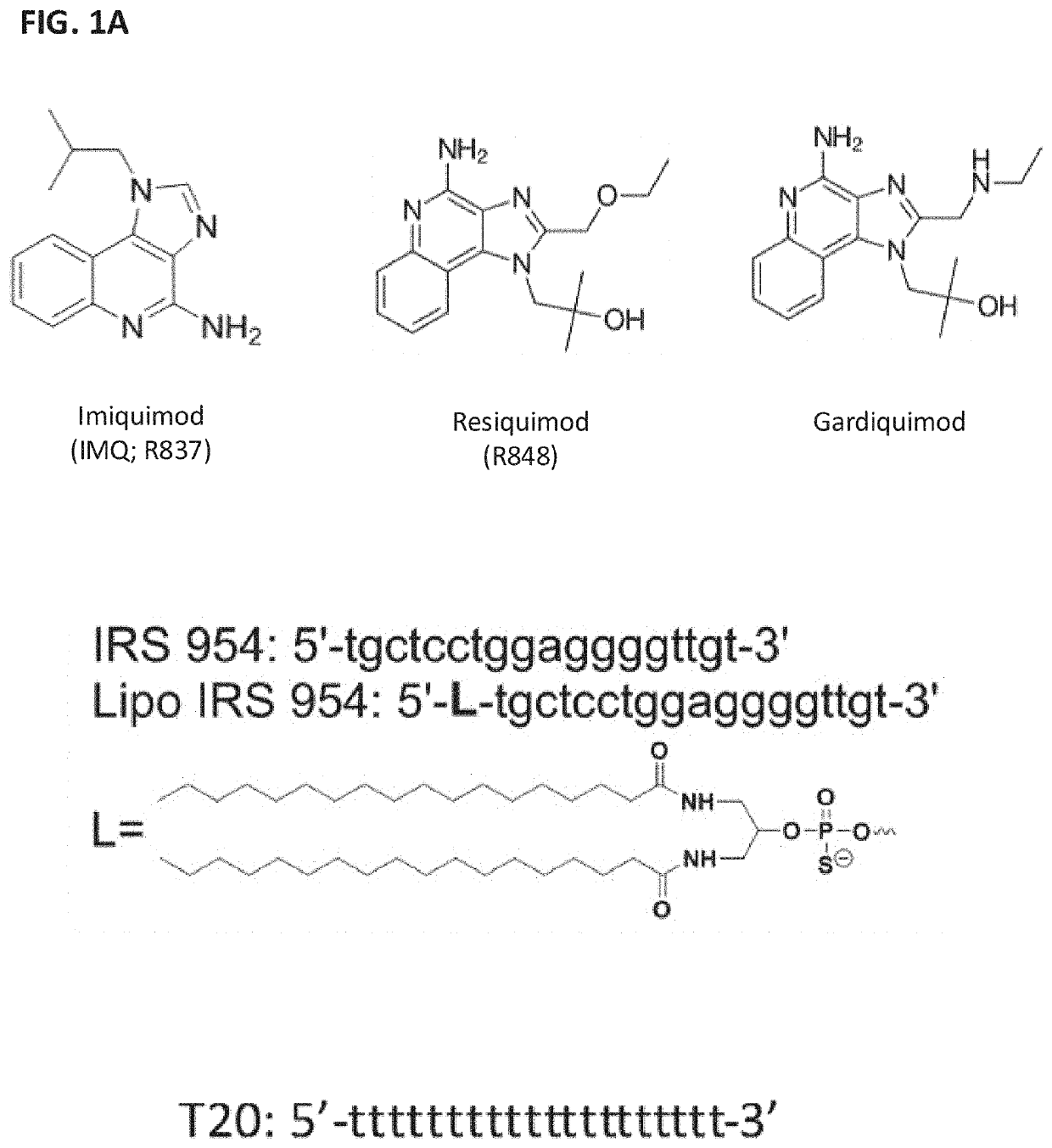
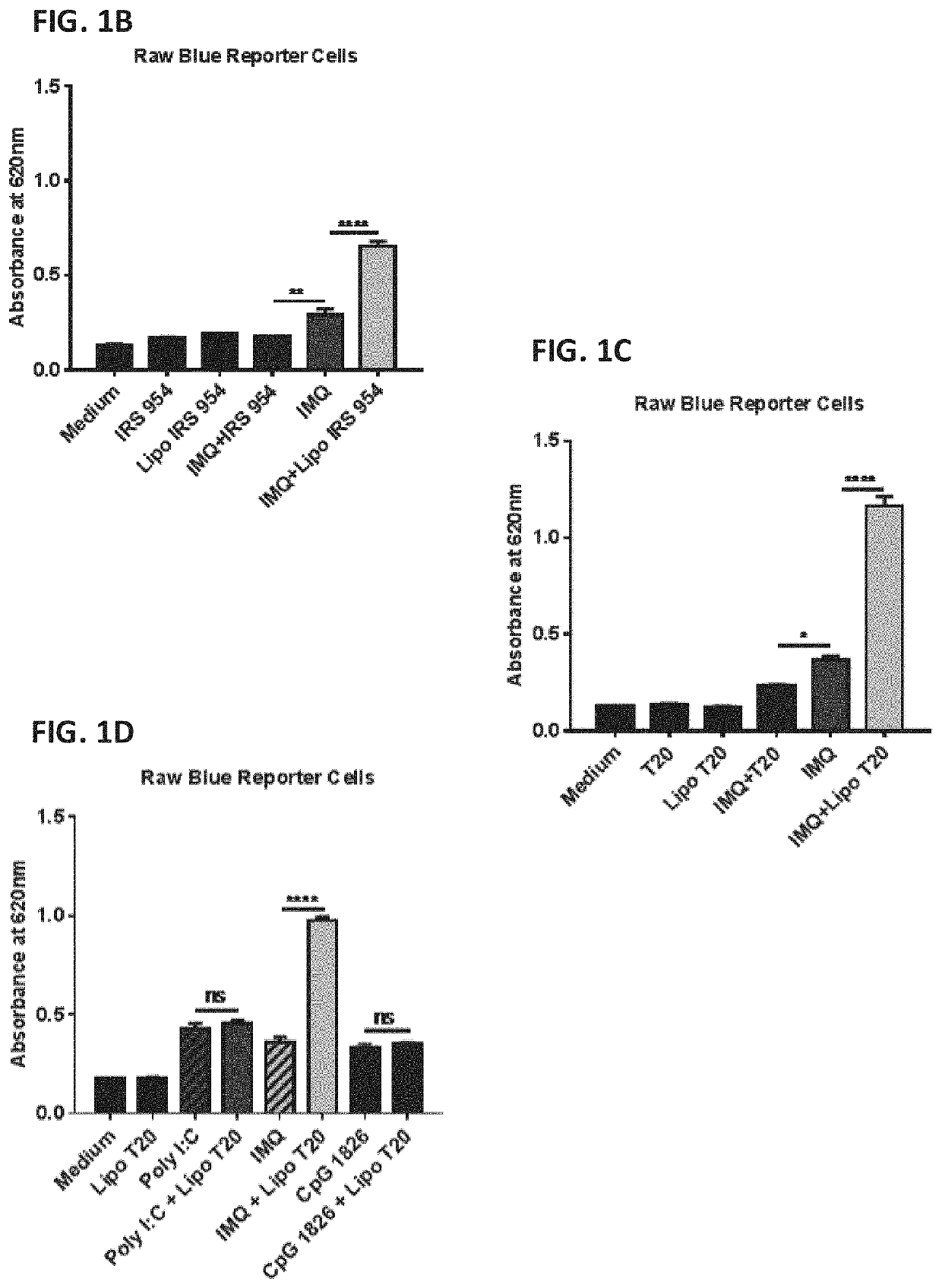
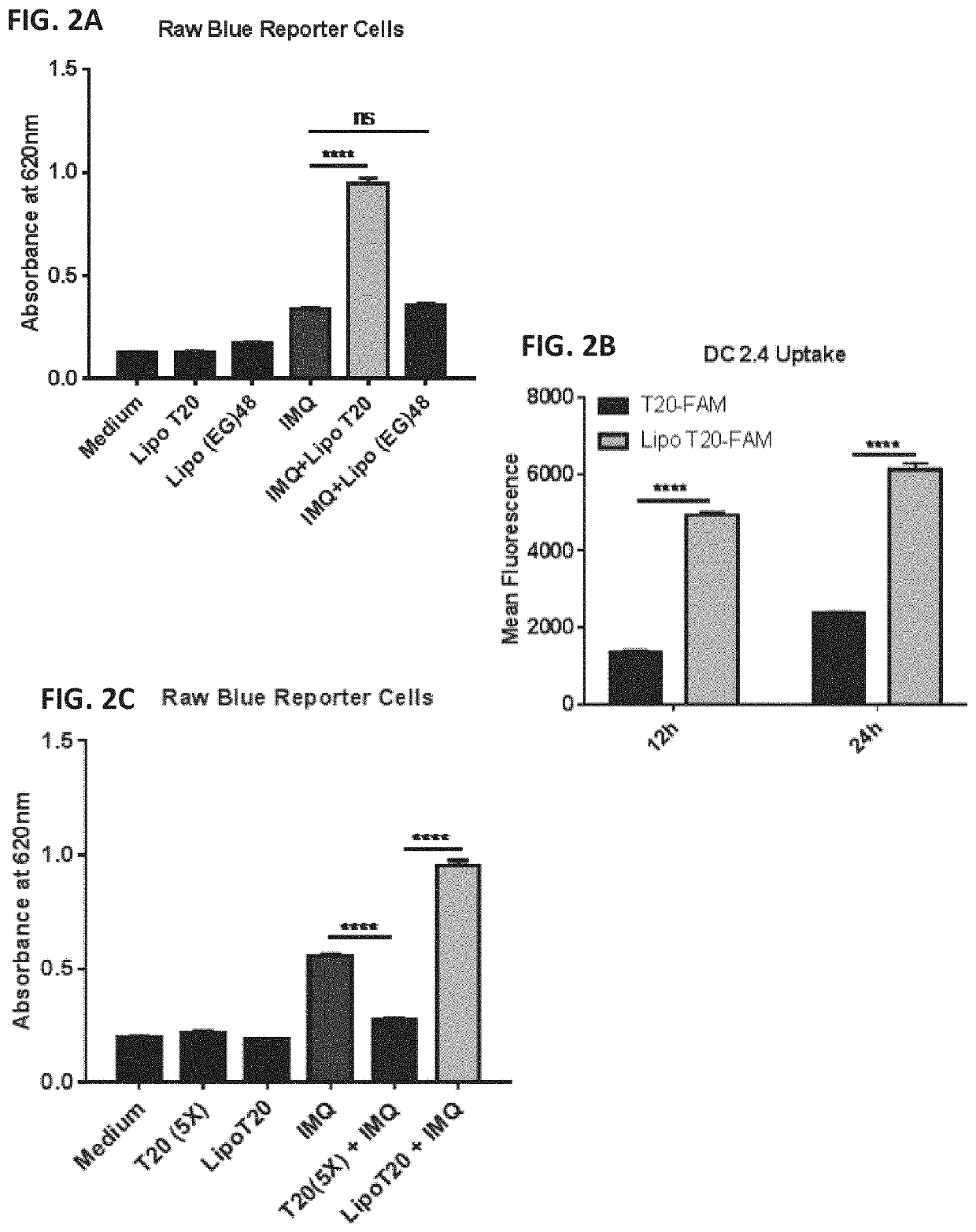
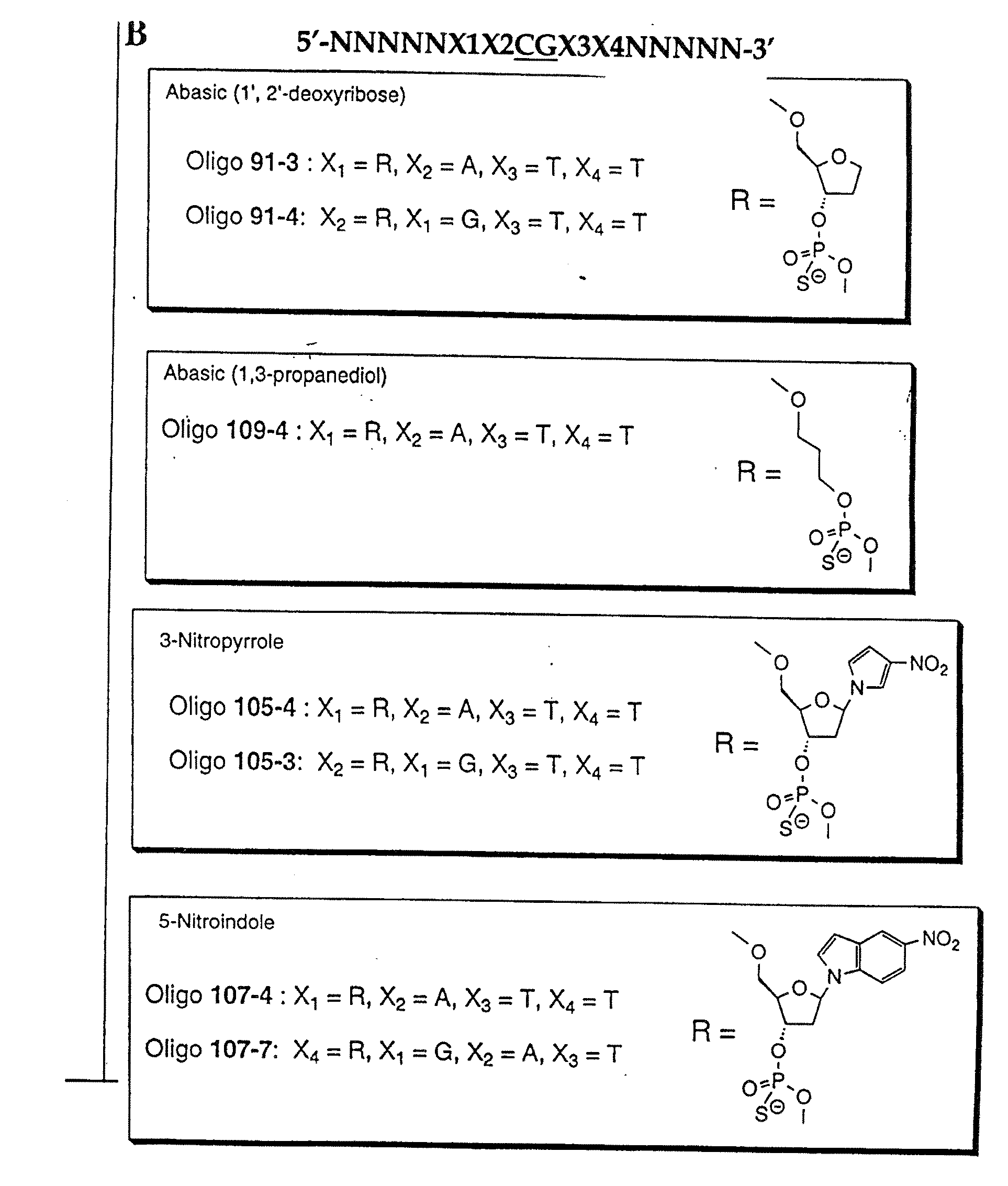
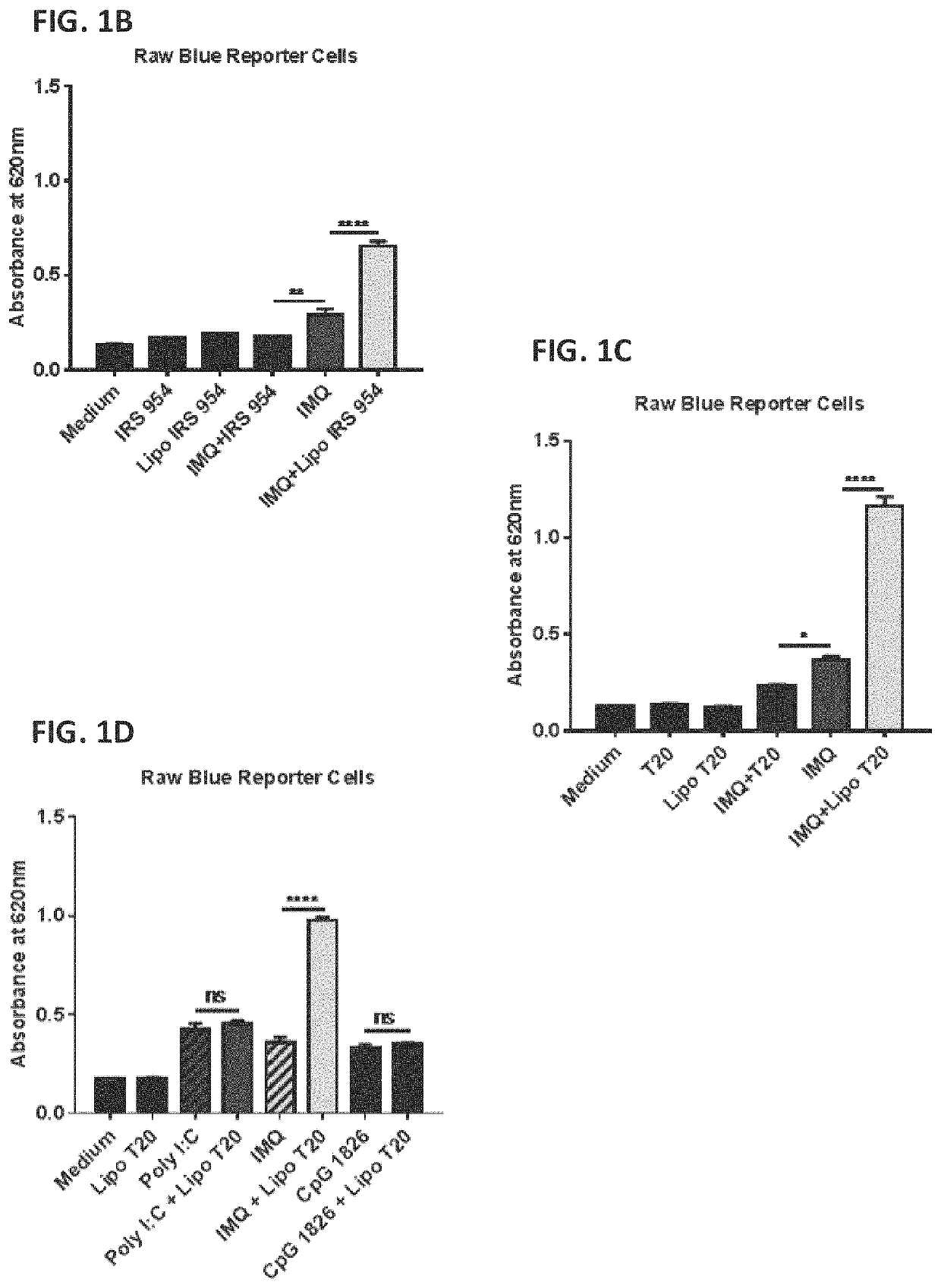
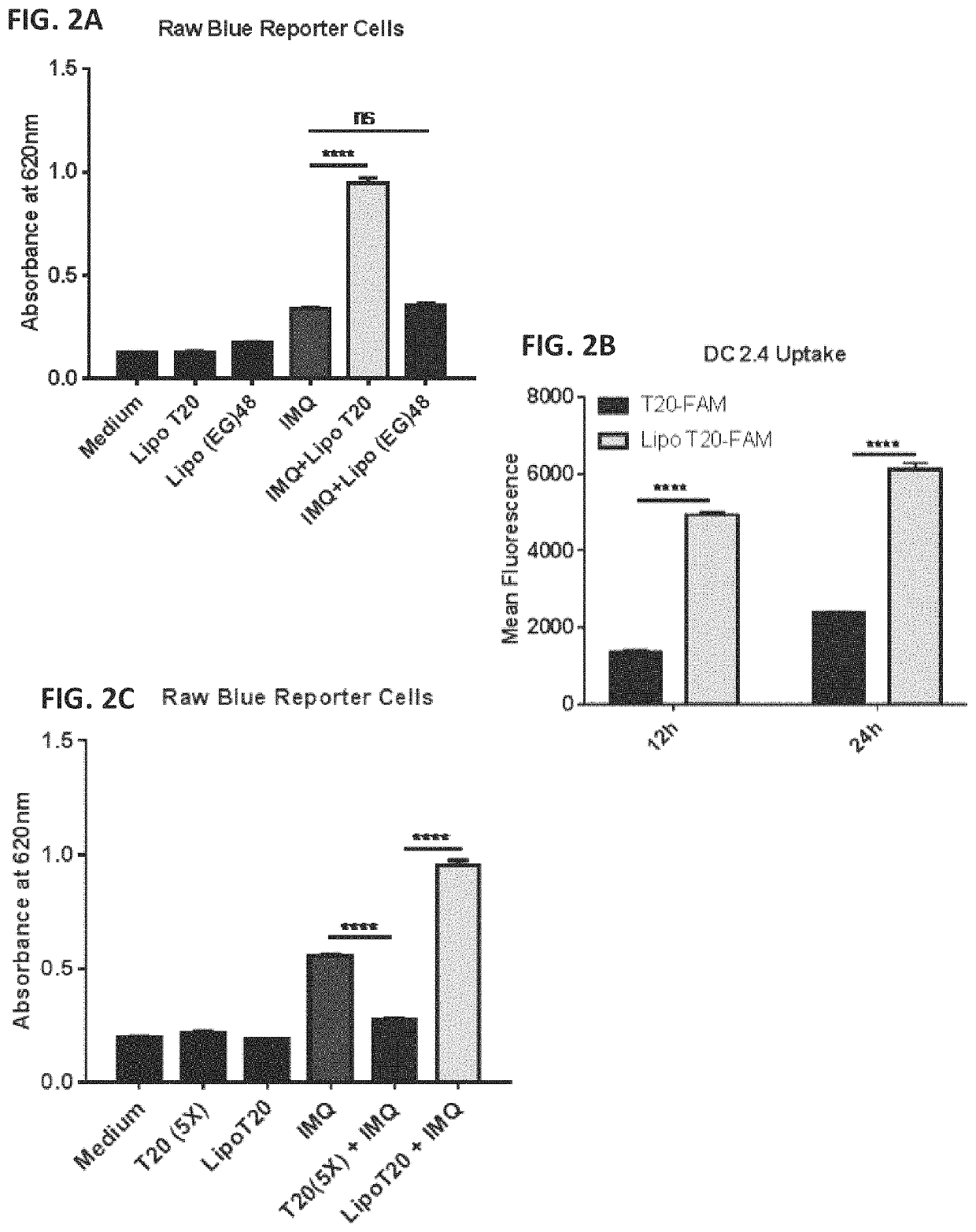
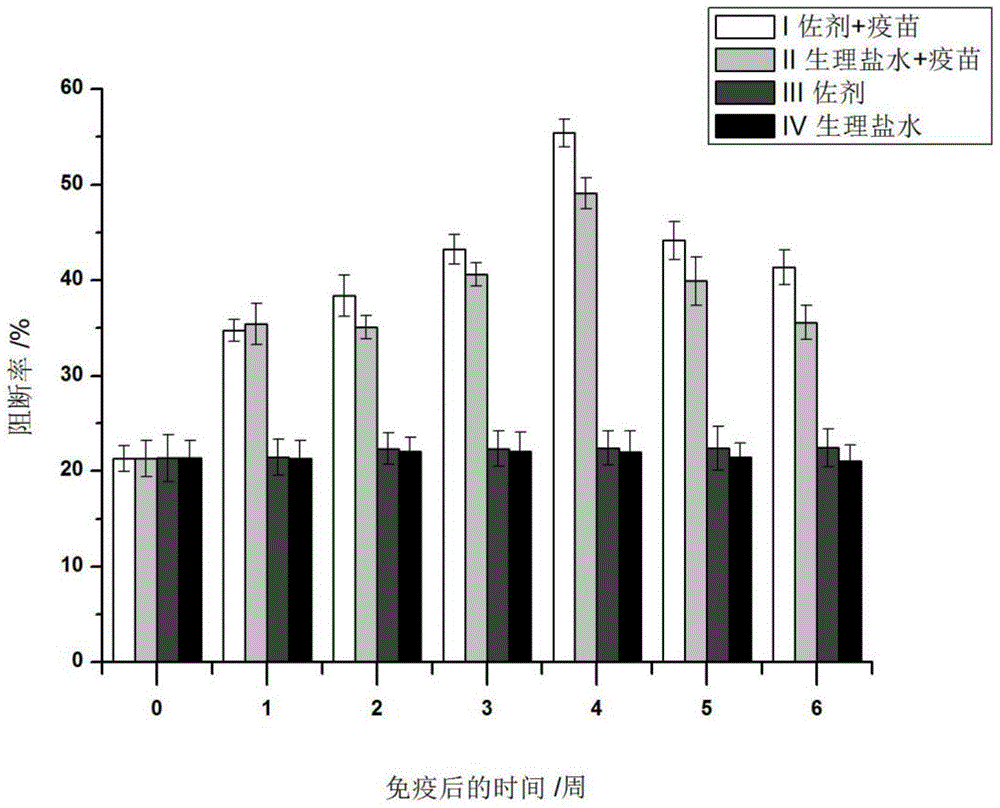
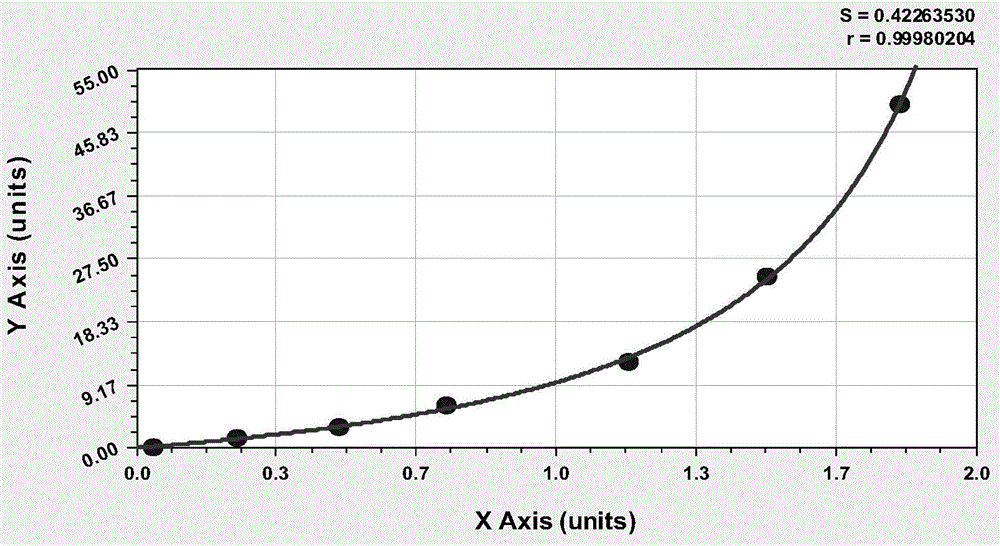
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37results about How to "Enhance immune stimulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
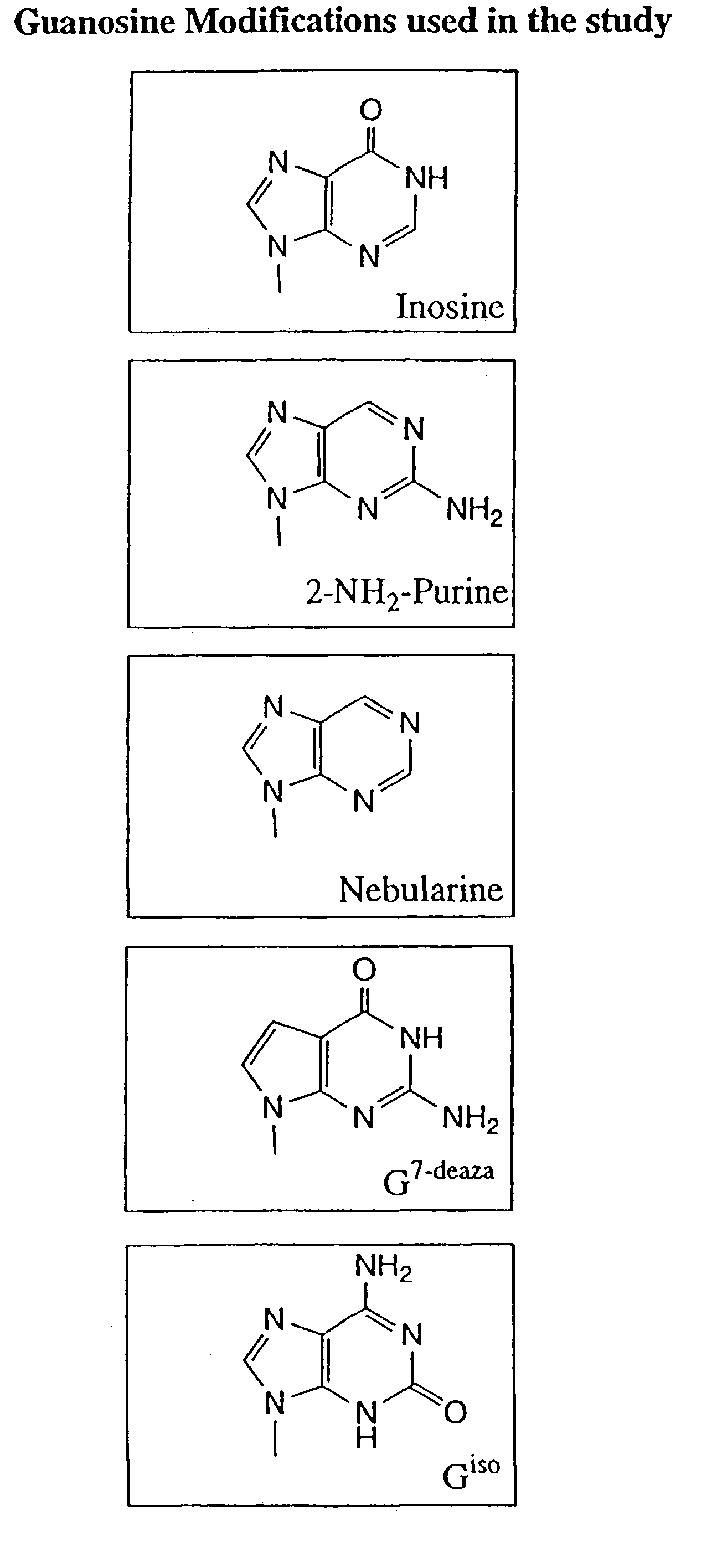
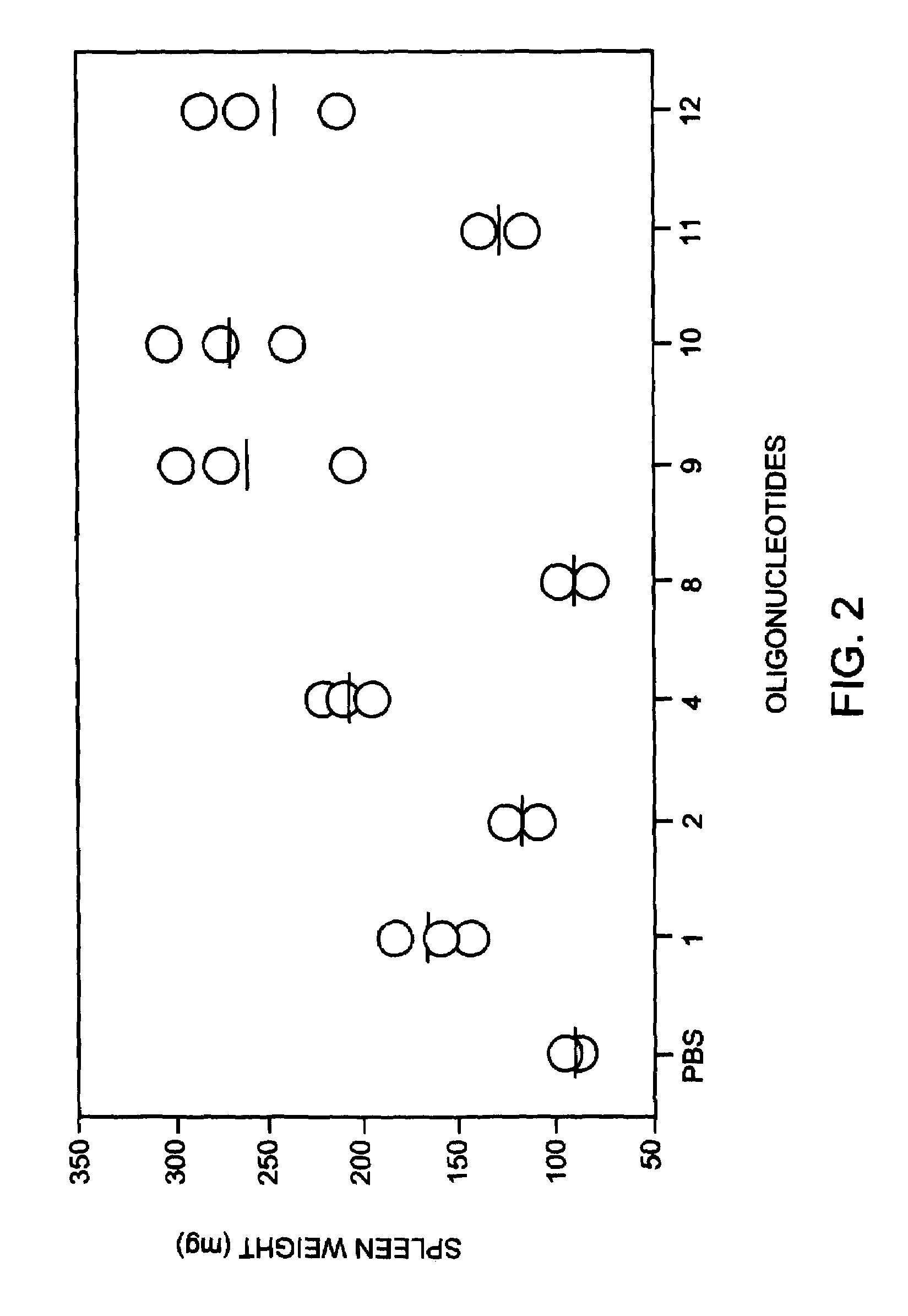
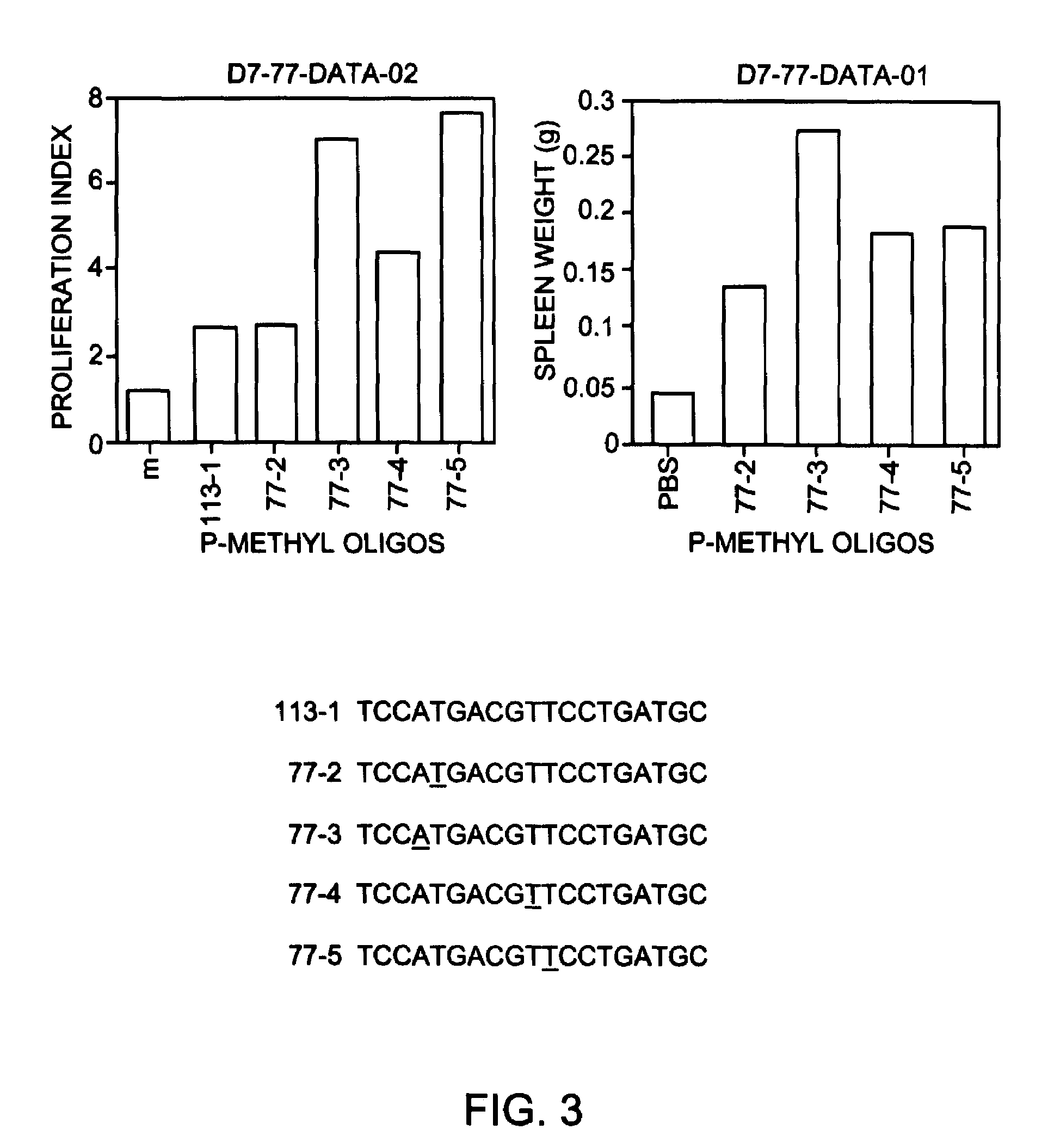
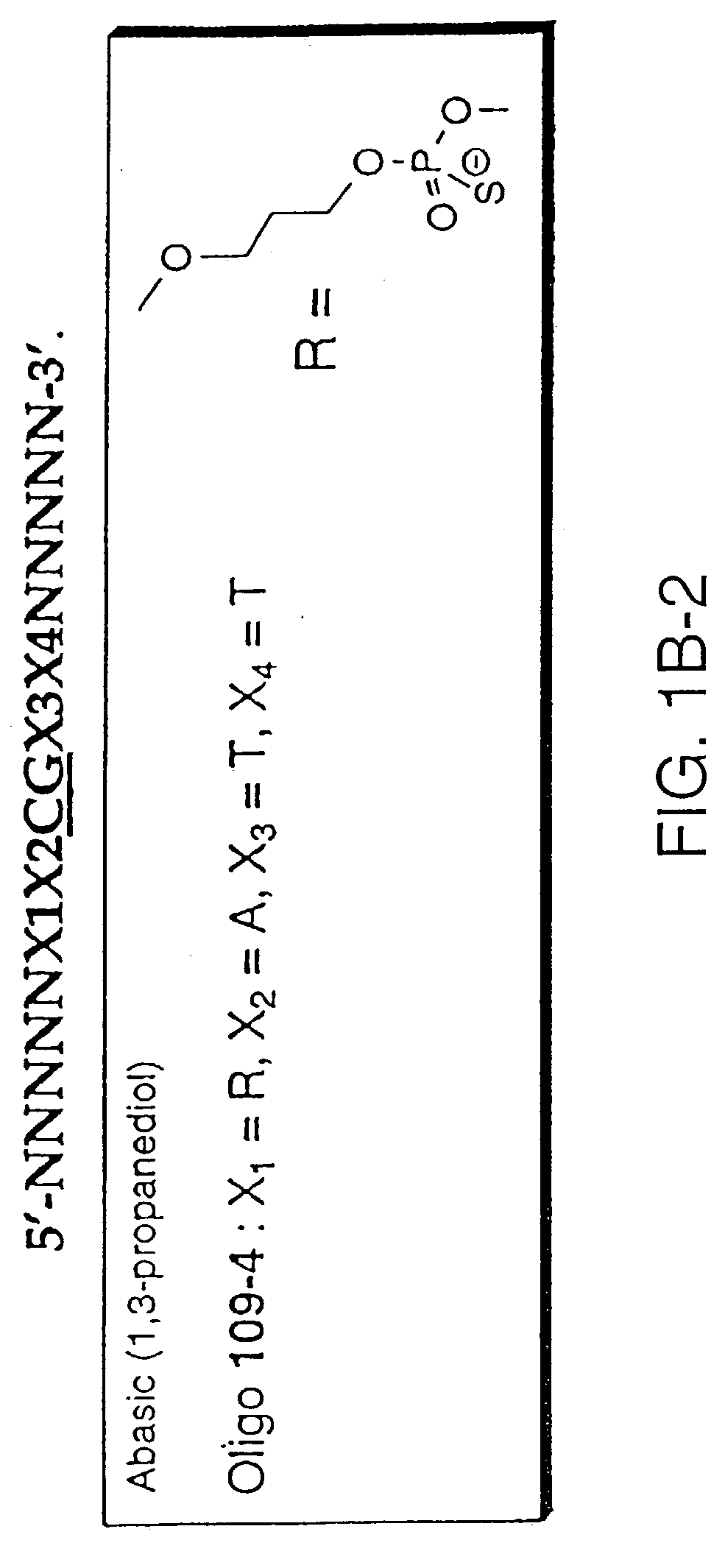
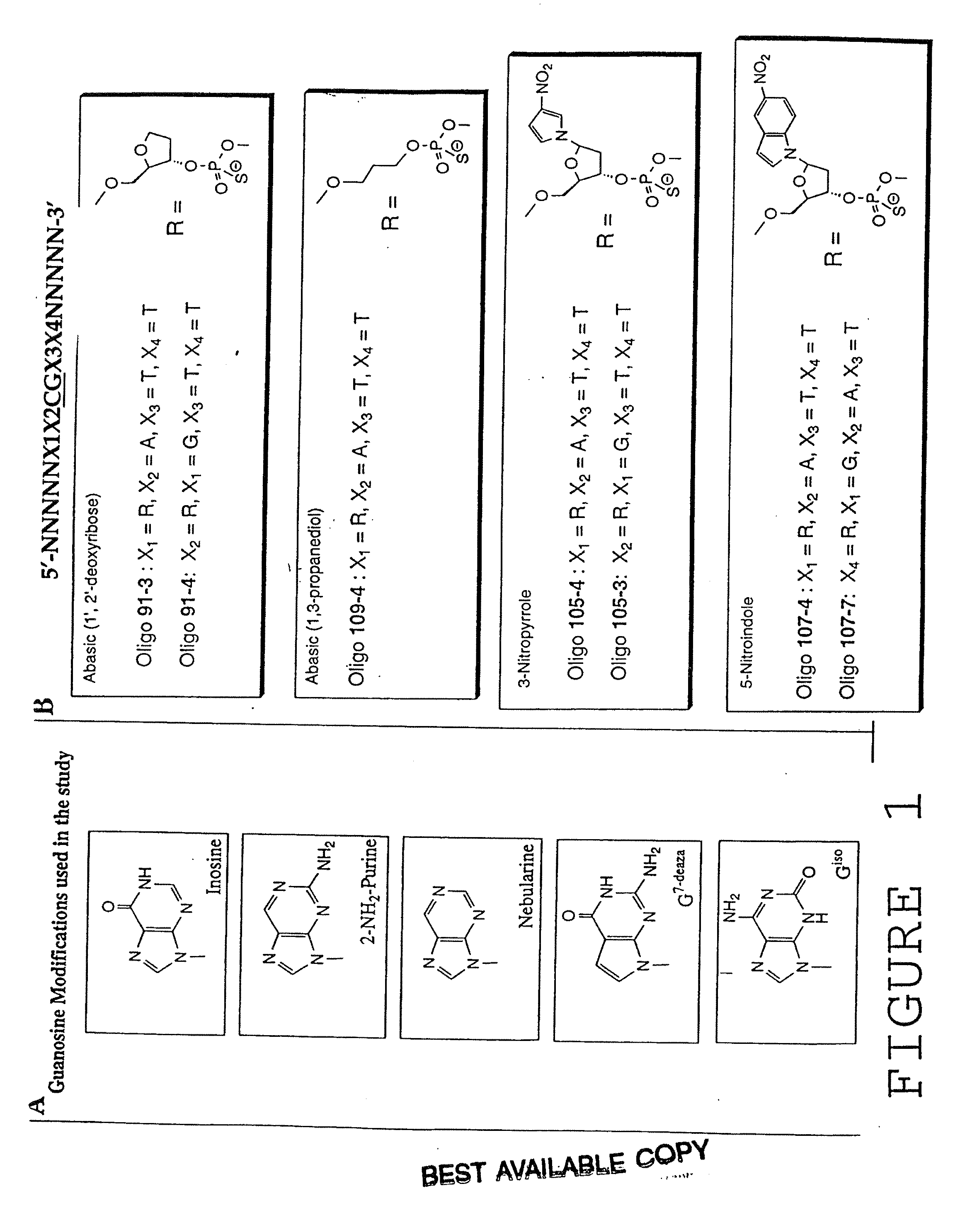
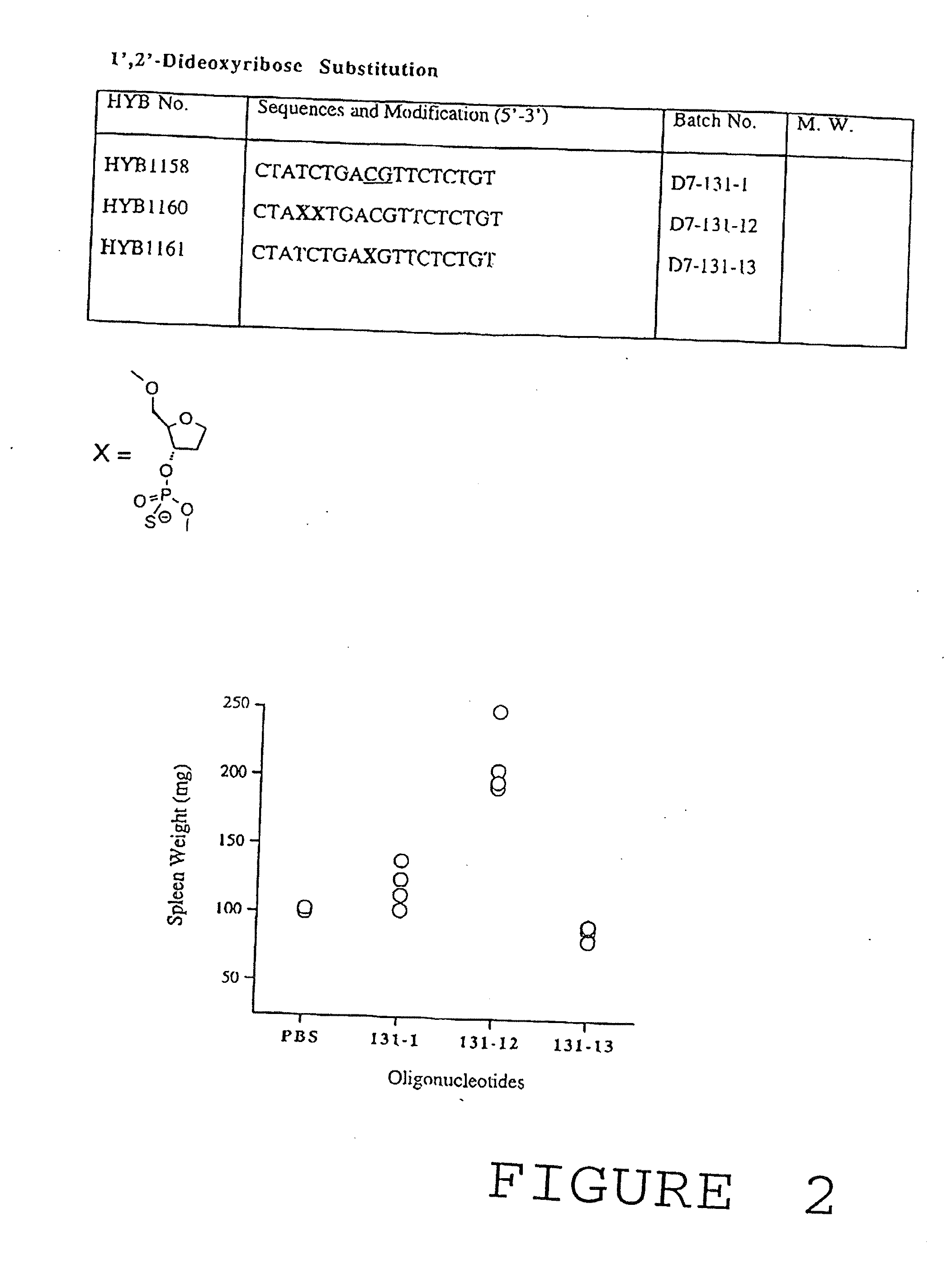
Modulation of oligonucleotide CpG-mediated immune stimulation by positional modification of nucleosides
InactiveUS7105495B2Decreasing immunostimulatory effectEnhance immune stimulationUltrasonic/sonic/infrasonic diagnosticsBiocideImmune StimulationPyrimidine Nucleotides
The invention provides methods for modulating the immune response caused by CpG dinucleotide-containing compounds. The methods according to the invention enables both decreasing the immunostimulatory effect for antisense applications, as well as increasing the immunostimulatory effect for immunotherapy applications.
Owner:IDERA PHARMA INC
Modulation of oligonucleotide CpG-mediated immune stimulation by positional modification of nucleosides
InactiveUS7329648B2Good effectDecreasing immunostimulatory effectBiocideOrganic active ingredientsImmune StimulationImmunotherapy
The invention provides methods for modulating the immune response caused by CpG-containing oligonucleotides. The methods according to the invention enable both decreasing the immunostimulatory effect for antisense applications, as well as increasing the immunostimulatory effect for immunotherapy applications.
Owner:IDERA PHARMA INC
Probiotic solid powder and preparation method thereof
ActiveCN102008016AHigh activityQuality improvementAnimal feeding stuffAccessory food factorsSodium BentoniteGram
The invention discloses probiotic solid powder and a preparation method thereof. The probiotic solid powder comprises a mixture of active probiotics and active zeolite, wherein the active probiotic content is between 12 and 50 billion colony-forming units (CFU) / gram; and the active zeolite mixture comprises the following components in percentage by weight: 30 to 50 percent of zeolite, 20 to 40 percent of attapulgite clay and 10 to 30 percent of bentonite. The probiotic solid powder provides a high-quality and low-cost antibiotic substitute product for livestock breeding; and the probiotic solid powder with 1 to 4 million CFU of live bacteria is added into each gram of feed, so the growth speed of chickens, pigs and fish can be improved by 2.0 to 15.6 percent, the conversion rate of the feed can be improved by 1.1 to 9.3 percent, and the diarrhea rate and the death rate of animals can be reduced obviously. The preparation method of the probiotic solid powder is simple, has low cost andcan reduce environmental pollution; and the content of the obtained product bacteria is high.
Owner:NANJING AGRICULTURAL UNIVERSITY
Vaccine adjuvant of swine mycoplasmal pneumonia live vaccine, and preparation method and application thereof
ActiveCN101954079AEnhance immune responseImmune activationAntibacterial agentsBacterial antigen ingredientsPhosphateLevamisole
Owner:JIANGSU ACAD OF AGRI SCI
Novel Trans-Adjuvant System
InactiveUS20100015214A1Enhancing and potentiating immune effector cell activationEnhance immune stimulationSsRNA viruses negative-senseViral antigen ingredientsAntigenVirosome
The present invention relates to a novel adjuvant system that generates an efficient immune response against antigens of various origins while reducing the risk of toxic side effects attendant to the use of known adjuvants. The novel adjuvant of the present invention comprises virosomes and allows antigenic molecules to be bound to or encapsulated in a variety of delivery vehicles which are easier to prepare for virosomes.
Owner:PEVION BIOTECH
Compositions and methods for the potentiation of immune responses against target antigens
InactiveUS20050196383A1Improving immunogenicityEnhancing and potentiating immune effector cell activationBiocidePeptide/protein ingredientsAdjuvantTarget antigen
The present invention provides novel adjuvant systems for the potentiation of immune responses against antigenic targets.
Owner:INTERCELL AG
Modulation of oligonucleotide CpG-mediated immune stimulation by positional modification of nucleosides
InactiveUS20030236211A1Decreasing immunostimulatory effectEnhance immune stimulationUltrasonic/sonic/infrasonic diagnosticsBiocideImmune StimulationPyrimidine Nucleotides
The invention provides methods for modulating the immune response caused by CpG dinucleotide-containing compounds. The methods according to the invention enables both decreasing the immunostimulatory effect for antisense applications, as well as increasing the immunostimulatory effect for immunotherapy applications.
Owner:IDERA PHARMA INC
Involucrin-driven retroviral expression cassettes encoding human immunodeficiency virus envelope glycoproteins
ActiveUS9730996B2Improve barrier propertiesEnhance immune stimulationViral antigen ingredientsGenetic material ingredientsGene deliveryImmunodeficiency virus
The present invention provides for novel compositions and methods for delivering genes of interest to stem cells using vectors that contain differentiation-specific transcriptional regulatory elements. For example, stem cells in the internal epithelia could be transfected with a vaccine construct, which has an epithelial cell differentiation-specific promoter driving the expression of viral envelope proteins. When the promoter used is specific for terminally differentiated epithelial cells, then the viral envelope proteins will be expressed only in the upper part of the epithelia and therefore, stimulate the immune response. The infected epithelial stem cells in the basal layer will continue to produce new antigen-expressing cells, without being eliminated by the immune response. This invention will be useful in the development of vaccines against viral agents that target the internal mucosa like HIV.
Owner:TEXAS BIOMEDICAL RES INST
Immunostimulatory composition and use thereof
PendingCN112972670AStrong immune responseEnhance immune stimulationAntibacterial agentsOrganic active ingredientsDiseaseAdjuvant
The invention provides an immunostimulatory composition, the immunostimulatory composition comprises saponin and CpG oligodeoxynucleotide, or the immunostimulatory composition is composed of an adjuvant containing the saponin, and the CpG oligodeoxynucleotide, wherein the CpG oligodeoxynucleotide sequence is provided with two or more copies of a 5'-TTCGTT-3' sequence motif or a 5'-TCGTCGTCG-3 'sequence motif. The invention provides the use of the immunostimulatory composition in the preparation of a medicament for the treatment of diseases.
Owner:JIANGSU THERAVAC BIO PHARMA CO LTD
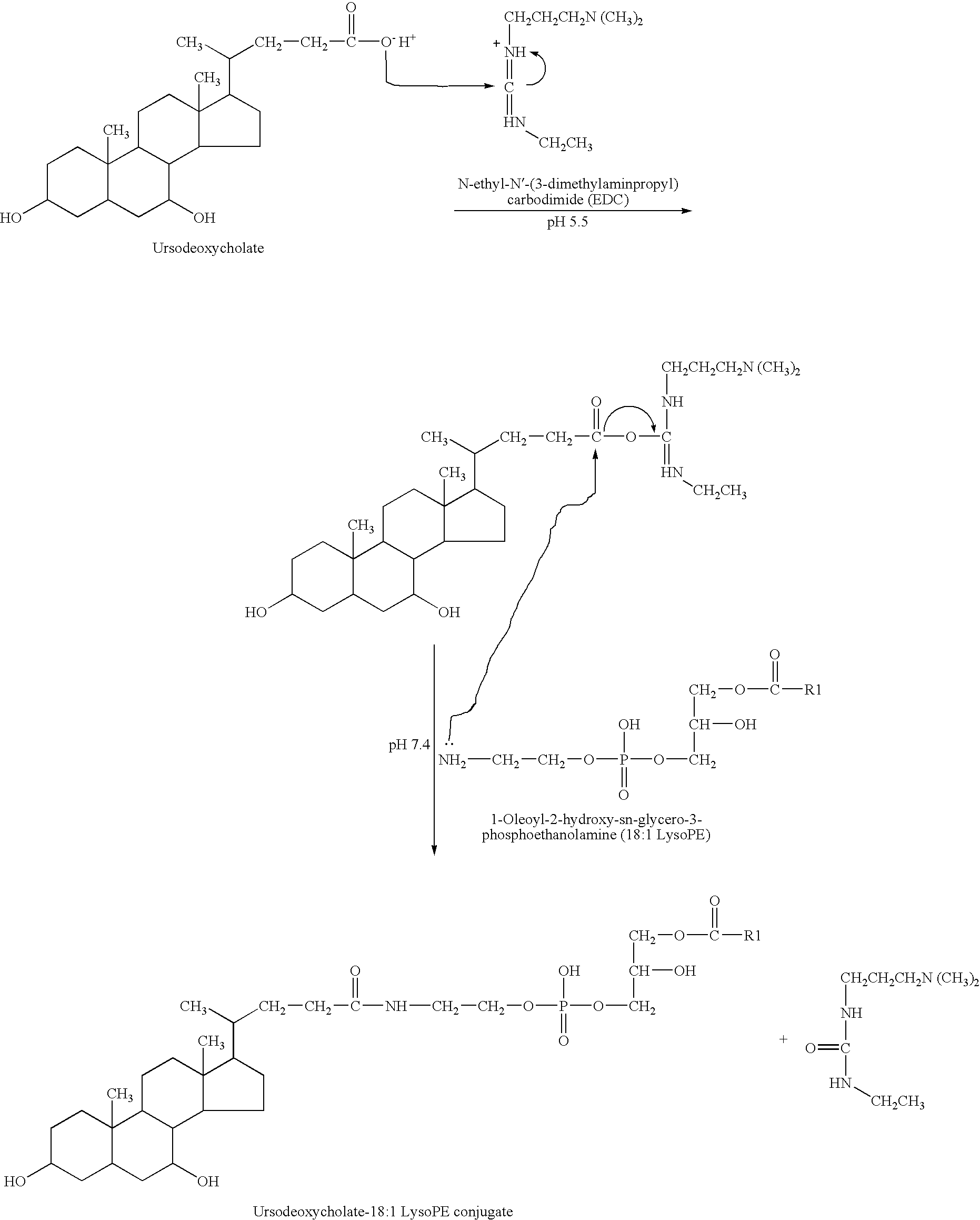
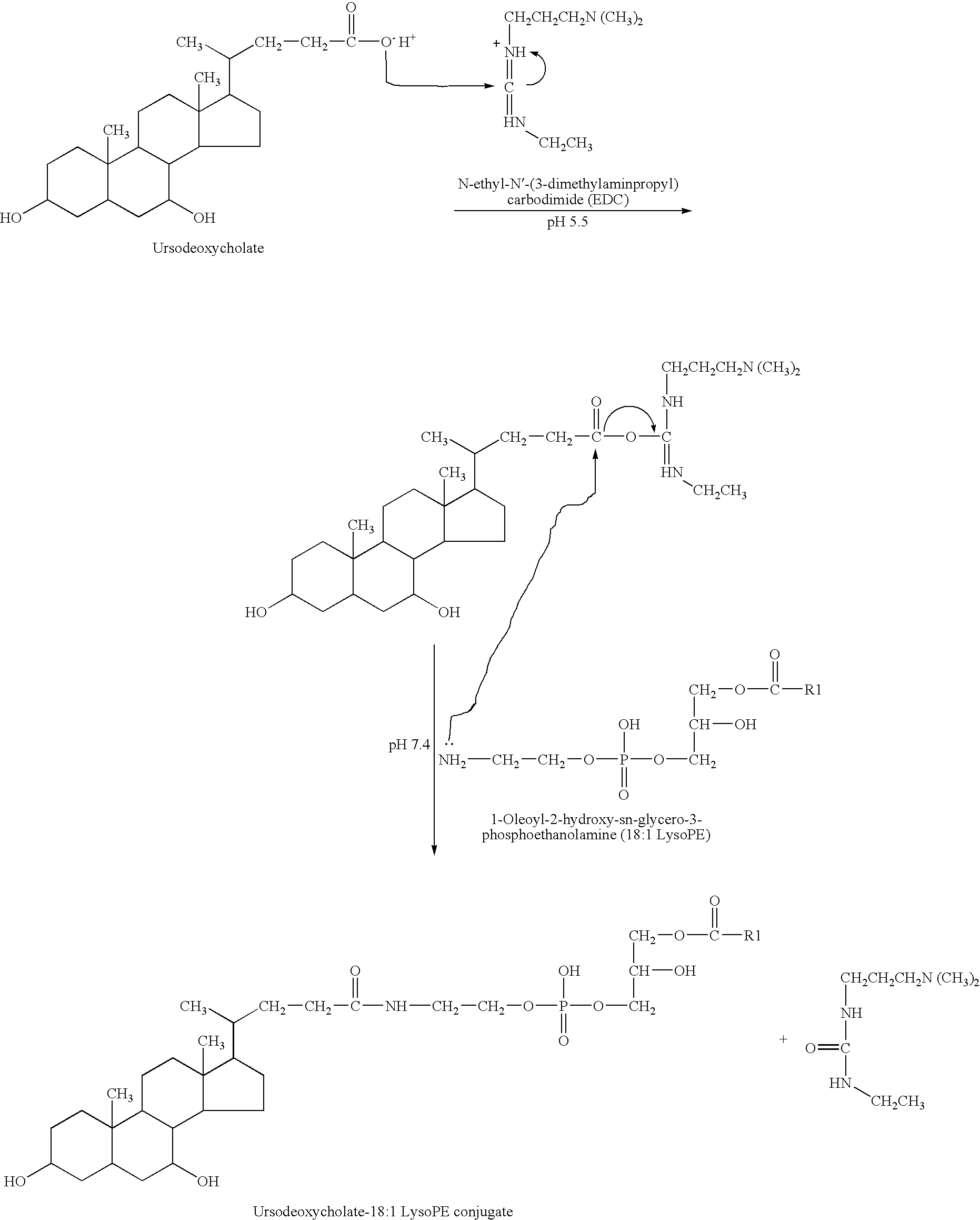
Use of therapeutically effective lipids and method for producing organ-/tissue-specific therapeutically effective lipids
InactiveUS20080166401A1Good clinical efficacyLittle and no side effectOrganic active ingredientsPeptide/protein ingredientsLipid formationCell specific
The use of therapeutically active lipids for organ / tissue-specific enrichment for the treatment of inflammatory, ischemic or degenerative disorders and / or for stimulating a regeneration is arranged and developed such that the lipids are bound on application to carrier molecules for which cell-specific uptake systems in the cells of the organs and / or tissue exist. In addition, a method of producing organ / tissue-specific therapeutically active lipids for treatment of inflammatory, ischemic or degenerative disorders and / or stimulation of a regeneration, in particular for treating inflammatory liver disorders, is claimed, which is arranged and developed such that lysophosphatidylethanolamine (LysoPE) is coupled to the carboxyl group of ursodeoxycholate (UrsoDOCA) converted to an ester to give a LysoPE-DOCA compound.
Owner:PAT
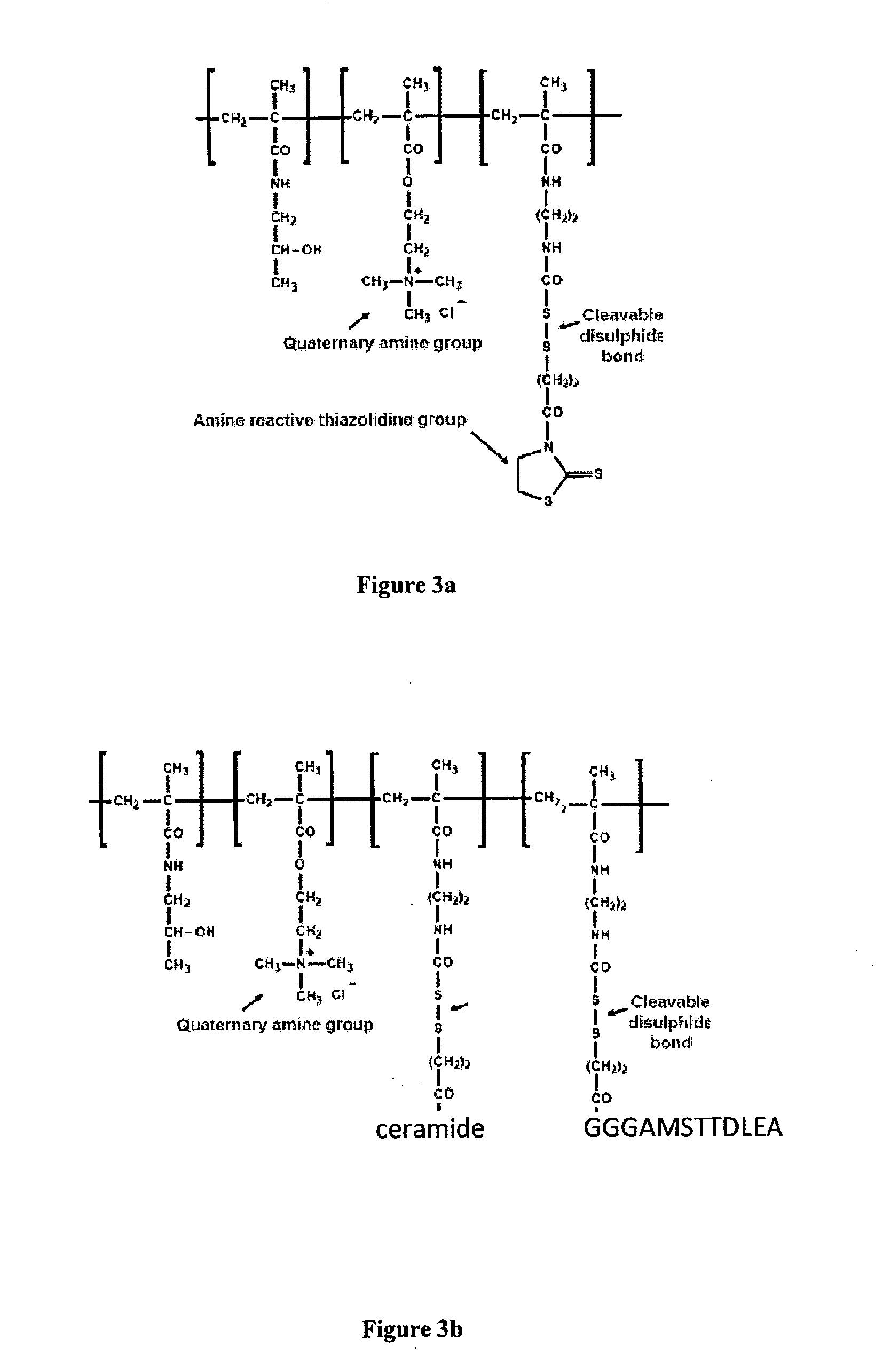
Multi-valent adjuvant display
InactiveUS20120141409A1Enhance immune stimulationImprove receptor aviditySsRNA viruses negative-senseCancer antigen ingredientsAdjuvantBackbone chain
The present invention provides an adjuvant-polymer construct comprising a polymer backbone which is covalently linked to 3 or more adjuvants, wherein the 3 or more adjuvants are each present in a pendant side chain, the adjuvants being connected to the polymer backbone either directly or via a spacer group.
Owner:PSIOXUS THERAPEUTICS LTD
Preparation method and application of graphene-CpG
InactiveCN103861118AGrowth inhibitionEnhance immune stimulationOrganic active ingredientsPharmaceutical non-active ingredientsPhosphatePolyethylene glycol
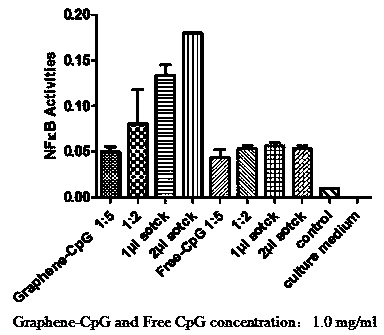
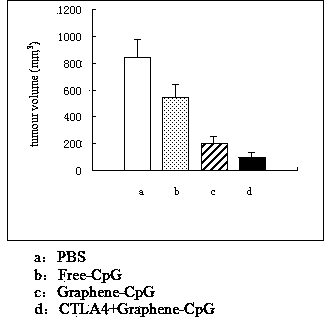
The invention relates to a preparation method and an application of a graphene-CpG. The preparation method is characterized by specifically comprising the steps of functionalizing the graphene, weighing 1mg of graphene and 5mg of PL-PEG5000-Amine (Biotin-Poly Ethylene Glycol) or PL-PEG2000-Amine and adding the two materials to a 20ml straight thick-wall glass tube, adding 5ml of distilled water to the 20ml straight thick-wall glass tube and putting the 20ml straight thick-wall glass tube in a shaker for shaking until DSPE-PEG (Distearoyl Phosphatidyl Ethanolamine-Poly Ethylene Glycol) is completely dissolved; centrifuging a graphene suspension at the room temperature for 3 hours at a rotating speed of 24000g and collecting the supernate, and recording the UV-VIS-NIR (Ultraviolet-Visual-Near Infrared) absorption spectrum of the obtained graphene solution, wherein the final concentration of the graphene is in a normal rang of 40-70mg / L; and preserving at 4 DEG C, and then bonding the graphene with an oligonucleotide CpG. The preparation method is unique and capable of obviously inhibiting the growth of the tumor of breast and the tumor of colorectum; compared with a PBS (Phosphate Buffer Solution) group, the graphene-CpG prepared by the preparation method disclosed by the invention has the advantages that the difference is significant and the growth of the tumors is obviously suppressed; besides, the preparation method has statistical significance.
Owner:JILIN UNIV
Nucleoside analogue as well as preparation method and application thereof
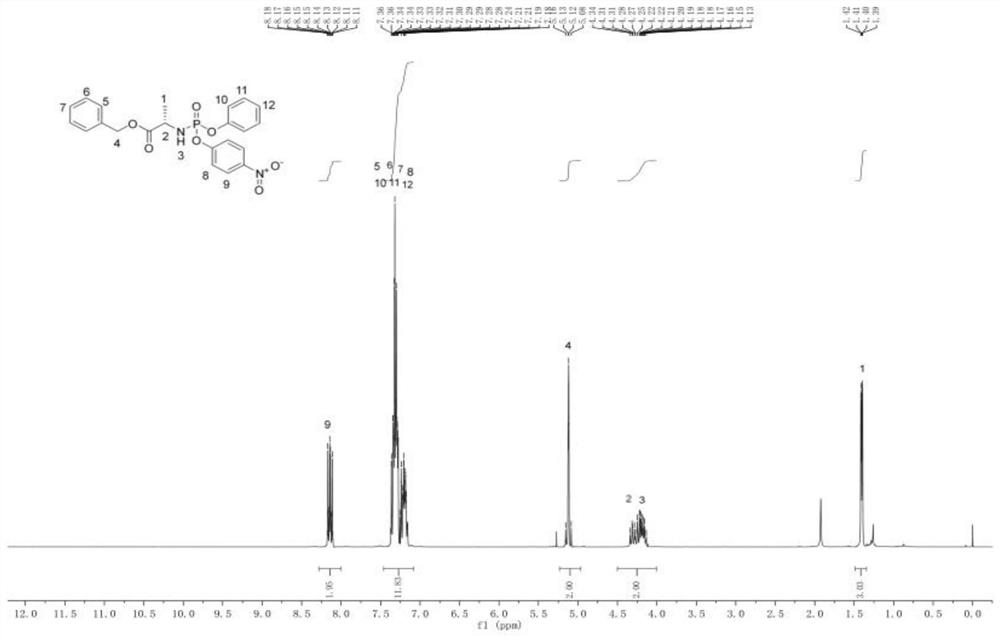
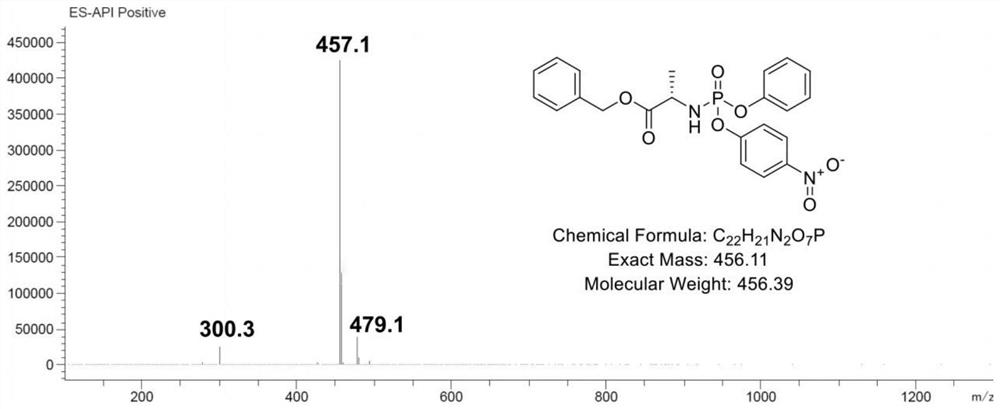
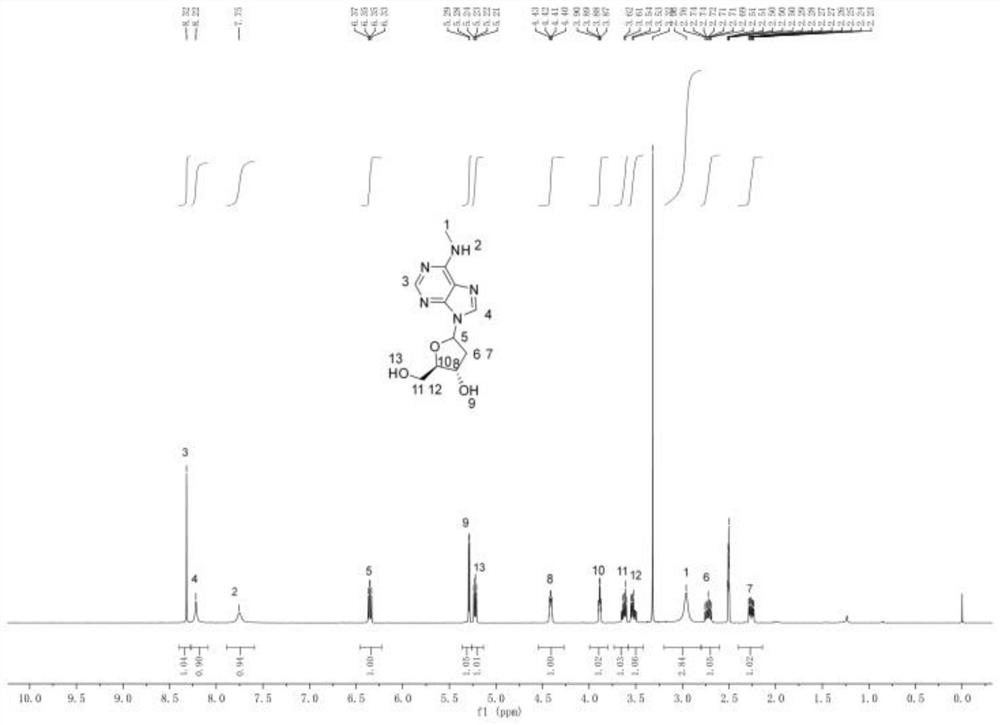
ActiveCN112778388AImprove bioavailabilityAnti-cancerSugar derivativesAntiviralsImmunocompetencePurine
The invention relates to a nucleoside analogue as well as a preparation method and application thereof, and belongs to the technical field of antiviral drugs. The nucleoside analogue disclosed by the invention is benzyl(((((2R,3S)-3-hydroxy-5-(6-(methylamino)-9H-purine-9-yl) tetrahydrofuran-2-yl) methoxy)(phenoxy) phosphoryl)-L-alaninate, and the structural formula of the nucleoside analogue is shown as a formula I in the specification. The nucleoside analogue disclosed by the invention is high in bioavailability, has anti-cancer and anti-virus effects, and can also improve the inherent immunity.
Owner:DALIAN MEDICAL UNIVERSITY
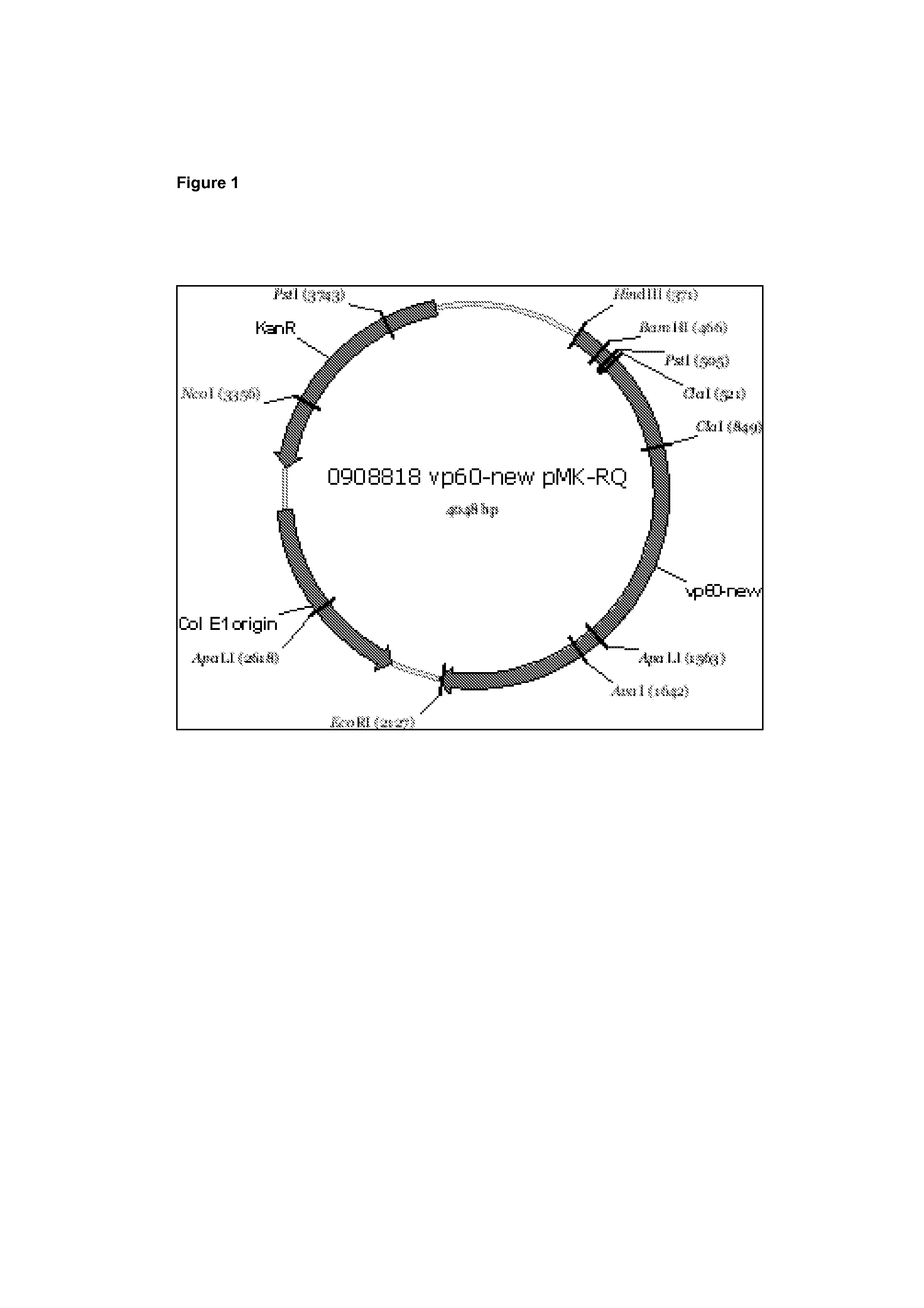
Parapoxvirus expressing the vp60 major capsid protein of the rabbit haemorrhagic disease virus
InactiveUS20130177582A1Speed up the descentUnable can be reproducedSsRNA viruses positive-senseSugar derivativesParapoxvirusProtein C
The invention describes a therapeutic agent capable of treating and / or preventing rabbit haemorrhagic disease virus infection in rabbits, namely a recombinant parapoxvirus, characterized in that the VP60 major capsid protein from the rabbit haemorrhagic disease virus (RHDV) is expressed from a foreign nucleic acid.
Owner:RIEMSER PHARMA
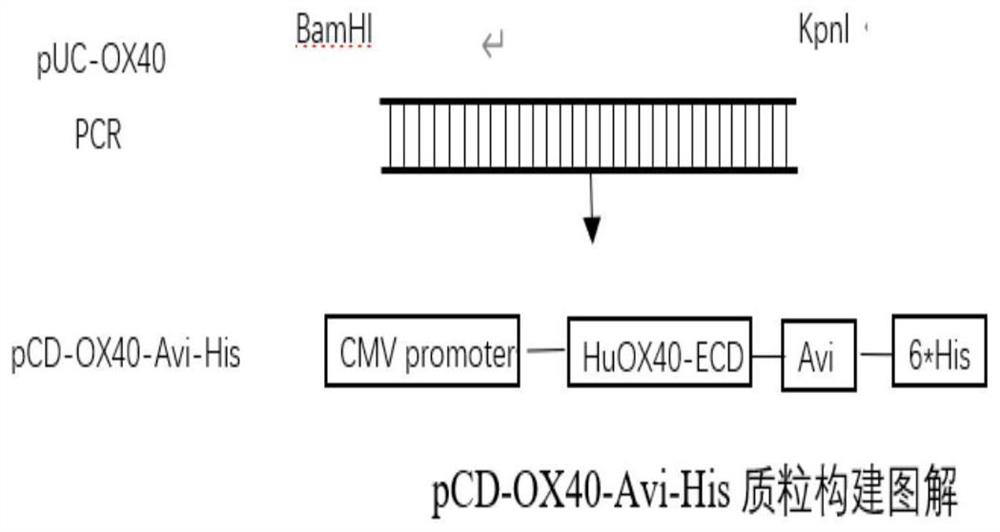
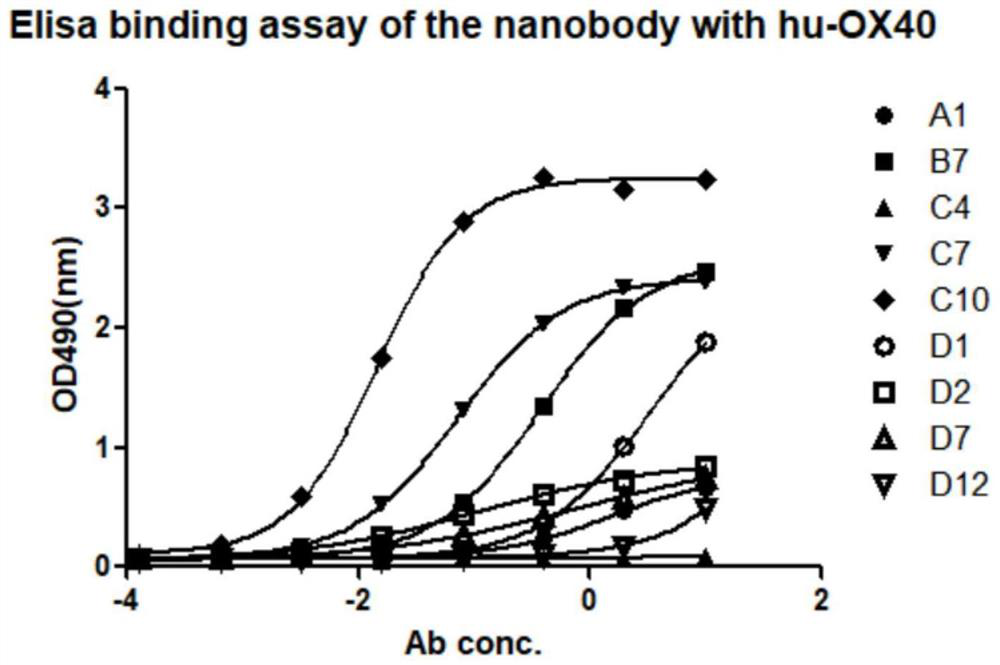
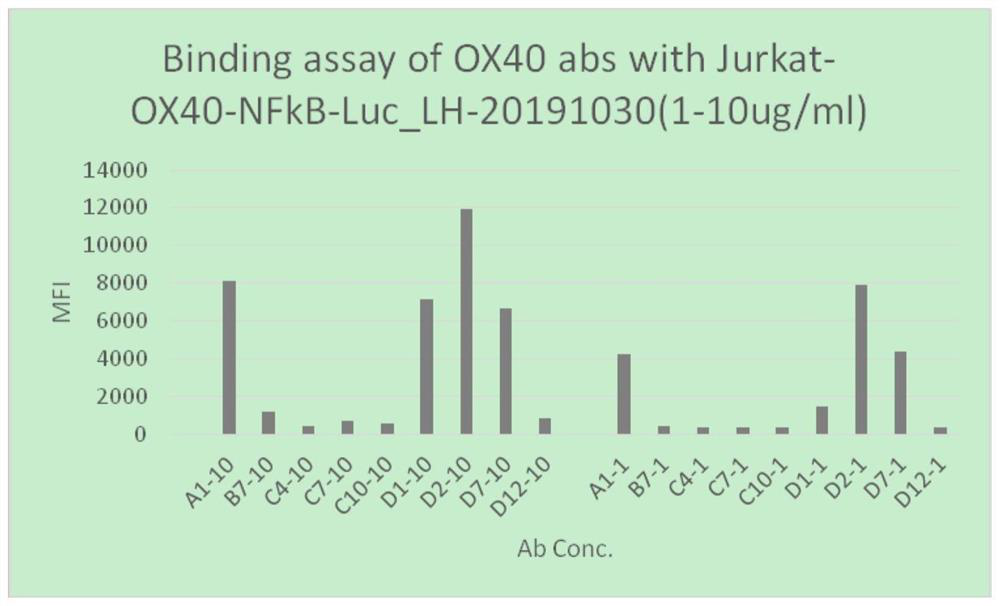
Anti-hu-OX40 antigen nano antibody and application thereof
PendingCN114656564AEnhance immune stimulationPromote secretionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenPhage antibodies
The invention belongs to the technical field of biology, and particularly relates to an anti-hu-OX40 antigen nano antibody which is a monoclonal nano antibody and comprises a heavy chain variable region; the heavy chain variable region comprises a CDR1 region, a CDR2 region and a CDR3 region, wherein the CDR1 region, the CDR2 region and the CDR3 region respectively comprise any one of amino acid sequences as shown in SEQ ID NO: 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 and 39 or a homologous sequence with at least 80% sequence identity with any one of the amino acid sequences as shown in SEQ ID NO: 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 and 39. According to the invention, 9 specific full-human anti-human OX40 nano antibodies A1, B7, C4, C7, C10, D1, D2, D7 and D12 are screened and optimized from a constructed large-capacity full-synthetic human phage antibody library, so that the immunostimulation of T effector cells can be enhanced, the secretion of cell factors can be promoted, and a remarkable tumor inhibition effect is shown in a mouse tumor model.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
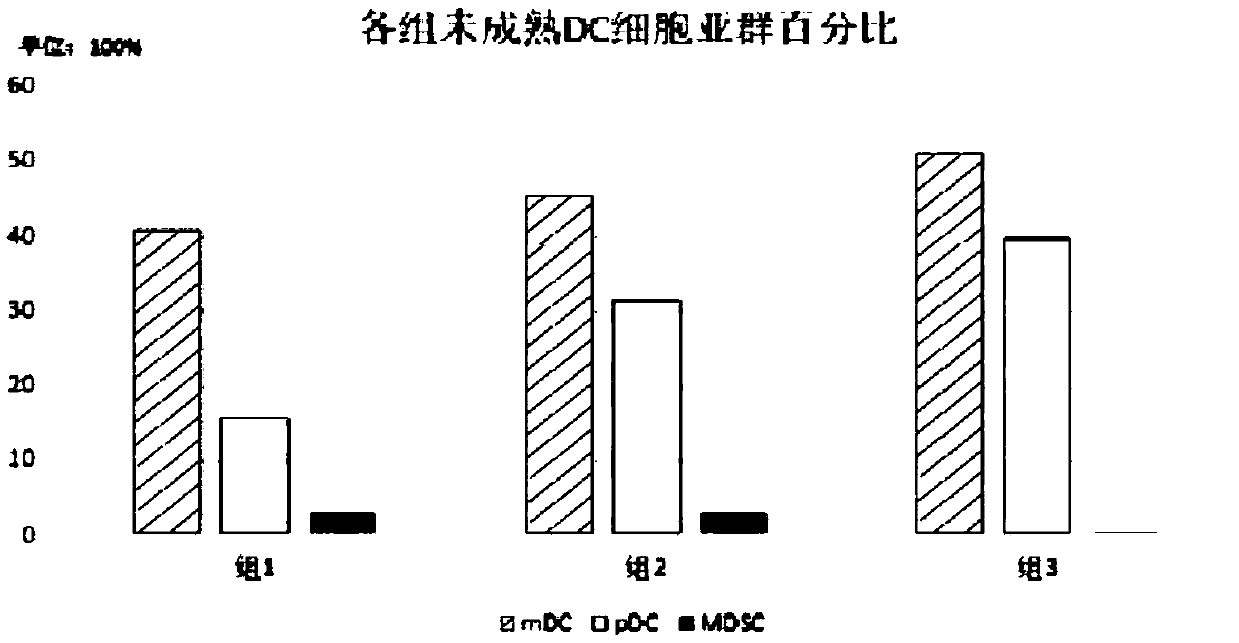
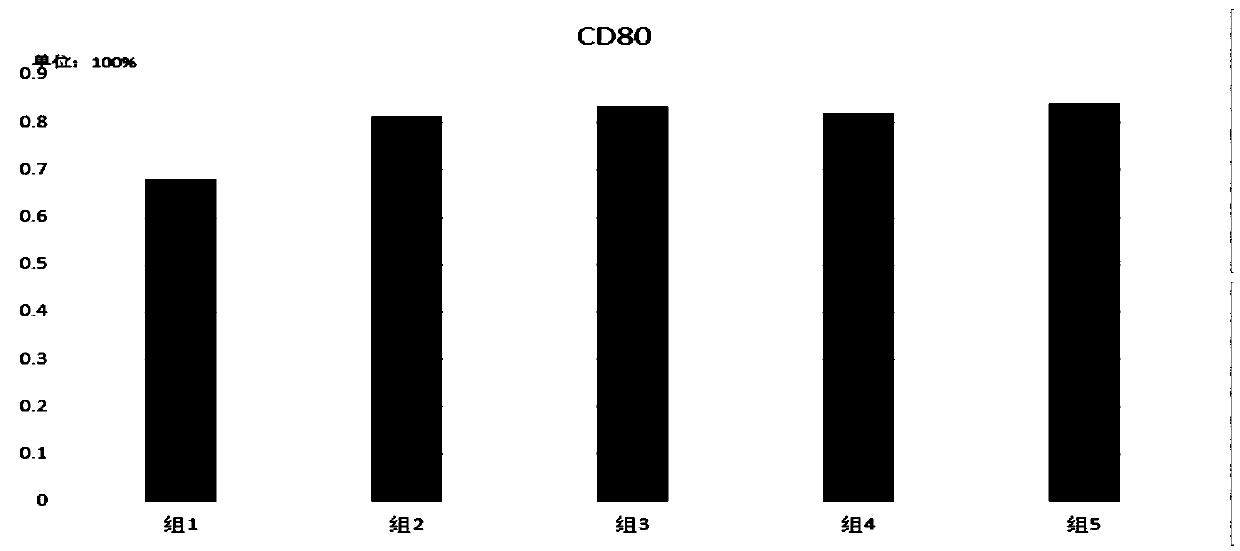
Kit of high-efficiency novel DC autovaccine, method for preparing high-efficiency novel DC autovaccine and application of DC autovaccine
ActiveCN105535952AEliminate negative regulationPromote maturityCancer antigen ingredientsBlood/immune system cellsDc vaccineAll-trans-Tretinoin
The invention discloses a kit, method and application of a high-efficiency novel DC autovaccine, and belongs to the technical field of biology. The kit comprises a cytokine combination of GM-CSF, IL-4, FLT3-L and ATRA. According to the method, an existing GM-CSF+IL-4 cultivation technique is improved, is combined with FLT3-L to promote generation of pDC, mononuclear cells are increased to convert DC, generation of MDSC is inhibited by utilizing all-transretinoic acid (ATRA) to eliminate the negative adjustment effect of MDSC in preparation of DC vaccines, and an OK432 and CPH ODN co-stimulation mode is adopted to effectively promote mature of mDC and pDC and enhance the DC immunostimulation function.
Owner:普济生物科技发展(山东)有限责任公司
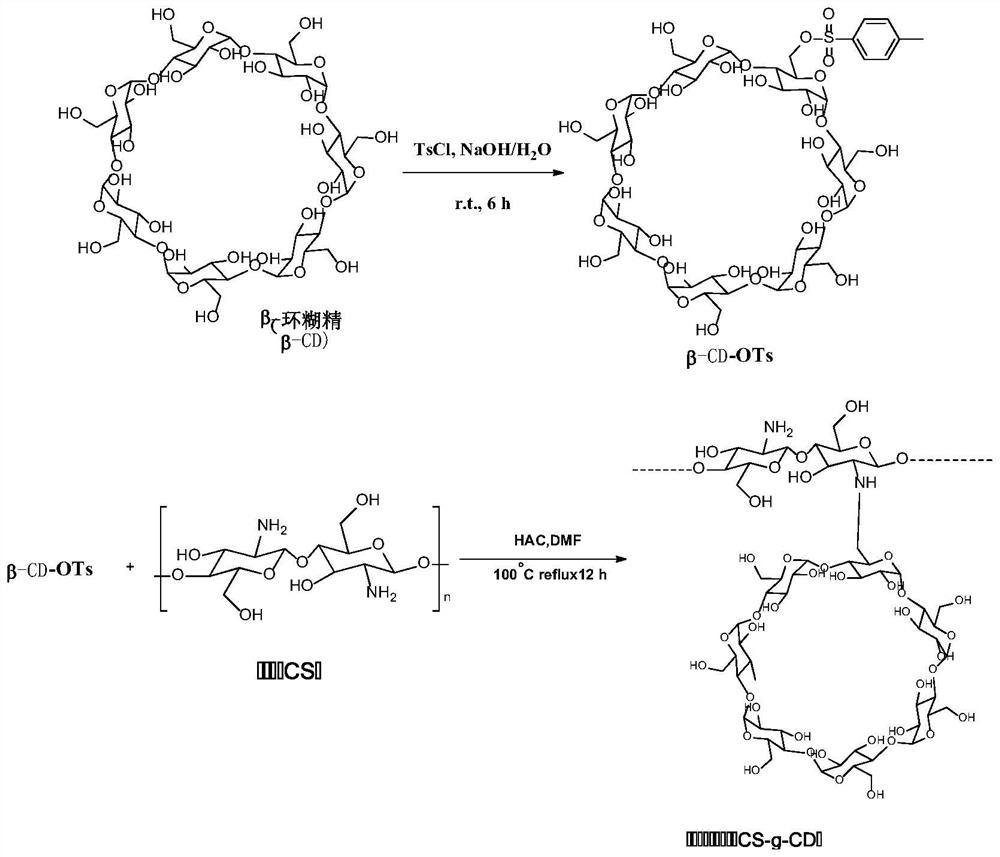
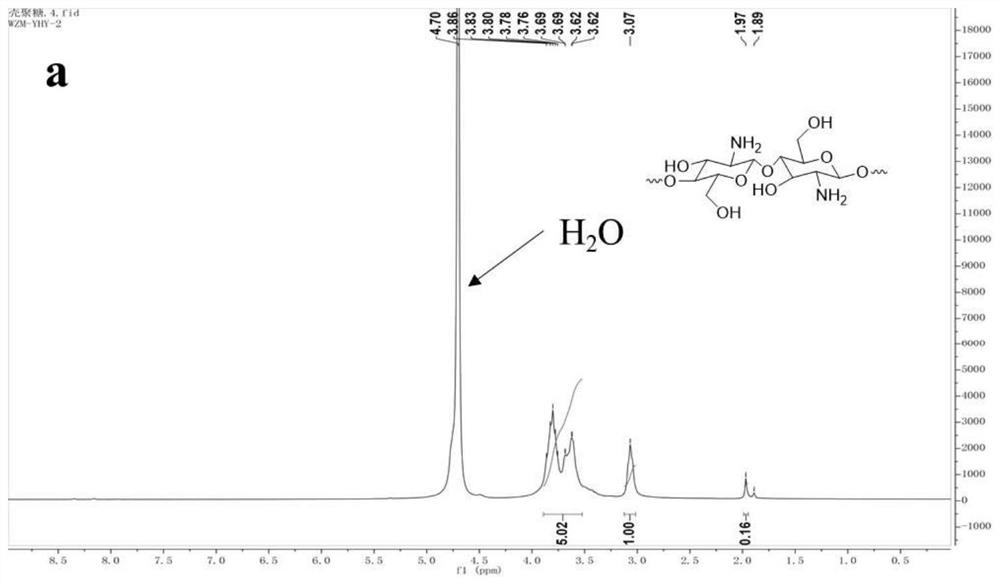
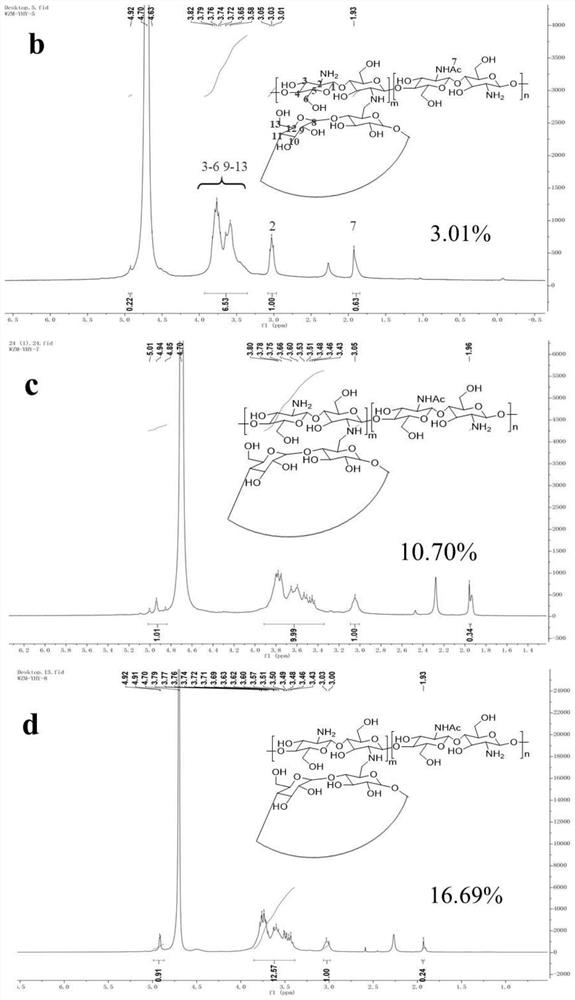
Vaccine based on cyclodextrin grafted chitosan, preparation method and application
PendingCN113995834AImprove solubilityImprove stabilityCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsBinding siteCyclodextrin
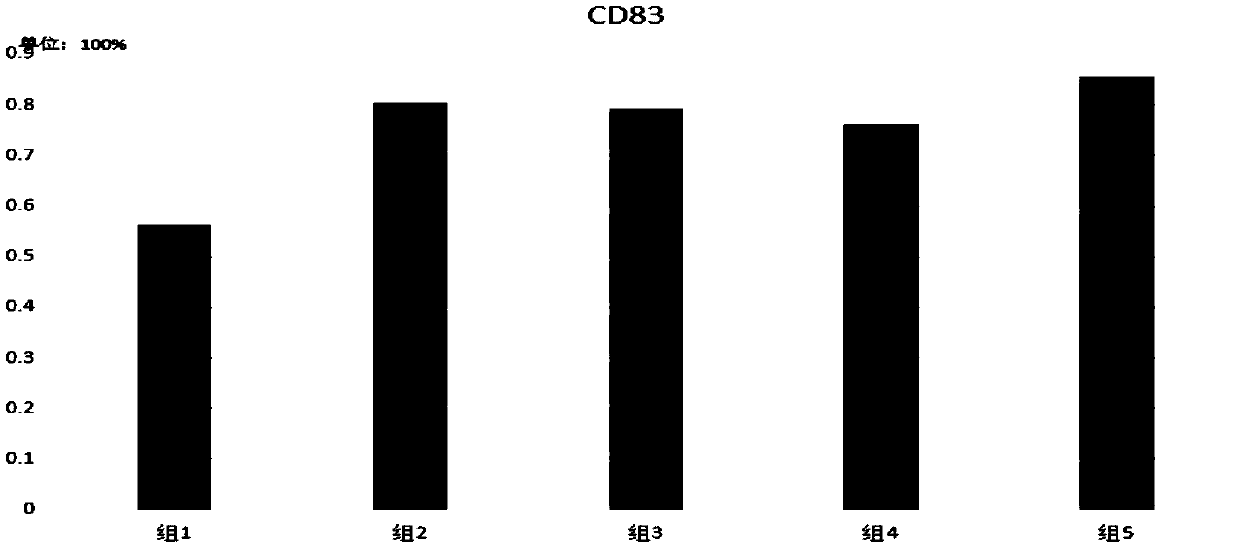
The invention discloses a vaccine based on cyclodextrin grafted chitosan (CS-g-CD), a preparation method and application, and belongs to the field of biomedical engineering. Antigen molecules, immunologic stimulants and the like are combined with CS-g-CD in a subject-object self-assembly mode to form supramolecules, and the supramolecules can be further prepared into nano-particles through methods such as sodium tripolyphosphate to serve as vaccine drugs. The cyclodextrin grafted chitosan provided by the invention has better solubility, and the cyclodextrin modified on the cyclodextrin grafted chitosan provides host-guest binding sites, so that the cyclodextrin grafted chitosan can be more easily used for interacting with antigen molecules to form a high-stability self-assembled supramolecular vaccine preparation, and is beneficial to better in-vivo delivery and antigen storage, thereby being beneficial to improving the immune stimulation ability. The vaccine preparation prepared in a subject-object self-assembly mode can be used as a platform for vaccine development, and is suitable for wider vaccine development.
Owner:JIANGNAN UNIV
Probiotic solid powder and preparation method thereof
ActiveCN102008016BHigh activityQuality improvementAnimal feeding stuffAccessory food factorsLivestock breedingGram
The invention discloses probiotic solid powder and a preparation method thereof. The probiotic solid powder comprises a mixture of active probiotics and active zeolite, wherein the active probiotic content is between 12 and 50 billion colony-forming units (CFU) / gram; and the active zeolite mixture comprises the following components in percentage by weight: 30 to 50 percent of zeolite, 20 to 40 percent of attapulgite clay and 10 to 30 percent of bentonite. The probiotic solid powder provides a high-quality and low-cost antibiotic substitute product for livestock breeding; and the probiotic solid powder with 1 to 4 million CFU of live bacteria is added into each gram of feed, so the growth speed of chickens, pigs and fish can be improved by 2.0 to 15.6 percent, the conversion rate of the feed can be improved by 1.1 to 9.3 percent, and the diarrhea rate and the death rate of animals can be reduced obviously. The preparation method of the probiotic solid powder is simple, has low cost andcan reduce environmental pollution; and the content of the obtained product bacteria is high.
Owner:NANJING AGRICULTURAL UNIVERSITY
Amphiphilic oligodeoxynucleotide conjugates as adjuvant enhancers
ActiveUS20200268879A1Increased activationEnhances cytokine productionActivity regulationPharmaceutical non-active ingredientsTLR8Antigen
Amphiphilic oligonucleotide conjugates that enhance adjuvant function are disclosed. The conjugates typically include: a lipophilic component, and conjugated thereto (directly or indirectly) an immunomodulating oligonucleotide that, if it were not conjugated to the lipophilic component, would suppress TLR7 and / or TLR8 stimulation. In the presence of albumin, these conjugates significantly enhance adjuvant function, in particular the function of TLR7 / 8-mediated adjuvants such as an imidazoquinolinamine. The conjugates can be administered, along with an adjuvant compound, to a subject in order to cause and / or enhance an immune response (for instance, to an infectious agent or a cancer antigen) in the subject.
Owner:WAYNE STATE UNIV
Modulation of oligonucleotide cpg-mediated immune stimulation by positional modification of nucleosides
InactiveUS20110059067A1Regulate immune responseDecreasing immunostimulatory effectOrganic active ingredientsPeptide/protein ingredientsImmune StimulationPyrimidine Nucleotides
The invention provides methods for modulating the immune response caused by CpG dinucleotide-containing compounds. The methods according to the invention enables both decreasing the immunostimulatory effect for antisense applications, as well as increasing the immunostimulatory effect for immunotherapy applications.
Owner:IDERA PHARMA INC
Use of therapeutically effective lipids and method for producing organ-/tissue-specific therapeutically effective lipids
InactiveUS8883426B2Anti-inflammatory effect can developAvoid developmentOrganic active ingredientsPeptide/protein ingredientsLipid formationCell specific
The use of therapeutically active lipids for organ / tissue-specific enrichment for the treatment of inflammatory, ischemic or degenerative disorders and / or for stimulating a regeneration is arranged and developed such that the lipids are bound on application to carrier molecules for which cell-specific uptake systems in the cells of the organs and / or tissue exist. In addition, a method of producing organ / tissue-specific therapeutically active lipids for treatment of inflammatory, ischemic or degenerative disorders and / or stimulation of a regeneration, in particular for treating inflammatory liver disorders, is claimed, which is arranged and developed such that lysophosphatidylethanolamine (LysoPE) is coupled to the carboxyl group of ursodeoxycholate (UrsoDOCA) converted to an ester to give a LysoPE-DOCA compound.
Owner:PAT
Multi-valent adjuvant display
InactiveUS20170112923A1Encourages receptor cross-linking and signallingReduced dosSsRNA viruses negative-senseCancer antigen ingredientsPolymer scienceAdjuvant
Owner:PSIOXUS THERAPEUTICS LTD
Amphiphilic oligodeoxynucleotide conjugates as adjuvant enhancers
ActiveUS11504425B2Enhance immune stimulationAmplify magnitudeSugar derivativesActivity regulationTLR8Antigen
Owner:WAYNE STATE UNIV
A kind of vaccine adjuvant, its preparation method and application
ActiveCN104147599BImprove the effectiveness of anti-virus protectionEnhance immune stimulationAntiviralsEmulsion deliveryOil phaseHigh pressure
The invention discloses an oil-in-water type vaccine adjuvant as well as a preparation method and application thereof. The oil-in-water type nano-emulsion vaccine adjuvant comprises the following components in percentage by mass: 0.1-10 percent of oil phase, 0.1-10 percent of emulsifier, 0.1-3 percent of stabilizer, 0.1-3 percent of complexing agent and 0.01-10 percent of immunopotentiator. The preparation method comprises the following steps: (1) uniformly dispersing the immunopotentiator, the stabilizer and the complexing agent in water, thereby obtaining an aqueous phase; (2) mixing an oil phase and an emulsifier, thereby obtaining an oil phase; (3) slowly adding the oil phase into the aqueous phase, and continuously stirring, thereby forming a stable emulsion; (4) regulating the pH value of the emulsion, and fixing the volume to obtain a primary emulsion; and (5) performing high-speed shearing and high-pressure homogenizing on the primary emulsion. The vaccine adjuvant provided by the invention is simple in preparation, convenient to use and small in side reactions, is used for diluting vaccines, particularly swine fever live vaccines and is high in stability and good in immune effect.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Vaccine adjuvant of swine mycoplasmal pneumonia live vaccine, and preparation method and application thereof
ActiveCN101954079BEnhance immune responseImmune activationAntibacterial agentsBacterial antigen ingredientsPhosphateLevamisole
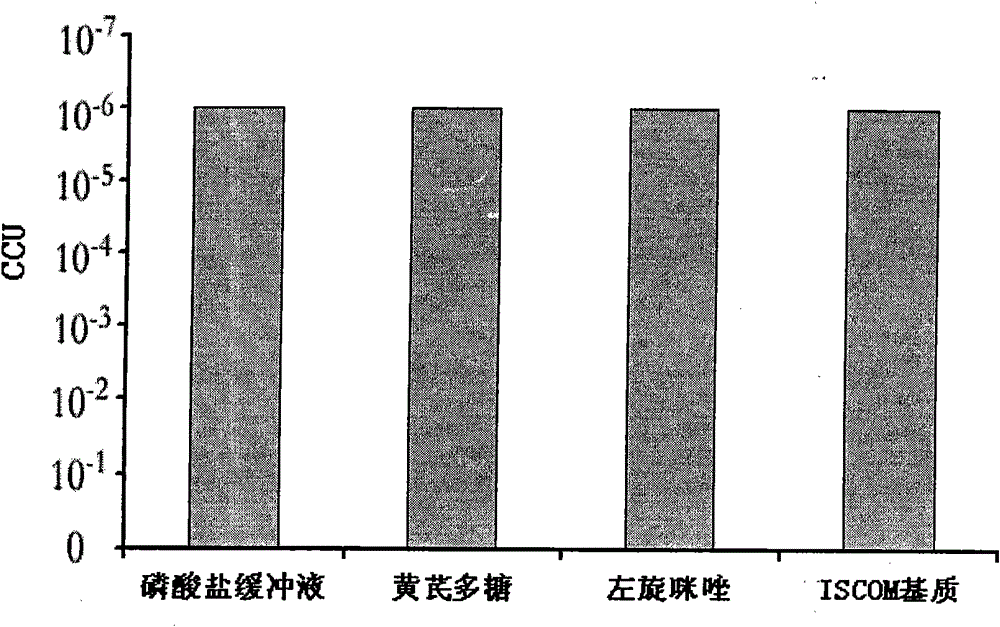
The invention discloses a vaccine adjuvant of swine mycoplasmal pneumonia live vaccine, which is mainly prepared from an ISCOM substrate, levamisole, traditional Chinese herbal polysaccharide and phosphate buffer. Each ml of adjuvant contains 0.1 to 2mg of Quil A in the ISCOM substrate, 2 to 10mg of levamisole, 20 to 100mg of traditional Chinese herbal polysaccharide and the balance of phosphate buffer. The combination of the ISCOM substrate, the levamisole and the traditional Chinese herbal polysaccharide can well make the best of respective advantages and complement each other, so that the adjuvant can strengthen the immune stimulating ability of the swine mycoplasmal pneumonia live vaccine and strengthen the immune effect of the vaccine, and has the advantages of simple and convenient preparation method, stable performance, convenient mass production and the like. When the vaccine adjuvant is applied, intramuscular injection immunization can be performed by blending a proper proportion of swine mycoplasmal pneumonia live vaccine and the adjuvant of the invention; the intramuscular injection immunization of the swine mycoplasmal pneumonia live vaccine is realized; and the defects of the traditional intrapulmonary injection immunization are overcome.
Owner:JIANGSU ACAD OF AGRI SCI
Bactericidal/permeability increasing protein for use in a method of immunization, preferably as an adjuvant in a method of vaccination
PendingUS20210299252A1Enhance immune stimulationHigh activityBacterial antigen ingredientsImmunological disordersBactericidal/permeability-increasing proteinAdjuvant
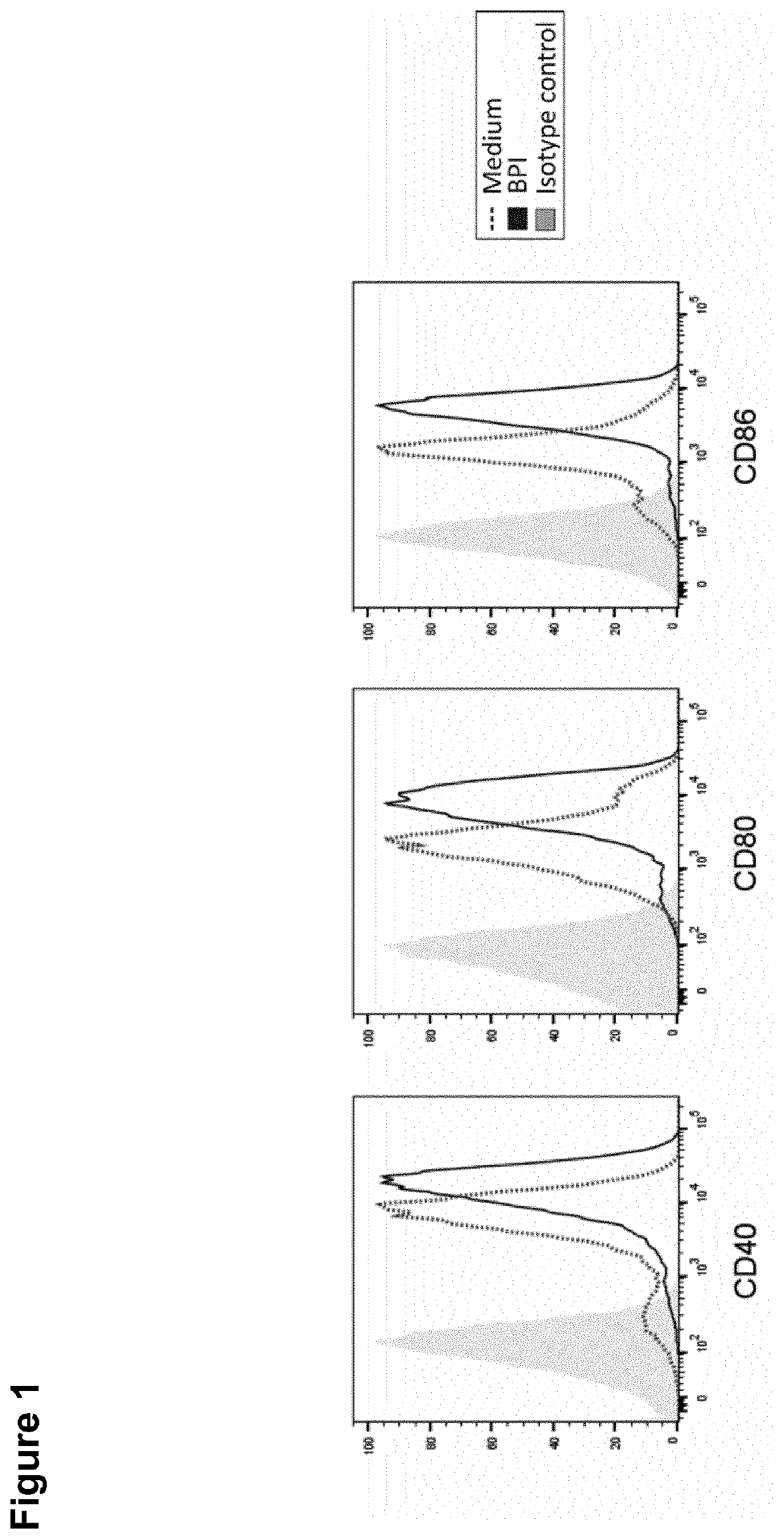
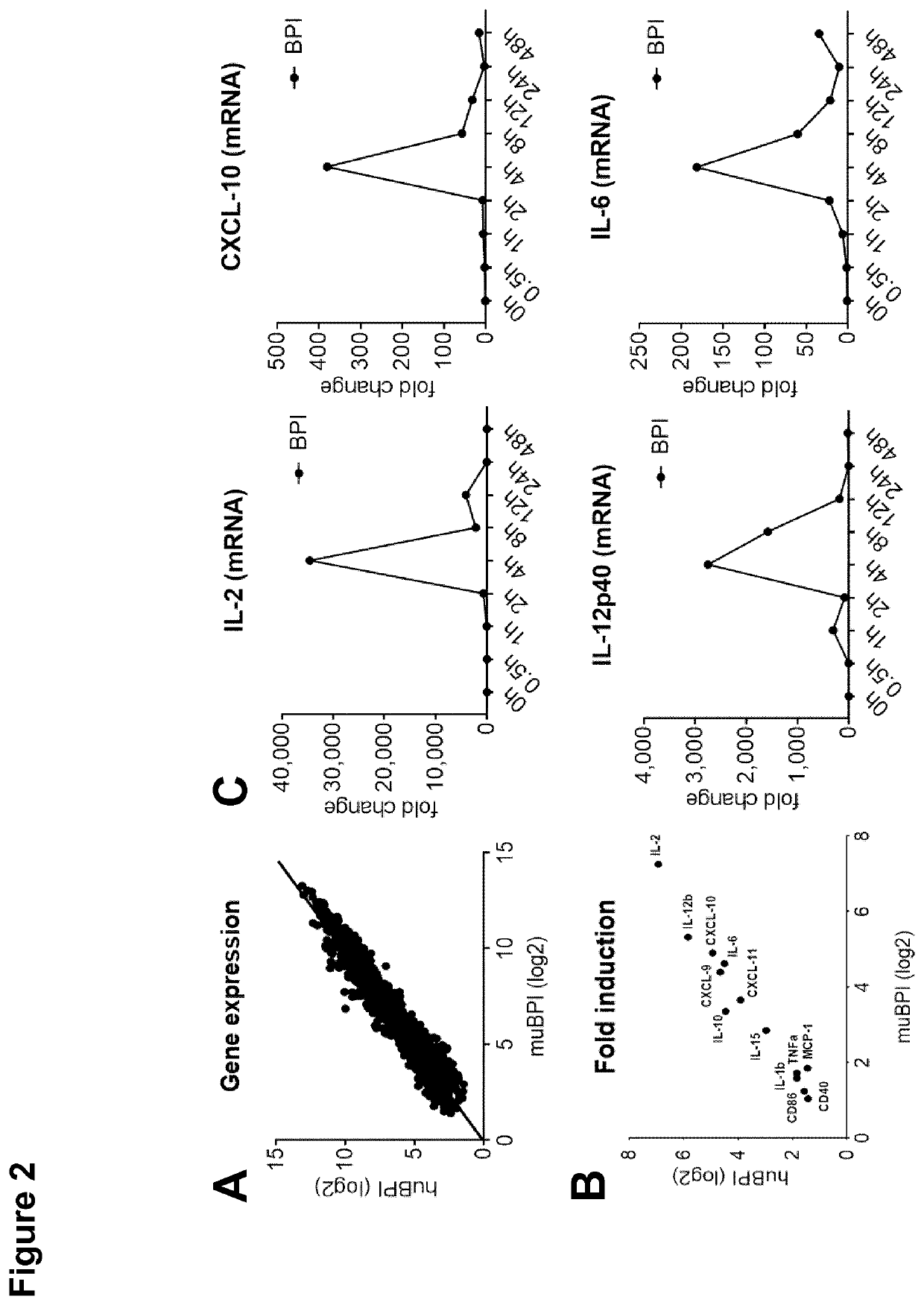
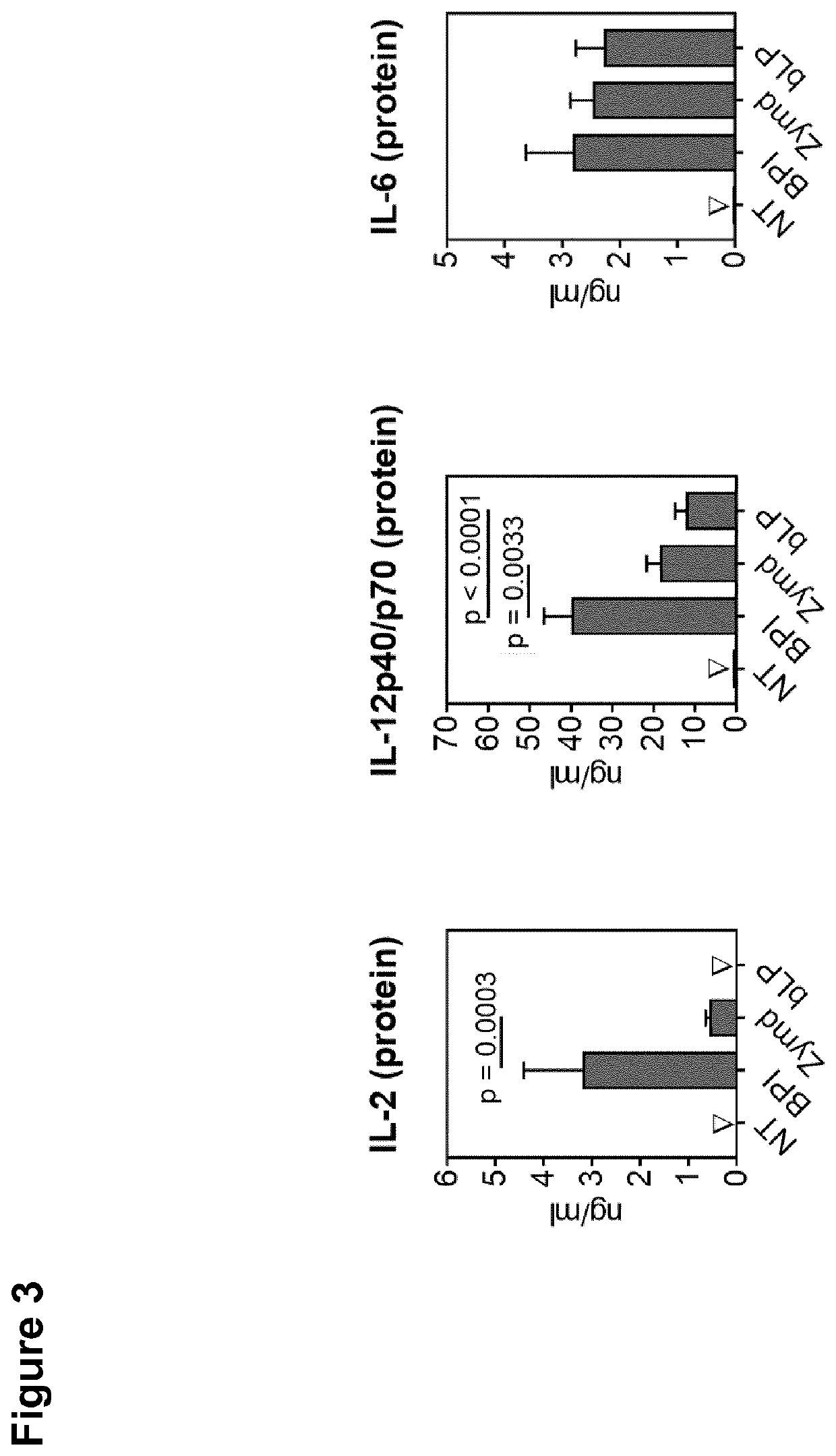
The present invention relates to bactericidal / permeability increasing protein (BPI) for use in a method of immunization of a patient, preferably as an adjuvant in a method of vaccination. The present invention also relates to a preparation comprising BPI for use in a method of immunization of a patient, and optionally an immunomodulatory agent. The present invention further relates to a process of producing a preparation including BPI for use in a method of immunization of a patient.
Owner:UNIV REGENSBURG
Compound microecological preparation for breeding stichopus japonicus and inhibiting pathogenic vibrio
ActiveCN114223777APromote growthPromote the conversion of butyric acidBacteriaMicroorganism based processesBiotechnologyUltrasound - action
The invention discloses a compound microecological preparation for inhibiting pathogenic vibrio for breeding stichopus japonicus, which is prepared by mixing rhodopseudomonas palustris, clostridium butyricum, oak leaf extract and aloe leaf extract into a feed additive, and the addition amount of the rhodopseudomonas palustris, the clostridium butyricum, the oak leaf extract and the aloe leaf extract in each gram of feed is 105-107 cfu, 105-107 cfu, 1-2 mg and 4-8 mg; the preparation method of the oak leaf extract and the aloe leaf extract comprises the following steps: respectively cleaning oak leaves and aloe leaves with distilled water, and ventilating and drying in the shade at 25 DEG C; the preparation method comprises the following steps: respectively homogenizing and crushing oak leaves and aloe leaves by using a homogenizer, and adding an ethanol solution with the volume fraction of 65-85% according to the material-liquid ratio of 0.05-0.1 g / mL; extracting for 1-2.5 hours at normal temperature under the action of ultrasonic waves with the working frequency of 40 KHZ and the power of 80 W; and filling the extract into an ultrafiltration membrane device, filtering to remove impurities under the pressure condition of 0.06 Mpa, and carrying out vacuum concentration and freeze drying to obtain freeze-dried powder.
Owner:LIAONING OCEAN & FISHERIES SCI RES INST
Kit, method and application for preparing highly efficient novel autologous dc vaccine
ActiveCN105535952BPromote conversionEnhance immune stimulationCancer antigen ingredientsBlood/immune system cellsDc vaccineAll-trans-Tretinoin
The invention discloses a kit, method and application of a high-efficiency novel DC autovaccine, and belongs to the technical field of biology. The kit comprises a cytokine combination of GM-CSF, IL-4, FLT3-L and ATRA. According to the method, an existing GM-CSF+IL-4 cultivation technique is improved, is combined with FLT3-L to promote generation of pDC, mononuclear cells are increased to convert DC, generation of MDSC is inhibited by utilizing all-transretinoic acid (ATRA) to eliminate the negative adjustment effect of MDSC in preparation of DC vaccines, and an OK432 and CPH ODN co-stimulation mode is adopted to effectively promote mature of mDC and pDC and enhance the DC immunostimulation function.
Owner:普济生物科技发展(山东)有限责任公司
Oil-in-water adjuvant used for animal vaccines and preparation method for oil-in-water adjuvant
PendingCN111110840AHigh purityReduce dosageEmulsion deliveryAntibody medical ingredientsBiotechnologyPolyoxyethylene castor oil
The invention provides an oil-in-water adjuvant used for animal vaccines and a preparation method for the oil-in-water adjuvant, and belongs to the technical field of biological product-like animal vaccines. The oil-in-water adjuvant used for the animal vaccines comprises the following raw materials by weight percent: 80.7%-89.5% of mineral oil, 2.1%-2.5% of span 80, 3.0%-5.3% of tween 85, 1.5%-3.5% of lauroyl macrogolglycerides, 0.5%-4.7% of polyglyceryl-6 caprylate and 2.2%-3.6% of polyoxyethylated castor oil. The oil-in-water adjuvant is simple and convenient to prepare, easy to obtain andlow in cost; prepared vaccines are low in viscosity, easy and safe to inject, stable in quality and small in grain size, can reach a nanoscale, and can induce temporary and permanent immunity; and theoil-in-water adjuvant is a safe adjuvant which can be used for poultry and livestock vaccines.
Owner:四川诺顺科技有限公司
Tumor vaccine based on injectable hydrogel as well as preparation method and application of tumor vaccine
PendingCN113663062ARetain biological activityLong-term sustained releaseAerosol deliveryOintment deliveryPolyethylene glycolTumor antigen
The invention provides a tumor vaccine based on injectable hydrogel as well as a preparation method and application of the tumor vaccine. The tumor vaccine is prepared from the following raw materials: a tumor antigen, an immunologic adjuvant, multi-arm polyethylene glycol and multi-aldehyde polysaccharide. The tumor vaccine can be constructed in a mild aqueous solution, can efficiently load tumor-associated antigens, adjuvants and the like, can protect the activity of the antigens, the adjuvants and the like, can maintain the stability of the antigens, the adjuvants and the like, realizes long-acting local slow release of the antigens and the adjuvants, and remarkably improves the immunostimulation effect.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com